Abstract
Large structural allografts used for reconstruction of bone defects after revision arthroplasty and tumor resection fracture up to 27% of the time from osteolytic resorption around the fixation screw holes and tendon or ligament attachment sites. Treating structural allografts before implantation with bisphosphonates may inhibit local osteoclastic processes and prevent bone resorption and the development of stress risers, thereby reducing the long-term fracture rate. Taking advantage of allografts’ open-pore structure, we asked whether passive soaking or positive-pressure pumping was a more efficient technique for delivering bisphosphonates. We treated matched pairs of ovine tibial allografts with fluids containing Tc-99m pamidronate and toluidine blue stain to facilitate indicator distribution analysis via microSPECT-microCT imaging and light microscopy, respectively. Surfactants octylphenoxy polyethoxy ethanol or beractant were added to the treatment fluids to reduce flow resistance of solutions pumped through the allografts. Indicator distribution after 1 hour of soaking produced a thin ring around periosteal and endosteal surfaces, while pumping for 10 minutes produced a more even distribution throughout the allograft. Flow resistance was reduced with octylphenoxy polyethoxy ethanol but unaffected with beractant. Pumped allografts displayed a more homogeneous indicator distribution in less time than soaking while surfactants enhanced fluid movement.
Introduction
Implantation of large structural bone allografts is one method to reconstruct bony defects created by the resection of primary and metastatic bone tumors. Such allografts have a long-term fracture rate as high as 27% [2, 3, 7, 8, 20, 24]. The osteoclastic resorption occurring around allograft screw holes leads to the development of stress risers that weaken the bone and cause it to fracture. We theorized local treatment of allograft bone with antiresorptive agents would inhibit local osteoclastic effects, prevent bone resorption, and minimize the magnitude of stress risers.
Bisphosphonates are used clinically to inhibit osteoclast-mediated bone resorption associated with many pathologic processes of bone [10, 11, 18, 19, 25]. They bind tightly to the hydroxyapatite crystals in mineralized bone [14, 23] with a half-time measured in years and matching the bone’s turnover period [18]. Frozen and freeze-dried cortical bulk allografts are not vascularized, and thus systemically administered bisphosphonates must passively diffuse into them from adjacent tissues. Similarly, soaking bone in solutions containing antiresorptive drugs is suitable for treating small pieces of bone, especially cancellous morsels whose surface-to-volume and surface-to-mass ratios are large. Because bone is an open-pore material [21], as an alternative strategy, we adapted a positive-pressure “water flooding” technique [5, 6] to pump fluids containing antiresorptive agents through the allograft.
Water flooding is a secondary recovery process used to extract the oil within the porous rock beneath an oil well whose internal pressure has been reduced following primary recovery. Aqueous fluids are pumped under pressure into regions surrounding the well and move through the porous rock displacing the oil towards the well head which pumps out a water-oil mixture. Fluid movement through the rock is facilitated by the inclusion of surfactants [12] to reduce the water’s surface tension so it will move more easily through the pores (ie, lowers the fluid’s flow resistance) and enhances its ability to displace oil within smaller pores and its distribution throughout the rock matrix. The net effect is to increase the oil recovery yield.
We conducted a preliminary study to investigate the feasibility of applying water flooding to bone using a 0.9% saline-toluidine blue dye-Tc-99m methyldiphosphonate solution and four matched pairs of canine femoral allografts. Tracer delivery with positive-pressure pumping was compared to simple soaking to determine which approach was more efficient for tracer and ultimately drug deposition throughout the porous bone matrix. Femoral allografts treated by pumping qualitatively had a more uniform tracer distribution and required a shorter treatment time than passive soaking.
We therefore tested the following hypotheses: (1) positive-pressure pumping of treatment fluids through ovine tibial allografts delivers drug throughout the bone matrix more rapidly than passive soaking, (2) the total quantity of drug delivered to bone is greater using positive-pressure pumping than passive soaking, and (3) addition of surfactants (ie, octylphenoxy polyethoxy ethanol [OPE] or beractant) to the treatment fluids reduces the fluids’ flow resistance when pumped through allografts.
Materials and Methods
We utilized 17 pairs of tibia harvested from mature female sheep; five pairs for the drug distribution experiment and 12 for the surfactant flow resistance experiment. All study protocols, including the tissue harvest, were approved by the MSKCC Institutional Animal Care and Use Committee.
The tibias were stripped of all soft tissues and cut transversely with a band saw at 3 cm from the plafond end. The medullary canal was cleaned using pressurized distilled water, and the bones were soaked in 70% ethanol for 1 hour to sterilize and defat the bone. This sequence is similar to that used for human allografts [9, 15, 26]. The weight and displacement volume of each bone was measured to determine its bulk density. Each bone was wrapped in saline-soaked gauze and stored at −26°C. The mean ± standard deviations of the tibias’ weight, length, and bulk density were 125 ± 19 g, 19 ± 2 cm, and 1.57 ± 0.1 g/cc, respectively; no difference (p > 0.64) between the right and left tibia was observed in any of these dimensional parameters. For the dye and Tc-99m APD distribution experiments, tibial pairs were thawed in distilled water; one was randomly selected for treatment by pumping and the contralateral by soaking.
The treatment media consisted of 0.9% NaCl solution (pH 7.0) containing 0.2% (w/v) toluidine blue (MW = 306) (Aldrich Chemical Co, Milwaukee, WI) with 25 mCi of Tc-99m pamidronate (APD) (T1/2 = 6.01 hours, 140 keV γ-rays). Toluidine blue is a cationic stain routinely used in pathologic processing of bone that binds to anionic moieties in the matrix. Tc-99m APD (MW = 279) [16] was used in this study in anticipation of its use as a SPECT tracer for nonradioactive pamidronate binding during the treatment of ovine tibial allografts that will be investigated as part of an in vivo study to determine the influence of antiresorptive drug-treated allografts on graft incorporation to host bone and their resistance to local osteolytic processes. The radiotracer was prepared by the Memorial Sloan-Kettering Cancer Center radiochemistry service and had an average radiochemical purity of 95%.
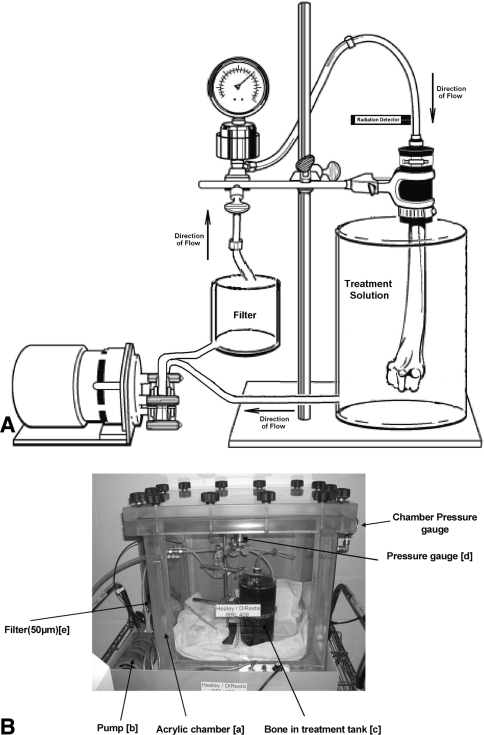
The apparatus used for positive-pressure pumping (Fig. 1A–B) consists of an acrylic chamber, fluid handling components, and pressure and radiation detection and recording systems. The chamber has a wall thickness of 3.5 cm capable of withstanding an internal vacuum pressure of −100 mmHg and includes a vacuum/pressure gauge and regulator. All exterior surfaces were covered with 1 mm lead to provide shielding against the Tc-99m’s 140 keV γ-ray emission. The fluid handling components include a 7553-20 MasterFlex® pump (Cole-Parmer Instrument Co, Vernon Hills, IL) with a 7015 peristaltic pump head and a 50-μm filter (Model 038B-2300; Keller Products, Lexington, MA), both external to the acrylic chamber which housed the rubber coupling for connecting the allograft to the fluid stream, and a 316 SS tall cylinder filled with fluid. A positive-pressure recirculation pumping scheme was used to treat the allograft. Fluid from within the cylinder was sucked into the pump head’s negative pressure inlet and then pushed through the filter directly to the bone. Pressurized fluid entered the bone through the cut end and medullary canal, moved through the matrix, and exited through the external surfaces back into the cylinder. Continuous measurements of fluid pressure were made at the rubber coupling-bone junction using a pressure gauge (Model 2-11-05-05-1, Cole-Parmer Instrument Co.) and pressure transducer designed and fabricated in-house that was attached to a DT154RS serial A/D module (Dataq, Inc., Akron, OH) connected to an IBM Model 770X laptop computer. A digital Geiger counter Model GCA-04 (Images SI, Inc., Staten Island, NY) was used to follow the time-dependent uptake of Tc-99m APD in bone by recording the activity of the recirculated treatment fluid. Its probe was placed inside a pinhole collimator and positioned against the fluid line feeding the allograft. The counter’s serial output was continuously acquired on the laptop computer.
Fig. 1A–B.
(A) The drawing presents the fluid handling components, in recirculation mode, with bone attached to rubber coupling. (B) Photograph of the acrylic pumping chamber ready to start pumping treatment. Lead shield is removed to visualize the fluid handling components.
The high-pressure treatment protocol began with securing the bone to the rubber coupling, closing the chamber, and identifying the flow rate to be used for treating the bone with the dye-Tc-99m APD solution. This was determined by first pumping saline through the bone to identify a flow rate such that the pressure at the bone-coupling junction (ie, back pressure) did not exceed 250 mmHg, the systolic arterial pressure. For our group of ovine tibia, this resulted in a typical flow rate of 1.2 mL/second. Once determined, the saline was replaced with 200 mL treatment solution. Vacuum was then applied to the chamber and regulated to a level of −50 mmHg as indicated by the chamber’s vacuum/pressure gauge. Pumping of the treatment solution through the tibia was then started. The fluid activity was followed with the Geiger counter system until the activity level stabilized, typically 20 minutes. At this point, the treatment solution was pumped out and replaced with 70% ethanol to remove the unbound dye and activity; this solution was not recirculated. The chamber was then opened and the bone removed. The surface fluid was pat dried and the bone was placed into a plastic specimen bag. The bone’s total bound Tc-99m APD activity was measured with a dose calibrator (Squibb Model CRC 17 Radioisotope Calibrator; ER Squibb and Sons, Inc., Princeton, NJ) and submitted for imaging in the XSPECT system (Gamma Medica, Northridge, CA), a small-animal microSPECT-microCT scanner.
The contralateral tibia was submerged in treatment solution for 1 hour [17]. Fluid within the canal was drained by gravity and the bone was then placed into a container of 70% ethanol for 5 minutes. The bone was removed from the ethanol, pat dried of its surface fluid, placed into a specimen bag, and assayed in the dose calibrator to measure its total bound Tc99m-APD activity. The bone was then imaged in the microSPECT-microCT scanner.
SPECT imaging was performed using pinhole collimation and scanning times were selected to acquire 100 K counts. Registered CT and SPECT images were obtained at 3 and 15 cm from the proximal cut end and at 1 cm from the distal end. Image quantitation was performed using a system calibration factor measured with a 50-mL cylindrical annular acrylic phantom with outer and inner diameters that produced a chamber thickness approximating that of the tibia’s cortex. The phantom was loaded with 20 g hydroxyapatite powder (Sigma Aldrich, St Louis, MO) and 20 mL saline. Tc99m activity (typically 0.8–1.2 mCi) was loaded into the phantom and mixed into the powder-saline solution. To simulate the porous cortical bone, the phantom was then placed into a centrifuge and spun for 5 minutes at 2000 rpm to compact the powder into a tight matrix. The phantom was placed into the dose calibrator to measure its activity. The pore volume over the SPECT’s axial segment was computed by knowing the hydroxyapatite particle and bulk densities, phantom annular geometry, and SPECT transverse section thickness. The phantom was placed into the microSPECT-microCT and imaged overnight at one location at several time points to develop a standard curve of activity (mCi) versus count rate per voxel over a single transverse slice. This factor was then used to convert the bone count rate into mCi. The bone-simulating shape and composition of the phantom account for the effects of attenuation and scatter of the imaging signal. Counts were decay-corrected to the time of the start of the SPECT acquisition. After imaging, in anticipation of histologic sectioning, the tibias were stored at −26°C for 3 days (12 half-lives of the Tc-99m) to allow near-complete decay of radioactivity.
SPECT-CT imaging was used to noninvasively visualize Tc-99m APD distribution and quantitate its concentration within the bone. The SPECT scanner count rate data were decay-corrected, reconstructed, and automatically registered to the CT image using the system’s software. The count rate/voxel was converted to mCi/voxel using the phantom calibration factor. The CT images were then used to select anatomical regions of interest (ROI) within the section because its spatial resolution is approximately 200 μm. The SPECT spatial resolution, approximately 1 mm, is too coarse to identify anatomical ROIs alone. The SPECT ROI, which contains the bone-bound Tc-99m APD concentration, is matched to the selected CT ROI using the registered image. The percent of Tc-99m APD uptake within the image slice was computed as the quotient of voxels with counts above background within the ROI to the total number of voxels within the ROI, and subsequently correlated to the percentage of dye uptake measured within the corresponding histologic sections.
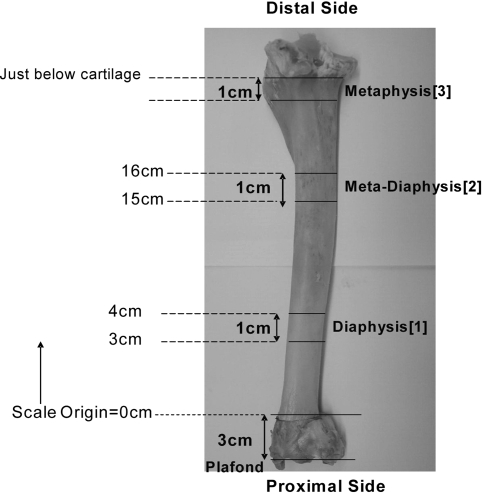
For histologic sectioning, we cut three 1-cm thick transverse sections from the frozen tibia using a band saw (Fig. 2) at the same locations selected for the SPECT imaging studies. Photographs of both sides of each section were acquired using a Coolpix® 880 digital camera (Nikon, Inc., Melville, NY) mounted to a Zeiss Model Urban Quadrascope® dissection microscope (Carl Zeiss Microimaging, Inc., Thornwood, NY) set to 20 × magnification. Model 4010 Micro-Mike® inspection and measuring microscope (DuMaurier, Inc, Virginia Beach, VA) was used to photograph the soaked bone.
Fig. 2.
Ovine tibia showing the cutting locations for the sections selected for histologic imaging of dye distribution.
Dye content within each bone section, reported as percent uptake, was determined as the quotient of dyed bone area to total bone area. The photomicrograph of each end of the section was segmented into four regions (anterior, medial, posterior, and lateral) to provide eight separate areas per segment. The dyed areas within each segment were measured using a manual, sequential color image analysis scheme with Photoshop v.7 (Adobe Systems, Inc., San Jose, CA) and SigmaScan Pro v5.0 (SPSS, Inc, Chicago, IL). Using Photoshop, the dyed pixels were converted to absolute black, and then the image was converted to a 256-level gray scale. This image was then transferred to SigmaScan Pro where total bone area and the absolute black area were determined by pixel count. The percent uptake within each section was computed as the mean of the eight regional percent uptake values.
Estimation of the time required for soaking to uniformly distribute tracer-dye throughout the allograft was performed using a mathematical simulation. The diffusion equation
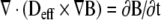 |
was used to estimate the time required for soaking to distribute dye throughout the bone. In this equation, Deff, is the effective diffusion coefficient (mm2/hr), t is time (hours), and B is toluidine blue dye concentration bound to cortical bone. The parameters estimation, performed with Flex PDEase (PDE Solutions, Inc., Spokane Valley, WA) used a dye image from a 1-hour soaked cortical segment.
We studied the influence of surfactants on flow resistance using 12 tibia treated with two surfactants, OPE (Triton-X-100®; Fisher Chemical, Fairlawn, NJ), a nonionic detergent, and beractant (Survanta®; Abbott Laboratories, Columbus, OH), a bovine lung extract used to treat pediatric respiratory distress syndrome. Beractant was chosen because it is an FDA-approved preparation that mimics the surface tension-lowering properties of normal lung surfactant. OPE (MW = 628) is a known wetting agent that reduces the surface tension of water [27]. It was prepared in two previously characterized [27] concentrations, 0.0001 mol/L and 0.003 mol/L in 0.9% NaCl adjusted to pH 7.0. One beractant concentration was prepared; 0.0628 g/mL which corresponds on a weight basis to the 0.0001 mol/L OPE solution. The control solution was 0.9% NaCl. No dye or isotope was included in the solution formulations. The measurements were performed using the acrylic treatment chamber described above within a 4°C cold room, however vacuum was not applied. Each bone was attached to the rubber coupling and fluids were pumped through the bone while backpressure was recorded continuously. The pumping flow rate, F, was varied between 0.3 to 5.0 mL/sec, a range that resulted in tibial backpressures that were within the pressure transducer’s dynamic range, 0 to 250 mm Hg. Since the fluid leaving the bone was at atmospheric pressure, the backpressure recorded is the differential pressure drop, ΔP, across the tibia. Using the fluid analog to Ohm’s law, ie, ΔP = F × R, the slope of the backpressure (ΔP) – flow (F) data is an estimate the bones’ flow resistance R to the treatment fluid.
Each of the 24 tibias was pumped first with control saline, then with 0.00001 mol/L OPE, and finally with 0.003 mol/L OPE. The backpressure was recorded as a function of flow rate for each bone. The flow rate was started at the low setting maintained and until the backpressure stabilized and then increased. Typically four to five flow rates were used; fluids were recirculated during these measurements. After the 0.003 mol/L OPE solution was used, the bone was flushed with saline without recirculation to prepare the bone for use with the beractant solution. Eight tibia were randomly selected from the 24 and treated with beractant. The bones were pumped with saline and then with the beractant solution as already described.
Comparisons of percentage dye, and percentage Tc-99m APD uptake within bone from pumping versus soaking study component were performed using Student’s t test. The slope, ie, R, associated with each ΔP – F data set obtained from the surfactant study component was estimated using linear regression analysis. The slope comparison method presented by Zar [28] was used to evaluate if surfactant presence reduced flow resistance between the control fluid versus fluids formulated with OPE or beractant All analyses were performed using Microsoft Excel XP (Microsoft Corp., Redmond, WA).
Results
We computed that effective diffusion coefficient is approximately 0.002 mm2/hr. With this value, we estimate more than 2000 hours of soaking would be required for uniform distribution of dye within the bone segment studied. This time is greater than the 20 minutes required to treat bone using high-pressure pumping.
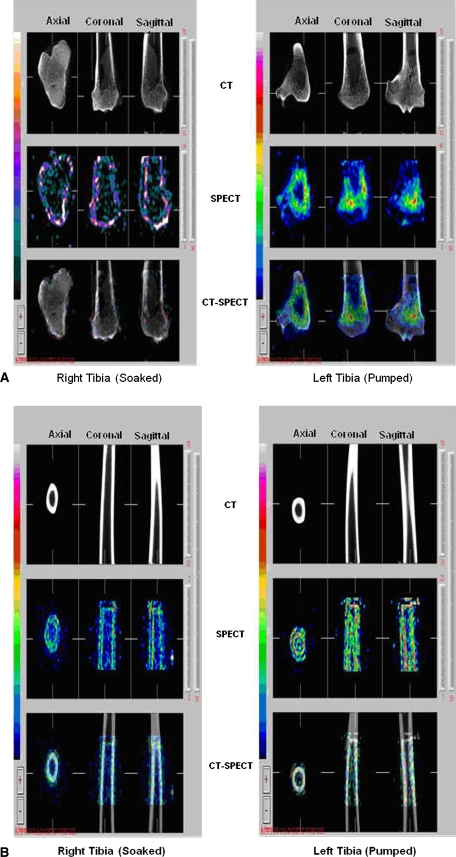
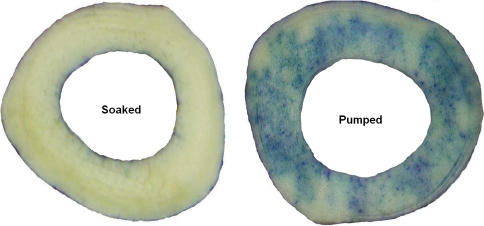
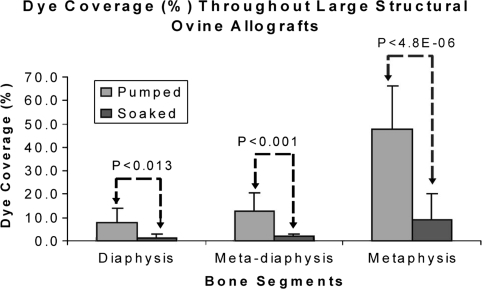
The total bone-bound Tc-99m APD activity in the pumped allografts (6.2 ± 2.6 μCi/g) was greater (p = 0.0247) than that in the soaked allografts (1.6 ± 0.6 μCi/g). SPECT-CT images were used to noninvasively assess the bound and unbound distribution of Tc-99m APD within the tibial allografts. The pumped bone had bound drug distributed throughout the medullary and cortical bone while the soaked bone only had a peripheral distribution of the radioactive tracer, ie, activity was restricted to the outer layers of the cortex. Representative CT, SPECT, and registered SPECT-CT images of metaphyseal (Fig. 3A) and diaphyseal regions (Fig. 3B) from two tibias illustrate the increased uptake of Tc-99m APD in the pumped bone over the soaked bone. Positive-pressurized pumping of the treatment solution delivered tracer to the endosteal and periosteal surfaces and to the inner cortical regions via the Haversian system. The soaking results demonstrated that after a 1-hour period, dye penetration is limited to thin bands along surfaces in direct contact with the treatment solution.; central portions of the cortical bone were undyed (Fig. 4). Dye content was greater (p < 0.001) in sections treated by pumping versus soaking in the all three zones (Fig. 5) with the greatest difference in the metaphyseal segments. A direct comparison of the percent uptake of Tc-99m APD to the percent uptake of dye at similar locations within the tibia (Table 1) demonstrate equivalent enhancement by pumping over passive soaking for both indicators. The percentage uptake was similar between the left tibia and right tibia for the two indicators (p > 0.25 and p > 0.67, respectively). Further, we observed no the difference in percentage uptake between soaking versus pumping using dye (p < 0.002) or Tc-99m APD (p < 0.003).
Fig. 3A–B.
(A) Soaked versus pumped tibia—metaphyseal CT image, metaphyseal SPECT image, metaphyseal registered CT-SPECT image. (B) Soaked versus pumped tibia—diaphyseal CT image, diaphyseal SPECT image, diaphyseal registered CT-SPECT image.
Fig. 4.
Photographic montage of section 1: Soaked bone (left image) and section 1: Pumped bone (right image) displaying the dye distribution throughout the section. These photographs are representative of the image data used in the percentage dye coverage analysis.
Fig. 5.
Comparison of percentage of dye uptake throughout soaked versus pumped tibia. In each section percentage of dye uptake was greater in pumped bone than in soaked bone.
Table 1.
Comparison of bone uptake of dye and radiolabeled pamidronate
| Bone (Treatment) | Dye (%) | Tc-99m Pamidronate (%) |
|---|---|---|
| Right tibia (soaked) | 5.5 ± 8.7 | 4.1 ± 4 |
| Left tibia (pumped) | 30.3 ± 22.7 | 22.8 ± 8.9 |
Values are expressed as mean ± standard deviation.
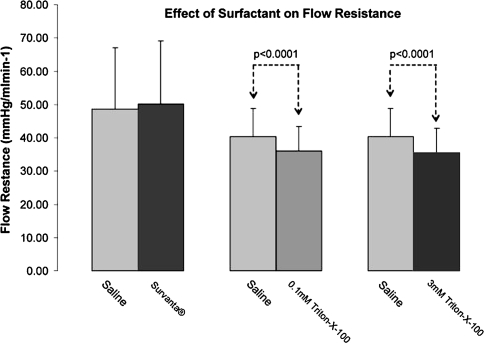
Both levels of OPE, ie, 0.0001 mol/L and 0.003 mol/L concentrations, produced a reduction (p < 0.001) in flow resistance over saline controls; however, the higher concentration produced no additional improvement (Fig. 6). Beractant, at the concentration used in this study, did not affect flow resistance (Fig. 6).
Fig. 6.
Comparison of surfactant. 0.9% NaCl solution formulations on flow resistance through ovine tibia showing. In both OPE solutions flow resistance was lower than in control solution.
Discussion
The dye and isotope uptake study component findings indicate high-pressure pumping of treatment fluids through large structural allografts is faster than soaking for delivering and distributing therapeutic agents within a bone matrix. The uptake advantage results because drug delivery proceeds by both convection and diffusion rather than by diffusion alone, a slow process. The surfactant study component findings indicate flow resistance will be reduced by their addition to a treatment solution; however, the choice of surfactant at the actual treatment temperature must be considered. Beractant did not reduce flow resistance through the bone. We suspect this may be due to its relative insolubility at 4°C given the milky appearance of the treatment solution; both OPE solutions were clear. The 4°C temperature was selected to reflect the treatment temperature used for processing freshly harvested tissue. Additional studies at 37°C, beractant’s recommended operating temperature, are required to investigate its ability to reduce surface tension.
The noninvasive microSPECT images of Tc-99m APD distribution within the bone (at a voxel resolution of 2–3 mm3) were corroborated by the histologic dye distribution images. While not a component of this study, these findings suggest SPECT imaging using a suitable bone phantom analytic approach can reliably quantitate the distribution of nonradioactive APD within allografts when tracer quantities of Tc99m-APD are added to the treatment.
Animal studies suggest pretreatment of bone allografts with bisphosphonates may be an effective therapeutic strategy for inhibiting osteoclastic resorption [1, 13, 22]. Observations from an early study demonstrated scapulae harvested from donor rats treated in vivo with the bisphosphonate methydiphosphonate and implanted into host rats experienced less resorption than scapulae harvested from untreated donor rats [22]. A subsequent rat study using donor cancellous bone allografts treated in vitro with alendronate and implanted into host rats determined the allografts were not resorbed and became infiltrated with the host rat’s bone [1]. However, a canine study using morselized allografts treated with alendronate determined that while osteoclastic resorption of the morsels was inhibited, their incorporation into host bone was also inhibited and new bone formation in regions adjacent to the morsels was blocked [13]. In each of these three studies, the amount of bisphosphonate bound to bone was not determined. The findings suggest a bisphosphonate-specific bone-bound level may exist that inhibits osteoclastic resorption with minimal effect on bone formation rate. Assuming this level was known for a specific bisphosphonate, the pretreatment of allografts with the bisphosphonate would require a procedure to rapidly deliver the drug uniformly throughout the bone, and have the ability to noninvasively monitor bound drug levels until the required level was achieved. Our observations suggest pumping treatment fluid containing tracer amounts of radiolabeled drugs through an allograft and monitoring its radioactive uptake of tracer drug using scintigraphic detectors, coupled with high resolution micro SPECT imaging could meet the methodologic requirements. Additionally, this technique, in conjunction with a suitable in vivo animal model [4], can be used to identify the bisphosphonate-specific bone-bound level required to pretreat an allograft to inhibit bone resorption with minimal adverse affects on its incorporation into the host skeleton.
We focused on a new delivery strategy for pretreating large allografts with drugs such as bisphosphonates and quantitating their bone-bound levels after treatment. Pumping fluids through the bone’s open pore and vascular structure is an alternative strategy to infusing drug to the cadaver prior to harvesting bone. The pumping fluid formulation is an important methodologic specification because in addition to the fluid’s drug concentration, its resistive properties (eg, viscosity, surface tension, etc) will influence the pumping pressure required to move the fluid through the porous media and the distribution homogeneity. We did not, however, consider the influence of bone-bound drug levels on inhibition of bone resorption following implantation or the influence other tissues within bone may exert on the drugs’ binding reaction. Addressing these important concerns requires a substantially more complex and costly in vivo study but is not germane to identifying the treatment fluid formulation and pumping specifications or posttreatment analysis. The findings demonstrate high-pressure pumping of aqueous solutions formulated with a suitable surfactant is a rapid technique for the local delivery and distribution of bisphosphonates or other biologics to structural allografts.
Acknowledgments
We thank Abbott Laboratories for donating the beractant (Survanta®), and Colorado State University College of Veterinary Medicine for donating the ovine sheep tibia.
Footnotes
One or more of the authors (CM, JHH) has received funding from the Orthopaedic Research and Education Foundation; the Musculoskeletal Transplant Foundation; and the Biomet Oncology Fellowship. We acknowledge technical services provided by the MSKCC Small-Animal Imaging Core Facility, supported in part by NIH Small Animal Imaging Research Program (SAIRP) grant #R24 CA83084 and NIH Center Grant #P30 CA 08748.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aspenberg P, Astrand J. Bone allografts pretreated with a bisphosphonate are not resorbed. Acta Orthop Scand. 2002;73:20–23. [DOI] [PubMed]
- 2.Berrey B, Lord C, Gebhardt M, Mankin H. Fractures of allografts, frequency, treatment, and end-results. J Bone Joint Surg Am. 1990;72:825–833. [PubMed]
- 3.Brigman B, Hornicek F, Gebhardt M, Mankin H. Allografts about the knee in young patients with high-grade sarcoma. Clin Orthop Relat Res. 2004;421:232–239. [DOI] [PubMed]
- 4.Donati D, Di Bella C. In vivo study on critical defects using the sheep model. Chir Organi Mov. 2005;90:31–39. [PubMed]
- 5.Enders A, Knight R. Incorporating pore geometry and fluid pressure communication into modeling the elastic behavior of porous rocks. Geophysics. 1997;62:106–117. [DOI]
- 6.Foster WR. Low-tension waterflooding process. J Petrol Tech. 1973:25:205–210.
- 7.Fox E, Hau M, Gebhardt M, Hornicek FJ, Tomford WW, Mankin HJ. Long-term followup of proximal femoral allografts. Clin Orthop Relat Res. 2002;97:106–113. [DOI] [PubMed]
- 8.Frenkel S, Jaffe WL, Valle CD, Jazrawi L, Maurer S, Baitner A, Wright K, Sala D, Hawkins M, Di Cesare PE. The effect of alendronate (fosamax) and implant surface on bone integration and remodeling in a canine model. J Biomed Mater Res. 2001;58:645–650. [DOI] [PubMed]
- 9.Friedlander G. Current concepts review bone-banking. J Bone Joint Surg Am. 1982;64:307–311. [PubMed]
- 10.Hortobagyi GN, Theriault RL, Lipton A, Porter L, Blayney D, Sinoff C, Wheeler H, Simeone JF, Seaman JJ, Knight RD, Heffernan M, Mellars K, Reitsma DJ. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. Protocol 19 Aredia Breast Cancer Study Group. J Clin Oncol. 1998;16:2038–2044. [DOI] [PubMed]
- 11.Hortobagyi GN, Theriault RL, Porter L, Blayney D, Lipton A, Sinoff C, Wheeler H, Simeone JF, Seaman J, Knight RD. Efficacy of pamidronate in reducing skeletal complications in patients with breast cancer and lytic bone metastases. Protocol 19 Aredia Breast Cancer Study Group. N Engl J Med. 1996;335:1785–1791. [DOI] [PubMed]
- 12.Hui M, Blunt M. Effects of wettability on three-phase flow in porous media. J Phys Chem B. 2000;104:3833–3845. [DOI]
- 13.Jakobsen T, Baas J, Bechtold JE, Elmengaard B, Saballe K. Soaking morselized allograft in bisphosphonate can impair implant fixation. Clin Orthop Relat Res. 2007;463:195–201. [DOI] [PubMed]
- 14.Jung A, Bisaz S, Fleisch H. The binding of pyrophosphate and two diphosphonates by hydroxyapatite crystals. Calc Tiss Res. 1973;11:269–280. [DOI] [PubMed]
- 15.Kainer M, Linden J, Whaley D, Holmes HT, Jarvis WR, Jernigan DB, Archibald LK. Clostridium infections associated with musculoskeletal-tissue allografts. N Engl J Med. 2004;350:2564–2571. [DOI] [PubMed]
- 16.Kumar D, Kumar V, Little DG, Howman-Giles RB, Wong E, Ali SO. Evaluation of biodistribution by local versus systemic administration of 99mTc-labeled pamidronate. J Orthop Sci. 2006;11:512–520. [DOI] [PubMed]
- 17.Leitman S, Tomford W, Gebhardt M, Springfield D, Mankin H. Complications of irradiated allografts in orthopaedic tumor surgery. Clin Orthop Relat Res. 2000;375:214–217. [DOI] [PubMed]
- 18.Lin J. Bisphosphonates: a review of their pharmocokinetic properties. Bone. 1996;18:75–85. [DOI] [PubMed]
- 19.Lipton A, Theriault RL, Hortobagyi GN, Simeone J, Knight RD, Mellars K, Reitsma DJ, Heffernan M, Seaman JJ. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer. 2000;88:1082–1090. [DOI] [PubMed]
- 20.Mankin H, Doppelt S, Tomford W. Clinical experience with allograft implantation. The first ten years. Clin Orthop Relat Res. 1983;174:69–86. [PubMed]
- 21.Martin B. Porosity and specific surface of bone. Crit Rev Biomed Eng. 1984;10:179–222. [PubMed]
- 22.Rosenquist JB, Baylink DJ. Resorption of bone implants. Int J Oral Surg. 1978;7:7–10. [DOI] [PubMed]
- 23.Russell RG, Rogers MJ, Frith JC, Luckman SP, Coxon FP, Benford HL, Croucher PI, Shipman C, Fleisch HA. The pharmacology of bisphosphonates and new insights into their mechanisms of action. J Bone Miner Res. 1999;14 Suppl 2:53–65. [DOI] [PubMed]
- 24.Sorger JI, Hornicek FJ, Zavatta M, Menzner JP, Gebhardt MC, Tomford WW, Mankin HJ. Allografts fractures revisited. Clin Orthop Relat Res. 2001;382:66–74. [DOI] [PubMed]
- 25.Theriault RL, Lipton A, Hortobagyi GN, Leff R, Glück S, Stewart JF, Costello S, Kennedy I, Simeone J, Seaman JJ, Knight RD, Mellars K, Heffernan M, Reitsma DJ. Pamidronate reduces skeletal morbidity in women with advanced breast cancer and lytic bone lesions: a randomized, placebo-controlled trial. J Clin Oncol. 1999;17:846–854. [DOI] [PubMed]
- 26.Tomford W, Doppelt S, Mankin H, Friedlander G. 1983 Bone Bank Procedures. Clin Orthop Relat Res. 1983;174:15–21. [PubMed]
- 27.Wu N, Dai J, Micale FJ. Dynamic surface tension measurement with a dynamic Wilhelmy plate technique J. Colloid Interface Sci. 1999;215:258–269. [DOI] [PubMed]
- 28.Zar H. Comparing simple linear regression equations. In: Zar JH. Biostatistical Analysis, 4th ed. Prentice-Hall: Upper Saddle River, NJ; 1999:360–376.