Abstract
Preservation of limb function in pediatric oncology patients is challenging with the ongoing growth of limbs contralateral to reconstructed limbs. We analyzed 22 patients younger than 10 years old who received an allograft after resection of a bone sarcoma with a minimum followup of 2 years (mean, 4 years; range, 2–14 years). The mean age was 7 years (range, 2–10 years). There were 16 boys and six girls with 17 osteosarcomas and five Ewing’s sarcomas. Thirteen reconstructions were performed with an intercalary allograft and nine with an osteoarticular allograft. Physes were uninvolved in five patients and one physis in 17. We documented outcomes using the Musculoskeletal Tumor Society functional and the International Society of Limb Salvage radiographic scoring systems. At last followup, three of the 22 patients died of their tumor, one was alive but with an amputation, and 18 retained their limbs. These 18 patients had an average functional score of 27 points and a mean radiographic score of 94%. Eight complications required a second surgery; in four, the allograft was removed (one infection, one local recurrence, two fractures) and in four, the allograft was preserved (two local recurrences, one fracture, one nonunion). We consider biologic reconstruction with allografts after sarcoma resection an appropriate reconstructive procedure in young children.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Bone sarcomas are presently treated mainly with preservation of the affected limb [10, 17, 28]. In addition, most patients with sarcoma are either adolescents or at a pediatric age and the tumor is located in an extremity, most frequently the lower extremity [4, 17, 28]. This is a demanding situation for surgeons treating those patients to reconstruct the limb with the most durable procedure with the least length discrepancy.
We asked whether: (1) allograft reconstruction is safe and reliable after limb-sparing surgery in growing children younger than 10 years of age; (2) we can achieve functional and radiographic results similar to those in skeletally mature patients; and (3) we can achieve limb-length equality when no physis is compromised by the resection and how much limb-length inequality should be expected when a single physis is sacrificed.
Materials and Methods
We retrospectively reviewed the records of 22 consecutive patients younger than 10 years old who received an allograft after resection of a bone sarcoma. The diagnoses included 17 osteosarcomas and five Ewing’s sarcomas. The mean age of the patients was 7 years with a range between 2 and 10 years. Sixteen were boys and six girls. The patients were followed for a minimum of 2 years (mean, 4 years; range, 2–14 years) (Table 1).
Table 1.
Demographics and clinical data
| Patient number | Age (years) | Gender | Stage | Followup (months) | Site | Tumor | Metastases | Type of allograft | Complication | Physis lost | Patient status | Allograft status | Limb status | Limb-length discrepancy (cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 | M | III | 30 | PH | Ewing | P | IA | No | D | Preserved | Preserved | — | |
| 2 | 6 | M | IIB | 25 | PH | OS | — | OA | 1 | ANED | Preserved | Preserved | 1 | |
| 3 | 2 | M | IIB | 24 | PF | Ewing | P | IA | Recurrence | No | AWED | Preserved | Preserved | 0 |
| 4 | 4 | M | IIB | 65 | PF | Ewing | — | OA | 1 | ANED | Preserved | Preserved | 3 | |
| 5 | 4 | M | IIB | 50 | DF | OS | — | IA | Fracture | No | ANED | Preserved | Preserved | 0 |
| 6 | 6 | M | IIB | 24 | DF | OS | P | OA | 1 | D | Preserved | Preserved | — | |
| 7 | 10 | M | III | 22 | DF | OS | P | OA | 1 | D | Preserved | Preserved | — | |
| 8 | 8 | F | IIB | 31 | DF | OS | — | IA | No | ANED | Preserved | Preserved | 0 | |
| 9 | 9 | F | III | 124 | DF | OS | — | IA | Fracture | 1 | ANED | Not preserved | Preserved | 3 |
| 10 | 10 | M | IIB | 80 | DF | OS | — | IA | Fracture | 1 | ANED | Not preserved | Preserved | 3 |
| 11 | 5 | M | IIB | 25 | DF | OS | — | IA | 1 | ANED | Preserved | Preserved | 2 | |
| 12 | 6 | M | IIB | 31 | DF | OS | — | IA | 1 | ANED | Preserved | Preserved | 1.5 | |
| 13 | 7 | F | IIB | 144 | DF | OS | — | OA | Nonunion | 1 | ANED | Preserved | Preserved | 6 |
| 14 | 7 | M | IIB | 24 | DF | OS | — | OA | 1 | ANED | Preserved | Preserved | 1 | |
| 15 | 8 | M | IIB | 26 | DF | OS | — | IA | 1 | ANED | Preserved | Preserved | 2 | |
| 16 | 9 | M | IIB | 42 | DF | OS | — | IA | Recurrence | 1 | ANED | Preserved | Preserved | 1 |
| 17 | 10 | F | III | 94 | DF | OS | — | IA | Recurrence | 1 | ANED | Preserved | Preserved | 3 |
| 18 | 10 | F | IIB | 78 | DF | OS | — | IA | 1 | ANED | Preserved | Preserved | 2 | |
| 19 | 10 | M | IIB | 24 | PT | OS | — | IA | No | ANED | Preserved | Preserved | 0 | |
| 20 | 9 | F | IIB | 110 | PT | OS | — | OA | Infection | 1 | ANED | Not preserved | Preserved | 1 |
| 21 | 9 | M | IIB | 32 | DT | Ewing | — | OA | 1 | ANED | Preserved | Preserved | 1 | |
| 22 | 7 | M | IIB | 69 | DT | Ewing | — | OA | Recurrence | 1 | ANED | Not preserved | Amputated | — |
M = male; F = female; PH = proximal humerus; DF = distal femur; PT = proximal tibia; DT = distal tibia; OS = osteosarcoma; P = proximal; IA = intercalary allograft; OA = osteoarticular allograft; D = dead; ANED = alive with no evidence of disease; AWED = alive with evidence of disease.
Operative treatment consisted of resection of the tumor and insertion of an allograft to reconstruct the bone defect. We harvested nonirradiated allografts under sterile conditions, which were stored frozen at −80°C in the bone bank that is established at the authors’ institution according to a technique that has been previously described [18]. No attempt was made to preserve the viability of the articular cartilage, and we performed bacteriologic and viral studies in accordance with the recommendations of the American Association of Tissue Banks and the tests available at the time.
The physes were unaffected by the procedure in five patients (Fig. 1), whereas in 17, only one physis was compromised after resection and reconstruction (Fig. 2). Sixteen were femur transplants (11 intercalary, five osteoarticular), four at the tibia (one intercalary, three osteoarticular), and two at the humerus (one intercalary, one osteoarticular). We did not perform arthrodesis reconstruction in any of these patients. After tumor resection, the grafts were taken out of their packaging and placed directly in a warm normal saline solution. In osteoarticular reconstructions, after being thawed, we cut the donor bone to the proper length and soft tissue structures were prepared for implantation. According to the reconstructed joint, the ligaments were reattached to the corresponding allograft tissues to improve stability. Reattachment of the allograft tissue to the host tissue was performed through a direct lateral-lateral continuous suture. In tibial allografts, the host meniscus was reattached to the osteoarticular allograft suturing both horn insertions and the articular capsule. We used a transverse osteotomy in every case. Twenty-one transplants were fixed with plates and screws, whereas in the remaining one, we obtained the fixation with an intramedullary interlocking nail. In all patients we attempted to overlengthen as much as possible to potentially compensate future limb length discrepancy. However, owing to the small limb size typically no more than two or three centimeters could be obtained.
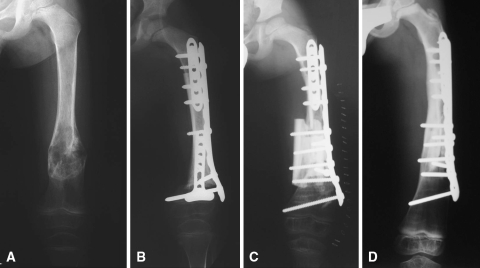
Fig. 1A–D.
Case 5 was a 4-year-old-boy with a diagnosis of diaphyseal osteosarcoma in whom, after chemotherapy and tumor resection, an intercalary allograft preserving both physes was performed. (A) The anteroposterior radiograph shows tumor extension. (B) This anteroposterior radiograph was made after resection of diaphyseal tumor and implantation of an intercalary allograft. Osteotomies were stabilized with two short anterior plates and one long lateral plate. (C) This anteroposterior radiograph was made after the second surgery as a result of allograft fracture. The distal anterior plate was extracted and two allograft struts were added to reinforce the original allograft. (D) This anteroposterior radiograph was made 4 years after original surgery showing incorporation and healing of the both allogeneic struts over the original allograft. Note growing of the distal femur physis.
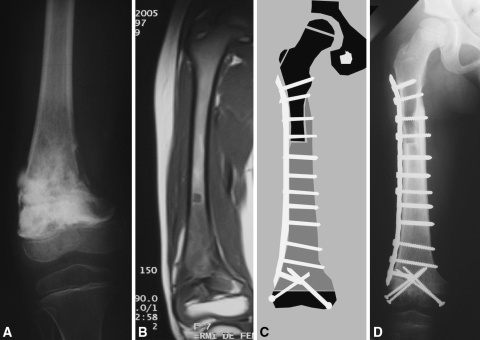
Fig. 2A–D.
Case 12 was a 6-year-old boy with a diagnosis of osteosarcoma in whom a transepiphyseal resection and reconstruction with an intercalary allograft was performed. (A) The anteroposterior radiograph of the knee after neoadjuvant chemotherapy shows the osteosarcoma compromises the medial cortex with a varus deformity. (B) A coronal T1-weighted MRI image shows the metaphyseal and diaphyseal extension. (C) A schematic drawing shows the preoperative planning of the reconstruction. (D) The anteroposterior radiograph shows the intercalary allograft after 2 years of followup.
Patients were seen postoperatively at 1 week, 2 weeks, 1 month, 2 months, and 3 months; every 3 months thereafter until 2 years; and then annually. Plain radiographs were made at every visit beginning 1 month after the operation.
The procedure was considered a failure when the allograft was removed either as a revision procedure or amputation. We recorded complications such as fracture, local recurrence, infection, nonunion, and limb-length discrepancy.
We performed the functional evaluation of the patients with the use of the revised 30-point functional classification system established by the Musculoskeletal Tumor Society [9]. This functional score measures six parameters: pain, function, emotional acceptance, use of walking supports, walking ability, and gait. Each parameter is given a value ranging from 0 to 5 according to specific criteria. The individual scores are added together to obtain an overall functional score with a maximum of 30 points. A score of at least 23 points is considered an excellent functional result, between 15 and 22 points a good result, between 8 and 14 points a fair result, and a score under 8 points a poor result. Orthopaedic surgeons (LAAT, GF) involved in clinical care interviewed patients by telephone or at their latest followup and completed a questionnaire with each patient. At the latest followup, a scanogram was performed to examine limb-length discrepancy between compromised and uncompromised limb and to assess physeal growth.
The plain radiographs were evaluated by two of us (LAAT, GF) according to the system established by the International Society of Limb Salvage [12], which is based on eight criteria: the healing of proximal or distal osteotomies, the contour of the graft, the fixation of the graft, the density of the graft, the stability of the joint, the diameter of the graft, and degeneration of the joint. Each parameter is given a value ranging from 0 to 5 according to specific criteria. We calculated the score by adding the value for each criterion and dividing the total maximum attainable score. The score is expressed as a percentage; the maximum possible score was 100%.
Results
Eighteen of the 22 patients (15 original allografts, three retransplanted; 17 lower and one upper limb) were alive with preservation of the limb and one was alive but had an amputation of the affected limb. Three patients died of tumor-related reasons without an allograft failure. In the remaining 19 patients, four allografts failed at an average of 13 months (range, 8–16 months) resulting from two fractures, one infection, and one local recurrence. In these two patients with fractures, removal of the allograft was necessary and a second allograft was performed. The patient who developed an acute deep infection was treated with removal of the allograft and implantation of a temporary cement spacer with antibiotics; after 6 weeks of intravenous antibiotics and 6 weeks of oral antibiotics, a second allograft was reimplanted. The remaining patient with local tumor recurrence underwent amputation.
The procedure seemed reliable: 15 of the 22 patients still had the original allograft at last followup, even after sustaining five complications that included three local recurrences, one fracture, and one diaphyseal nonunion. The local recurrences were in soft tissues with no contact with the reconstructions distant from the osteotomy sites. Two were resected with wide margins maintaining the original graft and the remaining one was treated with chemotherapy and radiotherapy. In the patient with the fracture, the internal fixation was replaced and two allograft struts were added to reinforce the original allograft (Fig. 1). In the diaphyseal nonunion, a single operation, in which the internal fixation was replaced and autogenous graft was added to the site, resulted in union at the host-donor junction.
At the latest followup examination, the average functional score was 27 points (range, 20–30 points). All patients referred no pain in the involved limb. Ten patients had no functional restrictions, and eight had restrictions in recreational activities. Fifteen patients walked without the use of supports, two used one cane, and the patient with the upper limb reconstruction was not able to put their hand above the shoulder. Eleven patients could walk an unlimited distance, six had some limitations in walking, and the patient with the upper limb sarcoma had normal dexterity. Seven patients had no discernible limp, nine had a minor cosmetic limp, one had a major cosmetic limp, and the patient with the upper limb sarcoma had limited lifting ability. The mean radiographic score for the 18 allografts evaluated was 94%, which represents an excellent radiographic result, with 17 grafts having scores between 80% and 100%.
We observed no limb-length discrepancy in four patients with both physes preserved. In the remaining 14 patients, limb-length discrepancy developed as a result of loss of one physis. The mean shortening was 2.1 cm (range, 1–6 cm). In these patients, the scanograms showed the adjacent physis continued to grow normally.
Discussion
One of the most controversial issues in limb salvage includes those patients with very immature skeletal age who have a sarcoma. One study suggests limb salvage surgery is relatively contraindicated in patients at a very young skeletal age (younger than 8 years old) [28]. We therefore asked whether (1) allograft reconstruction is safe and reliable after limb-sparing surgery in growing children younger than 10 years of age; (2) we can achieve functional and radiographic results similar to those in skeletally mature patients; and (3) we can achieve limb-length equality when no physis is compromised by the resection and how much limb-length inequality should be expected when a single physis is sacrificed.
Limitations of this study include its retrospective nature and no comparison with other types of reconstructions. However, these are demanding and somewhat individualized surgical procedures for a limited patient population and it would be difficult to compare substantially different techniques at the same institution. Another limitation is a relatively short followup and six of the patients reached skeletal maturity as a result of their very young age at the time of surgery and short followup. We therefore cannot establish any final limb-length discrepancy and our results should be considered preliminary. A longer followup is necessary to establish longer-term survival of the reconstructions and final limb-length discrepancies.
Surgical options for patients at this very early age include amputation, rotationplasty [6, 13, 26], or tumor resection followed by some type of reconstruction: expandable prosthesis [1, 3, 8, 14, 22, 23, 27], distraction osteogenesis [11], autologous grafts [11], or allografts [2, 5, 7, 15, 16, 19, 24, 25]. Although amputation is still an option in those patients with extensive sarcoma and who are nonresponsive to chemotherapy, cosmetic, emotional, and functional limitations are obvious [28]. Rotationplasty had been reported as a good alternative with excellent function but with questionable emotional and cosmetic acceptability for many patients [13, 26]. The technology of an expandable prosthesis has improved greatly in recent years [1, 3, 22, 23]; however, potential complications such as soft tissue retraction and limited articular function still remain [1]. In addition, because those patients surviving their disease have at least six decades of life expectancy, it is very difficult to say any prosthetic reconstruction could achieve that durability at the present time. Biologic reconstructions are for those reasons an alternative that should be considered by surgeons treating patients with sarcoma at such an early age.
The use of allograft reconstruction in a pediatric sarcoma population had been previously reported [2, 5, 15, 16, 24]. However, there are very few references in the literature [25] considering exclusively patients younger than 10 years old, a population that for reasons previously addressed is a particular difficulty for reconstructive surgeons. At last followup, 18 of our 22 patients had an average functional score of 27 points (range, 20–30 points). The mean radiographic score was 94%, and only one patient required amputation. Four complications required resection of the allograft and five other complications did not compromise the reconstruction. Although there are reports [2, 5] showing a higher complication rate in the pediatric population after allograft reconstruction, we found in this study a similar complication rate as the adult population [7, 20, 21]. This may be explained by children’s better biologic integration resulting from the high remodeling capacity at this very young age.
One of the main concerns when treating this young patient population is how many physes are compromised by tumor resection. Usually, reconstruction with a prosthesis compromises two growing physes; the growth physis of the segment is compromised by the tumor and the physis of the opposite side of the joint may be physiologically altered in its growing potential by the intramedullary stem perforation. Reconstruction with allografts sacrifices only the growth physis of the segment compromised by the tumor. Additionally, in selected diaphyseal lesions, the tumor resection could even preserve both growing physes and the affected segment could be reconstructed with an intercalary allograft. In our series, we found no limb-length discrepancy in those five patients who had both physes preserved. In the other patients in whom one physis was lost, we found a mean shortening of 2.1 cm (range, 1–6 cm) with the limited followup of this study. In our patients, the adjacent physis continued to grow normally (Fig. 3).
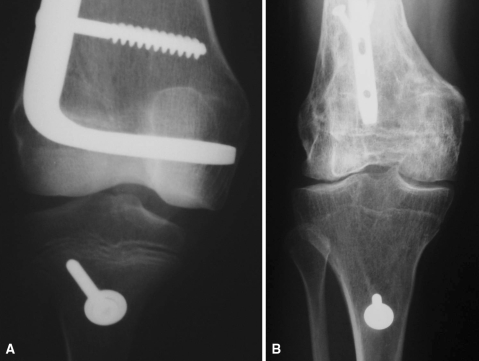
Fig. 3A–B.
Case 13 was a 7-year-old girl with an osteosarcoma in whom an osteoarticular allograft was performed. The patient had a diaphyseal nonunion in which the internal fixation was replaced and autogenous graft was added to the site, resulting in union at the host-donor junction. (A) This anteroposterior radiograph shows the knee immediately after initial surgery. Note purposely selected size disparity between the transplanted distal femur and the recipient proximal tibia considering the patient’s future growth. (B) This anteroposterior radiograph of the knee was made 10 years after original surgery. Note internal fixation changed from plate to nail to treat the nonunion and remodeling of the proximal tibia according to distal femur transplant size as a result of preservation of tibial growing physis.
Our data suggest, at least in short to intermediate followup, resection of bone sarcomas and reconstruction with an allograft in patients younger than 10 years old is acceptable considering complication rates, functional-radiographic scores, and allograft survival. Longer followup is needed to evaluate final limb-length discrepancy in those patients in whom growth potential was altered as a result of the loss of one physis during tumor resection.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his institution has approved the reporting of this case report and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Abudu A, Grimer R, Tillman R, Carter S. The use of prostheses in skeletally immature patients. Orthop Clin North Am. 2006;37:75–84. [DOI] [PubMed]
- 2.Alman BA, de Bari A, Krajbich JI. Massive allografts in the treatment of osteosarcoma and Ewing sarcoma in children and adolescents. J Bone Joint Surg Am. 1995;77:54–64. [DOI] [PubMed]
- 3.Arkader A, Viola D, Morris CD, Boland PJ, Healey JH. Coaxial extendible knee equalizes limb length in children with osteogenic sarcoma. Clin Orthop Relat Res. 2007;459:60–65. [DOI] [PubMed]
- 4.Arndt CA, Crist WM. Common musculoskeletal tumors of childhood and adolescence. N Engl J Med. 1999;341:342–352. [DOI] [PubMed]
- 5.Brigman BE, Hornicek FJ, Gebhardt MC, Mankin HJ. Allografts about the knee in young patients with high-grade sarcoma. Clin Orthop Relat Res. 2004,421:232–239. [DOI] [PubMed]
- 6.Cammisa FP Jr, Glasser DB, Otis JC, Kroll MA, Lane JM, Healey JH. The Van Nes tibial rotationplasty: a functionally viable reconstructive procedure in children who have a tumor of the distal end of the femur. J Bone Joint Surg Am. 1990;72:1541–1547. [PubMed]
- 7.Dick HM, Malinin TI, Mnaymneh WA. Massive allograft implantation following radical resection of high-grade tumors requiring adjuvant chemotherapy treatment. Clin Orthop Relat Res. 1985;197:88–95. [PubMed]
- 8.Eckardt JJ, Kabo JM, Kelley CM, Ward WG Sr, Asavamongkolkul A, Wirganowicz PZ, Yang RS, Elber FR. Expandable endoprosthesis reconstruction in skeletally immature patients with tumors. Clin Orthop Relat Res. 2000;373:51–61. [DOI] [PubMed]
- 9.Enneking WF, Dunham W, Gebhardt MC, Malawer M, Prichard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed]
- 10.Finn HA, Simon MA. Limb-salvage surgery in the treatment of osteosarcoma in skeletally immature individuals. Clin Orthop Relat Res. 1991;262:108–118. [PubMed]
- 11.Futani H, Minamizaki T, Nishimoto Y, Abe S, Yabe H, Ueda T. Long-term follow-up after limb salvage in skeletally immature children with a primary malignant tumor of the distal end of the femur. J Bone Joint Surg Am. 2006;88:595–603. [DOI] [PubMed]
- 12.Glasser D, Langlais F. The ISOLS radiological implant evaluation system. In: Langlais F, Tomeno B, eds. Limb Salvage: Major Reconstructions in Oncologic, Nontumoral Conditions. Heidelberg, Germany: Springer-Verlag; 1991.
- 13.Hillmann A, Hoffman C, Gosheger G, Krakau H, Winkelmann W. Malignant tumor of the distal part of the femur or the proximal part of the tibia: endoprosthetic replacement or rotationplasty. Functional outcome and quality-of-life measurements. J Bone Joint Surg Am. 1999;81:462–468. [DOI] [PubMed]
- 14.Kenan S, Bloom M, Lewis MM. Limb-sparing surgery in skeletally immature patients with osteosarcoma. The use of an expandable prosthesis. Clin Orthop Relat Res. 1991;270:223–230. [PubMed]
- 15.Kohler R, Lorge F, Brunat-Mentigny M, Noyer D, Patricot L. Massive bone allografts in children. Int Orthop. 1990;14:249–253. [DOI] [PubMed]
- 16.Manfrini M, Gasbarrini A, Malaguti C, Ceruso M, Innocenti M, Bini S, Capanna R, Campanacci M. Intraepiphyseal resection of the proximal tibia and its impact on lower limb growth. Clin Orthop Relat Res. 1999;358:111–119. [DOI] [PubMed]
- 17.Mercuri M, Capanna R, Manfrini M, Bacci G, Picci P, Ruggieri P, Ferruzzi A, Ferraro A, Donati D, Biagini R, De Maio M, Cazzola A, Campanacci M. The management of malignant bone tumors in children and adolescents. Clin Orthop Relat Res. 1991;264:156–168. [PubMed]
- 18.Muscolo DL, Ayerza MA, Aponte-Tinao LA. Massive allograft use in orthopedic oncology. Orthop Clin North Am. 2006;37:65–74. [DOI] [PubMed]
- 19.Muscolo DL, Ayerza MA, Aponte-Tinao LA, Ranalletta M. Partial epiphyseal preservation and intercalary allograft reconstruction in high-grade metaphyseal osteosarcoma of the knee. J Bone Joint Surg Am. 2004;86:2686–2693. [DOI] [PubMed]
- 20.Muscolo DL, Ayerza MA, Aponte-Tinao LA, Ranalletta M. Use of distal femoral osteoarticular allografts in limb salvage surgery. J Bone Joint Surg Am. 2005;87:2449–2455. [DOI] [PubMed]
- 21.Muscolo DL, Ayerza MA, Aponte-Tinao L, Ranalletta M, Abalo E. Intercalary femur and tibia segmental allografts provide an acceptable alternative in reconstructing tumor resections. Clin Orthop Relat Res. 2004;426:97–102. [DOI] [PubMed]
- 22.Neel MD, Heck R, Britton L, Daw N, Rao BN. Use of a smooth press-fit stem preserves physeal growth after tumor resection. Clin Orthop Relat Res. 2004;426:125–128. [DOI] [PubMed]
- 23.Neel MD, Wilkins RM, Rao BN, Kelly CM. Early multicenter experience with a noninvasive expandable prosthesis. Clin Orthop Relat Res. 2003;415:72–81. [DOI] [PubMed]
- 24.Ramseier LE, Malinin TI, Temple HT, Mnaymneh WA, Exner GU. Allograft reconstruction for bone sarcoma of the tibia in the growing child. J Bone Joint Surg Br. 2006;88:95–99. [DOI] [PubMed]
- 25.San-Julian M, Dolz R, Garcia-Barrecheguren E, Noain E, Sierrasesumaga L, Canadell J. Limb salvage in bone sarcomas in patients younger than age 10: a 20-year experience. J Pediatr Orthop. 2003;23:753–762. [DOI] [PubMed]
- 26.Veenstra KM, Sprangers MA, van der Eyken JW, Taminiau AH. Quality of life in survivors with a Van Ness-Borggreve rotationplasty after bone tumour resection. J Surg Oncol. 2000;73:192–197. [DOI] [PubMed]
- 27.Ward WG, Yang RS, Eckardt JJ. Endoprosthetic bone reconstruction following malignant tumor resection in skeletally immature patients. Orthop Clin North Am. 1996;27:493–502. [PubMed]
- 28.Weisstein JS, Goldsby RE, O’Donnell RJ. Oncologic approaches to pediatric limb preservation. J Am Acad Orthop Surg. 2005;13:544–554. [DOI] [PubMed]