Abstract
Fresh osteochondral allograft transplantation has been an effective treatment option with promising long-term clinical outcomes for focal posttraumatic defects in the knee for young, active individuals. We examined histologic features of 35 fresh osteochondral allograft specimens retrieved at the time of subsequent graft revision, osteotomy, or TKA. Graft survival time ranged from 1 to 25 years based on their time to reoperation. Histologic features of early graft failures were lack of chondrocyte viability and loss of matrix cationic staining. Histologic features of late graft failures were fracture through the graft, active and incomplete remodeling of the graft bone by the host bone, and resorption of the graft tissue by synovial inflammatory activity at graft edges. Histologic features associated with long-term allograft survival included viable chondrocytes, functional preservation of matrix, and complete replacement of the graft bone with the host bone. Given chondrocyte viability, long-term allograft survival depends on graft stability by rigid fixation of host bone to graft bone. With the stable osseous graft base, the hyaline cartilage portion of the allograft can survive and function for 25 years or more.
Introduction
Focal osteochondral defects of the knee in young, active patients can be a debilitating condition posing a complex treatment challenge. Due to their young age and high demands in their activity level, arthroplasty or arthrodesis surgery are not generally regarded reasonable solutions. For posttraumatic osteochondral defects, more biologic options to date have included realignment osteotomy, microfracturing, mosaicplasty, periosteal grafts, autologous chondrocyte transplantation, and osteochondral allograft transplantation. These procedures offer a biologic solution rather than an artificial bearing surface replacement with its inherent risks of early loosening and loss of bone stock for future surgeries.
Realignment osteotomies of the distal femur or the proximal tibia do not address the actual osteochondral defects and may be limited by under- or overcorrection of the mechanical axis and ligamentous pseudolaxity [28, 37]. Microfracturing procedures induce fibrocartilage tissue coverage over hyaline cartilage defects [6, 11, 47, 57]. Mosaicplasty is not a viable option for large osteochondral defects (greater than 3 cm in diameter and 1 cm in depth) and results in donor site morbidity and healing seams at the recipient site [7, 27]. Periosteal grafting and autologous chondrocyte transplantation provide surface coverage but do not address associated bone defects [8, 42–44, 47, 48].
Over the last few decades, fresh cadaveric osteochondral allograft (FOCA) transplantation has become an important treatment alternative for full-thickness osteochondral defects. A number of studies have supported its clinical efficacy, particularly for osteochondral defects greater than 3 cm in diameter and 1 cm in depth [24, 26, 34, 39, 49, 62]. Since the inception of a FOCA transplant program in 1972, 364 allograft transplantation procedures have been performed for posttraumatic knee defects at our institute as of July 2007. In our previous series [25], we reported 95% graft survival at 5 years, 80% at 10 years, and 65% at 15 years (Fig. 1). As a histologic correlate, we recently reported a 25-year posttransplantation allograft specimen demonstrating viable chondrocytes [38].
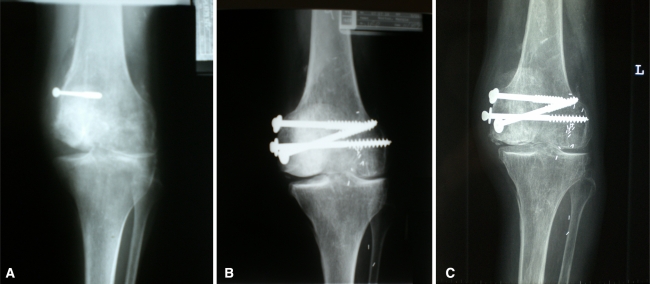
Fig. 1A–C.
(A) A radiograph shows traumatic loss of a medial femoral condyle in a 17-year-old girl. (B) A radiograph shows the same knee 10 years postreconstruction with FOCA. (C) A radiograph shows the same knee 20 years posttransplantation. There is now bicompartmental osteoarthritis.
Several previous studies suggest chondrocyte viability at the time of implantation is an important factor in ensuring long-term osteochondral allograft survival in vivo [2, 5]. Compared to frozen or cryopreserved allograft, fresh allograft offers superior chondrocyte viability (30% to 90%) up to 4 days when harvested within 24 hours of donor death and preserved at 4°C [14, 18, 51]. A number of studies confirm the longevity of the donor chondrocytes in the host environment [16, 17, 31–34, 40, 46]. This is thought to be mainly due to the avascular matrix that shields the donor chondrocytes from the host immune system [31–34]. In addition, the integrity of the osseous graft-host interface underlying the articular surface appears to play a critical role in successful clinical outcome [11, 15, 20]. The allograft bone, albeit necrotic, provides a structurally intact scaffold to support the overlying chondral surface [18, 46]. While chondrocyte viability is associated with successes, histologic characteristics of failures at varying times are unknown.
We therefore asked what histopathologic features characterized early and late failures of osteochondral allografts, and which characterized prolonged graft survival.
Materials and Methods
We reviewed the 69 known patients with allograft failure at our institution. Allografts from 41 patients were available for histopathologic examination. Of these 12 were biopsies and 29 consisted of allograft and host tissue retrieved at arthroplasty. On the basis of previous histologic observations, these were stratified as early graft retrieval (< 1 year; six patients; mean age, 53 years [range, 21–75 years]), mid-term graft retrieval (2–5 years; 11 patients; mean age, 45 years [range, 17–74 years]), and extended graft retrieval (> 6 years; 24 patients; mean age, 44 years [range, 17–78 years]).
Our histopathologic examination followed a standard protocol established with retrieval of the first graft in 1973 and modified only slightly since. All grafts were examined fresh immediately upon retrieval by the same team (AEG, KPHP). Immediately on removal, the graft surface was examined macroscopically to determine graft integrity, cartilage erosion, and synovial reaction.
To assess chondrocyte viability, sections were taken for electron microscopy. Where graft cartilage could be clearly identified, the sample taken for electron microscopic studies was usually near the midcoronal plane toward the lateral edge of the graft. We presumed this area representative of graft hyaline cartilage and least disturbed by mechanical forces. As well, the site was adjacent but did not involve tissue selected for light microscopy. For transmission electron microscopic studies, a selected sample of the articular cartilage was sectioned into five 1-mm blocks to select cartilage tissue. One-μm sections, stained with toluidine blue, were prepared. For electron microscopy, thin sections were then cut, stained with uranyl acetate and lead nitrate, and examined in a Philips 301 (Eindhoven, the Netherlands) or in later years a Philips CM100 electron microscope (Eindhoven, the Netherlands). We then examined the sections for cellular features of intact, active chondrocytes, including intact cellular membranes and organelles. The relationships of chondrocytes to the surface (cell polarity) and the surrounding matrix (staining) were also observed (Table 1).
Table 1.
Articular cartilage allografts: histologic findings
| Tissue examined | Early retrieval (< 1 year) (six cases) | Midterm retrieval (2–5 years; average, 2.9 years) (11 cases) | Long-term retrieval (> 5 years; average, 12 years) (24 cases) |
|---|---|---|---|
| Cartilage | Normal thickness and architecture | Normal thickness and architecture | Normal thickness and architecture |
| Retention of matrix and proteoglycan staining | Loss of matrix staining in the superficial and upper mid zones | Matrix staining normal except for superficial layer and upper mid zone | |
| Viable chondrocytes | Multiple chondrocytes within chondrons and some loss of chondrocyte polarity | Mostly viable chondrocytes with chondrocyte clusters and loss of chondrocyte polarity | |
| Bone | Graft bone structurally intact | Host bone extends to subchondral plate with orderly resorption of graft bone by host bone | Host bone extends to and is apposed to calcified cartilage zone but variable remnants of dead bone surrounded by live bone persist |
| No osteocytes in lacunae | |||
| Union of graft with host bone by 6 months |
Subsequently, a midcoronal cross-sectional block was taken through the allograft and adjacent host tissues. These blocks were radiographed using a Faxitron 43855B x-ray imaging system (Chicago, IL, USA). The radiograph was mutually compared to the tissue block and subsequently to the stained slides by one observer (KP). Features of interest noted included: bone density (mineralization) graft host interface integrity in bone and cartilage and cartilage erosion.
The block was then fixed in 10% neutral buffered formalin and decalcified in formic acid. Subsequently, the blocks were processed in graded ethanol solutions and embedded in paraffin. Five-micrometer sections of each block were cut, stained with hematoxylin and eosin, toluidine blue, and safranin O. We (KPHP, FLH) examined these sections by light and polarized light microscopy to assess preservation and morphologic features of graft articular cartilage: presence of degenerative arthritis, the morphology of graft cartilage of graft bone interface, the cartilage and bone graft host interface, and the subjacent host bone. One to three additional blocks of the graft were taken as required to clarify the features of the graft-host interface with cartilage and bone. As controls, midcoronal blocks were also routinely taken of the articular cartilage and bone of other compartments from the same joints. All blocks contained articular cartilage and subchondral bone.
Results
Among the six allografts examined within 1 year of transplantation, one showed hyaline articular cartilage in which there was necrosis and obliteration of all chondrocytes (Table 1). Another had focal lymphocytic inflammatory infiltrate in subchondral bone marrow. In that same patient within the synovial lining, there was focal lymphocyte aggregation beneath the synovial lining intermixed with shards of hyaline cartilage matrix. Initially, this was ascribed to an immune reaction against the graft, but subsequent followup revealed the inflammation represented chronic inflammatory arthritis in this patient since the patient had inflammatory joint disease. This is the only patient in our series in whom a prominent chronic inflammatory reaction was present in the grafts. In most of the early cases where a portion of the graft survived, the articular cartilage graft retained its surface thickness, architecture, and viable chondrocytes. Chondrocyte viability was demonstrated by morphologic features including intact nucleus and cytoplasm, ultrastructural features of intact cells with well-formed organelles and cytoplasmic processes, and retention of cartilage matrix proteoglycans as seen by intense cationic staining. Within 6 months posttransplantation, host appositional new bone formation was observed on the graft trabecular bone. The graft trabecular bone retained its structural integrity, but the bone was necrotic as indicated by absence of osteocytes in the bone lacunae. In the subchondral bone marrow, extensive fibrovascular tissue between the graft and host was observed, indicative of nonunion. Above the bone trabeculae nonunion areas, articular cartilage resorption could be observed.
In the midterm retrieval group of 11 patients, mean graft survival before biopsy/retrieval was 2.9 years. The intact graft cartilage showed focal loss of proteoglycan staining in the superficial and upper mid zones. Multiple chondrocytes were seen frequently within the chondrons, reflecting proliferative activity. Loss of chondrocyte polarity was observed, as well. By 5 years posttransplantation, within the bone, host appositional new bone formation extending to the subchondral plate with orderly resorption of the graft bone was commonly observed. At the graft periphery, graft trabeculae separated by fibrovascular tissue were seen. The bone trabeculae on each side of the fibrous tissue were mixed, consisting of necrotic graft bone on which viable appositional new bone was present. Superficial to the bone with incomplete repair, erosion of the graft hyaline articular cartilage was observed. Frequently, this was capped by fibrovascular connective tissue growing from the adjacent synovial margin.
In the group with extended survival grafts (24 patients), mean graft survival before biopsy/retrieval was 12 years. These grafts showed hyaline cartilage, which frequently retained its original intact surface thickness and architecture (Fig. 1, 2A–B). Cationic matrix staining was present except for the superficial layer and upper third of the mid zone (Fig. 2C). Although sporadic chondrocyte necrosis was observed, viable graft chondrocytes were present in a pattern similar to that observed in the mid-term grafts. By 6 years, the host bone was observed extending to the articular plate and was often seen apposed to the intact graft calcified cartilage (Fig. 2D). Even at 25 years posttransplantation, however, focal remnants of necrotic graft bone could be observed within the articular plate (Fig. 2E). At the lateral graft margin, host fibrocartilage could be seen closely apposed to the graft hyaline cartilage along its full thickness. However, a demarcation line could usually be discerned between the graft hyaline cartilage and host fibrocartilage, representing incomplete integration of the collagenous matrix between host and graft cartilage. Peripherally, host osteophyte formation could be seen adjacent to the host fibrocartilage (Figs. 1, 2). In the subchondral bone in the less stable areas, fibrovascular tissue and active host bone with osteoblasts and occasional osteoclasts were observed. In the least stable areas, host fibrovascular tissue extending from the synovial lining could be seen opposed to the graft hyaline cartilage. However, less erosion was seen than in the grafts that survived less than 6 years.
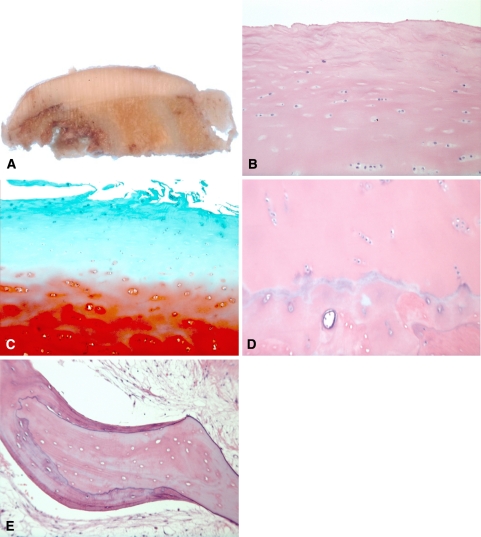
Fig. 2A–E.
The histopathologic findings of this patient at 20 years posttransplantation are shown. (A) A photograph shows a cross-section of the cartilage graft. Note preservation of graft thickness and integration of graft bone with host bone. (B) A photomicrograph shows the surface and superficial zone of the cartilage allograft (Stain, hematoxylin and eosin; original magnification, ×10). Note focal fallout of chondrocytes in the superficial zone and preservation of chondrocytes in the upper zone. The chondrocytes normally oriented “radially” are aligned more horizontally. (C) A photomicrograph shows the superficial zone of the cartilage allograft (Stain, safranin O [light green stain]; original magnification, ×10). The superficial cartilage matrix shows proteoglycan depletion. The deeper layer shows intense staining indicative of high proteoglycan concentration. (D) A photomicrograph shows the deep zone of the cartilage allograft (Stain, hematoxylin and eosin; original magnification, ×10). Note preservation of graft chondrocytes and tidemark. The bone subjacent to the tidemark is a mixture of necrotic graft bone and viable host bone. (E) A photomicrograph shows viable host appositional bone growing on necrotic graft trabecular bone (Stain, hematoxylin and eosin; original magnification, ×20).
In four of the 29 retrieved grafts in patients undergoing TKA, there was no remaining graft tissue present. The articular surface consisted of a mixture of fibrocartilage and host bone. All patients with this feature were observed within 2 years after transplantation. In two patients the fibrocartilage was of the same thickness as the original hyaline cartilage. The articular plate showed extensive fibrovascular connective tissue penetration through the bone and residual calcified cartilage.
Discussion
The fresh osteochondral transplant program was started at Mount Sinai Hospital, University of Toronto in 1972. The purpose of the program was to address osteochondral defects in patients who were too young for prosthetic replacement. Since then, other surgical modalities have been developed primarily using autograft tissue, but for large defects allograft tissue is necessary. We have always used fresh tissue because the chondrocytes are viable. This paper contains our data related to our retrieval studies which confirms the efficacy and safety of using fresh allograft tissue.
The major limitation of this study is that our results are related to only those patients that underwent further surgery, and the majority of our patients have never had tissue studies via biopsy or conversion to knee replacement. The data presented therefore does not reflect our best clinical results because most of the tissue is from patients who underwent subsequent surgery.
Osteochondral defects of the knee, regardless of etiology, have long been a therapeutic quandary for orthopaedic surgeons. Realignment osteotomy, microfracturing, mosaicplasty, periosteal grafts, and autologous chondrocyte transplantation are well-described surgical options for this difficult clinical entity [3, 6–9, 12, 23, 27, 36, 43, 45, 57, 59, 61]. Although these procedures have been implemented with varying degrees of success, no consensus exists on the gold-standard treatment. Technological advances continue to offer promising therapeutic options in the emerging field of biologic repair; however, a large osteochondral defect remains inadequately treated by the aforementioned procedures. This is principally due to the sheer bulk of both osseous and chondral tissues required to reconstitute the defect.
FOCA transplantation has yielded promising results over the last few decades [2, 4, 5, 13, 15, 16, 19–22, 24–26, 35, 39, 41, 52]. Our most recent series reported allograft survivorship of 95% at 5 year, 85% at 10 years, and 74% at 15 years for femoral condylar FOCA and 95% at 5 years, 80% at 10 years, and 65% at 15 years for tibial plateau FOCA [25]. These results also reflect the overall good to excellent functional and patient-based outcome scores reported in previous studies [5, 22, 25, 39, 56]. Our latest functional outcome study reported the mean modified Harris hip score of 85% and 86% good to excellent results at an average of 12 years (range, 5–24 years) in patients with intact FOCA [56].
As a clinical corollary to this study, patient-based outcome data were gathered from 40 age-matched cohorts who have not undergone further knee surgeries after their transplantation. Nineteen of 40 subjects were available for followup and showed a mean Oxford Knee Score of 17.5 (range, 12–40) at an average of 20.9 years (16–27 years) posttransplantation. Though many were lost to followup due to our international pool of cohorts and the lengths of followup, these excellent results underscore the potential longevity and efficacy of FOCA transplantation.
FOCA transplantation provides several distinct advantages of biologic joint reconstruction. A carefully matched and press-fitted (particularly by using a modern trephine technique) allograft plug results in an anatomic correction of an osteochondral lesion greater than 3 cm in diameter and 1 cm in depth. Healing seams such as those seen in mosaicplasty are thus eliminated. It also represents a biologic tissue that can accommodate meniscal or ligamentous tissue attachment. This in turn optimizes the potential for restoration of normal knee kinematics and stability to prolong joint preservation [1, 4, 5, 37]. Moreover, it does not cause any donor site morbidity associated with other biologic procedures. It can also decrease the complexity of subsequent knee arthroplasty by the restoration of the bone stock and the retention of ligamentous stabilizers that minimize joint malalignment.
FOCA transplantations have associated drawbacks as well. Risks of infectious disease transmission in fresh allograft specimens persist despite a strict adherence to the screening guidelines set forth by the American Association of Tissue Banks. The risks are less than or equal to those encountered in allogeneic packed red blood cell transfusion (1/493,000 for HIV, 1/103,000 for Hepatitis C, and 1/63,000 for Hepatitis B) [10, 29, 55]. Given the procedure is limited by the availability of donor tissue, it also requires an organized program that can facilitate prompt harvesting (within 24 hours postmortem), processing and storage of the allograft specimen, and emergent (less than 72 hours postharvest) transplantation into the host. Furthermore, potential complications of FOCA more pertinent to the focus of this study are allograft-host nonunion and allograft fracture or degeneration that ultimately lead to graft failure.
Previous studies have emphasized the importance of chondrocyte viability for FOCA survival [17, 39]. The main impetus behind the use of FOCA is its superior short- and long-term chondrocyte viability when compared to frozen or cryopreserved allograft [17, 38, 46, 53, 54, 58, 60]. This is believed a direct consequence of the chondrocyte loss being an early event related to suboptimal graft preservation such as in cryopreservation [20]. Correspondingly, our early failure specimens demonstrated the following key histopathologic features: lack of viable chondrocytes and cartilage matrix staining. Therefore, the importance of timely harvesting of allograft and transplantation, thus reducing the ex vivo, nutrient-deficient phase for the allograft, cannot be overemphasized. Interestingly, the presence of inflammatory cells and mediators associated with systemic inflammatory diseases was also noted in the early and late failure specimens. Histologically, resorption of the cartilage graft tissue by synovial inflammatory activity at the graft edges was observed. This, again, is not surprising given its detrimental effects on chondrocyte viability as seen in rheumatoid arthritis [34].
Chondrocyte viability continues to play an important role in the late graft failure as well. FOCA can undergo degeneration directly related to chondrocyte loss [40]. Clinically, a trend toward better FOCA survivorship with adjunct meniscal transplantation and realignment osteotomy (to shift weightbearing axis away from FOCA) was noted in our previous study [25]. We continue to recommend these adjunctive procedures with FOCA transplantation concurrently; their “antidegenerative” effects remain to be further defined with time.
Another key requirement of prolonged FOCA survival is the mechanical stability at the host-graft interface [30, 39, 50]. Only with a stable interface can the host bone grow appositionally on graft bone trabeculae and eventually replace the graft bone by creeping substitution, including the bone of the subchondral plate. Mechanical instability results in host-graft nonunion, fractures through the allograft, and active and incomplete remodeling of the allograft by the host bone. These are the characteristic histopathologic findings of late FOCA failures. In addition, the articular surface of failed FOCA commonly consisted of a thick fibrocartilage layer overlying the host bone with sclerosis. The presence of the fibrocartilage across the entire articular surface in this manner differs from that observed in osteoarthritic process. This finding suggests the integration of the graft bone into the host bone did not become complete. Subsequently, less stable host-graft interface produces the replacement of the entire hyaline cartilage surface with fibrocartilage. Thus, the fundamental cause of late FOCA failure appears to be graft instability leading to nonunion and continued remodeling at the host-graft interface, both bony and cartilaginous. From a technical point of view, a precisely matched and fitted allograft into the prepared host bed is of paramount importance in ensuring the stability.
Our data suggest chondrocyte viability and mechanical stability with replacement of graft bone by host bone at host-graft interface are crucial factors for long-term survival of FOCA. To promote long-term chondrocyte viability, we recommend prompt postmortem harvesting of the allograft and a short duration of ex vivo storage. Given chondrocyte viability, FOCA survival over the long term depends primarily on stable fixation of the allograft tissue to the host tissue by compression screws or more recently by press-fit dowels using a trephine technique. When these requirements are met, high clinical function can be expected over many years.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aubin PP, Cheah HK, Davis AM, Gross AE. Long-term follow-up of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2001;391:318–327. [DOI] [PubMed]
- 2.Bakay A, Csonge L, Papp G, Fekete L. Osteochondral resurfacing of the knee joint with allograft: clinical analysis of 33 cases. Int Orthop. 1998;22:277–281. [DOI] [PMC free article] [PubMed]
- 3.Bartlett W, Gooding CR, Carrington RW, Skinner JA, Briggs TW, Bentley G. Autologous chondrocyte implantation at the knee using a bilayer collagen membrane with bone graft.: a preliminary report. J Bone Joint Surg Br. 2005;87:330–332. [DOI] [PubMed]
- 4.Bayne O, Langer F, Pritzker KP, Houpt J, Gross AE. Osteochondral allografts in the treatment of osteonecrosis of the knee. Orthop Clin North Am. 1985;16:727–740. [PubMed]
- 5.Beaver RJ, Mahomed M, Backstein D, Davis A, Zukor DJ, Gross AE. Fresh osteochondral allografts for post-traumatic defects in the knee: a survivorship analysis. J Bone Joint Surg Br. 1992;74:105–110. [DOI] [PubMed]
- 6.Blevins FT, Steadman JR, Rodrigo JJ, Silliman J. Treatment of articular cartilage defects in athletes: an analysis of functional outcome and lesion appearance. Orthopedics. 1998;21:761–768. [DOI] [PubMed]
- 7.Bobic V, Carter T. Osteochondral autologous graft transfer. Oper Tech Sports Med. 2000;8:168–178. [DOI]
- 8.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. [DOI] [PubMed]
- 9.Brittberg M, Peterson L, Sjogren-Jansson E, Tallheden T, Lindahl A. Articular cartilage engineering with autologous chondrocyte transplantation: a review of recent developments. J Bone Joint Surg Am. 2003;85(Suppl 3):109–115. [DOI] [PubMed]
- 10.Buck BE, Malinin TI, Brown MD. Bone transplantation and human immunodeficiency virus: an estimate of risk of acquired immunodeficiency syndrome (AIDS). Clin Orthop Relat Res. 1989;240:129–136. [PubMed]
- 11.Buckwalter JA, Lohmander S. Operative treatment of osteoarthrosis: current practice and future development. J Bone Joint Surg Am. 1994;76:1405–1418. [DOI] [PubMed]
- 12.Buckwalter JA, Mankin HJ. Articular cartilage repair and transplantation. Arthritis Rheum. 1998;41:1331–1342. [DOI] [PubMed]
- 13.Bugbee WD, Convery FR. Osteochondral allograft transplantation. Clin Sports Med. 1999;18:67–75. [DOI] [PubMed]
- 14.Campbell CJ, Ishida H, Takahashi H, Kelly F. The transplantation of articular cartilage: an experimental study in dogs. J Bone Joint Surg Am. 1963;45:1579–1592. [PubMed]
- 15.Chu CR, Convery FR, Akeson WH, Meyers M, Amiel D. Articular cartilage transplantation: clinical results in the knee. Clin Orthop Relat Res. 1999;360:159–168. [DOI] [PubMed]
- 16.Convery FR, Meyers MH, Akeson WH. Fresh osteochondral allografting of the femoral condyle. Clin Orthop Relat Res. 1991;273:139–145. [PubMed]
- 17.Czitrom AA, Keating S, Gross AE. The viability of articular cartilage in fresh osteochondral allografts after clinical transplantation. J Bone Joint Surg Am. 1990;72:574–581. [PubMed]
- 18.Enneking WF, Mindell ER. Observations on massive retrieved human allografts. J Bone Joint Surg Am. 1991;73:1123–1142. [PubMed]
- 19.Fitzpatrick PL, Morgan DA. Fresh osteochondral allografts: a 6–10-year review. Aust N Z J Surg. 1998;68:573–579. [DOI] [PubMed]
- 20.Flynn JM, Springfield DS, Mankin HJ. Osteoarticular allografts to treat distal femoral osteonecrosis. Clin Orthop Relat Res. 1994;303:38–43. [PubMed]
- 21.Garrett JC. Fresh osteochondral allografts for treatment of articular defects in osteochondritis dissecans of the lateral femoral condyle in adults. Clin Orthop Relat Res. 1994;303:33–37. [PubMed]
- 22.Ghazavi MT, Pritzker KP, Davis AM, Gross AE. Fresh osteochondral allografts for post-traumatic osteochondral defects of the knee. J Bone Joint Surg Br. 1997;79:1008–1013. [DOI] [PubMed]
- 23.Gooding CR, Bartlett W, Bentley G, Skinner JA, Carrington R, Flanagan A. A prospective, randomised study comparing two techniques of autologous chondrocyte implantation for osteochondral defects in the knee: periosteum covered versus type I/III collagen covered. Knee. 2006;13:203–210. [DOI] [PubMed]
- 24.Gross AE, McKee NH, Pritzker KP, Langer F. Reconstruction of skeletal deficits at the knee: a comprehensive osteochondral transplant program. Clin Orthop Relat Res. 1983;174:96–106. [PubMed]
- 25.Gross AE, Shasha N, Aubin P. Long-term follow-up of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2005;435:79–87. [DOI] [PubMed]
- 26.Gross AE, Silverstein EA, Falk J, Falk R, Langer F. The allotransplantation of partial joints in the treatment of osteoarthritis of the knee. Clin Orthop Relat Res. 1975;108:7–14. [DOI] [PubMed]
- 27.Hangody LK, Karpati Z. Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects. Knee Surg Sports Traumatol Arthrosc. 1997;4:262–269. [DOI] [PubMed]
- 28.Insall JN, Joseph DM, Msika C. High tibial osteotomy for varus gonarthrosis: a long-term follow-up study. J Bone Joint Surg Am. 1984;66:1040–1048. [PubMed]
- 29.Jacobs NJ. Establishing a surgical bone bank. In Fawcett K, Barr HR, eds. Tissue Banking. Arlington VA: American Association of Blood Banks; 1987;67–96.
- 30.Kandel RA, Gross AE, Ganel A, McDermott AG, Langer F, Pritzker KP. Histopathology of failed osteoarticular shell allografts. Clin Orthop Relat Res. 1985;197:103–110. [PubMed]
- 31.Langer F, Czitrom A, Pritzker KP, Gross AE. The immunogenicity of fresh and frozen allogeneic bone. J Bone Joint Surg Am. 1975;57:216–220. [PubMed]
- 32.Langer F, Gross AE. Immunogenicity of allograft articular cartilage. J Bone Joint Surg Am. 1974;56:297–304. [PubMed]
- 33.Langer F, Gross AE, Greaves MF. The auto-immunogenicity of articular cortical. Clin Exp Immunol. 1974:12:31–37. [PMC free article] [PubMed]
- 34.Langer F, Gross AE, West M, Urovitz EP. The immunogenicity of allograft knee joint transplants. Clin Orthop Relat Res. 1978;132:155–162. [PubMed]
- 35.Locht RC, Gross AE, Langer F. Late osteochondral allograft resurfacing for tibial plateau fractures. J Bone Joint Surg Am. 1984;66:328–335. [PubMed]
- 36.Mandelbaum BR, Browne JE, Fu F, Micheli L, Mosely JB Jr, Erggelet C, Minas T, Peterson L. Articular cartilage lesions of the knee. Am J Sports Med. 1998;26:853–861. [DOI] [PubMed]
- 37.Matthews LS, Goldstein SA, Malvitz TA, Katz BP, Kaufer H. Proximal tibial osteotomy: factors that influence the duration of satisfactory function. Clin Orthop Relat Res. 1988;229:193–200. [PubMed]
- 38.Maury AC, Safir O, Las Heras F, Pritzker KP, Gross AE. Twenty-five year chondrocyte viability in fresh osteochondral allograft. J Bone Joint Surg Am. 2007;89:159–165. [DOI] [PubMed]
- 39.McDermott AG, Langer F, Pritzker KP, Gross AE. Fresh small-fragment osteochondral allografts: long-term follow-up study on first 100 cases. Clin Orthop Relat Res. 1985;197:96–102. [PubMed]
- 40.McGoveran BM, Pritzker KP, Shasha N, Price J, Gross AE. Long term chondrocyte viability in a fresh osteochondral allograft. Am J Knee Surg. 2002;15:97–100. [PubMed]
- 41.Meyers MH, Akeson W, Convery FR. Resurfacing of the knee with fresh osteochondral allograft. J Bone Joint Surg Am. 1989;71:704–713. [PubMed]
- 42.Minas T. Clinical methods of cartilage repair: autologous chondrocyte implantation for focal chondral defects of the knee. Clin Orthop Relat Res. 2001;391(Suppl):S349–S361. [DOI] [PubMed]
- 43.Minas TP. Autologous chondrocyte transplantation. Oper Tech Sports Med. 2000;8:144–157. [DOI]
- 44.Moran ME, Kim HK, Salter RB. Biological resurfacing of full-thickness defects in patellar articular cartilage of the rabbit: investigation of autogenous periosteal grafts subjected to continuous passive motion. J Bone Joint Surg Br. 1992;74:659–667. [DOI] [PubMed]
- 45.Newman AP. Articular cartilage repair. Am J Sports Med. 1998;26:309–324. [DOI] [PubMed]
- 46.Oakeshott RD, Farine I, Pritzker KP, Langer F, Gross AE. A clinical and histologic analysis of failed fresh osteochondral allografts. Clin Orthop Relat Res. 1988;233:283–294. [PubMed]
- 47.O’Driscoll SW. The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998;80:1795–1812. [PubMed]
- 48.O’Driscoll SW, Salter RB. The induction of neochondrogenesis in free intra-articular periosteal autografts under the influence of continuous passive motion: an experimental investigation in the rabbit. J Bone Joint Surg Am. 1984;66:1248–1257. [PubMed]
- 49.Parrish FF. Allograft replacement of all or part of the end of a long bone following excision of a tumor. J Bone Joint Surg Am. 1973;55:1–22. [PubMed]
- 50.Pritzker KP, Gross AE, Langer F, Luk SC, Houpt JB. Articular cartilage transplantation. Hum Pathol. 1977; 8:635–51. [DOI] [PubMed]
- 51.Rodrigo J, Thompson E., Travis C. 4 degree C preservation of avascular osteocartilaginous shell allografts in rats. Trans Orthop Res Soc. 1980;5:72. [PubMed]
- 52.Salai M, Ganel A, Horoszowski H. Fresh osteochondral allografts at the knee joint: good functional results in a follow-up study of more than 15 years. Arch Orthop Trauma Surg. 1997;116:423–425. [DOI] [PubMed]
- 53.Schachar N, McAllister D, Stevenson M, Novak K, McGann L. Metabolic and biochemical status of articular cartilage following cryopreservation and transplantation: a rabbit model. J Orthop Res. 1992;10:603–609. [DOI] [PubMed]
- 54.Schachar NS, McGann LE. Investigations of low-temperature storage of articular cartilage for transplantation. Clin Orthop Relat Res. 1986;208:146–150. [PubMed]
- 55.Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ. The risk of transfusion-transmitted viral infections. The Retrovirus Epidemiology Donor Study. N Engl J Med. 1996;334:1685–1690. [DOI] [PubMed]
- 56.Shasha N, Krywulak S, Backstein D, Pressman A, Gross AE. Long-term follow-up of fresh tibial osteochondral allografts for failed tibial plateau fractures. J Bone Joint Surg Am. 2003;85(Suppl 2):33–39. [DOI] [PubMed]
- 57.Steadman JR, Rodkey WG, Singelton SB, Briggs KK. Microfracture technique for full-thickness chondral defects: technique and clinical results. Oper Tech Orthop. 1997;7:300–305. [DOI]
- 58.Stevenson S, Dannucci GA, Sharkey NA, Pool RR. The fate of articular cartilage after transplantation of fresh and cryopreserved tissue-antigen-matched and mismatched osteochondral allografts in dogs. J Bone Joint Surg Am. 1989;71:1297–1307. [PubMed]
- 59.Tallheden T, Bengtsson C, Brantsing C, Sjogren-Jansson E, Carlsson L, Peterson L, Brittberg M, Lindahl A. Proliferation and differentiation potential of chondrocytes from osteoarthritic patients. Arthritis Res Ther. 2005;7:R560–R568. [DOI] [PMC free article] [PubMed]
- 60.Tomford WW, Duff GP, Mankin HJ. Experimental freeze-preservation of chondrocytes. Clin Orthop Relat Res. 1985;97:11–14. [PubMed]
- 61.Ulutas K, Menderes A, Karaca C, Ozkal S. Repair of cartilage defects with periosteal grafts. Br J Plast Surg. 2005;58:65–72. [DOI] [PubMed]
- 62.Volkov M. Allotransplantation of joints. J Bone Joint Surg Br. 1970;52:49–53. [PubMed]