Abstract
Recent endeavors in tissue engineering have attempted to identify the optimal parameters to create an artificial ligament. Both mechanical and biochemical stimulation have been used by others to independently modulate growth and differentiation, although few studies have explored their interactions. We applied previously described fabrication techniques to create a highly porous (90%–95% porosity, 212–300 μm), 3-D, bioabsorbable polymer scaffold (polycaprolactone). Scaffolds were coated with bovine collagen, and growth and differentiation factor 5 (GDF-5) was added to half of the scaffolds. Scaffolds were seeded with mesenchymal stem cells and cultured in a custom bioreactor under static or cyclic strain (10% strain, 0.33 Hz) conditions. After 48 hours, both mechanical stimulation and GDF-5 increased mRNA production of collagen I, II, and scleraxis compared to control; tenascin C production was not increased. Combining stimuli did not change gene expression; however, cellular metabolism was 1.7 times higher in scaffolds treated with both stimuli. We successfully grew a line of mesenchymal stem cells in 3-D culture, and our initial data indicate mechanical stimulation and GDF-5 influenced cellular activity and mRNA production; we did not, however, observe additive synergism with the mechanical and biological stimuli.
Introduction
The anterior cruciate ligament (ACL) is one of the most frequently injured ligaments in the knee, with over 100,000 reconstructions performed annually [40]. Poor results with nonoperative management [26], particularly in those who wish to remain active [4], has led to increased numbers of surgical reconstruction. Today, surgical reconstruction can restore function and prevent instability and early cartilage deterioration in the knee with 90% success reported in one study [49]. There are, however, limitations to autografts (donor site morbidity such as muscle weakness, patellar fracture, and anterior knee pain) [49] and allografts (donor availability, fear of disease transmission) [16, 49]. As a result, artificially engineered ligaments suitable for ACL reconstruction have emerged as one promising alternative.
Current approaches to ligament and tissue engineering are aimed at optimizing the growth of an autologous population of cells grown on a biocompatible scaffold. First, an appropriate cell source with high proliferative potential that can be easily harvested and cultured must be identified. These cells are then grown on a platform that enables cell adhesion, proliferation, and the bulk production of organized collagen matrix. However, the ideal growth conditions for ligamentogenesis must be still identified, and the interplay between different stimuli is largely unknown. The growth conditions are selected based on their ability to enhance proliferation, direct differentiation, and promote the production of an organized extracellular matrix. Ligaments and tendons have a specific alignment of their collagen fibrils and serve to transmit forces, and therefore, it is anticipated mechanical strain in the external environment can upregulate or direct ligamentogenesis. Indeed, cyclic strain is promising since it upregulates fibroblast markers [2, 41]. Another category of promising stimuli is growth factors, including members of the TGF-B superfamily such as bone morphogenic protein (BMP) and growth and differentiation factor (GDF). GDF-5, in particular, shows great promise in ligament- and tendon-specific differentiation [44, 50].
Using such an ex vivo ACL substitute we addressed three questions regarding this ligament engineering approach: (1) if a biopolymer scaffold would support the adherence and proliferation of a stem cell line; (2) whether mechanical stimulation and/or GDF-5 would influence cellular proliferation; and (3) whether mechanical stimulation and/or GDF-5 would influence cellular differentiation.
Materials and Methods
We fabricated bioabsorbable polymer scaffolds that we seeded with a multipotent bone marrow stromal cell line, cultured under experimental conditions for 48 hours, then removed for analysis. Four experimental groups were created. In group one (control), no mechanical strain or exogenous growth factor was used. In group two, cyclic uniaxial 10% strain at 0.33 Hz was applied to the scaffolds. In group three, GDF-5 (1600 ng/scaffold) was administered by adding it to the collagen coating of the scaffold. In group 4, both mechanical stimulation and exogenous growth factor were used. Two scaffolds from each group were randomly selected for qualitative histologic analysis in order to confirm the presence of cells and to observe the pattern of growth. Two scaffolds from each group were randomly selected for an MTS-based cellular proliferation assay (3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium—see below) to estimate the size of the live cell population. Two scaffolds from each group were randomly selected for RNA extraction and RT-PCR analysis for specific genes of interest (collagen I, collagen III, tenascin C, and scleraxis) to identify potential cellular differentiation and ECM production. The experiment was repeated for a total n = 3.
A multipotent mouse bone marrow stromal cell (BMSC) line was obtained from ATCC (Manassas, VA; Designation D1 ORL UVA). Cells were cultured in medium consisting of Dulbecco’s modified Eagle’s medium (DMEM) with high glucose, L-glutamine, and sodium pyruvate (Invitrogen, San Diego, CA). The medium was supplemented with 10% fetal bovine serum (Omega Scientific, Tarzana, CA) and Gibco 1% antibiotic-antimycotic solution (Invitrogen). Cells were cultured on standard cell-culture-treated plastic, and culture plates were maintained in a humidified incubator at 37°C and 5% CO2. The medium was changed every 3 to 4 days, and cells were split 5:1 when plates reached 70% to 80% confluence. At passages 4 and 6, cells were cryopreserved in 10% (v/v) dimethyl sulfoxide. All experiments were performed with cells from passages 8 and 9.
We prepared racetrack-shaped scaffolds according to previously reported techniques [41, 42]. Briefly, a 20% (w/w) solution was created by dissolving polycaprolactone (Birmingham Polymers, Birmingham, AL) in chloroform and stirring on an orbital shaker. Methanol was then added to create a 30% (w/w) methanol concentration, again mixed on an orbital shaker. Sucrose with grain sizes 212–300 μm was mixed in to provide a 90% to 95% porosity scaffold. This mixture was packed into machined Teflon® molds to create racetrack-shaped scaffolds. Scaffolds were then placed in a vacuum freeze-drier overnight and stored in a dessicator until use. Before seeding, sucrose particles were leached in deionized water for 24 hours. Scaffolds were then sterilized in 70% ethanol for 30 minutes, followed by five washes in sterile phosphate-buffered saline (PBS).
Cell adhesion and proliferation were enhanced by the noncovalent coating of bovine collagen and GDF-5 to the scaffolds as previously described [41]. Briefly, purified bovine dermal collagen (Cohesion Technologies, Palo Alto, CA) was neutralized to pH 7.4 with 10X PBS and NaOH in an 8:1:1 ratio. This was then diluted with sterile water to a final concentration of 0.25 mg/mL. For half the scaffolds, GDF-5 (R&D Systems, Minneapolis, MN) was then added. Under sterile conditions, 100 μL of the appropriate solution was then serially added to each arm of the scaffold, with 30 minutes of drying time between coats to allow for collagen and growth factor adhesion. A total of 0.125 mg collagen and 0 versus 800 ng GDF-5 was added to each scaffold arm.
After the final coat was dry, 1 × 106 BMSCs in 100 μL medium were applied to each scaffold arm, and scaffolds were incubated for 2 hours at 37°C. The scaffolds were then rotated 180° and the process was repeated for the other side of each scaffold arm. A total of 4 × 106 cells were seeded onto each scaffold.
Under sterile conditions, we then mounted scaffolds in a custom bioreactor. Each chamber of the bioreactor houses up to six scaffolds, and linear strain was applied to each chamber by a programmable linear stepper motor (Arrick Robotics, Tyler, TX). Ten percent strain was applied in a triangular waveform at 0.33 Hz. The bioreactor was maintained in a humidified incubator at 37°C and 5% CO2.
After incubation in the custom bioreactor for 48 hours, two scaffolds from each chamber were randomly selected for histologic sectioning, and they were fixed with 10% formalin for 24 hours. Individual scaffold arms were then excised en bloc using a sterile No. 15 scalpel, and samples were embedded using a gelatin embedding technique developed previously [8]. Briefly, scaffolds were placed in a 5% (w/w) porcine gelatin (G1890; Sigma-Aldrich, St. Louis, MO) with 5% (w/w) sucrose solution in disposable plastic molds and heated in a rocking convection oven at 45°C overnight. Samples were then immersed in an acetone and dry ice bath for rapid freezing and stored at −80°C until sectioned. Samples were taken from four areas throughout the depth of the scaffold. Each area was spaced 2.5 mm apart, and 10 sections were taken from each area. Samples were sectioned at 10 μm on a cryotome, mounted on Superfrost® Plus slides (VWR Scientific, West Chester, PA), and stained with hematoxylin (Fisherbrand Gill #3; Fisher Scientific, Pittsburgh, PA) and eosin (Sigma-Aldrich) by the primary author. We qualitatively examined slides with standard light microscopy.
To quantify relative cell number and metabolism, a modification of Promega’s MTS-based assay was used (Promega, Madison, WI). 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, also known as MTS, is a tetrazolium compound that undergoes colorimetric change when reduced. In the presence of reducing agents such as NADH and NADPH, MTS turns deep purple with a peak absorbance at 490 nm. Thus, this assay measures cellular reductive capacity as a surrogate for overall cellular metabolism and cell number. Individual scaffold arms were placed into each well of a 12-well plate. Scaffolds were then incubated in 1000 μL culture medium and 100 μL MTS at 37°C and 5% CO2 for 10 minutes. Media was then transferred into a 96-well plate, and absorbance at 490 nm was measured using a Tecan F-200 multiwell plate reader (Tecan Inc, San Jose, CA).
Scaffolds were randomly selected for RT-PCR analysis and immediately frozen in a liquid nitrogen vapor-phase storage tank. Frozen scaffolds were then excised and crushed, and RNA was extracted using an RNeasy® Mini Kit (Qiagen, Valencia, CA). Briefly, cells from crushed scaffolds were lysed and homogenized using the Qiashredder® (Qiagen) protocol, and the resulting lysate was serially processed and washed using the RNeasy® protocol. To prevent DNA contamination, we included the optional use of RNase-free DNase (Qiagen). Real-time RT-PCR was performed with a QuantiTect® RT-PCR kit (Qiagen) in a 96-well plate configuration. mRNA probes for Mus musculus genes of interest, including procollagen Type I, procollagen Type III, tenascin C, and scleraxis were obtained from Applied Biosystems (Foster City, CA). Internal control was performed with TaqMan® GAPDH rodent control reagents (Applied Biosystems). Quantitative real-time PCR was performed with an ABI Prism® 7900 Sequence Detection System (Applied Biosystems) at UCLA’s Sequencing and Genotyping Core facility.
Initial data analysis was performed with Microsoft Excel (Microsoft, Redmond, WA), and statistical analysis was performed with STATA (Stata Corp, College Station, TX). MTS results are reported as mean ± standard deviation relative to control, and one-way ANOVA was performed to assess for differences between treatment groups. Similarly, gene expression is reported as mean ± standard deviation relative to control, and one-way ANOVA was used to assess for differences. When a difference between groups was identified by ANOVA, a comparison of group means was performed using a Student’s t test.
Results
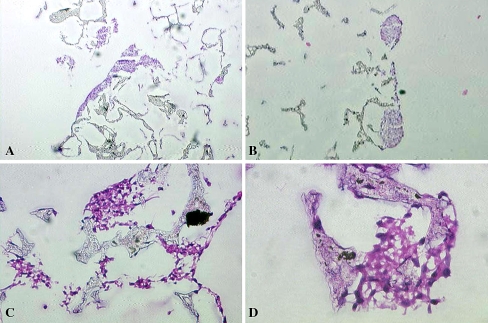
Histologic examination (Fig. 1) of the scaffolds demonstrated successful attachment and proliferation of cells in all groups. Qualitative observation suggested cell colonies tended to form along the surface of the scaffold, growing in large clusters. These clusters were noted in all treatment groups (Fig. 1A–B). Individual cells and small groups of cells were occasionally found throughout the scaffolds, including deep within the center of the scaffolds (Fig. 1C–D). Scaffolds treated with both GDF-5 and cyclic strain seemed to form the largest cell colonies, although quantitative analysis was not performed. Cell growth on the internal surfaces of scaffolds was most increased in the combined stimulus group, with groups of cells spanning between scaffold struts (Fig. 1C–D), although most of the cell growth remained confined to the superficial layers of the scaffold. At this early 48-hour time point, no obvious phenotypic changes were noted suggesting differentiation to a known cell or tissue type, and visible extracellular matrix production was essentially nonexistent.
Fig. 1A–D.
The cultured scaffolds demonstrated successful cell adhesion and growth (Stain, hematoxylin and eosin). (A) A low-power (×10) view of a scaffold treated with only mechanical stimulus shows a large cell colony on the surface of the scaffold. (B) A medium-power (×100) view of a scaffold treated with only GDF-5 also demonstrates a large cell colony on the surface of the scaffold. (C) High-power view (×500) of a scaffold treated with both cyclic strain and GDF-5 shows cell colonies within the substance of the scaffold, spanning scaffold struts. (D) Higher-power view (×1000) of a scaffold treated with both cyclic strain and GDF-5 shows a cell colony within the substance of the scaffold.
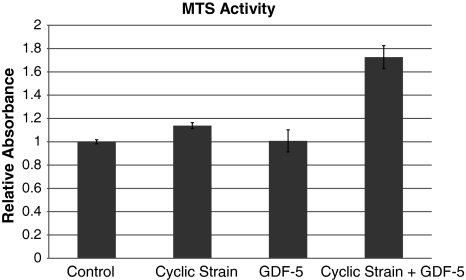
Incubation of MTS in the presence of multiple cell populations of known size confirmed the linear response of MTS to cell number. Reduction of MTS assay in the presence of cultured scaffolds confirmed the presence of live cells in the all scaffold groups but with differences (p = 0.02) between groups. Scaffolds treated by either cyclic strain or GDF-5 independently showed no increase (p > 0.05) in cellular activity compared to control (Fig. 2). However, scaffolds treated with both GDF-5 and cyclic strain demonstrated 1.7 times (p = 0.017) the reductive capacity as untreated controls.
Fig. 2.
Cellular proliferation was quantified by MTS reduction. Cultured scaffolds were incubated in the presence of MTS, which undergoes colorimetric change in the presence of cellular reductive agents. Scaffolds treated with both cyclic strain and GDF-5 demonstrated a 70% increase in cellular activity (p = 0.017). Values reported represent increases compared to nonstimulated controls. Error bars indicate 95% confidence interval of the mean.
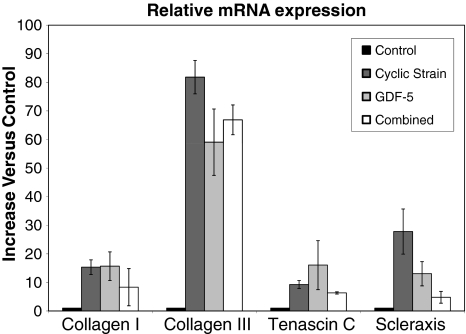
Production of mRNA was increased by both mechanical stimulation and GDF-5 when compared to unstimulated scaffolds (Fig. 3). Between the four groups we observed differences in collagen I (p = 0.0494), collagen III (p = 0.0009), and scleraxis production (p = 0.0096) but not tenascin C production (p = 0.07). Mechanical stimulation increased collagen I (15.3 ± 2.3; p = 0.012), collagen III (81.7 ± 5.2; p = 0.002), and scleraxis (27.8 ± 7; p = 0.03) mRNA production compared to control. GDF-5 increased collagen I (15.7 ± 4.4; p = 0.04), collagen III (59.1 ± 10.3; p = 0.01), and scleraxis (13 ± 3.8; p = 0.045) mRNA production. Combining the stimuli produced an increase (p = 0.004) only in the expression of collagen III (66.9 ± 4.6).
Fig. 3.
RT-PCR analysis of mRNA gene expression was performed on scaffolds that were either unstimulated or stimulated with uniaxial 10% cyclic strain at 0.33 Hz, GDF-5, or both. Either mechanical stimulation or GDF-5 increased gene expression of collagen I, collagen III, and scleraxis (p < 0.05), without increasing tenascin C (p > 0.05). Combined stimuli only increased expression of collagen III (p < 0.05). We observed no synergy. Values reported represent increases compared to nonstimulated controls. Error bars indicate standard deviation of the mean.
Discussion
The field of bioengineering may ultimately be able to supply artificially engineered tissues for use in humans; however, substantial challenges must be met before clinical use can be considered. Currently, there is no consensus regarding the ideal cell source, growth matrix, or growth conditions for ligament engineering. This study was undertaken to present the results of a novel technique using a pluripotent cell population, bioabsorbable scaffold, and promising combination of stimuli. Specifically, we wanted to evaluate the ability of cells to adhere to and survive on our 3-D matrix. Furthermore, we wanted to evaluate the influence of mechanical stretch and growth factor stimulation on cellular proliferation, as well as its ability to direct differentiation.
There are a number of limitations to our study that bear mentioning. First, the nature of the cell line selected for this study must be considered. As described below, the bone marrow stromal cell line used in this study is primarily osteogenic, though it has been used in adipogenic applications as well. We are not aware of any studies evaluating the tenocyte or fibroblast potential of this cell line. Indeed, one of the aims of our study is to identify the appropriate conditions for directing ligamentogenesis. Furthermore, the transformed nature of a cell line may limit the general applicability of any conclusions from this study, as a different cell population (such as harvested nonimmortalized cells) may respond differently to the stimuli used in this experiment. Still, the easy availability, relative robustness, and short doubling time of these cells compared to harvested cells makes them ideal for use in these types of investigative studies. Second, the conclusions we are able to draw from the histology are based on qualitative description only. The degree of cell clustering makes quantitative evaluation of cell area, aspect ratio, or cell number impossible. Third, the experimental design does not allow for any insight into the mechanism of action for these stimuli. For example, mechanical stimulation may upregulate growth by improving nutrient diffusion in the surrounding media rather than by direct stimulation of the cells. From the perspective of study design, however, the presence of a treatment effect must be established before the mechanism of action can be investigated; further research into the mechanism of action may be warranted in the future. Fourth, the experiment was limited to 48 hours of culture. Within this time frame, some cellular proliferation and early markers of lineage-specific differentiation may be present. Unfortunately, these early markers of tenocyte differentiation are not well-defined. Conclusive evidence of tenocyte formation and ligamentogenesis, such as the production of an organized collagen matrix, would not be expected at this early time point; longer culture periods are necessary. Naturally, we would like to observe these cells at multiple time points over extended culture; however, an exhaustive multivariable experiment is not technically feasible. Therefore, we chose to examine an early time point to ensure our selected stimuli did indeed have an effect on our cell population before moving to extended culture. Finally, for the purpose of these initial investigations, the number of genes assayed using RT-PCR technique used was limited. A complete panel could include other markers of differentiation (eg, bone specific markers); therefore, the ultimate differentiation pathway of these cells cannot be conclusively determined based on this gene profile. Future areas of investigation should be aimed at addressing these issues, including extended culture periods, evaluations of multiple time and doses, and additional genes of interest.
Because engineering an ACL substitute is one of the goals of ligament- and tendon-engineering projects, the ACL seems like a natural source for cells. However, in comparison to patellar tendon-derived fibroblasts [25], skin-derived fibroblasts [5], and MCL-derived fibroblasts [34], ACL-derived fibroblasts have limited proliferative capacity [5, 25, 34]. In fact, BMSCs may be superior to these candidates as targets for tissue-engineering applications, as BMSCs had higher collagen production and DNA content after seeding on polylactide/glycolide (PLGA) suture material than ACL or skin fibroblasts [47]. Similarly, others [28] have reported bone marrow mesenchymal stem cells had higher proliferation rate, collagen excretion, and durability in the knee when compared to ACL or MCL fibroblasts.
Therefore, most approaches have shifted to the use of mesenchymal stem cells, which can be harvested from the bone marrow. Mesenchymal stem cells have been used in multiple tissue-engineering applications, and they differentiate into multiple lineages, including osteoblasts, chondrocytes, adipocytes, and muscle/tendon forming cells [9–11, 23]. The multipotent, immortalized BMSC line used here is primarily osteogenic [24] and has been used in models of spinal fusion [19] and in the femur [22]. These cells also differentiate into adipocytes under the influence of steroids [20, 21] or alcohol [22]. The immortalized cell line used in this study was selected for its relative ease of use, rapid doubling time, and multilineage potential. We are not aware of any studies describing tenocyte differentiation of this cell line; indeed, one goal of ligament engineering as a field is to identify the necessary conditions to induce ligamentogenesis and tenocytic differentiation.
A multitude of different scaffold technologies have been investigated for use in tissue engineering. Some approaches involve materials currently used as suture material, including processed silk fibers [1, 12] and bioabsorbable polymers [7, 34]. Biologic substrates, including collagen gels [2, 6, 27], hyaluronan [17], and alginate/chitosan polymers [36] have also been used.
Our current scaffold structure is based on prior work in our laboratory [41, 42], which provides a platform for cell growth using a biodegradable material. Using these fabrication techniques, a wide variety of scaffold structures can be created using appropriately shaped molds. Unlike PLGA, polycaprolactone (PCL) has a low glass transition temperature of −60°C, and it is an amorphous solid at biologic temperatures. Therefore, it is capable of withstanding long-term cyclic strain. Furthermore, it is already an FDA-approved bioabsorbable polymer, sold under the brand name Monocryl® (Ethicon, Somerville, NJ). Our particle leaching technology provides 95% porous scaffold with known pore sizes, providing a high degree of interconnectivity to allow high uniform cell seeding and cell-cell interactions. The high porosity also helps with mass transport of nutrient and waste. Pilot studies have demonstrated our technique has 80% to 90% seeding efficiency using bone marrow stromal cells harvested from rats [41, 42].
Multiple growth factors have been used in tissue-engineering applications to stimulate proliferation and differentiation of cells, including FGF, TGF, platelet-derived growth factor (PDGF), EGF, and GDF [29, 30, 41]. However, it may be that a complicated sequence of growth factor administration is necessary to recapitulate the healing or embryogenesis of tendons and ligaments. One approach to addressing this multivariable problem has been fractional factorial design [39], which helps to identify the ideal media formulations and growth factor combinations for cell growth. Based on these results, a smaller group of growth factor combinations can be targeted for sequential administration to BMSCs; results from silk fiber matrices indicate mitogen/TGF-β-treated groups generally demonstrated both increased proliferation and collagen deposition in extended culture [38, 39].
Of the many proteins known to have mitogenic activity on mesenchymal tissue, the GDF-5, -6, and -7 (also known as BMP-14, -13, and -12, respectively) subfamily of proteins may be the most promising for applications involving tissue and ligament healing, regeneration, and engineering. As members of the TGF- β superfamily, these proteins induce tenocyte differentiation of bone marrow mesenchymal cells [48]. Receptor binding triggers members of the Smad family of nuclear transcription factors, including Smad-1, -5, and -8. Smad-8, in particular, promotes tenocyte differentiation of mesenchymal stem cells [32]. This family of proteins plays a clear role in the embryogenesis and differentiation of collagenous tissue, as GDF-deficient mice have abnormal tissue structure in the Achilles tendon [37] and in the tail [14]. More notably, decreased collagen content and tensile strength [37], as well as delayed healing, have been reported in the Achilles tendon of GDF-deficient mice [13]. Conversely, exogenous GDF protein delivered by carrier [3, 46], suture coating [43], or adenoviral transfection [44] improves tendon healing and tensile strength of transected tendons.
Even more promising, in vivo experiments of ectopic injection of GDF-5, -6, and -7 induces neotendon and ligament formation, suggesting GDFs act as signaling molecules during embryonic tendon and ligament formation [50]. Histologic examination of the induced tissue demonstrated organized collagen with regular periodicity, resembling neonatal tendon and ligament. Similar results have been reported with BMP-13 delivered via adenoviral transfection into athymic nude rats [31].
GDF-5 has been used recently for tissue engineering applications [33]. BMSCs treated with recombinant human TGF-β1 and GDF-5 were cultured on woven 3-D PLGA scaffolds over a period of 12 days. Both growth factors promoted cellular proliferation; however, only TGF-β1 increased collagen production.
Mechanical strain has been evaluated as a potential stimulus for cell proliferation and differentiation [2]. BMSCs seeded on collagen gels subjected to 10% longitudinal strain and 25% rotational strain at 0.0167 Hz for 21 days demonstrated an upregulation of ligament fibroblast markers, including collagen I, collagen III, and tenascin C. In these studies, there was no evidence of osteogenic or chondrogenic differentiation, with no upregulation of bone sialoprotein, collagen II, osteocalcin, or osteopontin. Similarly, dermal fibroblasts seeded on collagen gel constructs subjected to uniaxial cyclic tensile strain (10% strain, 1 Hz) had improved cell viability with cyclic strain; constructs preloaded at 2 mN also had increased collagen production [6].
As true ligament and tendon embryogenesis occurs in a complex environment with an array of external signals and stimuli, it is unlikely ligament engineering will depend on a single external influence. Rather, it will be necessary to identify the appropriate sequence of culture conditions, as well as the timing and nature of growth factors and nutrients. While some have examined growth factor combinations in 2-D systems [6], we are not aware of studies examining the interaction between mechanical stimulation and growth factor administration in a 3-D system. Previous work by our group [41, 42] has demonstrated both stimuli can independently enhance cellular differentiation and these independent effects can be maintained when both stimuli are used in concert.
The field of tissue and ligament engineering is still relatively young, and current techniques have yet to produce a suitable candidate for clinical ACL reconstruction. One problem has been the lack of clear, specific markers of tenocyte differentiation, although scleraxis [18] and tenomodulin appear promising candidates [45]. Future work must be performed to examine the effects of these stimuli in extended culture conditions, as the production of an organized collagen matrix will likely require longer culture periods.
We have developed a novel system allowing for the delivery of mechanical and biochemical stimuli to a 3-D construct seeded with candidate cells. This system has been successfully used to demonstrate the adhesion and proliferation of a multipotent mesenchymal cell line onto a bioabsorbable 3-D construct, with cellular viability throughout the scaffold. Furthermore, both mechanical stimulation and biochemical stimuli can be applied. Preliminary results suggest that, while mechanical stimulation and GDF-5 alone does not increase cellular proliferation, the combination of these factors can increase cell number after 48 hours of culture. Either mechanical stimuli or GDF-5 can increase expression of collagen I, collagen III, and scleraxis, although the combination of these stimuli does not appear to have any increased effect on the pattern of gene expression.
Acknowledgments
We thank Dr. Benjamin M. Wu, Dr. Michael Eagan, and Dr. Frank Petrigliano for their assistance and contributions to this work.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article. The institution of the authors has received funding from the Orthopaedic Hospital Foundation.
References
- 1.Altman GH, Horan RL, Lu HH, Moreau J, Martin I, Richmond JC, Kaplan DL. Silk matrix for tissue engineered anterior cruciate ligaments. Biomaterials. 2002;23:4131–4141. [DOI] [PubMed]
- 2.Altman GH, Horan RL, Martin I, Farhadi J, Stark PR, Volloch V, Richmond JC, Vunjak-Novakovic G, Kaplan DL. Cell differentiation by mechanical stress. Faseb J. 2002;16:270–272. [DOI] [PubMed]
- 3.Aspenberg P, Forslund C. Enhanced tendon healing with GDF 5 and 6. Acta Orthop Scand. 1999;70:51–54. [DOI] [PubMed]
- 4.Barrack RL, Bruckner JD, Kneisl J, Inman WS, Alexander AH. The outcome of nonoperatively treated complete tears of the anterior cruciate ligament in active young adults. Clin Orthop Relat Res. 1990;259:192–199. [PubMed]
- 5.Bellincampi LD, Closkey RF, Prasad R, Zawadsky JP, Dunn MG. Viability of fibroblast-seeded ligament analogs after autogenous implantation. J Orthop Res. 1998;16:414–420. [DOI] [PubMed]
- 6.Berry CC, Shelton JC, Bader DL, Lee DA. Influence of external uniaxial cyclic strain on oriented fibroblast-seeded collagen gels. Tissue Eng. 2003;9:613–624. [DOI] [PubMed]
- 7.Bourke SL, Kohn J, Dunn MG. Preliminary development of a novel resorbable synthetic polymer fiber scaffold for anterior cruciate ligament reconstruction. Tissue Eng. 2004;10:43–52. [DOI] [PubMed]
- 8.Brown DA, Chou YF, Beygui RE, Dunn JC, Wu BM. Gelatin-embedded cell-polymer constructs for histological cryosectioning. J Biomed Mater Res B Appl Biomater. 2005;72:79–85. [DOI] [PubMed]
- 9.Bruder SP, Fink DJ, Caplan AI. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem. 1994;56:283–294. [DOI] [PMC free article] [PubMed]
- 10.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. [DOI] [PubMed]
- 11.Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7:259–264. [DOI] [PubMed]
- 12.Chen J, Altman GH, Karageorgiou V, Horan R, Collette A, Volloch V, Colabro T, Kaplan DL. Human bone marrow stromal cell and ligament fibroblast responses on RGD-modified silk fibers. J Biomed Mater Res A. 2003;67:559–570. [DOI] [PubMed]
- 13.Chhabra A, Tsou D, Clark RT, Gaschen V, Hunziker EB, Mikic B. GDF-5 deficiency in mice delays Achilles tendon healing. J Orthop Res. 2003;21:826–835. [DOI] [PubMed]
- 14.Clark RT, Johnson TL, Schalet BJ, Davis L, Gaschen V, Hunziker EB, Oldberg A, Mikic B. GDF-5 deficiency in mice leads to disruption of tail tendon form and function. Connect Tissue Res. 2001;42:175–186. [DOI] [PubMed]
- 15.Cooper JA, Lu HH, Ko FK, Freeman JW, Laurencin CT. Fiber-based tissue-engineered scaffold for ligament replacement: design considerations and in vitro evaluation. Biomaterials. 2005;26:1523–1532. [DOI] [PubMed]
- 16.Crawford C, Kainer M, Jernigan D, Banerjee S, Friedman C, Ahmed F, Archibald LK. Investigation of postoperative allograft-associated infections in patients who underwent musculoskeletal allograft implantation. Clin Infect Dis. 2005;41:195–200. [DOI] [PubMed]
- 17.Cristino S, Grassi F, Toneguzzi S, Piacentini A, Grigolo B, Santi S, Riccio M, Tognana E, Facchini A, Lisignoli G. Analysis of mesenchymal stem cells grown on a three-dimensional HYAFF 11-based prototype ligament scaffold. J Biomed Mater Res A. 2005;73:275–283. [DOI] [PubMed]
- 18.Cserjesi P, Brown D, Ligon KL, Lyons GE, Copeland NG, Gilbert DJ, Jenkins NA, Olson EN. Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development. 1995;121:1099–1110. [DOI] [PubMed]
- 19.Cui Q, Ming Xiao Z, Balian G, Wang GJ. Comparison of lumbar spine fusion using mixed and cloned marrow cells. Spine. 2001;26:2305–2310. [DOI] [PubMed]
- 20.Cui Q, Wang GJ, Balian G. Pluripotential marrow cells produce adipocytes when transplanted into steroid-treated mice. Connect Tissue Res. 2000;41:45–56. [DOI] [PubMed]
- 21.Cui Q, Wang GJ, Balian G. Steroid-induced adipogenesis in a pluripotential cell line from bone marrow. J Bone Joint Surg Am. 1997;79:1054–1063. [DOI] [PubMed]
- 22.Cui Q, Wang Y, Saleh KJ, Wang GJ, Balian G. Alcohol-induced adipogenesis in a cloned bone-marrow stem cell. J Bone Joint Surg Am. 2006;88(Suppl 3):148–154. [DOI] [PubMed]
- 23.Dennis JE, Merriam A, Awadallah A, Yoo JU, Johnstone B, Caplan AI. A quadripotential mesenchymal progenitor cell isolated from the marrow of an adult mouse. J Bone Miner Res. 1999;14:700–709. [DOI] [PubMed]
- 24.Diduch DR, Coe MR, Joyner C, Owen ME, Balian G. Two cell lines from bone marrow that differ in terms of collagen synthesis, osteogenic characteristics, and matrix mineralization. J Bone Joint Surg Am. 1993;75:92–105. [DOI] [PubMed]
- 25.Dunn MG, Liesch JB, Tiku ML, Zawadsky JP. Development of fibroblast-seeded ligament analogs for ACL reconstruction. J Biomed Mater Res. 1995;29:1363–1371. [DOI] [PubMed]
- 26.Fetto JF, Marshall JL. The natural history and diagnosis of anterior cruciate ligament insufficiency. Clin Orthop Relat Res. 1980;147:29–38. [PubMed]
- 27.Garvin J, Qi J, Maloney M, Banes AJ. Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng. 2003;9:967–979. [DOI] [PubMed]
- 28.Ge Z, Goh JC, Lee EH. Selection of cell source for ligament tissue engineering. Cell Transplant. 2005;14:573–583. [DOI] [PubMed]
- 29.Goh JC, Ouyang HW, Teoh SH, Chan CK, Lee EH. Tissue-engineering approach to the repair and regeneration of tendons and ligaments. Tissue Eng. 2003;9:S31–44. [DOI] [PubMed]
- 30.Goh JC, Ouyang HW, Toh SL, Lee EH. Tissue engineering techniques in tendon, ligament replacement. Med J Malaysia. 2004;59(Suppl B):47–48. [PubMed]
- 31.Helm GA, Li JZ, Alden TD, Hudson SB, Beres EJ, Cunningham M, Mikkelsen MM, Pittman DD, Kerns KM, Kallmes DF. A light and electron microscopic study of ectopic tendon and ligament formation induced by bone morphogenetic protein-13 adenoviral gene therapy. J Neurosurg. 2001;95:298–307. [DOI] [PubMed]
- 32.Hoffmann A, Pelled G, Turgeman G, Eberle P, Zilberman Y, Shinar H, Keinan-Adamsky K, Winkel A, Shahab S, Navon G, Gross G, Gazit D. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J Clin Invest. 2006;116:940–952. [DOI] [PMC free article] [PubMed]
- 33.Jenner JM, van Eijk F, Saris DB, Willems WJ, Dhert WJ, Creemers LB. Effect of transforming growth factor-beta and growth differentiation factor-5 on proliferation and matrix production by human bone marrow stromal cells cultured on braided poly lactic-co-glycolic acid scaffolds for ligament tissue engineering. Tissue Eng. 2007;13:1573–1582. [DOI] [PubMed]
- 34.Lin VS, Lee MC, O’Neal S, McKean J, Sung KL. Ligament tissue engineering using synthetic biodegradable fiber scaffolds. Tissue Eng. 1999;5:443–452. [DOI] [PubMed]
- 35.Lou J, Tu Y, Burns M, Silva MJ, Manske P. BMP-12 gene transfer augmentation of lacerated tendon repair. J Orthop Res. 2001;19:1199–1202. [DOI] [PubMed]
- 36.Majima T, Funakosi T, Iwasaki N, Yamane ST, Harada K, Nonaka S, Minami A, Nishimura S. Alginate and chitosan polyion complex hybrid fibers for scaffolds in ligament and tendon tissue engineering. J Orthop Sci. 2005;10:302–307. [DOI] [PubMed]
- 37.Mikic B, Bierwert L, Tsou D. Achilles tendon characterization in GDF-7 deficient mice. J Orthop Res. 2006;24:831–841. [DOI] [PubMed]
- 38.Moreau J, Chen J, Kaplan D, Altman G. Sequential growth factor stimulation of bone marrow stromal cells in extended culture. Tissue Eng. 2006;12:2905–2912. [DOI] [PubMed]
- 39.Moreau JE, Chen J, Bramono DS, Volloch V, Chernoff H, Vunjak-Novakovic G, Richmond JC, Kaplan DL, Altman GH. Growth factor induced fibroblast differentiation from human bone marrow stromal cells in vitro. J Orthop Res. 2005;23:164–174. [DOI] [PubMed]
- 40.Owings MF, Kozak LJ. Ambulatory and inpatient procedures in the United States, 1996. Vital Health Stat. 1998;13:1–119. [PubMed]
- 41.Petrigliano FA, English CS, Barba D, Esmende S, Wu BM, McAllister DR. The effects of local bFGF release and uniaxial strain on cellular adaptation and gene expression in a 3D environment: implications for ligament tissue engineering. Tissue Eng. 2007;13:2721–2731. [DOI] [PubMed]
- 42.Puk CK, Miller DJ, Gamradt S, Wu BM, McAllister DR. The effects of short-term stimulation on fibroblast spreading in an in vitro 3D system. J Biomed Mater Res A. 2006;76:665–673. [DOI] [PubMed]
- 43.Rickert M, Jung M, Adiyaman M, Richter W, Simank HG. A growth and differentiation factor-5 (GDF-5)-coated suture stimulates tendon healing in an Achilles tendon model in rats. Growth Factors. 2001;19:115–126. [DOI] [PubMed]
- 44.Rickert M, Wang H, Wieloch P, Lorenz H, Steck E, Sabo D, Richter W. Adenovirus-mediated gene transfer of growth and differentiation factor-5 into tenocytes and the healing rat Achilles tendon. Connect Tissue Res. 2005;46:175–183. [DOI] [PubMed]
- 45.Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. [DOI] [PubMed]
- 46.Tashiro T, Hiraoka H, Ikeda Y, Ohnuki T, Suzuki R, Ochi T, Nakamura K, Fukui N. Effect of GDF-5 on ligament healing. J Orthop Res. 2006;24:71–79. [DOI] [PubMed]
- 47.Van Eijk F, Saris DB, Riesle J, Willems WJ, Van Blitterswijk CA, Verbout AJ, Dhert WJ. Tissue engineering of ligaments: a comparison of bone marrow stromal cells, anterior cruciate ligament, and skin fibroblasts as cell source. Tissue Eng. 2004;10:893–903. [DOI] [PubMed]
- 48.Wang QW, Chen ZL, Piao YJ. Mesenchymal stem cells differentiate into tenocytes by bone morphogenetic protein (BMP) 12 gene transfer. J Biosci Bioeng. 2005;100:418–422. [DOI] [PubMed]
- 49.West RV, Harner CD. Graft selection in anterior cruciate ligament reconstruction. J Am Acad Orthop Surg. 2005;13:197–207. [DOI] [PubMed]
- 50.Wolfman NM, Hattersley G, Cox K, Celeste AJ, Nelson R, Yamaji N, Dube JL, DiBlasio-Smith E, Nove J, Song JJ, Wozney JM, Rosen V. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest. 1997;100:321–330. [DOI] [PMC free article] [PubMed]