Abstract
Ionizing radiation could supplement tissue bank screening to further reduce the probability of diseases transmitted by allografts if denaturation effects can be minimized. It is important, however, such sterilization procedures be nondetrimental to tissues. We compared crosslinking and free radical scavenging potential methods to accomplish this task in tendon tissue. In addition, two forms of ionizing irradiation, gamma and electron beam (e-beam), were also compared. Crosslinkers included 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and glucose, which were used to add exogenous crosslinks to collagen. Free radical scavengers included mannitol, ascorbate, and riboflavin. Radioprotective effects were assessed through tensile testing and collagenase resistance testing after irradiation at 25 kGy and 50 kGy. Gamma and e-beam irradiation produced similar degenerative effects. Crosslinkers had the highest strength at 50 kGy, EDC treated tendons had 54% and 49% higher strength than untreated, for gamma and e-beam irradiation respectively. Free radical scavengers showed protective effects up to 25 kGy, especially for ascorbate and riboflavin. Crosslinked samples had higher resistance to collagenase and over a wider dose range than scavenger-treated. Of the options studied, the data suggest EDC precrosslinking or glucose treatment provides the best maintenance of native tendon properties after exposure to ionizing irradiation.
Introduction
Allografts have gained increasing popularity as treatment methods for musculoskeletal injuries. This is reflected by the 1.5 million bone and soft tissue allografts implanted yearly as of 2007 [23]. A major concern associated with allografts is the potential for disease transmission. Current tissue bank safety measures include investigations into donor history, detection assays, and aseptic handling [9]. Although these procedures minimize the probability of disease transmission, they are not infallible. There is a period in which an infection just preceding death could pass through these protocols undetected [9, 10]. The slight danger of disease transmission can be further decreased through terminal sterilization.
Gamma and electron beam (e-beam) irradiation are both forms of ionizing irradiation that are effective sterilization methods [2, 20]. In 1990, gamma irradiation at 25 kGy was recommended as the standard sterilization dose for tissue banks [16]. Conversely, there has been considerably less literature exploring the use of e-beam irradiation for tissue sterilization. Tissue banks that perform sterilization commonly do so at 10 kGy to 35 kGy [24], which is efficient against bacteria [24], but higher doses are required to neutralize HIV [22, 24] and more resistive pathogens such as mold spores [13].
This method of pathogen inactivation involves rupture of covalent bonds and, more prominently, modification by free radicals, which are both caused by high energy gamma rays or electrons [2]. Ionizing radiation has been successful for sterilization of medical products such as syringes, sutures, and instruments [2, 20]. Unfortunately, collagen, the major structural protein of musculoskeletal tissues, is susceptible to irradiation-induced free radical modification [2]. Gamma irradiation reduces crosslink density [21], as well as causes fragmentation of collagen [5, 15]. On a tissue level, high-dose sterilization would result in weakened mechanical properties, increased degradation by proteolytic enzymes, and ultimately premature failure postimplantation [5, 14, 21]. If methods to protect tissues from these detrimental effects can be developed, then radiation sterilization could be utilized for allografts.
This study considers gamma and e-beam irradiation conditions as well as crosslinking and free radical scavenging methods aimed at offsetting radiation effects in collagenous tissues. Crosslinkers selected to form exogenous crosslinks in collagen included glucose and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC). The main distinction between these crosslinkers is that EDC is a precrosslinking procedure and glucose crosslinking occurs at the time of irradiation. Free radical scavengers selected to sequester free radicals and limit chemical modifications to collagen included mannitol (MA), ascorbate (AS), and riboflavin (RB). We hypothesized gamma and e-beam irradiation would cause similar deleterious effects on mechanical properties and resistance to enzyme degradation of tendons. Additionally, we hypothesized these effects could be decreased by treating tendons with crosslinking or free radical scavenging.
Materials and Methods
We compared radiation dose response on mechanical properties and enzyme resistance of tendons exposed to either gamma or e-beam irradiation. This also provided a basis for our primary objective of evaluating crosslinking and free radical scavenging methods intended to minimize the denaturing effects of ionizing radiation.
Achilles tendons from mature (5–8 months) male and female New Zealand white rabbits were used as the model for tendon allografts. Frozen rabbit hind limbs were obtained from Pel-Freeze Biologicals (Rogers, AR). Tendons were dissected from hind limbs, wrapped in phosphate buffer solution (PBS)-soaked gauze, and placed in 15 mL polystyrene tubes. These tubes were stored in a −20°C freezer. Tendons were arbitrarily distributed into two parent groups. The first group was designated for gathering baseline data. More specifically, these were untreated tendons subjected to various doses of gamma or e-beam irradiation. The second group consisted of tendons that were treated by crosslinking or soaked in free radical scavenger solution prior to irradiation. There were a total of six groups: five treatment conditions and an untreated condition. There were also five radiation dose conditions. Ten tendons were used per group per dose condition, eight were used for mechanical testing and two for enzyme resistance testing for a total of 300 tendons. Power analysis yielded a value of 1.00 for all two-way ANOVA tests. Maintenance of native tendon properties was measured by comparing results from the treatment groups to the baseline groups.
Tendons to be irradiated were packaged on dry ice and sent to processing facilities for sterilization. Gamma irradiation was performed using a Co60 source by Sterigenics Inc. (Rockaway, NJ). E-beam irradiation was performed using a 5-MeV electron accelerator by E-beam Services Inc. (Cranbury, NJ). Tendons received an average absorbed dose of 25 kGy and 50 kGy. Untreated tendons were irradiated on dry ice. All treated tendons were intentionally shipped with a minimal amount of dry ice and remained in solution during irradiation. Tendons not subjected to irradiation were also kept on dry ice or in solution while the irradiated groups were being processed.
Crosslinking may bolster functional properties of tissues and compensate for radiation damage. Tendons were crosslinked two per 15 mL in a solution of 10 mM EDC (Sigma-Aldrich, St. Louis, MO) supplemented with 5 mM NHS (N-hydroxyl succinimide) (Sigma-Aldrich, St. Louis, MO) in deionized water. Tendons were set in crosslinking solution for 24 hours and washed three times in deionized water every 10 minutes. Tendons were then soaked in 0.1 M Na2HPO4 (Sigma-Aldrich, St. Louis, MO) solution for 2 hours. This was followed by soaking in deionized water for another 12 hours to complete the process. This protocol was developed previously in this lab for collagen scaffolds designed for anterior cruciate ligament reconstruction [4]. Tendons were stored at 4°C during crosslinking and washing steps. Preparation involved soaking in a 100-mM solution of D (+) glucose, 99.5% anhydrous (Sigma-Aldrich, St. Louis, MO), and PBS for 36 hours in 4°C. Tendons were soaked two per 15 mL and irradiated in a similar fashion. A pilot study was performed to determine the optimal concentrations for various treatments ranging from 50 mM to 500 mM (data not shown).
Binding of irradiation-derived free radicals by free radical scavengers could prevent them from participation in chemical modifications with collagen. Preparation was similar among the three scavengers: D-mannitol (Sigma-Aldrich, St. Louis, MO), (+) sodium ascorbate (Sigma-Aldrich, St. Louis, MO), and riboflavin (Sigma-Aldrich, St. Louis, MO). Two tendons per 15 mL were soaked in 500 mM mannitol solution, 100 mM ascorbate solution, or 100 mM riboflavin solution in PBS for 36 hours in solution at 4°C.
Tendons designated for mechanical testing were thawed out in PBS and left to soak for 30 minutes. Dimensional measurements were taken using a Z-mike 1202 (Dayton, OH) series laser micrometer. Tendons were mounted against the laser and held with minimal tension. Two measurements were taken for thickness and width, and the area was calculated assuming a rectangular cross-section. Tensile testing was performed using an Instron model 4204 (Canton, MA) testing machine with tendons mounted in cryogenic freeze clamps (Enduratec, Eden Prairie, MN). Tendons were allowed to freeze in the clamps until 1 mm of the tendons were visibly frozen outside of the clamps. PBS was also regularly applied to maintain hydration. Before failure testing, tendons were first preconditioned in tension at about 1.5 N to 3.5 N for five cycles. Gauge length was taken as the distance between the frozen ends. Tendons were then pulled in tension at a speed of 100 mm/min until failure. Tests that showed evidence of slipping were excluded from the data pool. Raw data was collected using a Smart Mother Board data collector (Microstrain Inc., Williston, VT). Structural and material parameters were calculated from raw data using a custom written MATLAB (Mathworks, Norwood, MA) program. All values were calculated to failure. Elastic modulus values were calculated from linear regions of the stress-strain curve. All values are reported as mean ± standard deviation.
Dissolution of collagen materials in collagenase is dependent on the amount of collagen nativity and crosslinks [25], which are both affected by ionizing irradiation. Collagenase digestion is impeded when active sites are obscured by native crosslinks. Tendons were lyophilized and then sectioned into 3.5-mg segments. These segments were placed in test tubes and soaked in 10 mL collagenase solution (400 units/mL) derived from clostridiopeptidase A (Sigma-Aldrich, St. Louis, MO). The collagenase resistance time (CRT) was determined in triplicate for each group, with collagen sponges included as positive controls. Test tubes were placed in a 37°C water bath and observed every half hour until the samples were no longer intact or for a maximum of 24 hours. The average dissolution time ± standard deviation was recorded for each group.
Individual tests were conducted for mechanical data as well as CRT data. We used Student-Neuman-Keuls (SNK) two-way analysis of variance; the two factors and corresponding levels included irradiation (type, unirradiated or gamma-/e-beam-irradiated at 25 kGy/50 kGy) and treatment (unteated or specific crosslinker/free radical scavenger). These tests assumed samples were normally distributed and with equal variance. These analytical tests were performed to determine if irradiation and treatment influenced mechanical properties and dissolution time in collagenase. We used SigmaStat (Systat Software Inc., San Jose, CA) software.
Results
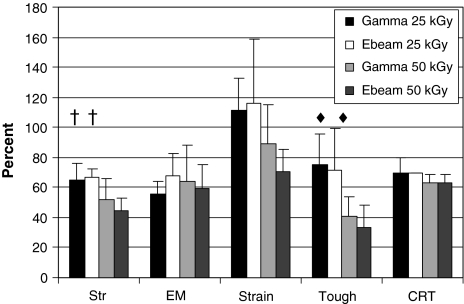
Gamma and e-beam irradiation caused similar reductions in tensile strength, elastic modulus, strain, and toughness of tendons. Upon comparing data at 25 kGy and 50 kGy, a clear dose-dependent decrease was evident for strength and toughness. There was an average of 36% and 55% loss in tensile strength at 25 kGy and 50 kGy compared to unsterilized controls (Fig. 1). Changes in strain were least noticeable between irradiated and unirradiated tendons at 25 kGy, and were slightly decreased at 50 kGy. Although radiation effects were least discernable on strain values, dose-related trends were similar to strength and toughness (Fig. 1). A decrease in elastic modulus was observed after irradiation, though dose related differences were not observed (Fig. 1). These trends were generally observed for all irradiated groups in this study. Gamma- and e-beam-irradiated tendons also showed similar resistance to collagenase (Fig. 1). The dose effect of irradiation on collagenase resistance was not as prominent for mechanical properties. There was a 10% decrease in collagenase resistance after irradiation at 50 kGy compared to 25 kGy (Fig. 1).
Fig. 1.
Data show there are no differences between gamma and e-beam effects on mechanical properties and collagenase resistance on untreated tendons. Dose-related reduction of native tendon properties were most evident for strength (Str), toughness (Tough), and collagenase resistance time (CRT), but least evident for elastic modulus (EM); for gamma- and e-beam-treated tendons, we observed decreases as dose was increased from 25 kGy to 50 kGy for strength and toughness. Trends showed that increased radiation dose produced greater reductions for these values. Strain values observed the least change, but had the same general dose-related trend. † = Ebeam 25 kGy versus Gamma/EBeam 50 kGy (p = .047, p = .004); † = Gamma 25 kGy versus Gamma/Ebeam 50 kGy (p = .033, p = .004); ◆ = Ebeam 25 kGy vs Gamma/Ebeam 50 kGy (p = .010, p = .004); ◆ = Gamma 25 kGy versus Gamma/Ebeam 50 kGy (p = .015, p = .007).
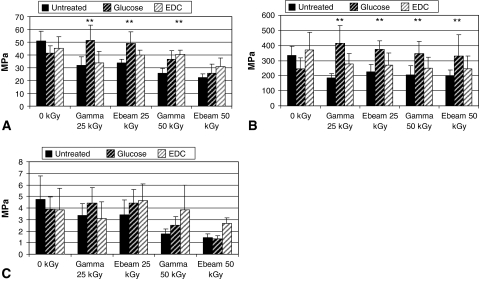
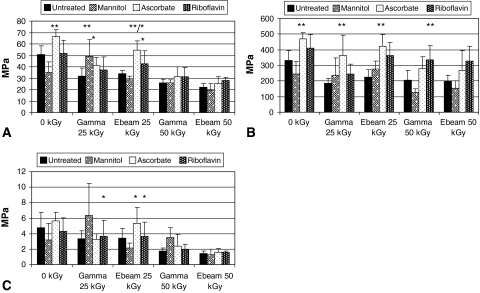
Several conditions in the crosslinker- and free radical scavenger-treated groups exhibited higher mechanical properties, especially strength and elastic modulus values. Crosslinked tendons had the highest strength values compared to untreated groups at 50 kGy. At this dose, EDC- and glucose-treated tendons had 32% and 22% higher average strength than untreated tendons. At 25-kGy gamma and e-beam irradiation, glucose-crosslinked tendons possessed higher tensile strength compared to untreated (Fig. 2A). Elastic modulus of crosslinked tendons was higher than untreated tendons over the entire radiation dose range (Fig. 2B). Among glucose-treated tendons, increases in strength and elastic modulus occurred when irradiation was applied at 25 kGy compared to unirradiated. Fewer differences were observed with toughness values, for which EDC treatments showed the most increase at 50 kGy (Fig. 2C). Free radical scavengers were more effective at 25 kGy, especially for ascorbate and riboflavin treatments. At 25 kGy, the gamma-irradiated ascorbate group was 40% stronger than the untreated group (Fig. 3A). Elastic moduli for ascorbate and riboflavin groups were also higher than untreated for all irradiation conditions (Fig. 3B). Effects of free radical scavengers diminished for tensile strength and toughness as radiation dose increased from 25 kGy to 50 kGy. Ascorbate-treated tendons irradiated at 50 kGy were lower compared to 25 kGy, and similar for toughness which was lower for e-beam irradiation at 25 kGy compared to 50 kGy. Riboflavin treatment also showed similar trends (Fig. 3C). Mannitol treatment appeared ineffective at most dose conditions except gamma-irradiated at 25 kGy.
Fig. 2A–C.
Protective effects generated from crosslinking treatment were most apparent for strength and elastic modulus. (A) Glucose crosslinked tendons had higher strength at 25 kGy than untreated tendons, and were comparable to native tendon. ** = significance compared to untreated; * = significance compared to irradiation dose; ** = P(GLUC) Gamma/Ebeam 25 kGy (p < .001, p = .003); ** = P(EDC) Gamma 50 kGy (p = .016). (B) Glucose crosslinked tendons also had higher elastic modulus compared to untreated. ** = P(GLUC) Gamma 25 kGy, Ebeam 25 kGy, Gamma 50 kGy (p < .001, p = .008, p = .007). (C) There were similar protective and dose-related trends among toughness values as seen with strength values, but they were not as distinct.
Fig. 3A–C.
Among free radical scavengers, the most effective protectors of mechanical properties were ascorbate and riboflavin treatments. Both of these treatments provided selective protection of strength and elastic modulus at 25 kGy. (A) Strength of ascorbate-treated tendons was higher than untreated at 25 kGy e-beam irradiation. ** = P(AS) 0 kGy, Ebeam 25 kGy (p < .001, p < .001); ** = P(MA) Gamma 25 kGy p = .003; * = P(AS) Ebeam 25 kGy versus Ebeam/Gamma 50 kGy (p < .001), Gamma 25 kGy versus Ebeam/Gamma 50 kGy (p < .001,p = .005); * = P(RB) Ebeam 25 versus Ebeam/Gamma 50 kGy (p = .010, p.030). (B) Similar to crosslinkers, elastic modulus values did not seem affected by radiation dose. ** = P(AS) 0 kGy, Gamma 25 kGy, Ebeam 25 kGy (p = .030, p < .001, p < .001). (C) Trends were less consistent with toughness, though dose related reduction similar to untreated and crosslinked tendons were again observed. Finally, there was not much of an effect by mannitol treatment. * = P(AS) Ebeam 25 kGy vs Gamma/Ebeam 50 kGy (p < .001); * = P(RB) Ebeam 25 kGy versus Gamma 50 kGy, Gamma 25 kGy versus Ebeam 50 kGy (p = .030, p = .043).
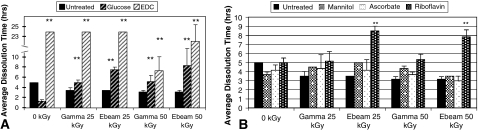
There was a greater positive effect by the crosslinkers compared to the free radical scavengers with regard to resistance against collagenase degradation. EDC crosslinked tendons were by far the most resistant to collagenase, and were the only group to remain intact throughout the 24 hour study (Fig. 4A). Glucose-crosslinked tendons also displayed higher resistance than the untreated groups for conditions involving radiation. Riboflavin treatments resulted in an increase in collagenase resistance for e-beam irradiation. Meanwhile, ascorbate and mannitol treatments provided moderate resistance at 25 kGy; however, at 50 kGy differences versus untreated controls were marginal (Fig. 4B).
Fig. 4A–B.
(A) Collagenase resistance time (CRT) showed that EDC treated tendons were the most resistant to collagenase, over the entire dose range. Additionally, glucose treatments displayed increased resistance when radiation was present. Free radical scavenger treatment resulted in slight increases in collagenase resistance at 25 kGy, and little effect at 50 kGy. ** = P(EDC) p < .001; ** = P(GLUC) p < .001. (B) Among scavengers, only riboflavin treatment showed increases. ** = P(RB) p < .001.
Discussion
Sterilization via ionizing irradiation can aid tissue banks to ensure safer allograft distribution by supplementing current screening protocols. This form of sterilization is not routinely performed because irradiation of allografts can damage structural proteins resulting in decreased strength resulting in premature failure. It was our hypothesis that crosslinking or free radical scavenging might offset the negative effects of irradiation on mechanical properties and enzyme resistance. Dose responses to gamma and e-beam irradiation were also compared. Successful protection of allografts would allow use of ionizing irradiation to reduce bioburden while maintaining functional properties.
There are several limitations to our study that should first be noted. Our results cannot predict postimplantation behavior of allografts, nor were validation studies performed to test sterility. These were preliminary experiments to be used as a guide for more involved studies in the future. Potential differences in processing protocols at gamma and e-beam facilities could have lead to variability associated with irradiation conditions, though nothing in the data suggests this occurred. It was also assumed that mechanical data and enzyme testing would yield the same trends. They are both considered methods of characterizing crosslinking and nativity of collagen. There were a few instances when divergence occurred, including the dose-dependent effects of radiation and protective effect of ascorbate, which were both seen in mechanical testing but not as distinct in collagenase resistance. This may be the result of using a high concentration of collagenase, which may accelerate degradation beyond the point where differences between irradiation doses would be clear. Alternatively, miniscule defects caused by irradiation are likely to have more effect on failure mechanisms during tensile testing compared to enzyme degradation. Despite these limitations, this study contributes novel data to sterilization and radioprotection literature including potential of EDC and glucose protection, and direct comparison of crosslinking and free radical scavenging, as well as e-beam and gamma irradiation.
Gamma irradiation has been noted for effective neutralization of pathogens as well as deep penetrability, about 30 cm through a density of water [6]. In comparison, e-beam irradiation possesses lower penetrability, only 8 cm through the density of water [6]. This may be an obstacle for cortical bone allografts, which has a density about twice that of water, and therefore half the penetration depth [6]. E-beam penetration is still sufficient enough for soft tissues. The advantage to e-beam irradiation is higher processing speed, on the order of seconds compared to hours, for gamma irradiation. With the high dose rate of e-beam irradiation, one concern is local variation of absorbed dose. Exposure to either gamma or e-beam irradiation produced decreased mechanical properties and enzyme resistance in tendons. According to our data, these effects were nearly identical for gamma or e-beam irradiation. Mechanical testing showed strength and toughness were the parameters most sensitive to radiation dose. Elastic modulus was marginally affected, and strain was the least affected. Lesions caused by direct impact of high-energy particles likely have the greatest impact on break load, with less of an influence on deformation. These data are also in agreement with those of Salehpour et al. [21], who reported greatest changes in toughness and the least change in elongation. There was also no difference between the effect of gamma or e-beam irradiation, suggesting either one could be used for sterilization.
Both treatment types protected mechanical properties at 25 kGy, but at 50 kGy crosslinkers were superior. Strength of glucose-treated tendons gamma and e-beam irradiated at 25 kGy were close to native tendon. Strength, modulus, and toughness were all increased at 25 kGy compared to untreated samples as well as unirradiated samples. These mechanical data suggest that glucose-derived crosslinking occurs, and furthermore during the irradiation process. This is consistent with the data of Ohan and Dunn [17], which also suggest that glucose crosslinking of collagen is driven by ionizing irradiation derived free radicals. This mechanism involves free radicals encouraging the linear configuration of glucose, which is necessary to participate in the crosslinking reaction [17]. Natural glucose crosslinking of collagen occurs through the Maillard reaction, which is a much slower process associated with aging [19]. Resistance to collagenase was also higher for irradiated tendons compared to unirradiated for glucose treatment. The mechanical properties of glucose-treated tendons decreased at 50 kGy compared to 25 kGy, suggesting exhaustion of crosslinking sites, or that the rate of crosslinking was exceeded by crosslink breakdown.
Free radical scavengers showed selective protective effects in mechanical data at 25 kGy, and were less pronounced at 50 kGy. Riboflavin and ascorbate showed the majority of these positive effects for strength and elastic modulus, while mannitol treatments were largely ineffective. Although mannitol possesses radical scavenging ability, hydroxyl radical attack of organic alcohols can give rise to reactive superoxide anions [8]. Superoxide anion has been identified as a radical capable of fragmenting collagen [15]. The production of these radicals may have negated scavenging effects.
Collagenase resistance data showed that crosslinked tendons were much more resistant than free radical scavenger-treated tendons, especially at higher doses. EDC crosslinked tendons possessed the highest resistance at all dose conditions. As mentioned glucose crosslinked tendons also showed improved collagenase resistance in the presence of irradiation. Interestingly, only riboflavin treatment provided increases in resistance among free radical scavengers. Past investigation of riboflavin suggests it also has the capacity to act as a crosslinker of collagen [11, 27], in addition to free radical scavenging [7]. Riboflavin treatment in combination with ultraviolet light successfully crosslinked human cornea, which is composed of type II collagen [27]. It is plausible that riboflavin is able to form crosslinks in tendon in the presence of ionizing irradiation in a similar fashion. Our data are unable to distinguish protective means, whether crosslinking or free radical scavenging. More studies would be needed to determine the function of riboflavin treatment on tendons during irradiation.
The literature pertaining to protection of irradiated allografts is limited, making comparison of results difficult. The majority of the studies have been on free radical scavengers, which have limited ionizing irradiation damage in proteins in aqueous environments [3, 12, 28]. Recently, however, Grieb et al. [9] reported that a radioprotective cocktail solution, which included mannitol, was successful in protecting mechanical properties of human semitendinosus tendon at 50 kGy under regulated conditions. Akkus et al. [1] reported use of another free radical scavenger, thiourea, resulted in increased postyield toughness at 36 kGy but not to the level of controls. Akkus and colleagues [1] tested bone allografts, which possess structural and compositional differences compared to soft tissue. These studies suggest that soft or hard tissue allografts may be stabilized by free radical scavenging during ionizing irradiation.
The two methods of treatment each have their advantages and disadvantages. The advantage to free radical scavengers is the limitation on chemical modification. An associated disadvantage is the possibility that scavenging may also protect bacteria or viruses within the tissue as well, thus requiring higher doses. This highlights the importance to conduct sterility validation studies. A possible drawback of crosslinking is the introduction of cytotoxic substrates, but EDC is a zero-order crosslinker, meaning no foreign subunits exist in crosslinks [18]. Wissink et al. [26] reported no issues with human umbilical vein endothelial cell attachment, morphology, or function to EDC/NHS crosslinked collagen scaffolds. Both crosslinking and scavenging demonstrated protective effects; however, crosslinkers provided the greatest range of radioprotection. At 50 kGy, EDC crosslinked tendons had notably high tensile strength. At 25 kGy, glucose-treated tendons had comparable strength, modulus, toughness, and strain to native tendon. In addition to proper mechanical function after implantation, preventing premature digestion by proteolytic enzymes is an equally important concern. Crosslinkers demonstrated success for both these characteristics. According to the data obtained in this study, EDC and glucose are the most capable at protecting allografts against ionizing irradiation over a range of doses.
Our data suggest the majority of treatments afforded improved mechanical properties and enzyme resistance at 25 kGy. At 50 kGy, crosslinkers were more effective than free radical scavengers, although neither were to the level of native tendon. Additionally, both gamma and e-beam irradiation had similar effects on tendons. This suggests that they can be used interchangeably with regard to mechanical properties. If the terminal dose could be lowered to 25 kGy, the treatments assessed in this study could be used to provide stabilization for tissue allografts. It may be possible to lower bioburden and, accordingly, the irradiation dose required for sterilization through decellularization methods. Another option could be to combine crosslinking and scavenging methods for a potentially greater protective effect. Furthermore, combinations of free radical scavengers have synergistic radioprotective effects [12]. Our long-term goal is to develop and validate terminal sterilization methods for soft tissue allografts without compromising their mechanical integrity and performance after implantation. This would improve the safety of allografts and further increase their acceptance by surgeons and patients.
Footnotes
One or more of the authors (MGD) have received funding from the Musculoskeletal Transplant Foundation Peer Reviewed Scientific Grants Program (January–December 2005).
References
- 1.Akkus O, Belaney RM, Das P. Free radical scavenging alleviates the biomechanical impairment of gamma radiation sterilized bone tissue. J Orthop Res. 2005;23:838–845. [DOI] [PubMed]
- 2.Block SS. Disinfection, Sterilization, and Preservation, 4th ed. Philadelphia, PA and London, England: Lea and Febiger; 1991.
- 3.Cai L, Koropatnick J, Cherian MG. Roles of vitamin C in radiation-induced DNA damage in presence and absence of copper. Chem Biol Interact. 2001;137:75–88. [DOI] [PubMed]
- 4.Caruso AB, Dunn MG. Functional evaluation of collagen fiber scaffolds for ACL reconstruction: cyclic loading in proteolytic enzyme solutions. J Biomed Mater Res A. 2004;69:164–171. [DOI] [PubMed]
- 5.Cheung DT, Perelman N, Tong D, Nimni ME. The effect of gamma-irradiation on collagen molecules, isolated alpha-chains, and crosslinked native fibers. J Biomed Mater Res. 1990;24:581–589. [DOI] [PubMed]
- 6.Dziedzic-Goclawska A, Kaminski A, Uhrynowska-Tyszkiewicz I, Stachowicz W. Irradiation as a safety procedure in tissue banking. Cell Tissue Bank. 2005;6:201–219. [DOI] [PubMed]
- 7.Fuga L, Kragl M, Getoff N. Vitamin B2 (riboflavin) and a mixture of vitamin B2 and C affects MMC efficiency in aerated media under irradiation. Anticancer Res. 2004;24:4031–4034. [PubMed]
- 8.Garrett RH, Grisham CM. Biochemistry, 2nd ed. Fort Worth, TX: Saunders College Publishing; 1999.
- 9.Grieb TA, Forng RY, Bogdansky S, Ronholdt C, Parks B, Drohan WN, Burgess WH, Lin J. High-dose gamma irradiation for soft tissue allografts: High margin of safety with biomechanical integrity. J Orthop Res. 2006;24:1011–1018. [DOI] [PubMed]
- 10.Hernigou P. Allograft sterility as exemplified by human immunodeficiency virus and sterilization by irradiation. J Arthroplasty. 2000;15:1051–1058. [DOI] [PubMed]
- 11.Kohlhaas M, Spoerl E, Schilde T, Unger G, Wittig C, Pillunat LE. Biomechanical evidence of the distribution of cross-links in corneas treated with riboflavin and ultraviolet A light. J Cataract Refract Surg. 2006;32:279–283. [DOI] [PubMed]
- 12.Konopacka M, Widel M, Rzeszowska-Wolny J. Modifying effect of vitamins C, E and beta-carotene against gamma-ray-induced DNA damage in mouse cells. Mutat Res. 1998;417:85–94. [DOI] [PubMed]
- 13.Li X, Trout JM, Jenkins MC, Palmer R, Fayer R. Effects of gamma radiation on viability of Encephalitozoon spores. J Parasitol. 2002;88:812–813. [DOI] [PubMed]
- 14.Lietman SA, Tomford WW, Gebhardt MC, Springfield DS, Mankin HJ. Complications of irradiated allografts in orthopaedic tumor surgery. Clin Orthop Relat Res. 2000;375:214–217. [DOI] [PubMed]
- 15.Monboisse JC, Borel JP. Oxidative damage to collagen. EXS. 1992;62:323–327. [DOI] [PubMed]
- 16.Nguyen H, Morgan DA, Forwood MR. Sterilization of allograft bone: is 25 kGy the gold standard for gamma irradiation? Cell Tissue Bank. 2007;8:81–91. [DOI] [PubMed]
- 17.Ohan MP, Dunn MG. Glucose stabilizes collagen sterilized with gamma irradiation. J Biomed Mater Res A. 2003;67:1188–1195. [DOI] [PubMed]
- 18.Powell HM, Boyce ST. EDC cross-linking improves skin substitute strength and stability. Biomaterials. 2006;27:5821–5827. [DOI] [PubMed]
- 19.Reddy GK. Glucose-mediated in vitro glycation modulates biomechanical integrity of the soft tissues but not hard tissues. J Orthop Res. 2003;21:738–743. [DOI] [PubMed]
- 20.Russell AD. Principles, Practice of Disinfection, Preservation, and Sterilization, 2nd ed. Oxford, UK: Blackwell Scientific Publications; 1992.
- 21.Salehpour A, Butler DL, Proch FS, Schwartz HE, Feder SM, Doxey CM, Ratcliffe A. Dose-dependent response of gamma irradiation on mechanical properties and related biochemical composition of goat bone-patellar tendon-bone allografts. J Orthop Res. 1995;13:898–906. [DOI] [PubMed]
- 22.Smith RA, Ingels J, Lochemes JJ, Dutkowsky JP, Pifer LL. Gamma irradiation of HIV-1. J Orthop Res. 2001;19:815–819. [DOI] [PubMed]
- 23.Suarez LS, Richmond JC. Overview of procurement, processing, and sterilization of soft tissue allografts for sports medicine. Sports Med Arthrosc. 2007;15:106–113. [DOI] [PubMed]
- 24.Vangsness CT Jr, Garcia IA, Mills CR, Kainer MA, Roberts MR, Moore TM. Allograft transplantation in the knee: tissue regulation, procurement, processing, and sterilization. Am J Sports Med. 2003;31:474–481. [DOI] [PubMed]
- 25.Weadock KS, Miller EJ, Keuffel EL, Dunn MG. Effect of physical crosslinking methods on collagen-fiber durability in proteolytic solutions. J Biomed Mater Res. 1996;32:221–226. [DOI] [PubMed]
- 26.Wissink MJ, van Luyn MJ, Beernink R, Dijk F, Poot AA, Engbers GH, Beugeling T, van Aken WG, Feijen J. Endothelial cell seeding on crosslinked collagen: effects of crosslinking on endothelial cell proliferation and functional parameters. Thromb Haemost. 2000;84:325–331. [PubMed]
- 27.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg. 2003;29:1780–1785. [DOI] [PubMed]
- 28.Zbikowska HM, Nowak P, Wachowicz B. Protein modification caused by a high dose of gamma irradiation in cryo-sterilized plasma: protective effects of ascorbate. Free Radic Biol Med. 2006;40:536–542. [DOI] [PubMed]