Abstract
Achieving union between host bone and massive structural allografts can be difficult. Donor and recipient human leukocyte antigen (HLA) mismatches and recipient antibody response to donor HLA antigens might affect union. In a prospective multiinstitutional study, we enrolled a consecutive series of patients receiving cortex-replacing, massive structural bone allografts to determine the rate of donor-specific HLA antibody sensitization and to investigate the potential effect of such HLA alloantibody sensitization on allograft incorporation. HLA typing of patients and donors was determined by molecular typing methods. Donor-specific HLA sensitization occurred in 57% of the patients but had no demonstrable effect on graft incorporation or union. The type of host-allograft junction did have a major effect on graft incorporation. Cortical-to-cortical allograft-to-host junctions healed more slowly (mean, 542 days) than corticocancellous to corticocancellous allograft-to-host junctions (mean, 243 days). Although HLA sensitization does not appear to delay structural allograft bone incorporation, further followup is required to determine if there is an association between HLA sensitization and long-term graft survival. Based on these preliminary data, measures to further minimize or modulate HLA sensitization or response are not indicated at present for the purposes of improving structural bone allograft union.
Level of Evidence: Level II, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Achieving union between host bone and massive structural allografts can be difficult. If sensitization by human leukocyte antigen (HLA) mismatches adversely affects allograft healing, it could have great impact on current clinical practices. In 2006, the Musculoskeletal Transplant Foundation alone distributed over 350,000 grafts for human implantation (Source: Musculoskeletal Transplant Foundation web site [http://www.mtf.org/who/fact.html]). Allograft bone procedures are performed without regard to HLA matching or allosensitization because tissue matching of bone between donors and recipients is currently considered unnecessary. However, an immunologic reaction to bone allografts has been recognized in both patients and in animal models [1, 3–24], prompting measures such as freezing and thawing of the donor bone to reduce the antigenicity of the allograft. The reported failure rates for massive structural allografts range from 10% to 50% [9, 10]. The most common causes for graft failure are limited incorporation of the allograft into host tissue, fractures, infections, or unopposed resorption. An alloimmune response from the host may contribute to suboptimal events such as rejection, delayed graft incorporation, osteolysis, bone erosions, articular degeneration, infection, and fracture. Animal models and human studies demonstrate HLA alloantibodies are induced by mismatched bone transplants [8–12, 15, 18, 20, 23, 24]. For example, Strong and coworkers reported an increase in HLA antibody sensitization from 39% to 67% associated with bone allografts, but they were unsure if there was any associated effect on allograft incorporation. Like most HLA bone allograft studies, their study did not test for donor-specific HLA antibody sensitization, only a global HLA sensitization recorded as present or absent [21]. The extent and importance of the antibody responses relative to the outcome of fresh, frozen human bone allograft implantation is not established.
One preliminary study reported donor-specific antibodies developed in 17 of 32 patients (53%) receiving massive structural allografts of which 11 of 17 were positive against Class I and II antigen mismatches, four of 17 were positive against Class I only, and two of 17 were positive against Class II only [23]. Perhaps owing to the limited data the authors found no correlation between HLA status and time to host-allograft union, but detected a trend that would require more time and/or patients to detect. Other than one paper published on the immunologic effects of bone-soluble proteins on allograft incorporation by VandeVord et al. [23], we are aware of no further publications on HLA sensitization and allograft bone incorporation. It remains unknown whether the observed HLA sensitizations reported in the past literature reflect donor-specific HLA sensitization, and whether donor-specific HLA sensitization influences the incorporation of bone allografts and their subsequent biologic performance.
We also sought to define the incidence and specificity of HLA antibody sensitization from cortical bone-replacing allograft procedures. Given the earlier reported trend, we hypothesized host HLA sensitization to major structural allograft antigens would delay healing. Therefore, our study was designed to answer the following two primary questions: (1) What was the rate or incidence of donor HLA antigen-specific HLA antibody sensitization induced in the host by these massive cortical and corticocancellous structural allografts? (2) What demonstrable effect on healing time (incorporation time) of those allografts did the HLA sensitization have? The study was also designed to answer the following additional questions to investigate factors that might independently affect healing: (3) Would differential healing rates based on the junction type (cancellous versus corticocancellous) reveal any subtle HLA healing effects? (4) Was the healing time influenced by the nature of the bone contact areas, comparing cortical with cortical junctions with corticocancellous to corticocancellous junctions? (5) Could we detect other factors that might affect allograft healing, including initial fixation devices, initial construct stability, need for additional surgeries, complication occurrences, bone bank allograft source, use of chemotherapy, use of radiation therapy, extent or quality of initial bone coaptation, allograft type, or the use of supplemental allograft or autograft at the junction site? (6) Would the exact definition of “healed” influence the results materially or would the results stand up regardless of how “healed” was defined, comparing the results based on the contributing surgeon’s clinical judgment regarding “healed” with a standardized radiographic interpretations of healing by individuals blinded to the patient’s HLA status? (7) Was there an HLA sensitization effect on allograft reconstruction longevity as measured by graft resorption, graft fracture, articular degeneration, or other complications?
Materials and Methods
In this ongoing prospective study, each of seven participating centers agreed to enroll all consenting patients older than 12 years scheduled for massive structural, cortex-replacing, cortical or corticocancellous fresh-frozen human bone allograft implantations into the study. All patients had reconstruction of the involved long bone with an intercalary graft, an osteoarticular graft, or an allograft-endoprosthesis composite graft of a resection-arthrodesis graft. All patients in the study were reconstructed after limb-salvaging resection of musculoskeletal tumors. The subjects self-randomized themselves into two groups based on their donor-specific alloantibody response to the structural bone allograft as determined by molecular testing of the donor allograft tissue and the host serum antibody testing methodology described subsequently. Determination of the healing and of the results was recorded by the contributing surgeons and a radiographic interpretation of all studies was also performed by two of the study investigators (WGW, CHB), all of whom remained blinded to the results of the HLA blood testing. We prospectively enrolled 57 patients who underwent implantation of massive cortical bone-replacing, weightbearing structural allografts, including both pure cortical allografts as well as corticocancellous allografts. We did not consider patients who did not require major weightbearing cortical or corticocancellous allograft bone grafts; thus, patients with cancellous-only and cavity-only defects were not considered. Furthermore, onlay allografts such as onlay struts were excluded. In other words, the graft itself had to restore a weightbearing cortical structure. We used no additional inclusion or exclusion criteria. Those grafts in the diaphyseal and proximal metaphyseal regions were classified as cortical only, and those in the epiphyseal and distal metaphyseal regions were arbitrarily classified as corticocancellous. At the time of writing, posttransplantation antibody sensitization data were available for 57 patients; 46 of these 57 patients also had radiographic followup of varying time intervals to determine radiographic healing (11 excluded as a result of absence of radiographic followup). Because of variable followup, survivorship analysis techniques (log rank tests) were used to evaluate the healing data. Among these 46 patients, 28 were men and 18 women; the average age at implantation was 29.1 years (range, 12–62 years). Allograft source was the Musculoskeletal Transplant Foundation, Edison, NJ (n = 29); Northwest Tissue Bank, Seattle, WA (n = 3); Regenerative Technologies, Cambridge, MA (n = 2); University of Miami Bone Bank, Miami, FL (n = 1); and unknown (n = 10). Time to union was the primary outcome used to determine whether sensitization to the HLA alloantigens correlated to the reduced likelihood of incorporation of the bone graft into the host bone. The minimum clinical and radiographic followup was 112 days (mean, 694 days; median, 763 days; range, 112–1547 days). The HLA-positive and HLA-negative patient groups are presented (Table 1).
Table 1.
Comparative characteristics of HLA groups
| Characteristics | HLA-negative patients (19) junctions (29) | HLA-positive patients (25) junctions (35) | p Value |
|---|---|---|---|
| Age, mean (SD) | 28.7 (14.0) years | 29.5 (14.4) years | 0.8607 |
| Gender, number (%) male | 14 (70.0%) | 14 (53.9%) | 0.2658 |
| Followup, mean (SD) | 724.9 (389.4) days | 670.9 (436.2) days | 0.6654 |
| Type of junction | 0.4176 | ||
| Cortical, number (%) | 21 (72.4%) | 22 (62.9%) | |
| Corticocancellous, number (%) | 8 (27.6%) | 13 (37.1%) | |
| Chemotherapy, number (%) | 14 (70%) | 14 (53.8%) | 0.2658 |
| Radiation therapy, number (%) | 2 (10.0%) | 2 (7.7%) | NA |
| Type of allograft | 0.6376 | ||
| Osteoarticular, number (%) | 6 (31.6%) | 7 (28.0%) | |
| Allograft-prosthetic composite, number (%) | 5 (26.3%) | 6 (24.0%) | |
| Intercalary, number (%) | 7 (36.8%) | 12 (48.0%) | |
| Resection arthrodesis, number (%) | 1 (5.3%) | 0 (0%) |
SD = standard deviation; NA = not available.
Before implantation of the allograft, blood samples were drawn and analyzed to determine each patient’s HLA antibody status. The HLA sensitization status of the patients was unknown at the time of surgery and remained unknown to all surgeons and investigators until final data analysis was completed. Patients completed a demographics form before surgery and again at each annual visit, including age, gender, race, education level, marital status, employment status, transfusion history and, in women, pregnancy history.
Physicians at each of the seven collaborating centers provided recipient blood samples and donor bone tissue samples to the HLA/Immunogenetics Laboratory, Wake Forest University Baptist Medical Center so HLA typings and anti-HLA antibody analysis could be performed. HLA Class I and Class II genotypes of patients and donors were obtained from polymerase chain reaction (PCR)-amplified DNA through sequence-specific primer SSP kits (Invitrogen, Carlsbad, CA). The DNA was isolated from peripheral blood buffy coat cells of graft recipients by Qiagen QIAmp (Valencia, CA) or Promega Maxwell 16 (Madison, WI) blood kits. DNA was isolated from residual tissue or from the discarded fatty bone marrow of submitted bones by tissue kits for QIAmp or Maxwell 16 systems.
Serum samples were tested for anti-Class I and anti-Class II HLA antibodies by solid phase techniques. Initially, GTI ELISA (Waukesha, WI) kits were used. From July 2004 to May 2005, solid phase flow cytometry beads from One Lambda, Inc (Canoga Park, CA) were used. After May 2005, Tepnel LifeMatch Luminex PRA (Stanford, CT) kits were used for anti-HLA antibody identification for Class I and Class II antibodies. The final analysis of HLA-antibody data evaluated the HLA genotype of bone allograft donors and recipients in an attempt to distinguish between antibodies induced by the bone allograft and those induced by transfusion or pregnancy. (For a more detailed description of the molecular PCR-SSP HLA typing methodology, see our prior description in Ward et al. [24].)
Each surgeon documented details of their reconstructive surgical procedure, including surgical site, length of resection, type of allograft (osteoarticular, allograft-prosthetic composite, intercalary, resection arthrodesis), fixation devices used (plates, rods, endoprostheses, interfragmentary screws, and cortical struts with cables), fixation/construct stability (solid, some motion—1 to 2 mm of motion which we view as substantial—will need bracing, or other), and utilization of host and/or allograft junctional supplemental grafting (Tables 2–6). At the bony junctions, supplemental host autograft was used in 10 cases (seven iliac crest bone grafts, three other undefined sources) and supplemental allograft was used at the bony junctions in 15 cases (including eight demineralized bone grafts, six morselized allografts, three cortical struts, two other). We did not gather specific data regarding the soft tissue envelope and periosteum preservation, but the periosteum is resected up to the bony resection margin in most tumor resections. Each patient’s chemotherapy and radiation therapy participation before and after surgery was documented as well. An immediate postoperative radiograph of the allograft site was obtained. Patients returned for followup examinations and radiographs at 3, 6, 9, 12, 18, and 24 months. Patients continued to be followed for another 12 months if the allograft site was not healed by 24 months. At each visit, the surgeon rated the allograft as clinically healed or not healed. At 6 and 12 months posttransplant, serum samples were collected for additional HLA antibody analysis.
Table 2.
Fixation device used and healing data
| Device used | Plates (percent healed) | Intramedullary rods (percent healed) | Endoprosthesis (percent healed) | Interfragmentary screws (percent healed) | Cortical struts with cables (percent healed) |
|---|---|---|---|---|---|
| Yes | 24 of 38 (63%) | 12 of 21 (57%) | 4 of 5 (80%) | 10 of 15 (63%) | 2 of 5 (40%) |
| No | 15 of 25 (60%) | 27 of 42 (64%) | 35 of 58 (60%) | 29 of 48 (60%) | 37 of 58 (64%) |
| p value | NS | NS | NS | NS | NS |
NS = nonsignificant.
Table 6.
Bone bank source and distribution of healing and HLA status
| Allograft source | Number (%) of patients (46) | Number (%) of junctions (64) | Percent healed | Percent HLA-positive | Percent HLA-negative |
|---|---|---|---|---|---|
| Allosource | 1 (2.8%) | 1 (1.9%) | 0 (0%) | 0 (0%) | 1 (100%) |
| Musculoskeletal Transplant Foundation | 29 (80.6%) | 42 (79.2%) | 27 (64.3%) | 24 (57.1%) | 18 (42.9%) |
| Northwest Tissue Center | 3 (8.3%) | 5 (9.4%) | 4 (80%) | 5 (100%) | 0 (0%) |
| Regeneration Technologies | 2 (5.6%) | 3 (5.7%) | 3 (100%) | 0 (0%) | 3 (100%) |
| University of Miami | 1 (2.8%) | 2 (3.8%) | 0 (0%) | 0 (0%) | 2 (100%) |
| 10 unknown* | 11 unknown* |
*Bone Bank Source was determined retrospectively; data were not available for all cases at the time of manuscript preparation.
All radiographs from each followup interval were interpreted by an orthopaedic oncologist (WGW) and a musculoskeletal radiologist (CHB), both of whom were blinded as to the HLA status of the patients through a standardized interpretation template (Appendix 1). Any differences were resolved by consensual agreement by having the two reviewers reevaluate the radiographs together and reconsider the data point(s) in question. All such discrepancies were thereby readily resolved by consensual agreement (we retrospectively estimate that fewer than 5% of determinations or readings involved such discrepancies).
The junctions were all categorized as either a cortical-cortical junction or as a corticocancellous to corticocancellous junction depending on the anatomic location of the junction (diaphyseal and proximal metaphysis = cortical; distal metaphyseal and diaphyseal = corticocancellous). All unicondylar or hemicondylar grafts were corticocancellous by definition. Healing was defined for purposes of data analysis when graded on the reading form as either “completely healed” or “probably healed” (Appendix 1). The host-allograft junction sites were evaluated for bone-to-bone healing (incorporation) by standard orthopaedic radiographic criteria; for example, cortical to cortical healing was judged to have occurred when the cortex of the allograft and of the host flowed together uninterruptedly without an intervening lucent line and corticocancellous when the cortex flowed together as described previously and there was trabecular bridging of the cancellous bone, all without an intervening lucent line. The vast majority of radiographs clearly fell into either the healed or unhealed categories, but as a result of the variability in anatomic locations, radiographic projections, and overlying metallic shadows, we had to place some indefinite readings into the probably healed or probably not healed categories. However, by simply looking at the prior and the subsequent radiograph, one could readily determine that the vast majority were properly categorized. Virtually all of the “probably not healed” ones were still unhealed by the next time interval and the “probably healed” ones were clearly healed by the next time interval, confirming our original interpretations.
We analyzed and compared healing times of the entire group based on their HLA status. We used the Student’s t-test to compare times to healing in patients without and with HLA antibodies in those that actually healed, excluding those without healing. We then used log rank to compare survival in the two groups using time to healing as the endpoint and including all cases up to the point of their latest radiographic followup. This allowed us to control for the variable followup in these patients with cancer, many of whom died or were otherwise lost to longer followup. By including all patients, we avoided the potential bias introduced by excluding patients whose grafts never healed. Simple proportional comparisons were analyzed by chi square analyses. As a result of the known tendency of corticocancellous junctions to heal more rapidly than pure cortical junctions, we analyzed the HLA sensitization effect on both types of interfaces to determine if HLA status affected either type of interface healing. We also assessed the HLA effect on healing by defining healing slightly differently to be certain bias was not arbitrarily introduced by our definition of healing. The original category defined as “healed” included only those rated as “definitely healed” and “probably healed” from the radiographic assessment form completed by the physicians blinded to the patient’s HLA status. We reanalyzed the HLA effect by combining “partial healing” with “definitely healed” and “probably healed” (Appendix 1, Item B) and by assessment of osseous integration rating rather than overall healing (Appendix 1, Item C). Finally, we evaluated time to clinical healing as identified by the contributing surgeon rather than by the two blinded radiographic reviewers. In all of these analyses, we compared the healing time of HLA-positive patients with that of HLA-negative patients. We also compared the cortical junction healing times with corticocancellous junction healing times. We also analyzed multiple other factors that could influence the healing times, including initial fixation devices, initial construct stability, need for additional surgeries, complication occurrences, bone bank allograft source, use of chemotherapy, use of radiation therapy, extent or quality of initial bone coaptation, allograft type, or the use of supplemental allograft or autograft at the junction site. All data were collected prospectively except the allograft bone bank source, which was collected retrospectively as requested during the process of manuscript revision.
Results
Donor-specific HLA-antibody sensitization occurred in 26 of the 46 patients (57%) who had both posttransplantation antibody screening and radiographic data. Of those sensitized, 20 of 26 (77%) were positive against Class I and II antigen mismatches, three of 26 (11.5%) were positive against Class I only, and three of 26 (11.5%) were positive against Class II only. In all cases in which patients were sensitized posttransplant, at least one antibody specific to an HLA antigen of the bone donor was identified (data not shown). Where a large number of antibody specificities were identified, additional antibodies were largely members of a crossreactive epitope group of one of the donor antigens (data not shown). Of 24 patients with a history of blood transfusions, two were HLA-positive preoperatively. Of 13 women with prior pregnancy data, only one of six with prior pregnancy was preoperatively HLA-positive and one of seven without prior pregnancy was preoperatively HLA-positive. The characteristics of the HLA-positive and the HLA-negative patient groupings are shown (Table 1). There were no differences in the two groups in terms of age, gender, followup, type of junction, use of adjuvant therapies, or type of allograft.
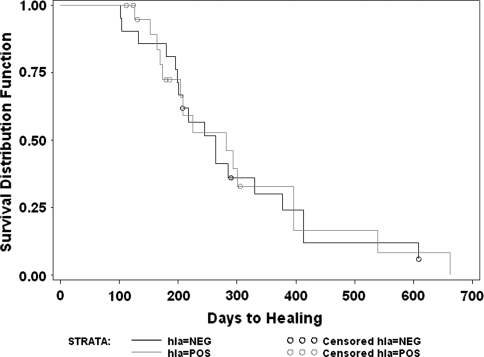
Analysis of HLA sensitization effect on allograft incorporation for the overall group revealed no differences with those developing HLA sensitization healing in an average of 451 days versus 450 days in those without HLA sensitization when comparing only those who healed during the study period (n = 27). A log rank survivorship analysis of all 46 patients also confirmed there was no difference in the two groups (Fig. 1) in time to healing.
Fig. 1.
This log rank survivorship analysis compares those who developed HLA antibodies (hla = POS) with those who did not develop HLA-specific antibodies (hla = NEG) for the overall group of all patients. Note the lack of difference between the two curves reflecting the lack of HLA effect on the ultimate healing times overall (p = nonsignificant).
Analysis of HLA sensitization on cortical-to-cortical junction (n = 43) allograft healing alone revealed HLA mismatch sensitization was not associated with an alteration of the healing time. The mean number of days to allograft-host bony union was 497 days in those without HLA sensitization mismatch versus 587 days in those with an HLA sensitization mismatch (p = 0.57, Student’s t-test) in the 22 cortical junctions that healed during the study period. We observed no difference (p = 0.61) in survival by HLA sensitization status when the 21 patients that were not healed at latest followup were included (Fig. 2).
Fig. 2.
This log rank survivorship analysis reveals no difference in the curves comparing healing-free survivorship of all cortical-to-cortical junctions when grouped by those who developed donor-specific HLA antibodies (hla = POS) versus those who did not develop donor-specific antibodies (hla = NEG) (p = nonsignificant).
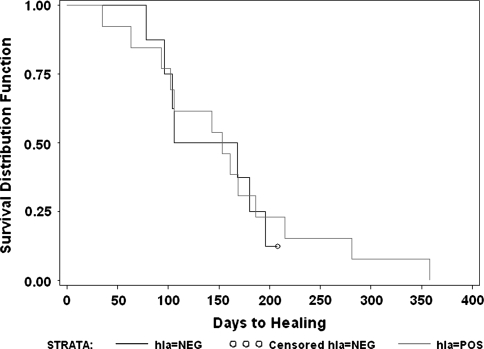
For allografts with corticocancellous junctions (n = 21), there was no difference (p = 0.85) in number of days to junctional healing for HLA-nonsensitized patients (mean, 252 days) compared with HLA-sensitized patients (mean, 238 days). We observed no difference in survival (p = 0.40) by HLA sensitization status, including the four patients who were not healed at latest followup (Fig. 3).
Fig. 3.
This log rank survivorship analysis reveals no difference in the curves comparing healing-free survivorship of all corticocancellous to corticocancellous junctions when grouped by those who developed donor-specific HLA antibodies (hla = POS) versus those who did not develop donor-specific antibodies (hla = NEG) (p = nonsignificant).
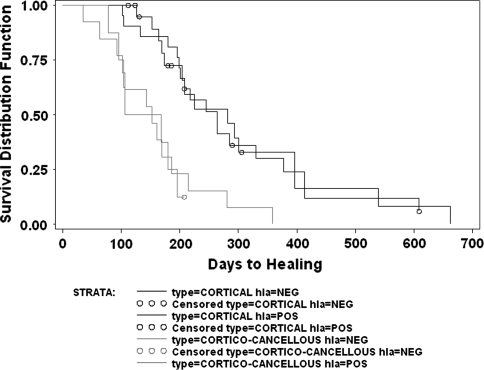
The type of junction (corticocancellous versus cortical) appeared to have a major association with healing. For those with HLA sensitization, corticocancellous junctions healed faster (p = 0.008) than cortical junctions (mean, 238 days versus 587 days) in the 21 junctions that healed during the study period. Survival analysis (n = 35) with time to union and including the 14 patients who were not healed at latest followup confirmed faster healing for corticocancellous junctions than cortical junctions in patients (p = 0.027) with HLA sensitization (Fig. 4). For those without HLA sensitization, corticocancellous junctions also healed faster (p = 0.08) than cortical junctions (252 days versus 497 days) for the 18 patients who healed during the study period. Survival with time to healing among these 29 patients including the 11 patients who had not healed at latest followup again confirmed the faster healing for corticocancellous junctions in patients (p = 0.0017) without HLA sensitization (Fig. 5). Overall survivorship analysis of all four groupings (HLA-positive, HLA-negative, cortical junctions, corticocancellous junctions) demonstrated the differences (p = 0.0014) regardless of the HLA sensitization status (Fig. 6) as does survivorship analysis of the healing differences of cortical-to-cortical versus corticocancellous to corticocancellous junctions, including all patients without regard to their HLA status (n = 64; p = 0.0002) (Fig. 7).
Fig. 4.
This log rank survivorship analysis depicts the difference between the cortical-to-cortical junctions versus the corticocancellous to corticocancellous junctions analyzing only those patients who developed donor-specific HLA antibodies. Note the more rapid (p = 0.027) healing of the corticocancellous junctions.
Fig. 5.
This log rank survivorship analysis depicts the more delayed healing of the cortical-to-cortical junctions compared with the corticocancellous to corticocancellous junctions analyzing only those patients who did not develop donor-specific HLA antibodies (p = 0.0017).
Fig. 6.
This log rank survivorship analysis depicts all four curves, including cortical-to-cortical junctions compared with corticocancellous junctions also considering those that developed donor-specific HLA antibodies (hla = POS) and those that did not develop donor-specific HLA antibodies (hla = NEG). The curves clearly demonstrate that the segregation is based on the junctional type rather than the HLA antibody status (p = 0.0014).
Fig. 7.
This log rank survivorship graph depicts the difference between cortical-to-cortical junctions versus corticocancellous to corticocancellous junctions considering the entire cohort without regard to HLA status. It clearly demonstrates the difference (p = 0.0002) between healing of the two groups with the more rapid healing of the corticocancellous junctions.
A number of other variables and factors were assessed, but none demonstrated an effect on the proportions of allograft-host junctions that healed. These include initial fixation devices used (Table 2), stability rating of the initial fixation (all but one rated solid by operating surgeon), need for or type of additional surgery required (Table 3), complications (including infection, nonunion, fracture of host bone, fracture of allograft, fixation failure, allograft resorption, wound dehiscence, tumor recurrence, and deep venous thrombosis) (Table 4), use of chemotherapy and radiation therapy (Table 5), or bone bank source of allograft (Table 6). The results for initial bony coaptation and ultimate healing show a trend with 11 of 14 (79%) of those with excellent coaptation healing compared with 18 of 37 (49%) of those with good or fair coaptation healing (p = 0.054), but by survivorship analysis, there was no difference or trend detectable (Appendix 1, Item A). The bony coaptation ratings were roughly evenly distributed across the HLA groups but slightly favored the HLA-negative group; 30% of those who were HLA-positive had excellent coaptation ratings compared with 43% of those who were HLA-negative, and 70% of those who were HLA-positive had good and fair ratings compared with 57% of those who were HLA-negative (p = 0.044). The use of any form of autograft supplementation at the junctional zones was associated with a higher proportion (p = 0.024) of junctions that healed (13 of 15 healed [87%]) compared with those without supplementation (26 of 48 healed [54%]), but by survivorship analysis of time to healing, there were no differences between the two groups. The use of supplemental allograft at the junctions showed no association with the proportion of junctions healing because 13 of 23 (54%) healed with grafting versus 26 of 40 (65%) without grafting. Supplemental allografting did not alter survivorship using healing as an endpoint. The widely varied anatomic distribution of the allografts precluded analysis by location with the current numbers. Healing by graft type was reflective of the cortical versus corticocancellous junctional nature. A greater (p = 0.015) percentage of osteoarticular allografts united to the host bone compared to intercalary allografts (92% versus 60%, respectively); 42% of host allograft junctions in allograft-prosthetic composites united. However, survival with time to healing as the endpoint was similar among the three groups (see Table 7).
Table 3.
Indications for additional surgery and healing data
| Condition present | Nonunion/delayed union (percent healed) | Fracture (percent healed) | Wound dehiscence (percent healed) | Fixation failure (percent healed) | Tumor recurrence (percent healed) | Infection (percent healed) | Other (percent healed) | Any (percent healed) |
|---|---|---|---|---|---|---|---|---|
| Yes | 0 of 2 (0%) | 1 of 1 (100%) | 3 of 4 (75%) | 1 of 2 (50%) | 2 of 4 (50%) | 2 of 4 (50%) | 9 of 15 (60%) | 12 of 20 (60%) |
| No | 39 of 62 (63% | 38 of 63 (60%) | 36 of 60 (60%) | 38 of 62 (61%) | 37 of 60 (62%) | 37 of 60 (62%) | 30 of 49 (61%) | 27 of 44 (61%) |
Table 4.
Complication incidence and healing data
| Complication present | None (percent healed) | Superficial infection (percent healed) | Deep infection (percent healed) | Nonunion/delayed union (percent healed) | Fracture (host bone; percent healed) | Fracture (allograft bone; percent healed) | Fixation failure (percent healed) | Allograft resorption (percent healed) | Wound dehiscence (percent healed) | Tumor recurrence (percent healed) | Deep venous thrombosis (percent healed) | Other % healed | Any (percent healed) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | 10 of 16 (63%) | 2 of 3 (67%) | None | 2 of 5 (40%) | 1 of 2 (50%) | None | 3 of 4 (75%) | 1 of 1 (100%) | 1 of 1 (100%) | 0 of 1 (0%) | 0 of 1 (0%) | 6 of 9 (67%) | 20 of 34 (59%) |
| No | 29 of 48 (60%) | 37 of 61 (61%) | 39 of 64 (61%) | 37 of 59 (63%) | 38 of 62 (61%) | 39 of 64 (61%) | 36 of 60 (60%) | 38 of 63 (60%) | 38 of 63 (60%) | 39 of 63 (62%) | 39 of 63 (62%) | 33 of 55 (60%) | 19 of 30 (63%) |
Table 5.
Chemotherapy and radiation therapy incidence and healing data
| Treatment | Chemotherapy (percent healed) | Radiation (percent healed) |
|---|---|---|
| Yes | 26 of 42 (62%) | 2 of 6 (33%) |
| No | 13 of 22 (59%) | 37 of 58 (64%) |
Table 7.
Allograft reconstruction type and healing data
| Allograft reconstruction type | Number (%) of patients (46) | Number (%) of junctions (64) | Healing rates (p = 0.0154) | Mean healing time (p = 0.6044) | Percent HLA-positive | Percent HLA-negative |
|---|---|---|---|---|---|---|
| 1 = Osteoarticular | 13 (28%) | 13 (21%) | 12 (92%) | 489 days | 7 (20.6%) | 6 (21.4%) |
| 2 = Allograft-prosthetic | 11 (24%) | 12 (19%) | 5 (42%) | 440 days | 6 (17.7%) | 6 (21.4%) |
| 3 = Intercalary | 19 (41%) | 35 (56%) | 21 (60%) | 370 days | 21 (60.0%) | 14 (50.0%) |
| 4 = Resection-arthrodesis | 1 (2%) | 2 (3%) | 0 (0%) | 0 (0%) | 2 (7.1%) |
We also assessed healing by several other defined healing end points without finding any difference in the results obtained from the analyses presented previously. Combining “probable” with “complete” and “partial” healing for analysis purposes again confirmed the findings reported previously as did the use of the contributing surgeons’ assessment of time to union for analysis. Even with the alternate assessment of healing, HLA status did not influence overall incorporation (Fig. 8), cortical-to-cortical junction healing (Fig. 9), or corticocancellous junctional healing (Fig. 10). Corticocancellous to corticocancellous junctions healed more quickly (p < 0.0001) than cortical to cortical junctions even with this alternate radiographic definition of healing (Fig. 11). Thus, no matter how defined, the results were the same, whether based on contributing surgeon’s evaluations of healing or by any variation of the definition of healed in our blinded radiographic interpretations.
Fig. 8.
This log rank survivorship analysis demonstrates there in no effect on time to allograft healing comparing those that develop HLA antibodies (hla = POS) with those that do not develop HLA antibodies (hla = NEG) even when the definition of radiographic healing is expanded to include the categories of “possibly healed” in addition to “completely healed” and “probably healed.”
Fig. 9.
This log rank survivorship analysis of cortical to cortical junction healing times, including completely healed, probably healed, and possibly healed in the radiographic definition of healing, again confirms an absence of any detectable effect of HLA sensitization (hla = POS) or the lack of HLA sensitization (hla = NEG) on healing in cortical junctions.
Fig. 10.
This log rank survivorship analysis on the timing of healing of corticocancellous to corticocancellous junctions includes completely healed, probably healed, and possibly healed in the definition of healing. There is no detectable effect of HLA sensitization (hla = POS) or the lack thereof (hla = NEG) on time to allograft healing in these corticocancellous junction sites.
Fig. 11.
This series of log rank survivorship curves, stratified by cortical-to-cortical junctions and corticocancellous to corticocancellous junctions as well as HLA-positive status (hla = POS) and HLA-negative status (hla = NEG), clearly demonstrates that the only factor effecting healing is the junctional type. The corticocancellous to corticocancellous junctions healed faster (p = 0.0001) than the cortical-to-cortical junctions.
There were too few failures of the allografts in this short followup timeframe to perform any analyses of potential HLA influence on such events (Tables 3, 4).
Discussion
The rationale of this study was to determine the rate of HLA sensitization in these cortex-replacing structural massive fresh-frozen allografts and to determine if the subsequent HLA sensitization had a detectable effect on allograft healing or longevity. By way of review, the HLA system’s purpose is to present antigens to CD-8+ T-cells (Class I) and CD-4+ T-cells (Class II) among other functions. Class I HLA is found on just about every nucleated cell of the body. Antibodies to Class I molecules are the main effectors of hyperacute rejection and one of the main effectors of acute rejection. Antibodies to foreign HLA can only be developed by three mechanisms: transplant, transfusion, or pregnancy. A person’s response to foreign HLA is not monolithic. A previous response to a particular HLA antigen will not preclude a response to a different HLA antigen in a subsequent exposure. Indeed, two patients in this report who had anti-HLA antibodies pretransplant developed additional antibodies specific to their donor’s HLA (and other specificities) posttransplant. Our results strongly suggest bone grafts can cause sensitization to HLA antibodies that may result in a patient being at a higher risk for any subsequent organ or tissue transplant. With regard to the bony allograft reconstructive process, if a deleterious effect of HLA sensitization on allograft incorporation was detected, then modifications to allograft use would be indicated, potentially including HLA crossmatching or immune system manipulations. Although the rate of HLA sensitization was 57%, for the purposes of effecting improved rates allograft healing, no benefit from HLA crossmatching or immune system manipulation would be predicted from this study.
We had a heterogeneous patient population (including anatomic locations, surgical bone defects, surgical techniques, and bone fixation hardware variations) with variables that can influence healing and healing rates. The allograft constructs reflect the widely varying needs of the individual patients. We assume these variables would not influence differences based on HLA status. The use of such massive, weightsharing cortical or corticocancellous allografts is so uncommon that such a heterogeneous population is required to assemble sufficient cases for analysis. However, all cases involved structural cortical bone loss requiring cortex-containing structural allograft reconstructions. It required a multiinstitutional contribution to gather sufficient cases for meaningful analysis. Limiting the study to one anatomic area would be desirable, but acquiring sufficient numbers would be difficult. Another inherent weakness is the difficulty in accurately and precisely defining allograft incorporation or healing. However, no matter what exact definition we used to define “healed,” the results were unchanged and never demonstrated any HLA effect on healing. A potential source of research weakness is the variability of the grafts themselves in terms of processing, implantation, fixation techniques, fixation quality, complications, radiation use, chemotherapy use, soft tissue envelope, periosteal preservation, supplemental bone grafting at the junctions, and anatomic distribution. We addressed these concerns as best we could with the limited numbers available (Tables 1–7), but the limited numbers do not allow definitive statements to be made regarding any of these variables. Although some of the allografts may have undergone slightly differing processing techniques based on their bone bank of origin, because at least 81% of the allografts in which we could determine the source bank were from one bank, and because there was an even distribution of HLA status across this bank, it is unlikely processing affected the healing results in a major material manner for the purposes of this study (Table 6).
Although we were unable to demonstrate any clinically detectable effect of HLA sensitization on allograft incorporation to date, junction type (cortical versus corticocancellous) demonstrated a substantial effect on allograft incorporation. This effect was present regardless of HLA sensitization status. Cortical allografts, not surprisingly, had greater difficulty in achieving host-allograft union. Measures to further reduce sensitization and immunogenicity for the purposes of improving allograft incorporation rates may not affect allograft incorporation and are not supported by these data. There could be other benefits from minimizing the HLA sensitization rate. There might be less potential interference with future eligibility for receipt of organ transplantation. There could be a decrease in other as yet undefined potential adverse effects of HLA sensitization, including potential long-term effects on the allograft. However, at this point in time, no definite benefit from modulation of HLA sensitization in terms of structural allograft incorporation would be predicted from our data. Further research is indicated to determine the advantages and disadvantages of these potential interventions as well as that of other factors influencing allograft success.
The overall HLA sensitization rate of 57% has one important negative implication. Sensitized patients will have greater difficulty in finding suitably matched organs should they ever need a living tissue organ transplantation such as a kidney or heart transplant. The sensitization might accentuate their rejection of such organ transplants. Measures to reduce the sensitization rate of bone allograft patients is indicated for this purpose. A similar effect has been accomplished with packed red blood cell transfusion with the introduction of leukocyte cleansing, which decreased the HLA sensitization rate of blood donation from 31% to 11% [2].
General knowledge regarding bone graft incorporation predicts corticocancellous grafts should heal much more readily than pure cortical-to-cortical junction allografts; thus, the results of this study are in keeping with general expectations arising from bone graft biology, reflecting this cohort of patients provides a reasonably representative group of patients for allograft science investigations. The lack of any detectable effect of HLA sensitization on time to healing of cortical or corticocancellous allografts suggests the effect, if any, of HLA sensitization on allograft incorporation is small and of less importance than other biologic processes such as junction characteristics. This argument is strengthened by the simple observation that 17 of 21 corticocancellous junctions healed within the timeframe of this study compared with only 22 of 43 (51%) cortical junctions within this study’s current timeframe (despite an average radiographic followup of 391 days in the 21 unhealed cortical junctions). There was no association between type of fixation and healing.
The majority of the healed allografts survived for the minimum 3-year followup period of this study. As of the time of this writing, there is no correlation between HLA status and other complications, revisions, or articular deterioration. Therefore, a study to investigate the potential longer-term effects of HLA sensitization such as articular destruction, degenerative wear, allograft fracture, allograft dissolution, allograft resorption, and other potential factors will require longer followup and greater numbers based on the low incidence of such events within the first 3 years of followup. Such long-term followup should provide additional valuable information about the effects of HLA mismatch on graft survival.
We believe it is important to determine if HLA sensitization affects the long-term survival of massive allografts such as allograft fracture, allograft resorption, or articular degeneration. We observed a low rate of baseline preoperative HLA sensitization. Furthermore, every patient who demonstrated HLA sensitization postoperatively had at least one antibody specifically directed toward at least one of the donor HLA antigens. No patient was HLA-positive without having at least one specific antidonor antibody. Therefore, it stands to reason that a future study could be performed without requiring preoperative testing. One could simply compare the rate of HLA antibody sensitization in patients with allograft complications with the rate of HLA antibody sensitization in those without complications to provide reasonable evidence regarding the effect, if any, of HLA sensitization on such complications. Such a study could be performed in a multiinstitutional manner without the need for HLA typing of the donor bone and without the need to analyze for donor-specific antibodies. Although this would lack the one-to-one antigen to antibody specificity of this study, the acceptance of this implication would greatly enhance the feasibility of such a study as long as the study group is composed of a population similar to that studied here.
These preliminary results confirm that HLA sensitization occurs in over half of the patients who receive a massive bone-replacing allograft. Although the data suggest it might be reasonable to ignore HLA matching with current allografts for purposes of graft incorporation, there may be other important issues that the sensitization does cause for individual patients such as presensitizing patients who may need a living organ transplantation in the future. It is important to continue to enroll and study patients with massive structural allografts to determine what HLA-associated effects might be detectable with longer followup in a larger series.
Acknowledgments
We thank Beth Smith, PhD, for guidance and copyediting, Leah Passmore, for her statistical analysis, Debbie Bullard for research coordination, Matthew Cline, MS III, for his valuable assistance as medical student research project assistant, and Kathy Riggs for typing the manuscript. We also thank David Kiger, CHS, Elaine Forrest, CHS, and the staff of the Wake Forest University/Baptist Medical Center’s HLA/Immunogenetics Laboratory for their dedication and professionalism in dealing with less-than-ideal specimens in an expeditious fashion. The following individuals and institutions were contributing members of the HLA Allograft Project Team and each contributed completed cases to the study: Mark Scarborough, MD, University of Florida, Gainesville, FL; Lor Randall, MD, University of Utah, Salt Lake City, UT; Michael Joyce, MD, Cleveland Clinic, Cleveland, OH; Gary Bos, MD, University of North Carolina, Chapel Hill, NC; Richard Nicholas, MD, University of Arkansas, Little Rock, AR; Luke Vaughan, MD, Scripps Research Institute, La Jolla, CA, Scott C. Wilson, MD, Wake Forest University Baptist Medical Center, Winston-Salem, NC; and William G. Ward, MD, Wake Forest University Baptist Medical Center, Winston-Salem, NC.
Appendix 1. Radiographic evaluation form
| A. Bony coaptation is: |
| 1 Excellent (difficult to accurately see interface—hard to determine healing) |
| 2 Good—mostly in contact but easy to detect interface (less than 4 mm maximum) |
| 3 Fair—gap 2 to 4 mm or angulated; easy to detect gap |
| 4 Poor—wide obvious gap |
| 5 Unable to determine |
| B. The radiographic appearance is: |
| 1 Completely healed (united) |
| 2 Probably healed |
| 3 Partially healed |
| 4 Undecided about healing |
| 5 Probably not healed |
| 6 Definitely not healed |
| 7 Definitely not healed |
| 8 Unable to determine |
| C. Overall osseous integration is: |
| 1 Complete (essentially all areas [greater than 75%]) |
| 2 Partial—some healed interface (at least 25%) and some unhealed interface |
| 3 Minimal—less than 25% healed or united |
| 4 Absent—no interface osseous integration |
| 5 Not applicable (baseline, etc) |
| 6 Unable to determine |
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patient/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author and contributor of patient material certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent was obtained for participating in the study.
References
- 1.Aho AJ, Eskola J, Ekfors T, Manner I, Kouri T, Hollmen T. Immune responses and clinical outcome of massive human osteoarticular allografts. Clin Orthop Relat Res. 1997;346:196–206. [PubMed]
- 2.Andrew G, Dewailly J, Leberre C, Quarre MC, Bidet ML, Tardivel R, Devers L, Lam Y, Soreau E, Boccaccio C. Prevention of HLA immunization with leukocyte-poor packed red cells and platelet concentrates obtained by filtration. Blood. 1988;72:964–969. [PubMed]
- 3.Aro HT, Aho AJ. Clinical use of bone allografts. Ann Med. 1993;25:403–412. [DOI] [PubMed]
- 4.Bos GD, Goldberg VM, Powell AE, Heiple KG, Zika JM. The effect of histocompatibility matching on canine frozen bone allografts. J Bone Joint Surg Am. 1983;65:89–96. [PubMed]
- 5.Burchardt H, Enneking WF. Transplantation of bone. Surg Clin North Am. 1978;58:403–427. [DOI] [PubMed]
- 6.Czitrom AA. The immune response: the afferent arm. Clin Orthop Relat Res. 1996;326:11–24. [DOI] [PubMed]
- 7.Enneking WF, Mindell ER. Observations on massive retrieved human allografts. J Bone Joint Surg Am. 1991;73:1123–1142. [PubMed]
- 8.Friedlander GE. Biological and immunological aspects of allogeneic bone transplantation. In: Lindholm S, ed. New Trends in Bone Grafting. Tampere: University of Tampere; 1976:169–175.
- 9.Freidlander GE. Immune responses to osteochondral allografts. Current knowledge and future directions. Clin Orthop Relat Res. 1983;174:58–68. [PubMed]
- 10.Friedlander GE, Strong DM, Sell KW. Studies on the antigenicity of bone. I: freeze-dried and deep frozen allografts in rabbits. J Bone Joint Surg Am. 1976;58:854–858. [PubMed]
- 11.Horowitz MC, Friedlander GE. Immunologic aspects of bone transplantation: a rationale for future studies. Orthop Clin North Am. 1987;18:227–233. [PubMed]
- 12.Horowitz MC, Friedlander GE. Induction of specific T-cell responsiveness to allogeneic bone. J Bone Joint Surg Am. 1991;73:1157–1168. [PubMed]
- 13.Lee MY, Finn HA, Lazda VA, Thistlethwaite JR Jr, Simon MA. Bone allografts are immunogenic and may preclude subsequent organ. Clin Orthop Relat Res. 1997;340:215–219. [DOI] [PubMed]
- 14.Muscolo DL, Ayerza MA, Calabrese ME, Redal MA, Santini AS. Human leukocyte antigen matching, radiographic score and histologic findings in massive frozen bone allograft. Clin Orthop Relat Res. 1996;326:115–126. [DOI] [PubMed]
- 15.Rodrigo JJ, Fuller TC, Mankin HG. Cytotoxic HLA antibodies in patients with bone and cartilage allografts. Trans Orthop Res Soc. 1976;1:131.
- 16.Skjodt H, Hughes DE, Dobson PRM, Russell RGG. Constitutive and inducible expression of HLA class II determinants by human osteoblast-like cells in vivo. J Clin Invest. 1990;85:1421–1426. [DOI] [PMC free article] [PubMed]
- 17.Stevenson S, Emery DW, Goldberg VM. Factors affecting bone graft incorporation. Clin Orthop Relat Res. 1996;323:66–74. [DOI] [PubMed]
- 18.Stevenson S, Li XQ, Martin B. The fate of cancellous and cortical bone after transplantation of fresh and frozen tissue antigens matched and mismatched osteochondral allografts in dogs. J Bone Joint Surg Am. 1991;73:1143–1156. [PubMed]
- 19.Stevenson S, Qing X, Davy DT, Klein L, Goldberg AM. Critical biological determinants of incorporation of non-vascularized cortical bone grafts. J Bone Joint Surg Am. 1997;79:1–16. [DOI] [PubMed]
- 20.Stevenson S, Shaffer JW. The humoral response to vascular and nonvascular allografts of bone. Clin Orthop Relat Res. 1996;326:86–95. [DOI] [PubMed]
- 21.Strong DM, Friedlander GE, Tomford WW, Springfield DS, Shives TC, Burchardt H, Enneking WF, Mankin HJ. Immunologic responses in human recipients of osseous and osteochondral allografts. Clin Orthop Relat Res. 1996;326:107–114. [DOI] [PubMed]
- 22.Tan MH, Mankin HJ. Blood transfusion and bone allografts. Clin Orthop Relat Res. 1997;340:207–214. [DOI] [PubMed]
- 23.VandeVord P, Nassar S, Wooley PH. Immunological responses to bone soluble proteins in recipients of bone allografts. J Orthop Res. 2005;23:1059–1064. [DOI] [PubMed]
- 24.Ward WG, Heise E, Boles C, Kiger D, Gautreaux M, Rushing J, Smith BP, Bullard D. Human leukocyte antigen sensitization after structural cortical allograft implantations. Clin Orthop Relat Res. 2005;435:31–35. [DOI] [PubMed]