Abstract
Gamma radiation is widely used to sterilize bone allografts but may impair their strength. While radioprotectant use may reduce radiation damage they may compromise sterility by protecting pathogens. We assessed the radioprotective potential of various agents (L-cysteine, N-acetyl-L-cysteine, L-cysteine-ethyl-ester and L-cysteine-methyl-ester) to identify those which do not protect spores of Bacillus subtilis. We hypothesized charge of these agents will affect their ability to radioprotect spores. We also determined ability of these radioprotectants and a radiosensitizer (nitroimidazole-linked phenanthridinium) to selectively sensitize spores to radiation damage by intercalating into the nucleic acid of spores. Spores were treated either directly in solutions of these agents or treated after being embedded and sealed in bone to assess the ability of these agents to diffuse into bone. L-cysteine and L-cysteine-ethyl-ester did not provide radioprotection. Positively charged L-cysteine-methyl-ester protected the spores, whereas positively charged L-cysteine-ethyl-ester did not, indicating charge does not determine the extent of radioprotection. The spores were sensitized to radiation damage when irradiated in nitroimidazole-linked phenanthridinium solution and sensitization disappeared after rinsing, suggesting nitroimidazole-linked phenanthridinium was unable to intercalate into the nucleic acid of the spores. Some cysteine-derived radioprotectants do not shield bacterial spores against gamma radiation and may be suitable for curbing the radiation damage to bone grafts while achieving sterility.
Introduction
In 2002, an estimated 1.2 million bone allograft procedures were performed in the United States for repair or reconstruction of skeletal defects [19]. Although allografts are generally considered safe, isolated reports on transmission of viral and bacterial pathogens through allograft transplantation have been well documented, especially for osteochondral and soft tissue grafts which undergo more limited aseptic processing than bones [6, 10, 12–17, 20, 45, 48]. The current practice for minimization of disease transmission and infection includes donor screening, serology testing, and treatment with antimicrobial solutions. Donor history is not always available in detail or the available knowledge does not always preclude the risk of contamination. The serology testing is not free of errors such that it may provide false negatives or bacteriostasis may elude serological tests. On certain occasions, even if the source tissue is clean, infectious agents could be introduced to the graft during handling, processing or storage. Therefore, the aseptic processing is sometimes supplemented with terminal sterilization to assure the sterility.
Gamma radiation has been a popular method for sterilization of allografts as a result of its efficacy in viral and bacterial bioburden reduction with high penetration depth [8, 9, 11]. However, in vitro mechanical tests document that gamma radiation impairs the mechanical properties of bone [2, 4, 8, 33, 39] in a dose-dependent [5, 25] and state-dependent fashion [32]. Clinical followup of irradiated massive allografts has confirmed these in vitro tests by reporting a greater incidence of failure for radiated allografts [36]. Therefore, beneficial effects of gamma radiation on sterility must be balanced with its deleterious effects on graft stability. The major damage to biomolecules is induced by free radicals resulting from the radiolysis of water molecules during gamma radiation [31, 46] which react with target molecules and produce irrecoverable changes in the target molecules’ chemical structure [31]. In particular, the hydroxyl (-OH•) free radical induces the greater portion of damage during gamma radiation [3, 45, 46, 50]. Although the exact mechanisms by which gamma radiation impairs bone matrix are not completely understood, recent research supports the hypothesis that gamma radiation-induced allograft damage is caused, in part, by free radical attack to the molecular structure of collagen [3, 34].
Targeting the damaging free radicals with the use of radioprotecting free radical scavengers is emerging as a method for countering the damaging effects of gamma radiation [3, 29, 30, 42]. Cortical bone irradiated in the presence of the radioprotectant thiourea has superior biochemical and biomechanical properties compared to cortical bone irradiated in the absence of thiourea [3]. The beneficial effect of radioprotectants notwithstanding, there also exists the potential unintended consequence of radioprotection of pathogenic organisms that should be eradicated. In the light of the existing knowledge, we are faced with the problem of identifying whether radioprotectants impair the sterility of allografts.
Our first hypothesis was the potential of a given chemical agent to radioprotect bacterial spores (measured in terms of spore counts) will be a function of the chemical agent’s charge. The second hypothesis was bacterial spores can be selectively rendered susceptible to radiation damage by using radiosensitizers which target the DNA/RNA of spores. Finally, we had the technical aim of assessing the ability of several radioprotectants and a radiosensitizer agent to diffuse through bone matrix to elicit their effects on bacterial spores embedded in bone.
Materials and Methods
The key dependent variable involved in this study was the spore count and the independent variables were radiation, chemical treatment and the mode of treatment (in solution versus embedded in bone). Experiments were performed to assess (1) the baseline effects of cysteine derivatives and nitroimidazole-linked phenanthridinium (NLP) on bacterial spores, (2) the radioprotective and radiosensitizer effects on spores when irradiated while immersed in solutions and (3) the effects of radioprotectant and radiosensitizer on the spores embedded and isolated in bone and then immersed in solution (thereby access of agent to strip is by way of diffusion through the bone matrix). Should these agents diffuse through the matrix, then the baseline effects of agents would be similar. The power provided by four samples/group for comparison of five groups was obtained as 84% assuming the following: the maximum difference between any two groups is 3-logs and typical standard deviation was about 1-log. These assumptions were corroborated by the later results.
The ideal radioprotectant for bone allograft treatment should: (1) be biocompatible; (2) diffuse into bone matrix; (3) not protect the pathogens; and (4) dissolve in water. L-cysteine, which is water-soluble (according to the 2001 Merck Index), is a naturally occurring amino acid and an essential nutrient. Cysteine is a noncarcinogenic compound and has no toxic effects when administered at reasonable concentration levels on a daily basis. Its molecular weight is less than 300 Da. Compounds above this molecular weight cannot penetrate the fabric of bone [47]. The -SH group on the side chain of cysteine provides free radical scavenging capacity. Our earlier study [3] confirmed the radioprotective effect of thiourea on bone tissue, which also has an -SH group as the radioprotecting element. Thus, N-acetyl-L-cysteine (NAC; negative), L-cysteine-ethyl-ester (LCEE; positive), and L-cysteine-methyl-ester (LCME; positive) derivatives of L-cysteine (LC, zwitterionic) with varying charges were evaluated as radioprotectants. LC, LCME, LCEE and NAC (Acros Organics, Geel, Belgium; Sigma Aldrich, St Louis, MO) have comparable reaction rate constants with the hydroxyl radical ranging from 7.9 to 35 × 109 M−1 s−1 [7, 28, 41, 44, 49].
NLP is a bifunctional agent used in chemotherapy, which has a DNA/RNA intercalating phenanthridine group and an oxygen singlet generating nitroimidazole group on radiation [22–24, 37, 43]. The selective damage to DNA by means of these intercalating agents poses the major advantage of maintaining sterilization at lower doses of radiation. NLP has selective toxicity to hypoxic cells at 37°C, and it has been used to increase radiation damage to cancer cells [18, 21]. Therefore, NLP may potentially be used to enhance the targeting of microbial DNA/RNA during gamma-radiation sterilization. NLP is not commercially available. Therefore, it was synthesized (JS) following previously published protocols [27]. The sodium salt of azomycin (2-nitroimidazole) was treated with two-fold excess of dibromobutane in dimethylsulfoxide and removed by distillation. The residual oil was purified by chromatography over silica gel. This compound was refluxed in acetonitrile for 24 hours with phenanthridine. 5-[4-(2-nitro-1-imidazoyl)-butyl]-phenanthridinium bromide precipitated out of hot acetonitrile and was then isolated by filtration and further recrystallized from methanol/ether to get the water-soluble NLP salt. The structure of the newly synthesized NLP was characterized using proton nuclear magnetic resonance spectroscopy (VXRS-400 MHz, Varian Inc., Palo Alto, CA, USA) and mass spectrometry (Esquire, Bruker, Madison, WI, USA). The purity of synthetic intermediates and the target compounds was verified by thin layer chromatography, high-performance liquid chromatography, and elemental analysis.
The effect of LC, LCEE, LCME, or NAC and NLP on bacterial spores (Bacillus subtilis, 1.4 × 106 spores/strip, NAMSA, Northwood, OH, USA) was assessed. The LC, LCEE, LCME, and NAC solutions were prepared at 0.1 M in PBS (pH 7.3) and NLP was prepared at 0.2 mM in PBS. Experiments with higher concentrations of LCEE (0.5 M and 1 M) were also conducted to assess whether radioprotection has dose-response. The samples treated in PBS only were considered as controls. Specifically, PBS-treated irradiated samples were referred to as irradiated controls, whereas those treated with PBS only (without irradiation) were referred to as nonirradiated controls. All solutions were filtered with a 0.2-μm Whatman filter (Fisher Scientific, Pittsburgh, PA, USA) to remove extraneous bacteria. The spore strips were placed in 20 mL of radioprotectant or radiosensitizer solution of known concentration (four strips per solution) for 24 hours at room temperature to assess baseline effects.
Spore strips were irradiated in the range of 0.64 Mrad to 2.5 Mrad using a Co-60 radiation source (Gammacell 220; Nordion International Inc, Kanata, Canada) in radioprotectant and radiosensitizer solutions (Table 1). The applied radiation dosage was monitored using perspex dosimeters (Type 3042, Harwell Ltd., Oxfordshire, UK). The dose range utilized in this study extends below the range of 1 Mrad to 2.5 Mrad [1, 40] which is used in the tissue-banking practice. We utilized lower radiation dosage than usual practice because a radiation level of 0.5 Mrad is sufficient to kill most bacteria in the vegetative form [12] whereas 5-log reduction is reported in the viability of B. subtilis spores when irradiated at 0.78 Mrad [26]. Under certain treatment conditions, the spore strips which were irradiated at 2.5 Mrads were occasionally totally inactivated and did not yield spore counts. Accounting for the possibility that 2.5 Mrad would be an excessive dose and mask the potential protective effect of the radioprotectant, we utilized lower dose levels of 0.64 Mrad and 1.3 Mrad, as well. A greater dose that eliminates the bioburden (such as 2.5 Mrad) may not have allowed capturing a potential rebound in the viability of pathogens due to radioprotection. While 2.5 Mrad had occasionally inactivated B. subtilis in the current study, it is insufficient to inactivate HIV [35].
Table 1.
Representation of treatment groups treated with radioprotectants and a radiosensitizer at different doses of radiation
Following treatments, spore strips were incubated in a rotating incubator (Thermoforma, Thermo Scientific, Waltham, MA, USA) in 20 mL tryptic soy broth (TSB) media (Mobio Laboratories Inc., Carlsbad, CA, USA) for 14 days at 35°C. The incubated samples were serially diluted in TSB media and plated on Petri dishes with tryptic soybean-casein digest agar (Mobio Laboratories Inc.) (n = 4/dilution), and incubated for 24 hours at 37°C in the incubator after which colony counts were performed.
The ability of the radioprotectants and the radiosensitizer to penetrate the cortical bone matrix to induce the desired effect was assessed by embedding and sealing spore strips in cortical bone segments. In this case, the chemical treatment was conducted after sealing the spores in the cortical bone. Diaphyseal segments of cortical bone 40 mm long were sawed from the diaphysis at the midshaft regions of human femurs (Musculoskeletal Tissue Foundation, Edison, NJ, USA) using a low-speed saw (Isomet, Buehler, Lake Bluff, IL, USA). One-millimeter diameter through-holes were drilled at the midcortical thickness between the periosteum and endosteum. The longer axes of holes were parallel to the long axis of the diaphyseal shaft. Each treatment group (Table 2) was assigned one segment of cortical bone and each segment had four holes: one on anterior, posterior, medial and lateral sides of the bone. The midcortical thickness within a given segment varied from 4 mm to 6 mm, which amounted to a solution penetration distance of 1.5 mm to 2.5 mm. Before the insertion of spores, diaphyseal segments were immersed in 70% ethyl alcohol to minimize the presence of pathogens other than those on the spore strip. We did not encounter bacterial growth from these controls when they were incubated in the TSB media.
Table 2.
Treatment protocol for bone segments with embedded spore strips
| Treatment groups | Day 1 | Day 2 | Day 3 | Radiation |
|---|---|---|---|---|
| Nonradiated control | Phosphate buffered saline | Phosphate buffered saline | Phosphate buffered saline | 0 Megarad |
| Radiated control | Phosphate buffered saline | Phosphate buffered saline | Phosphate buffered saline | 0.64 Megarad |
| L-cysteine-treated-nonradiated | Phosphate buffered saline | Phosphate buffered saline | L-cysteine | 0 Megarad |
| L-cysteine-treated-radiated | Phosphate buffered saline | Phosphate buffered saline | L-cysteine | 0.64 Megarad |
| Nitroimidazole-linked-phenanthridinium-treated-nonradiated | Nitroimidazole-linked-phenanthridinium | Phosphate buffered saline | Phosphate buffered saline | 0 Megarad |
| Nitroimidazole-linked-phenanthridinium-treated-radiated | Nitroimidazole-linked-phenanthridinium | Phosphate buffered saline | Phosphate buffered saline | 0.64 Megarad |
A mini spore strip (Bacillus subtilis, 1.1 × 106 spores/strip, NAMSA, Northwood, OH, USA) was inserted into each hole under aseptic conditions. Both ends of the holes were sealed with self-tapping screws 6 mm in length. An O-ring was present between the cap of each screw and the surface of the bone to eliminate the entry of treatment solution to the hole. Therefore, the only means for the treatment solution to reach the spore strip was by diffusion through the cortex (ie, diffusion from periosteum to midcortex and endosteum to midcortex) when the segment was placed in treatment solution.
Diaphyseal segments were immersed in various solutions (Table 2) in a desiccator at −4°C for 24 hours. The control group was handled similar to treatment groups and it was suspended in PBS only. To evaluate intercalation ability of the radiosensitizer, we introduced an additional rinsing stage by suspending bone segments in PBS for 24 hours to remove the excess and unbound NLP molecules. The segments were wrapped in sterile gauze and irradiated in a sealed container packed with dry ice. A radiation dose of 0.64 Mrad was applied. Nonirradiated controls were kept at −4°C while other groups were irradiated. Following treatment, the mini spore strips were removed under aseptic conditions and incubated in 20 mL TSB media for 14 days at 35°C. The incubated samples were diluted serially, plated (n = 4/dilution) and colony counts were performed.
The colony count data obtained in this study is presented in the logarithmic scale and difference between the two groups is expressed at the level of log10. One-log reduction indicates a tenfold reduction in spore count compared to the respective control count. The data expressed as “baseline effect” reflect the effect of the radioprotectant or radiosensitizer alone on the viability of bacterial spores without any radiation being applied. The difference between groups was assessed by a Kruskall-Wallis test. When the Kruskal-Wallis test indicated a difference (p < 0.05), we performed a post hoc Mann-Whitney U-test to compare groups pairwise. The tests were performed using Minitab software (Minitab Inc., State College, PA, USA).
Results
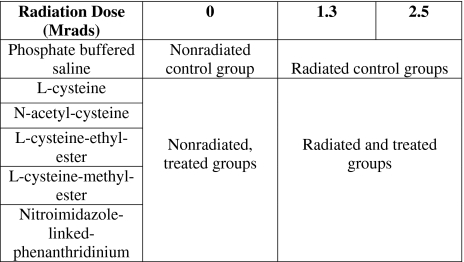
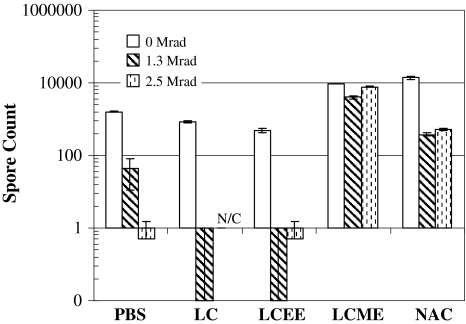
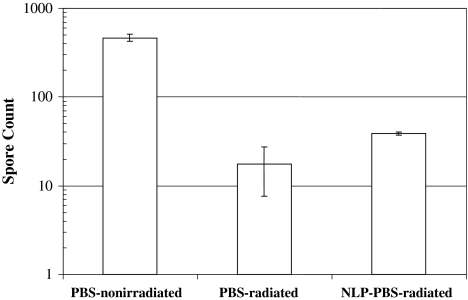
When the spore strips were treated with radioprotectants in the absence of radiation, the spore count in LCME- and NAC-treated spores increased (p = 0.007) by 1-log (Fig. 1) compared to control, thus LCME and NAC benefited the viability of bacteria. On the other hand, spore counts of LC- (p = 0.021) and LCEE-treated (p = 0.020) spore strips were decreased compared to controls. When the spore strips were irradiated in the absence of cysteine derivatives, the spore counts of radiated controls were reduced by 1.5-logs (p = 0.021) and 3-logs (p = 0.020) after gamma radiation at 1.3 Mrads and 2.5 Mrads respectively, when compared to nonradiated controls (Fig. 1). Spore counts of LCME-treated strips stayed at the same log level (p = 0.007) at both radiation levels, indicating LCME provided radioprotection. Spore counts from NAC-treated strips were reduced (p = 0.021) by 1.5-logs at 2.5 Mrad, which also indicates a radioprotective effect because the matching radiated controls displayed a 3-log reduction (p = 0.021) at the same radiation level. LCEE had 3-log reduction (p = 0.018) at 2.5 Mrad compared to nonradiated control and LC-treated spores at 2.5 Mrad were inactivated, yielding no counts, indicating that LC, LCEE did not provide radioprotection. Therefore, with all concentrations being equal, we chose to proceed with LC and LCEE because they neither protected the spores against radiation nor increased the baseline viability. The radiosensitizer affected the spore count as follows. NLP itself induced a reduction (p = 0.028) in baseline counts. Untreated radiated controls had approximately a 1-log reduction (p = 0.029) at 1.3 Mrad compared to spore count of nonirradiated controls, whereas strips irradiated in NLP at 1.3 Mrad had 2.5-logs of reduction (p = 0.021) compared to nonirradiated control (Fig. 2). This result indicates NLP sensitizes the spores to radiation damage when the spore strip is immersed in solution. At 2.5 Mrads of radiation, all groups attained total sterility regardless of NLP treatment.
Fig. 1.
At 0 Mrad, LCME and NAC increased spore count while LC and LCEE did not. At 1.3 Mrad and 2.5 Mrad, LC and LCEE did not elicit radioprotection whereas LCME and NAC protected the spores against radiation. N/C indicates that spores treated with LC and irradiated at 2.5 Mrad were inactivated, yielding no counts.
Fig. 2.
In the presence of NLP, gamma radiation-related reduction in the spore counts were more pronounced when compared to groups which were radiated in the absence of NLP.
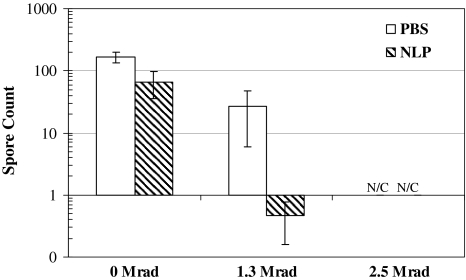
When comparing the radioprotection/radiosensitization of spore strips isolated in the bone matrix, a control bone without any strips embedded had no bacterial growth, confirmed the absence of bacterial pathogens in bone segments prior to our further experiments. Spore counts of NLP-treated bone-embedded strips were approximately half-log less (p = 0.021) than the strips immersed in NLP. NLP had some baseline toxic effect regardless of whether the strips were embedded in bone or suspended in solution (Fig. 3). Bone-embedded spore strips treated with NLP and rinsed with PBS were not radiosensitized because the log value was at the same order of magnitude (p = 0.021) with irradiated controls (Fig. 4). This observation suggests NLP is unable to bind to the nucleic material of spores because its sensitization effect was negated after rinsing.
Fig. 3.
The effects of LC and NLP on spore strips while immersed in solution were similar to spores treated while embedded in bone. Therefore, the agents were able to diffuse through bone to induce their effects. N/C indicates that spores irradiated in both PBS and NLP at 2.5 Mrad were inactivated, yielding no counts.
Fig. 4.
The radiosensitizing effect of NLP vanished after an additional PBS rinsing stage since the colony counts of NLP treated-rinsed samples were not smaller than the irradiated controls. Therefore, it does not seem likely that NLP binds to the DNA of spores.
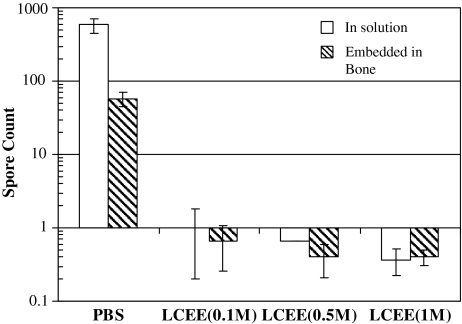
Neither LC nor LCEE protected bacterial spores (Fig. 1); the 0.1 M of concentration used may be an insufficient amount of radioprotectant to deliver a shield to bone matrix against radiation damage. Since, the solubility of LC is limited to 0.1 M; strips were irradiated in higher concentrations of LCEE solution. The spore count of LCEE-treated strips were smaller (p = 0.021 at 0.1 M, p = 0.020 at 0.5 M, p = 0.020 at 1 M) than irradiated controls (at 1.3 Mrads) irrespective of LCEE concentration (Fig. 5). This suggests higher concentrations of LCEE also do not have a radioprotective effect.
Fig. 5.
LCEE did not provide radioprotection at concentrations up to 1 M. Strips in both solution and embedded in bone were irradiated at 1.3 Mrad.
Discussion
While gamma radiation is widely used for sterilizing bone allografts it may impair their strength, and while radioprotectant use may reduce radiation damage it may compromise sterility by protecting pathogens. We sought to identify radioprotecting and radiosensitizing agents which can efficiently perfuse bone yet minimally protect pathogens. We therefore hypothesized the ability of a radioprotectant to shield bacterial spores is a function of its charge. Therefore, cysteine derivatives having similar free radical scavenging ability but with varying charges were utilized.
Several important limitations of the study should be considered in interpreting the outcomes. We assessed spores of an aerobic bacterial strain and further research is essential to test the approach on other types of spores, viral pathogens, and prions. Bacteria in vegetative form were not investigated. However, spores are more relevant to the tissue banking practice and spores are also much more resistant to radiation damage or excipient treatment. The radiation was administered at levels relevant to tissue-banking practice for some of the reported data; however, the dose application rate that can be provided by our radiation device is slower (total dose applied in days) than can be applied by commercial radiation sources (total dose applied in hours). It is known that applying the same dose in a slower fashion reduces the extent of gamma-radiation damage [38]. However, this was a comparison-based study and application of the radiation at a faster rate should not affect the overall outcome.
The baseline toxic effect of cysteine derivatives ranged from mildly cytotoxic (LC, LCEE) to nontoxic (LCME, NAC). This finding suggests that the chemical modifications, which changed the charge structures of these agents, also created other venues by which the spores were biologically affected. Although LC and LCEE had mild negative effects, LCME and NAC increased baseline viability tenfold, rendering LCME and NAC ineligible for further consideration. In assessing the radioprotective effect of an agent, a radioprotectant-treated irradiated group that had a higher or similar log value than its corresponding control-irradiated group indicated that protection of the pathogen has occurred. LCME and NAC were both unfavorable in this regard as they failed to reduce the spore count. LC and LCEE did not elicit radioprotection. These four scavengers have comparable affinities to OH radical [7, 28, 41, 44, 49] and therefore the absence of radioprotection of LC and LCEE can be explained by their inability to get within or around the spores. Our initial expectation was that charge-based differences could explain the differences in the radioprotective potential of these agents. However, both LCME and LCEE are positively charged molecules and their radioprotective effects on spores during irradiation were quite the opposite. Therefore, it seems there are factors involved other than the charge of the agent. The endospore-forming Bacillus subtilis spores are highly resistant to radiation and impermeable to most of the chemicals. Therefore, in the current experiments, LCEE may have failed to penetrate the extra protecting layers around the spore.
The results of radioprotectant and radiosensitizer on the spores in solutions and spores embedded in bone were compared to assess the penetration of these agents through the bone matrix. Our data suggest cysteine derivatives and NLP penetrate bone matrix and NLP is able to sensitize the spores to radiation damage. The radiosensitizer of our selection is used for cancer treatment [18]. NLP has been demonstrated to bind to the DNA of mammalian cells and generate oxygen singlets on radiation, rendering selective damage to the nucleic bases. This approach, within a sterilization context, would require treatment of the allograft with the nucleic acid-targeting agent. With the assumption that NLP binds to DNA, a successive rinsing procedure should remove the sensitizer from elsewhere but not from pathogen. Therefore, the radiosensitization effects should be limited to the pathogens, alleviating undesired radiosensitization of the matrix. The radiosensitization of NLP was confirmed when spore strips were irradiated immersed in NLP solution. However, when a subsequent rinsing process was applied, the radiosensitization effect vanished, suggesting that the expected intercalation of the agent to the nucleic material did not take place.
Radioprotectants of similar free-radical-scavenging capacities may differ in their radioprotective potential on spores. Therefore, a systematic evaluation of a wide variety of radioprotectants should be conducted to identify those compounds that can protect graft integrity without compromising sterility. The DNA intercalating radiosensitizer approach, at least for NLP, is not feasible. Further experiments will be essential to assess the effect of LC or LCEE on graft strength using biomechanical tests. These concepts are not limited to bone as a tissue, but any connective tissue would be expected to benefit from scavenger and radiosensitizer treatment.
Acknowledgments
We thank the Musculoskeletal Transplant Foundation for providing human femurs. We also thank Arun Mohan for assistance in preparing bone samples.
Footnotes
One or more of the authors (OA, JS, SAK) has received funding from a research grant from the Musculoskeletal Transplant Foundation.
References
- 1.AAMI Technical Information Report. AAMI TIR33:2005 – Sterilization of Health Care Products - Radiation - Substantiation of a Selected Sterilization Dose - Method VDmax. Arlington, VA: Association for the Advancement of Medical Instrumentation; 2006.
- 2.Akkus O, Belaney RM. Sterilization by gamma radiation impairs the tensile fatigue life of cortical bone by two orders of magnitude. J Orthop Res. 2005;23:1054–1058. [DOI] [PubMed]
- 3.Akkus O, Belaney RM, Das P. Free radical scavenging alleviates the biomechanical impairment of gamma radiation sterilized bone tissue. J Orthop Res. 2005;23:838–845. [DOI] [PubMed]
- 4.Akkus O, Rimnac CM. Fracture resistance of gamma radiation sterilized cortical bone allografts. J Orthop Res. 2001;19:927–934. [DOI] [PubMed]
- 5.Anderson MJ, Keyak JH, Skinner HB. Compressive mechanical properties of human cancellous bone after gamma irradiation. J Bone Joint Surg Am. 1992;74:747–752. [PubMed]
- 6.Asselmeier MA, Caspari RB, Bottenfield S. A review of allograft processing and sterilization techniques and their role in transmission of the human immunodeficiency virus. Am J Sports Med. 1993;21:170–175. [DOI] [PubMed]
- 7.Braams R. Rate constants of hydrated electron reactions with amino acids. Radiat Res. 1966;27:319–329. [DOI] [PubMed]
- 8.Bright RW, Burchardt H. The biomechanical properties of preserved bone grafts. In: Friedlander GE, Mankin HJ, Sell KW, eds. Osteochondral Allografts: Biology, Banking and Clinical Applications. Boston, MA: Little, Brown; 1984:241–247.
- 9.Bright RW, Smarsh JD, Gambill VM. Sterilization of human bone by irradiation. In: Friedlander GE, Mankin HJ, Sell KW, eds. Osteochondral Allografts: Biology, Banking and Clinical Applications. Boston, MA: Little, Brown; 1984:223–232.
- 10.Buck BE, Resnick L, Shah SM, Malinin TI. Human immunodeficiency virus cultured from bone. Implications for transplantation. Clin Orthop Relat Res. 1990;251:249–253. [PubMed]
- 11.Campbell DG, Li P, Stephenson AJ, Oakeshott RD. Sterilization of HIV by gamma irradiation. A bone allograft model. Int Orthop. 1994;18:172–176. [DOI] [PubMed]
- 12.CDC: Centers for Disease Control and Prevention. Hepatitis C virus transmission from an antibody-negative organ and tissue donor- United States, 2000–2002. MMWR Morb Mortal Wkly Rep. 2003;52:273–276. [PubMed]
- 13.CDC: Centers for Disease Control and Prevention. Invasive streptococcus pyogenes after allograft implantation-Colorado, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:1173–1176. [PubMed]
- 14.CDC: Centers for Disease Control and Prevention. Septic arthritis following anterior cruciate ligament reconstruction using tendon allografts — Florida and Louisiana, 2000. MMWR Morb Mortal Wkly Rep. 2001;50:1081–1083. [PubMed]
- 15.CDC: Centers for Disease Control and Prevention. Transmission of HIV through bone transplantation: case report and public health recommendations. MMWR Morb Mortal Wkly Rep. 1988;37:597–599. [PubMed]
- 16.CDC: Centers for Disease Control and Prevention. Unexplained deaths following knee surgery - Minnesota, 2001. MMWR Morb Mortal Wkly Rep. 2001;50:1080. [PubMed]
- 17.CDC: Centers for Disease Control and Prevention. Update: allograft associated bacterial infections-United States 2002. MMWR Morb Mortal Wkly Rep. 2002;51:207–210. [PubMed]
- 18.Chan P, Milosevic M, Fyles A, Carson J, Pintilie M, Rauth M, Thomas G. A phase III randomized study of misonidazole plus radiation vs. radiation alone for cervix cancer. Radiother Oncol. 2004;70:295–299. [DOI] [PubMed]
- 19.Committee on Research: Science and Therapy of the American Academy of Periodontology. Tissue banking of bone allografts used in periodontal regeneration. J Periodontol. 2001;72:834–838. [DOI] [PubMed]
- 20.Conrad EU, Gretch DR, Obermeyer KR, Moogk MS, Sayers M, Wilson JJ, Strong DM. Transmission of the hepatitis C virus by tissue transplantation. J Bone Joint Surg Am. 1995;77:214–224. [DOI] [PubMed]
- 21.Cowan DS, Kanagasabapathy VM, McClelland RA, Rauth AM. Mechanistic studies of enhanced in vitro radiosensitization and hypoxic cell cytotoxicity by targeting radiosensitizers to DNA via intercalation. Int J Radiat Oncol Biol Phys. 1992;22:541–544. [DOI] [PubMed]
- 22.Cowan DS, Matejovic JF, McClelland RA, Rauth AM. DNA-targeted 2-nitroimidazoles: in vitro and in vivo studies. Br J Cancer. 1994;70:1067–1074. [DOI] [PMC free article] [PubMed]
- 23.Cowan DS, Matejovic JF, Wardman P, McClelland RA, Rauth AM. Radiosensitizing and cytotoxic properties of DNA targeted phenanthridine-linked nitroheterocycles of varying electron affinities. Int J Radiat Biol. 1994;66:729–738. [PubMed]
- 24.Cowan DS, Panicucci R, McClelland RA, Rauth AM. Targeting radiosensitizers to DNA by attachment of an intercalating group: nitroimidazole-linked phenanthridines. Radiat Res. 1991;127:81–89. [DOI] [PubMed]
- 25.Currey JD, Foreman J, Laketic I, Mitchell J. Effects of ionizing radiation on the mechanical properties of human bone. J Orthop Res. 1997;15:111–117. [DOI] [PubMed]
- 26.DeLara J, Fernandez PS, Periago PM. Irradiation of spores of Bacillus cereus and Bacillus subtilis with electron beams. Innovat Food Sci EmergTechnol. 2002;3:379–384. [DOI]
- 27.Denny WA, Roberts PB, Anderson RF, Brown JM, Phil D, Wilson WR. NLA-1: a 2-nitroimidazole radiosensitizer targeted to DNA by intercalation. Int J Radiat Oncol Biol Phys. 1992;22:553–556. [DOI] [PubMed]
- 28.Enescu M, Cardey B. Mechanism of cysteine oxidation by a hydroxyl radical: A theoretical study. Chemphyschem. 2006;7:912–919. [DOI] [PubMed]
- 29.Grieb TA, Forng RY, Bogdansky S, Ronholdt C, Parks B, Drohan WN, Burgess WH, Lin J. High-dose gamma irradiation for soft tissue allografts: High margin of safety with biomechanical integrity. J Orthop Res. 2006;24:1011–1018. [DOI] [PubMed]
- 30.Grieb TA, Forng RY, Stafford RE, Lin J, Almeida J, Bogdansky S, Ronholdt C, Drohan WN, Burgess WH. Effective use of optimized, high-dose (50 kGy) gamma irradiation for pathogen inactivation of human bone allografts. Biomaterial. 2005;26:2033–2042. [DOI] [PubMed]
- 31.Halliwel B, Gutteridge JMC. Free Radicals in Biology, Medicine. Oxford UK: Clarendon Press; 1989.
- 32.Hamer AJ, Stockley I, Elson RA. Changes in allograft bone irradiated at different temperatures. J Bone Joint Surg Br. 1999;81:342–344. [DOI] [PubMed]
- 33.Hamer AJ, Strachan JR, Black MM, Ibbotson CJ, Stockley I, Elson RA. Biomechanical properties of cortical allograft bone using a new method of bone strength measurement. J Bone Joint Surg Br. 1996;78:363–368. [PubMed]
- 34.Hawkins CL, Davies MJ. Oxidative damage to collagen and related substrates by metal ion/hydrogen peroxide systems: random attack or site-specific damage? Biochimica et Biophysica Acta. 1997;1360:84–96. [DOI] [PubMed]
- 35.Kitchen AD, Mann GF, Harrison JF, Zuckerman AJ. Effect of gamma irradiation on the human immunodeficiency virus and human coagulation proteins. Vox Sang. 1989;56:223–229. [DOI] [PubMed]
- 36.Lietman SA, Tomford WW, Gebhardt MC, Springfield DS, Mankin HJ. Complications of irradiated allografts in orthopaedic tumor surgery. Clin Orthop Relat Res. 2000:214–217. [DOI] [PubMed]
- 37.Marciniec B, Ogrodowczyk M, Ambroz H, Przybytniak G. The effect of gamma-radiation on nitroimidazole derivatives. Acta Pol Pharm. 2000;57:95–99. [PubMed]
- 38.Miekka SI, Forng RY, Rohwer RG, MacAuley C, Stafford RE, Flack SL, MacPhee M, Kent RS, Drohan WN. Inactivation of viral and prion pathogens by gamma-irradiation under conditions that maintain the integrity of human albumin. Vox Sang. 2003;84:36–44. [DOI] [PubMed]
- 39.Mitchell EJ, Stawarz AM, Kayacan R, Rimnac CM. The effect of gamma radiation sterilization on the fatigue crack propagation resistance of human cortical bone. J Bone Joint Surg Am. 2004;86:2648–2657. [DOI] [PubMed]
- 40.Nguyen H, Morgan DA, Forwood MR. Sterilization of allograft bone: is 25 kGy the gold standard for gamma irradiation? Cell Tissue Bank. 2007;8:81–91. [DOI] [PubMed]
- 41.Nucifora G, Smaller B, Avery EC, Remko R. Transient radicals of DNA bases by pulse radiolysis - effects of cysteine and cysteamine as radioprotectors. Radiat Res. 1972;49:96–111. [DOI] [PubMed]
- 42.Ohan MP, Dunn MG. Glucose stabilizes collagen sterilized with gamma irradiation. J Biomed Mater Res A. 2003;67:1188–1195. [DOI] [PubMed]
- 43.Panicucci R, Heal R, Laderoute K, Cowan D, McClelland RA, Rauth AM. NLP-1: a DNA intercalating hypoxic cell radiosensitizer and cytotoxin. Int J Radiat Oncol Biol Phys. 1989;16:1039–1043. [DOI] [PubMed]
- 44.Rougee M, Bensasson RV, Land EJ, Pariente R. Deactivation of singlet molecular-oxygen by thiols and related compounds, possible protectors against skin photosensitivity. Photochem Photobiol. 1988;47:485–489. [DOI] [PubMed]
- 45.Simonds RJ, Holmberg SD, Hurwitz RL, Coleman TR, Bottenfield S, Conley LJ, Kohlenberg SH, Castro KG, Dahan BA, Schable CA. Transmission of human immunodeficiency virus type 1 from a seronegative organ and tissue donor. N Engl J Med. 1992;326:726–732. [DOI] [PubMed]
- 46.Stadtman ER. Oxidation of free amino acids and aminoacid residues by radiolysis and by metal-catalyzed reactions. Ann Rev Biochem. 1993;62:797–821. [DOI] [PubMed]
- 47.Tami AE, Schaffler MB, Knothe Tate ML. Probing the tissue to subcellular level structure underlying bone’s molecular sieving function. Biorheology. 2003;40:577–590. [PubMed]
- 48.Tomford WW, Mankin HJ, Friedlaender GE, Doppelt SH, Gebhardt MC. Methods of banking bone and cartilage for allograft transplantation. Orthop Clin North Am. 1987;18:241–247. [PubMed]
- 49.Varmenot N, Remita S, Abedinzadeh Z, Wisniowski P. Strzelczak G. Bobrowski K. Oxidation processes of N,S-diacetyl-L-cysteine ethyl ester: Influence of S-acetylation. J Phys Chem. 2001;105:6867–6875.
- 50.Ward JF. The yield of DNA double strand breaks produce intracellularly by ionizing radiation. Int J Radiat Biol. 1990;57:1141–1150. [DOI] [PubMed]