Abstract
Fibrin, a homologous polymer, is the natural scaffold of wound healing and therefore a candidate as a carrier for cell transplantation. We explored a novel matrix-based implant cartilage repair composed of both fibrin and hyaluronan in a defined ratio that takes advantage of the biological and mechanical properties of these two elements. The matrix was seeded with autologous chondrocytes expanded in the presence of a proprietary growth factor variant designed to preserve their chondrogenic potential. We prospectively followed eight patients with symptomatic-chronic cartilage defects treated with this carrier. Patients had arthroscopy to harvest autologous chondrocytes then grown in autologous serum. Chondrocytes were cultured in the presence of the FGF variant and then seeded on the fibrin-hyaluronan matrix. About 4 weeks following biopsy, the patients underwent implantation of the constructs by miniarthrotomy. Three of the eight patients had transient effusion. Clinical performance was measured by Lysholm and IKDC scores, MRI, and the need for secondary surgery. The clinical outcome of a 1-year followup demonstrated increase of clinical scores. The MRI followup showed good filling of the defect with tissue having the imaging appearance of cartilage in all patients. Apart from the transient effusion in three patients we observed no other adverse events during the followup.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Articular cartilage injuries of the knee are common, especially among physically active people, and are a frequent cause of pain and loss of joint function. Injured articular cartilage has a limited capability of self-repair; lesions of 5-mm diameter lead to persistent damage of the cartilage. Lesions greater than 2 cm2 are described as the critical size for treatment [14]. Potential long-term problems include early osteoarthritis and disability, frequently leading to total joint replacement [5, 15].
Repair options for damaged articular cartilage are limited. The main approaches currently in use in clinical practice are bone marrow stimulation techniques such as microfracture [32, 33], osteochondral graft transplantation [3, 12, 16], and autologous chondrocyte transplantation (ACT) [4]. Microfracture is an efficient one-step procedure but produces mainly fibrocartilage repair tissue with often inadequate filling of the defect and limited loading capacity [17]. Recent studies suggest decreasing joint function 18 months after microfracture especially in the case of larger lesions and in elderly patients [18]. Osteochondral transplantation is limited by the number and sorts of grafts available, unstable fixation and uneven surfaces in multiple grafting, and potential cell damage due to the forces required in implantation [16, 26, 35]. ACT as developed by Brittberg et al. [4] requires implantation of a cultured autologous cell suspension under an excised periosteal flap. This treatment has been used for cartilage repair in more than 30,000 patients worldwide [30]. However, ACT involves a complex surgery in which, in addition to harvesting and implanting the chondrocytes, the surgeon must remove a periosteal flap from a healthy site and suture the flap to the defect borders to create a watertight compartment. This not only damages healthy tissue but also lengthens and complicates the surgery. In some cases, where articular cartilage transplantation with a periosteal flap was employed, cartilage overgrowth, delamination, or fibrous degeneration of the newly formed tissue was observed [26]. The unpredictable mechanical and biological properties and limitations in size and thickness have led to replacement of the periosteal patch approach by biomatrices, at least in Europe. Initially, these matrices were used as a patch [34] but recently have been used as a cell-support device. Basically, chondrocytes are precultured on the matrix and are implanted as a cell-seeded construct [2, 27]. This approach allows a more predictable transport of the cells into the defect without the need for suturing. Most of the biomaterials used in these techniques are natural biopolymers such as collagen [7, 8, 24] or hyaluronan [10, 21, 27] derived from animals or synthetic materials like polylactides [6].
Fibrin, a natural homologous biopolymer, constitutes part of the normal scaffold of wound healing and therefore is a promising candidate for cell transplantation [29]. It can be copolymerized with hyaluronan and produced as a matrix to serve as a 3-D scaffold for chondrocyte transplantation and closely approximates that of cartilage. This biocompatible, 3-D scaffold has been tested preclinically in conjunction with autologous cells with high chondrogenic potential obtained with the use of a proprietary FGF variant but this is the first report of the use in a clinical setting. Other studies on matrix assisted chondrocyte transplantation suggest an improvement of function and pain [21, 27, 28]. Apart from biopsies, high-resolution MRI and well-defined MRI variables are a reliable, reproducible and accurate tool for assessing cartilage repair tissue [22].
We asked the following questions: (1) Will implantation of the novel scaffold into femoral cartilage defects improve clinical scores at 1 year? (2) Will the scaffold-implanted defect appear healed by MRI? (3) Will the technique be associated with any adverse effects?
Materials and Methods
We prospectively followed eight patients who underwent implantation of the 3-D fibrin-hyaluronan scaffold seeded with autologous chondrocytes (Table 1). We selected patients with a single symptomatic chronic cartilage defect in the femoral condyle related to trauma or osteochondritis dissecans with sizes ranging from 1 to 8 cm² and no more than 5 mm deep. We excluded patients with multiple or larger defects, signs of osteoarthritis, resected menisci, malalignment of the leg, history of rheumatoid arthritis, or other general diseases with chronic medication. Of the eight patients, two had undergone previous surgery; patient 00102 had had diagnostic arthroscopy without any further procedures and patient 00106 had a failed autologous cartilage transplantation with a hyaluronan scaffold in another hospital. In six patients, the complaints began gradually without specific injury; however, three patients had a history of sport accidents and one had an earlier accident at work. Two patients had acute onset of symptoms after/during work or sport. The mean age was 30.4 years (range, 19–40 years) with a standard deviation of 6.7 years. Average lesion sizes were 2 cm (lesion length) and 2 cm (lesion width) with an average lesion area of 3 cm2. The average depth of treated lesions was 4.4 mm. The protocol was written according to GCP principles and the declaration of Helsinki and was approved by the ethics committee of the University of Vienna (Prot.-Nr.: 358/2003).
Table 1.
Patients and defect characteristics
| Patient | Age | Gender | Knee | Onset of symptoms | Etiology | Localization | Lesion (cm2) |
|---|---|---|---|---|---|---|---|
| 00101 | 31 | Male | Right | Gradual | Daily activities | Medial condyle | 4.9 |
| 00102 | 26 | Male | Left | Gradual | Daily activities | Medial condyle | 1.8 |
| 00103 | 38 | Male | Right | Acute | Sports | Medial condyle | 3.1 |
| 00104 | 19 | Male | Left | Gradual | Sports | Trochlea | 4.9 |
| 00105 | 30 | Male | Left | Acute | Work | Medial condyle | 3.1 |
| 00106 | 27 | Female | Right | Gradual | Other | Medial condyle | 7.1 |
| 00107 | 32 | Male | Left | Gradual | Daily activities | Medial condyle | 4.9 |
| 00108 | 40 | Male | Left | Gradual | Sports | Medial condyle | 7.1 |
The fibrin-hyaluronan matrix BioCart™II (ProChon BioTech Ltd., Ness Ziona, Israel) is produced by copolymerization of homologous human fibrinogen (Omrix Biopharmaceuticals, New York, NY) and recombinant hyaluronan (Ferring Pharmaceuticals, Switzerland) and is subsequently freeze-dried to produce a sponge-like 3-D structure (Figs. 1, 2).
Fig. 1.
The macroscopic aspect of the fibrin hyaluronan scaffold is pictured.
Fig. 2.
The microscopic structure of the freeze dried 3-D porous scaffold is shown.
The patients underwent arthroscopic surgery in which several (two to three) small cartilage biopsies of about 150 mg were removed from the nonweightbearing area in the intercondylar notch and stored in a sterile transport container. Blood (140 mL) was collected from the patient and centrifuged to prepare autologous serum. The cartilage biopsy in sterile DMEM (Invitrogen USA) and serum samples were sent by courier in a temperature controlled cooled package to the GMP facility at ProChon BioTech (Ness Ziona, Israel) for autologous cell isolation and expansion. BioCart™II was assembled by seeding the autologous chondrocytes into the fibrin hyaluronan-based matrix 3 to 4 days prior to the planned surgery during which sterility, endotoxin levels and QC tests were performed.
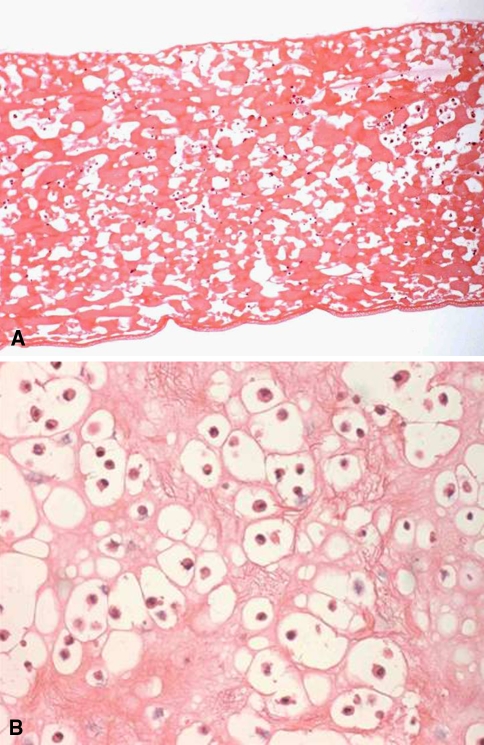
The biopsies were enzymatically degraded with collagenase to provide a homogenous population of isolated chondrocytes. Growth medium was supplemented with autologous human serum and a recombinant fibroblast growth factor 2 variant (FGF2v, 10 ng/mL) prepared by ProChon. The cells were injected into the scaffold using a syringe. Each implant contained autologous cartilage cells at a concentration of 0.4 + 0.1 × 106 cells/cm2. The fibrin-based matrix has uniform open pores, allowing a homogeneous cell distribution. A typical scaffold absorbs more than 90% of the seeded cells (as judged by digesting seeded scaffolds with collagenase and then counting the number of cells released) and the cells remain viable in the scaffold for at least 1 month (Fig. 3A–B).
Fig. 3A–B.
(A) Histology of the cell loaded fibrin-based matrix is shown (stain, hematoxylin and eosin; original magnification × 10). (B) Higher magnification of the fibrin-based matrix reveals the chondrocytic phenotype of the cells (stain, hematoxylin and eosin; original magnification × 40).
Scaffold samples were placed in 4% formalin dehydrated through graded concentrations of ethanol and xylene and embedded in paraffin. Morphology of the seeded matrix was evaluated by staining paraffin sections (4 μm) with hematoxylin (10 minutes incubation) and then 1 minute in eosin stain (Pioneer Research Chemicals Ltd., Colchester, Essex, UK). Images of tissue sections were viewed by oil immersion microscopy.
Three to four weeks following cell harvest, patients were hospitalized for implantation. The implantation was performed through a miniarthrotomy according to the side of the defect on the femoral condyle. We did not use a tourniquet. The defect was débrided to the adjacent healthy, stable cartilage, and a template of the defect prepared from sterile aluminum foil. The cell-seeded implant was removed from the sterile transport container, put on a plastic dish and cut to size with scissors according to the template. The cell-loaded construct was then implanted into the defect by press-fitting and fibrin gluing at the borders of the defect. After achieving control of bleeding, the arthrotomy was closed in layers with no suction drainage.
Immediately postoperatively a hinged knee brace was applied to limit postoperative range of motion from 0 to 30°. After the first postoperative day we instituted continuous passive motion (CPM) from full to as much as 30° of flexion, gradually increasing flexion after 4 weeks. CPM devices were prescribed for home use at discharge. The patients began touch weightbearing with crutches after 2 days postoperatively, and continued for 6 weeks; weightbearing was gradually increased until free walking without assists was allowed at 12 weeks. No treatments or medications were prohibited during the study.
Following surgery, patients were evaluated at weeks 1, 2, 6 and 12, and after 6 and 12 months. We recorded any adverse events, and performed hematology and biochemistry tests, including renal, liver, electrolytes and inflammatory parameters to exclude systemic reactions to the biomaterial. The name and duration of treatment of all medications taken at the time of the inclusion or during the study were recorded on the case report form (CRF). We (CC, SN) obtained scores using the form recommended by the International Knee Documentation Committee (IKDC) [13] at baseline and 6 and 12 months after implantation. IKDC knee examination scores include the following components: effusion, passive motion deficit, ligament examination, compartment findings, harvest site pathology, radiographic findings, and functional tests; these parameters are graded and converted to a scale that ranges between 0 and 100, where a higher score indicates a response presenting a high level of function or low level of symptoms. We determined modified Lysholm score [19] at baseline, 6-month, and 12-month intervals.
MRI analysis was performed at 6 weeks, 6 months, and 12 months following matrix implantation. MRI analysis was performed using the MOCART evaluation system [22] and scans performed on a 3T MR unit (Magnetom Trio; Siemens Erlangen, Germany) with a gradient strength of 40 mT/m using an eight-channel phased array knee coil (In vivo, Gainesville, FL). For morphological evaluation we used an isotropic 3-D Double Echo Steady State (DESS) sequence with a TR of 15.1 ms, TE of 5.11 ms, and a flip angle of 25°. The field of view was 150 mm × 150 mm with a 250 × 250 pixel matrix and a slice thickness of 0.6 mm with an in-plane resolution of 0.6 mm × 0.6 mm. An acceleration factor of two was applied; the total scan time was 6 minutes and 32 seconds.
We assessed a number of repair parameters using the MOCART system [22], which assesses the degree of defect repair, the interface between the implant, cartilage and bone, the surface and structure of the implant, the condition of the subchondral lamina and bone, the signal intensity of the implant and the presence of adhesions or joint effusion (Table 2).
Table 2.
MRI findings at 12 months followup using MOCART evaluation system
| Parameter | Description | Patients (N = 8) |
|---|---|---|
| Degree of defect repair | Complete (100%) | 2 |
| Hypertrophy (> 100%) | 4 | |
| Incomplete (75–100%) | 2 | |
| Cartilage interface | Intact | 7 |
| Incomplete with a split-like border | 1 | |
| Bone interface | Intact (one delamination resolved after 6 months) | 8 |
| Surface | Intact | 6 |
| Damaged, fissures less than 50% of cartilage thickness | 2 | |
| Structure | Homogeneous | 5 |
| Inhomogeneous | 3 | |
| Subchondral lamina | Intact | 6 |
| Central osteophyte formation | 2 | |
| Subchondral bone | Normal | 4 |
| Minor edema (< 2 cm) granulation tissue and a small cyst | 1 | |
| Minor edema and granulation tissue | 1 | |
| Minor edema | 2 | |
| Signal intensity | Isointense on FSE | 3 |
| Hyperintense on FSE | 5 | |
| Isointense on 3D-GRE | 4 | |
| Hypointense on 3D-GRE | 4 | |
| Adhesion | None | 8 |
| Joint effusion | None | 6 |
| Present | 2 |
Results
The treatment of single cartilage defects of the femoral condyle with a fibrin-hyaluronan matrix improved function and reduced pain. The mean Lysholm score at baseline (56.38 ± 14.40) improved at 6 months (82.75 ± 6.45) and 12 months (84.50 ± 6.02) (Table 3). The IKDC score also improved at 6 and 12 months followup (Table 3).
Table 3.
Lysholm and IKDC scores at baseline, 6 months and 12 months
| Patient number | Lysholm score | IKDC score | ||||
|---|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | Baseline | 6 months | 12 months | |
| 00101 | 38 | 90 | 90 | D | A | A |
| 00102 | 62 | 75 | 76 | C | C | B |
| 00103 | 65 | 85 | 85 | B | A | C |
| 00104 | 61 | 86 | 85 | C | B | A |
| 00105 | 48 | 90 | 90 | D | B | A |
| 00106 | 35 | 75 | 75 | D | B | C |
| 00107 | 67 | 85 | 90 | C | A | A |
| 00108 | 75 | 76 | 85 | B | D | A |
MRI at the end of the 12-month followup after implantation demonstrated a good defect repair with 75% to 100% filling in all patients (Table 2). No adhesions were reported. When implant hypertrophy was observed it appeared minimal. The cartilage and bone interface was intact in all patients except one, who had a gap between the adjacent normal cartilage on one side of the implant. Minimal subchondral edema was present in four of the eight patients with one patient demonstrating an additional cyst.
We observed no serious adverse event during the 12-month followup. Five episodes of effusion were reported in three patients, all of which were considered moderate, did not require any medical action, and resolved without any sequelae. Medications taken by the patients during the study were within the normal range of postoperative medication. The laboratory results were within normal ranges as well as vital signs. No allergic or toxic reactions were recorded.
Discussion
The aim of our study was to determine the clinical feasibility of implanting a fibrin-based matrix as a scaffold for autologous chondrocyte transplantation and preliminary clinical and MRI outcomes after one year followup in a pilot trial. We asked the following questions: (1) Will implantation of the novel scaffold into femoral cartilage defects improve clinical scores at 1 year? (2) Will the scaffold-implanted defect appear healed by MRI? (3) Will the technique be associated with any adverse effects?
The results are limited by the small number of patients and present preliminary outcomes with no relevance for long-term performance. However, the study design relates to a phase one trial of medical devices to demonstrate feasibility in clinical situation and primary safety issues with respect to major and minor adverse events. The cell-loaded construct could be manipulated sufficiently and it was surgically feasible to implant the cell-seeded construct in isolated cartilage defects in the condyle of the knee. No major adverse events or undue biological reactions were reported during the observation period. The clinical results demonstrated increased clinical performance and sufficient fill of the defect in the majority of the patients in the MRI followup.
Analysis of bioactivity of chondrocytes in in vitro experiments using the fibrin matrix have shown promising results with respect to bioactivity of the cells. By using FGF and animal experiments sufficient cartilage repair was revealed in a goat model, however, the results are not published yet with respect to proprietary rights. In vitro experiments for other biomaterials like a nonwoven hyaluronan mesh derived from rooster’s comb [11, 31] and collagen matrices [23, 24] demonstrate chondrocytes can reexpress their chondrocytic phenotype in the biomaterial presented by their round phenotype and the Type 2 collagen synthesis. The use of the FGF growth factor optimized the culture conditions of the cells in our study and allowed a shorter timeframe to implantation of the cell-seeded construct by increasing the proliferation rate and revealed a high cell quality with respect to chondrogenic markers.
However, the use of a predominately homologous biomaterial for chondrocyte transplantation is a major advantage in the matrix assisted transplantation of chondrocytes, especially with respect to regulation concerning animal derived or synthetic materials. Furthermore, a complete autologous system using autologous fibrin with autologous cells could be the ultimate goal of this technique. Successful applications of biodegradable matrices for chondrocyte transplantation utilizing a nonwoven mesh of hyaluronan has proven that hyaluronan can serve as a scaffold for cultured chondrocytes and allows implantation by gluing the cell-seeded constructs with fibrin. Clinical studies with midterm results confirm improvement of the clinical performance comparable with ACT, but with less surgical trauma with respect to the incision and time of surgery by avoiding suturing of the implant [9, 20, 21, 27]. Additionally, the fibrin-hyaluronan matrix with its unique structure presents an important advantage with a high seeding efficacy and maintenance of the chondrocytic phenotype. Also, a collagen membrane comprising type 1 and 3 collagen can be used to replace the periosteal patch and allow a more adequate implantation without harvesting periosteum from the adjacent tibia; however, it still needs suture fixation in most cases. The clinical results in these studies meet the success rate of the classic chondrocyte transplantation using the periosteal patch of about 90% good and excellent results for isolated femoral lesions [1, 2]. In comparative studies collagen membranes have proven that the periosteal patch can be replaced by a collagen membrane sutured to the defect [34].
The clinical results of our study demonstrated an improvement in the Lysholm scores and a reduction in the IKDC knee evaluation score at 6 and 12 months after implantation as compared to baseline in all patients. Despite the small number of patients in our study, the data are consistent with other studies utilizing hyaluronan [21, 27] or collagen matrices in longer-term followups [2]. However, BioCart™II is a human-derived product that is self-adhesive due to its high-interval fibrinogen content, and implantation can be achieved with a minimally invasive surgical approach because there is no need to suture the graft to the defect site; all implants were suitably fixed to the adjacent cartilage with fibrin glue.
MRI proved a stable fixation of the fibrin matrix at the early control 6 weeks after surgery. Adverse effects such as hypertrophy of the graft or delamination which are often associated with failure of the periosteal patch technique were not recorded [25]. MRI findings demonstrated an adequate repair with a 75% to 100% defect-fill in all patients. Compared to outcomes with microfracture our approach resulted in increased filling of the defects [9, 22], presumably an important factor in long-term clinical success. MRIs showed no displacement of the graft and revealed stable integration to the adjacent cartilage after 1 year. Most of the repair tissue fulfilled the criteria of hyaline tissue, which is the prerequisite of a long-lasting regeneration and improved functionality [30]. While long-term studies utilizing biomaterial as a scaffold for cell transplantation are not available, preliminary midterm data up to 5 years suggest improvement as compared with classic ACT [2].
The fibrin matrix did not give rise to any serious adverse events during the followup period. Five events of mild effusion were reported by three patients and resolved without any further medical action. This is comparable to complications after implantation of other cell augmented matrices [2, 27, 34]. No clinically important changes in the blood chemistry and hematology results were recorded in any of the patients participating in the study. As reported for other biomaterials used as scaffolds for cell transplantation, the fibrin-hyaluronan matrix did not produce any undue biological, immunological, or allergic response during the followup period. We observed no graft hypertrophy or delamination, which are often associated with failure of the periosteal patch technique [25].
The fibrin-hyaluronan matrix integrates a 3-D structure that mimics the normal cartilage with a high quality of autologous chondrocytes obtained in a relatively short time from biopsy with support of a growth factor, thus preserving the chondrogenic potential of the cells. This pilot study demonstrated the feasibility of the implantation of a cell-seeded fibrin-hyaluronan matrix in a clinical setting and allowed a successful treatment of cartilage defects in a small number of patients. Further studies will be required to evaluate the long-term performance and outcomes after the implantation of this cell-seeded construct.
Acknowledgments
We thank Roni Wechsler and Ann Avron for critical review of the manuscript. Furthermore we thank Christoph Buchta for preparing the patients’ serum and Siegfried Trattnig for reviewing the MRI studies.
Footnotes
One or more of the authors (SN) certifies that he or she has received funding from ProChon to support this project. Two of the authors (AY, HB) are salaried employees of ProChon.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, Bentley G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87:640–645. [DOI] [PubMed]
- 2.Behrens P, Bitter T, Kurz B, Russlies M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI) - 5-year follow-up. Knee. 2006;13:194–202. [DOI] [PubMed]
- 3.Bobic V. Autologous osteo-chondral grafts in the management of articular cartilage lesions [in German]. Orthopade. 1999;28:19–25. [DOI] [PubMed]
- 4.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. [DOI] [PubMed]
- 5.Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect. 1998;47:487–504. [PubMed]
- 6.Chu CR, Coutts RD, Yoshioka M, Harwood FL, Monosov AZ, Amiel D. Articular cartilage repair using allogeneic perichondrocyte-seeded biodegradable porous polylactic acid (PLA): a tissue-engineering study. J Biomed Mater Res. 1995;29:1147–1154. [DOI] [PubMed]
- 7.Dorotka R, Bindreiter U, Macfelda K, Windberger U, Nehrer S. Marrow stimulation and chondrocyte transplantation using a collagen matrix for cartilage repair. Osteoarthritis Cartilage. 2005;13:655–664. [DOI] [PubMed]
- 8.Dorotka R, Windberger U, Macfelda K, Bindreiter U, Toma C, Nehrer S. Repair of articular cartilage defects treated by microfracture and a three-dimensional collagen matrix. Biomaterials. 2005;26:3617–3629. [DOI] [PubMed]
- 9.Gobbi A, Kon E, Berruto M, Francisco R, Filardo G, Marcacci M. Patellofemoral full-thickness chondral defects treated with Hyalograft-C: a clinical, arthroscopic, and histologic review. Am J Sports Med. 2006;34:1763–1773. [DOI] [PubMed]
- 10.Grigolo B, Roseti L, Fiorini M, Fini M, Giavaresi G, Aldini NN, Giardino R, Facchini A. Transplantation of chondrocytes seeded on a hyaluronan derivative (hyaff-11) into cartilage defects in rabbits. Biomaterials. 2001;22:2417–2424. [DOI] [PubMed]
- 11.Grigolo B, Lisignoli G, Piacentini A, Fiorini M, Gobbi P, Mazzotti G, Duca M, Pavesio A, Facchini A. Evidence for redifferentiation of human chondrocytes grown on a hyaluronan-based biomaterial (HYAff 11): molecular, immunohistochemical and ultrastructural analysis. Biomaterials. 2002;23:1187–1195. [DOI] [PubMed]
- 12.Hangody L, Feczko P, Bartha L, Bodo G, Kish G. Mosaicplasty for the treatment of articular defects of the knee and ankle. Clin Orthop Relat Res. 2001;391(Suppl):S328–336. [DOI] [PubMed]
- 13.Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the IKDC form. Knee Surg Sports Traumatol Arthrosc. 1993;1:226–234. [DOI] [PubMed]
- 14.Homminga GN, Bulstra SK, Bouwmeester PS, van der Linden AJ. Perichondral grafting for cartilage lesions of the knee. J Bone Joint Surg Br. 1990;72:1003–1007. [DOI] [PubMed]
- 15.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–463. [DOI] [PubMed]
- 16.Jakob RP, Franz T, Gautier E, Mainil-Varlet P. Autologous osteochondral grafting in the knee: indication, results, and reflections. Clin Orthop Relat Res. 2002;401:170–184. [DOI] [PubMed]
- 17.Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grontvedt T, Solheim E, Strand T, Roberts S, Isaksen V, Johansen O. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86:455–464. [DOI] [PubMed]
- 18.Kreuz PC, Steinwachs MR, Erggelet C, Krause SJ, Konrad G, Uhl M, Sudkamp N. Results after microfracture of full-thickness chondral defects in different compartments in the knee. Osteoarthritis Cartilage. 2006;14:1119–1125. [DOI] [PubMed]
- 19.Lysholm J, Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10:150–154. [DOI] [PubMed]
- 20.Manfredini M, Zerbinati F, Gildone A, Faccini R. Autologous chondrocyte implantation: a comparison between an open periosteal-covered and an arthroscopic matrix-guided technique. Acta Orthop Belg. 2007;73:207–218. [PubMed]
- 21.Marcacci M, Berruto M, Brocchetta D, Delcogliano A, Ghinelli D, Gobbi A, Kon E, Pederzini L, Rosa D, Sacchetti GL, Stefani G, Zanasi S. Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin Orthop Relat Res. 2005;435:96–105. [DOI] [PubMed]
- 22.Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57:16–23. [DOI] [PubMed]
- 23.Nehrer S, Breinan HA, Ramappa A, Shortkroff S, Young G, Minas T, Sledge CB, Yannas IV, Spector M. Canine chondrocytes seeded in type I and type II collagen implants investigated in vitro. J Biomed Mater Res. 1997;38:95–104. [DOI] [PubMed]
- 24.Nehrer S, Breinan HA, Ramappa A, Hsu HP, Minas T, Shortkroff S, Sledge CB, Yannas IV, Spector M. Chondrocyte-seeded collagen matrices implanted in a chondral defect in a canine model. Biomaterials. 1998;19:2313–2328. [DOI] [PubMed]
- 25.Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop Relat Res. 1999;365:149–162. [DOI] [PubMed]
- 26.Nehrer S, Minas T. Treatment of articular cartilage defects. Invest Radiol. 2000;35:639–646. [DOI] [PubMed]
- 27.Nehrer S, Domayer S, Dorotka R, Schatz K, Bindreiter U, Kotz R. Three-year clinical outcome after chondrocyte transplantation using a hyaluronan matrix for cartilage repair. Eur J Radiol. 2006;57:3–8. [DOI] [PubMed]
- 28.Ossendorf C, Kaps C, Kreuz PC, Burmester GR, Sittinger M, Erggelet C. Treatment of posttraumatic and focal osteoarthritic cartilage defects of the knee with autologous polymer-based three-dimensional chondrocyte grafts: 2-year clinical results. Arthritis Res Ther. 2007;9:R41. [DOI] [PMC free article] [PubMed]
- 29.Paletta GA, Arnoczky SP, Warren RF. The repair of osteochondral defects using an exogenous fibrin clot. An experimental study in dogs. Am J Sports Med. 1992;20:725–731. [DOI] [PubMed]
- 30.Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212–234. [DOI] [PubMed]
- 31.Solchaga LA, Dennis JE, Goldberg VM, Caplan AI. Hyaluronic acid-based polymers as cell carriers for tissue-engineered repair of bone and cartilage. J Orthop Res. 1999;17:205–213. [DOI] [PubMed]
- 32.Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391(Suppl):S362–369. [DOI] [PubMed]
- 33.Steadman JR, Rodkey WG, Briggs KK. Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg. 2002;15:170–176. [PubMed]
- 34.Steinwachs M, Kreuz PC. Autologous chondrocyte implantation in chondral defects of the knee with a type I/III collagen membrane: a prospective study with a 3-year follow-up. Arthroscopy. 2007;23:381–387. [DOI] [PubMed]
- 35.Szerb I, Hangody L, Duska Z, Kaposi NP. Mosaicplasty: long-term follow-up. Bull Hosp Jt Dis. 2005;63:54–62. [PubMed]