Abstract
Myocyte nuclear factor (MNF) is a winged helix transcription factor that is expressed selectively in myogenic stem cells (satellite cells) of adult animals. Using a gene knockout strategy to generate a functional null allele at the Mnf locus, we observed that mice lacking MNF are viable, but severely runted. Skeletal muscles of Mnf−/− animals are atrophic, and satellite cell function is impaired. Muscle regeneration after injury is delayed and incomplete, and the normal timing of expression of cell cycle regulators and myogenic determination genes is dysregulated. Mnf mutant mice were intercrossed with mdx mice that lack dystrophin and exhibit only a subtle myopathic phenotype. In contrast, mdx mice that also lack MNF die in the first few weeks of life with a severe myopathy. Haploinsufficiency at the Mnf locus (Mnf+/−) also exacerbates the mdx phenotype to more closely resemble Duchenne's muscular dystrophy in humans. We conclude that MNF acts to regulate genes that coordinate the proliferation and differentiation of myogenic stem cells after muscle injury. Animals deficient in MNF may prove useful for evaluation of potential therapeutic interventions to promote muscle regeneration for patients having Duchenne's muscular dystrophy.
Duchenne muscular dystrophy (DMD) is a common, X-linked disease that results in progressive muscle wasting and death of affected male patients by their early twenties due to respiratory or cardiac failure (1, 2). DMD is caused by mutations in the gene encoding dystrophin, a large membrane-associated protein expressed in both cardiac and skeletal muscle (3–5). Clinical deterioration in adolescent patients with DMD is associated with muscle necrosis and fibrosis, in association with exhaustion of the myogenic stem cell pool (satellite cells) that supports muscle regeneration in younger patients (1, 2, 6).
Satellite cells of adult skeletal muscles constitute a self-renewing stem cell pool capable of regenerating skeletal myofibers in response to muscle injury (7–9). In addition, satellite cells participate in the hypertrophic response of skeletal muscles to functional overload (10) and are required to maintain muscle mass during normal aging (11). These cells are committed to a myogenic cell fate, but in the quiescent state they do not express myogenic determination genes of the MyoD or MEF2 families or other known markers of terminal differentiation (12–14). We previously determined that myocyte nuclear factor (MNF), a member of the forkhead/winged helix transcription factor family, is localized to this myogenic stem cell population in adult skeletal muscles (15). Two protein isoforms (MNF-α and MNF-β) arise by alternative splicing of primary transcripts derived from a single Mnf gene (16, 17). During embryogenesis, MNF is expressed predominately within the myotome of somites, within the developing myocardium, and in certain regions of the brain. In adult mice, however, MNF is restricted to the satellite cell population (13).
Materials and Methods
Generation of Mnf−/− Mice.
An Mnf genomic clone was isolated by screening a P1 genomic library with a complementary DNA fragment that included the DNA binding domain of the Mnf gene (17). The targeting vector was constructed by replacing a BamHI–HindIII fragment containing the winged helix domain with an RNA polymerase II promoter driving the neomycin expression cassette. The targeting vector consisted of 1.0 kb of 5′ Mnf sequence, the neomycin cassette, 7.1 kb of 3′ Mnf sequence, and a plasmid encoding double thymidine kinase drug-resistance cassettes. After electroporation into J1-embryonic stem cells, colonies were selected after incubation with G418 and gancyclovir. Homologous recombinants were identified by digesting genomic DNA with SacI and hybridizing with the 0.8-kb EcoRI/BamHI 5′ probe (hybridizing to the Mnf gene outside of the targeting vector). Offspring were genotyped by Southern blot analysis. Breeding pairs of mdx mice (C57BL/10ScSn) were purchased from Jackson Laboratories (Bar Harbor, ME). The genotypic assay used to assay DNA from tail biopsies of the mdx mice was the amplification refractory mutation system PCR assay to screen for the mdx allele (18).
RNA Isolation and Reverse Transcription (RT)-PCR.
Total RNA was isolated from adult skeletal muscle or cultured myoblasts or myotubes by using the Tripure isolation kit (Boehringer Mannheim). Four micrograms of total RNA was used in each RT reaction (Retro-script, Ambion). Complementary DNA (2 μl) then was used as a template for the PCR in a 20-μl reaction volume including 100 ng of each primer, 2 mM MgCl2, Taq buffer, and 1 unit of Taq polymerase (GIBCO/BRL). Fifteen microliters of each PCR was loaded on a 2% agarose gel as described (15, 16). Semiquantitative RT-PCR using RNA isolated from skeletal muscle or isolated myoblasts/myotubes was performed as previously described. PCR primer pairs used for this study include glyceraldehyde-3-phosphate dehydrogenase (GAPDH): F, 5′-GTGGCAAAGTGGAGATTGTTGCC-3′, R, 5′-GATGATGACCCGTTTGGCTCC-3′; MNF-α: F, 5′TACTTCATCAAAGTCCCTCGGTC-3′, R, 5′-GTACTCTGGAACAGAGGCTAACTT-3′; MNF-β: F, 5′-TACTTCATCAAAGTCCCTGCCTC-3′, R, 5′-GTGCGCGCGCGCATGTGGGC-3′; cdc-2: F, 5′-CGTTACTCCACTCCGGTTGACATC-3′, R, 5′-GACATCCATCCAGAGGGCTACATC-3′; c-myc: F, 5′-CAGGACTGTATGTGGAGCCGTTTC-3′, R, 5′-CTGGTGGTGGGCGGTGTCTCC-3′; MyoD: F, 5′-GCAGGCTCTGCTGCGCGACC-3′, R, 5′-TGCAGTCGATCTCTCAAAGCACC-3′; Myf5: F, 5′-TGAATGTAACAGCCCTGTCTGGTC-3′, R, 5′-CGTGATAGATAAGTCTGGAGCTGG-3′; and myogenin: F, 5′-GAGCGCGATCTCCGCTACAGAGG-3′, R, 5′-TCTGGCTTGCAGCCCAGG-3′.
Cardiotoxin-Induced Muscle Injury.
As described (15), cardiotoxin, diluted in PBS, was delivered i.m. to the hind limbs of adult mice. At specified intervals, mice were killed, and hind limb skeletal muscles were fixed in 4% paraformaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin and eosin.
Histological Analysis and Immunohistochemistry.
Skeletal muscles from wild-type, Mnf−/−, mdx, Mnf+/−:mdx, and Mnf−/−:mdx littermates were examined by light microscopy. Age distribution of the pairs was examined from 1–2 weeks of age through 6 months of age. Skeletal muscles were frozen in liquid nitrogen-cooled isopentane, and 6-μm thick sections were obtained. For bright-field microscopy, sections were fixed and stained with hematoxylin and eosin or Masson trichrome stain. For immunohistochemistry, sections were incubated overnight at 4°C with the primary antibody that was detected with a fluorophor-conjugated secondary antiserum as described (15).
In Vivo Membrane Permeability Assay.
Age-matched wild-type, Mnf−/−, mdx, and double mutant mice were injected with Evans blue, a dye that binds to albumin, fluoresces red, and is selectively taken up by cells with leaky plasma membranes. Briefly, 50 μl of Evans blue dye (500 μg/50 μl PBS) per 10 g of body weight was injected i.v. (dorsal tail vein), and 3 h after the injection the animals were killed and skeletal muscle was harvested, frozen in liquid nitrogen, and cryosectioned. Mice that were successfully injected with Evans blue dye had blue ears and blue feet as described (19–21, 23, 24).
Cell Culture and Proliferation Assays.
Muscle cells were isolated from wild-type, Mnf+/−, and Mnf−/− mice 3 days after birth according to the protocol of Pavlath et al. (24). Cell proliferation assays were performed on 1 × 104 cells plated in a 96-well plate and grown for 48 h at 37°C in a humidified 5% CO2 incubator. After addition of 20 μl of CellTiter96 Aqueous One Solution Reagent (Promega), absorbance was measured at a wavelength of 490 nm at 1-h time intervals by using a Bio-Tek plate reader. All results were corrected for total cell number as outlined in the Promega protocol.
Image Analysis.
Stained sections were examined with a Bio-Rad MRC 1024 confocal microscope equipped with a krypton/argon laser (Bio-Rad Life Science Group). Image processing was completed by using Adobe photoshop 5.0 and printed by using a Kodak XLS 8600 PS printer.
Statistical Analysis.
Statistical analysis used a multiway ANOVA for repeated measures with Duncan's posthoc analysis (BMDP Software, Supulvida, CA).
Results
MNF-α and MNF-β Are Reciprocally Expressed During the Sequential Stages of Muscle Regeneration.
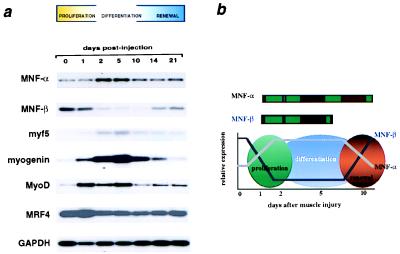
In the present study, we observed that the two isoforms of MNF are reciprocally regulated as satellite cells withdraw from a quiescent state, proliferate, and differentiate into multinucleated myofibers and re-establish a quiescent stem cell pool (Fig. 1a). Expression of MNF proteins was assessed by a RT-PCR assay using primers specific to each isoform (primers for MNF-α are outside the winged helix domain). MNF-β is the principal form expressed in quiescent satellite cells, whereas MNF-α predominates in proliferating myoblasts derived from these cells after cardiotoxin injury. This reciprocal expression pattern (shown schematically in Fig. 1b) suggests that the two isoforms of MNF may exert opposing effects on expression of target genes active at discrete steps in muscle regeneration or in renewal of the satellite cell pool.
Figure 1.
Skeletal muscle gene expression after cardiotoxin injury. (a) RT-PCR/Southern blot assay of the expression of MNF-α, MNF-β, and basic helix–loop–helix proteins of the MyoD family in regenerating muscles after cardiotoxin injury. MNF-α and MNF-β are reciprocally expressed in myogenic cells during regeneration, with MNF-β expressed in the quiescent stem cell and MNF-α expressed during stem cell proliferation. (b) Schematic of MNF-α and MNF-β expression during muscle regeneration.
Mice Without MNF Exhibit Growth Retardation and Impaired Muscle Regeneration.
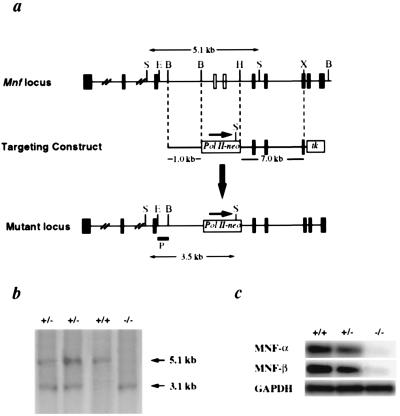
To assess the functional importance of MNF for muscle regeneration, we used homologous recombination to generate a functional null allele at the Mnf locus, ultimately generating mice lacking both isoforms of MNF. The gene targeting strategy deleted the winged helix region (DNA binding domain) common to MNF-α and MNF-β, which was replaced with a neomycin-resistance cassette (Fig. 2a) and progeny from Mnf+/− intercrosses were analyzed by using Southern analysis (Fig. 2b). Neither MNF transcripts (PCR primers for MNF-α were outside the deleted winged helix domain) nor MNF proteins could be detected by RT-PCR (Fig. 2c) or immunohistochemical assays (data not shown) in animals genotyped as Mnf−/−.
Figure 2.
Targeted disruption of Mnf. (a) Restriction map of the Mnf locus and gene targeting strategy. The targeting vector was constructed by replacing a BamHI–HindIII genomic fragment containing exons encoding the winged helix domain of MNF with a neomycin expression cassette, driven by an RNA polymerase II promoter. The targeting vector included a 1.0-kb 5′ Mnf sequence, 7.1 kb of 3′ Mnf sequence, and two thymidine kinase drug-resistance cassettes. Homologous recombinants were identified by Southern blot analysis using an 0.8-kb EcoRI/BamHI genomic fragment as the probe (hybridizing to the Mnf gene 5′ to the targeted region). Exons are represented as filled boxes. E, EcoRI; B, BamHI; H, HindIII; X, XhoI. (b) Offspring were genotyped by Southern blot analysis. (c) RT-PCR analysis of adult skeletal muscle reveals an absence of MNF-α and MNF-β transcripts in mutant mice.
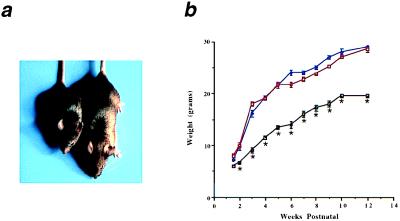
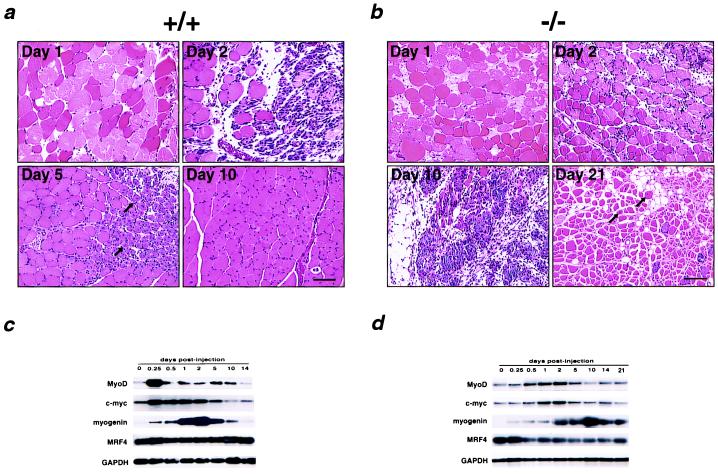
Mice lacking MNF display significant growth retardation that is evident at birth and persists into adulthood. Mnf−/− mice are approximately 60% the size of gender-matched Mnf+/+ littermates (Fig. 3a) at ages up to 1 year (Fig. 3b). When animals are challenged to regenerate muscle fibers after injection of cardiotoxin, Mnf−/− animals exhibit impaired muscle repair. In wild-type mice, skeletal muscle architecture is largely restored within 10 days of injury, whereas muscles of Mnf−/− animals manifest a hypercellular, myonecrotic response even 3 weeks after the initial injury (Fig. 4). Furthermore, muscle regeneration that eventually occurs is accompanied by extensive replacement of muscle by fat.
Figure 3.
Growth retardation of Mnf mutant mice. (a) The Mnf−/− mouse (Left) is approximately 60% the size of its wild-type, gender-matched (male) littermate (Right) at 6 weeks of age. (b) Mice without MNF are significantly smaller at each age up to a 1-year period compared with gender-matched littermates that are heterozygous for the targeted allele or wild type (Mnf+/+, blue line; Mnf+/−, red line; Mnf−/−, black line; n = 6 male mice at each age; *, P < 0.05). There were no significant differences noted between the heterozygotes and wild-type mice at 6 months of age, but significant differences were observed at 12 months of age (Mnf−/−, 26.4 ± 1.3 g vs. Mnf+/−, 36.2 ± 1.9 g vs. Mnf+/+, 41.7 ± 2.5 g; n = 6 pair, P < 0.05).
Figure 4.
Morphological and molecular assessment of the skeletal muscle after cardiotoxin delivery in Mnf+/+ and Mnf−/− mice. (a) In the Mnf+/+ mouse after cardiotoxin delivery, edematous myofibers are evident by day 1, an extensive cellular proliferative response by day 2, regenerating myofibers with central nuclei (arrows) by day 5, and restoration of the hind limb architecture by 10 days (12, 15). (b) In contrast, the Mnf−/− skeletal muscle is characterized by a persistent hypercellular response that remains 10 days after injury, the delayed appearance of regenerating myofibers at 21 days, and restoration of muscle architecture only after 28 days (n = 3). Note focal regions of myofiber replacement by fat (days 10 and 21) in regenerating Mnf mutant skeletal muscle. (Bar = 100 μ.) RT-PCR/Southern analysis using primers specific for MyoD, c-myc, MRF4, myogenin, or GAPDH sequences from total RNA (10 μg) isolated from hind limbs of control (c) or Mnf-null (d) mice at the indicated times after injection of cardiotoxin. Peak expression of MyoD and c-myc mRNA after cardiotoxin injury is delayed compared with the wild-type controls. Myogenin and MRF4 also are dysregulated in the Mnf−/− mouse (n = 3).
Expression of Myogenic Determination Genes Is Dysregulated in the Absence of MNF.
At this time, the biologically important target genes controlled directly by MNF are not known. However, the expression of myogenic determination genes (MyoD, myogenin, and MRF4) and cell cycle regulatory genes (c-myc) after cardiotoxin injury is perturbed in the Mnf mutant animals compared with wild-type controls (Fig. 4).
Perturbations in gene expression also are evident in myoblasts isolated from hind limbs of young (3-day-old) mice lacking MNF. Myoblasts from Mnf−/− animals are capable of forming differentiated myotubes after the removal of serum (Fig. 5a). However, in media supplemented with high concentrations of serum (20%), Mnf−/− cells grow more slowly than Mnf+/− or Mnf+/+ cells (Fig. 5b) and exhibit dysregulated expression of myogenic determination genes, most notably MRF4 (Fig. 5c). The functional significance of increased MRF4 expression in the Mnf−/− myotubes is unclear, but preliminary experiments have shown a modest delay in muscle regeneration in the MRF4 knockout mouse (data not shown).
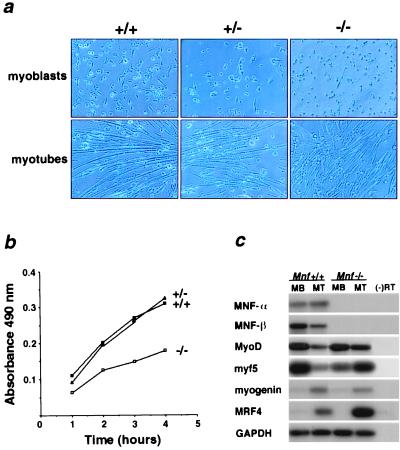
Figure 5.
Growth inhibition and differential gene expression of cultured muscle cells lacking MNF. (a) Muscle cells isolated from 3-day-old wild-type (+/+), heterozygous (+/−), and Mnf null (−/−) neonates were cultured in growth medium (myoblasts) and differentiation medium (myotubes). Mnf mutant myoblasts are capable of forming differentiated myotubes under low serum conditions. (b) Cell proliferation assays were performed on muscle cells isolated from 3-day-old neonatal wild-type (+/+), Mnf-heterologous (+/−), and Mnf-null (−/−) mice (performed in triplicate). (c) Semiquantitative RT-PCR/Southern blot analysis of MNF isoforms and MyoD family members in wild-type myoblasts (MB) and myotubes (MT) compared with Mnf−/− myoblasts (MB) and myotubes (MT) (performed in quadruplicate).
Myopathy in Animals Lacking Dystrophin Becomes Severe and Lethal in the Absence of MNF.
A defect in muscle regeneration in animals lacking MNF also is evident when these animals are intercrossed with the mdx strain, which lacks dystrophin (19, 25, 26). Mice deficient for both MNF and dystrophin (Mnf−/−:mdx) are smaller even than the runted Mnf−/− animals (Fig. 6a). The doubly mutant mice are less active, fragile, and weak compared with age- and sex-matched littermates of either the Mnf−/− or mdx genotype. Morphological examination of skeletal muscles from these animals revealed myonecrosis and fibrosis of the chest wall and diaphragm (Fig. 6). Doubly mutant animals die within the first few weeks of postnatal life. Of a colony of 336 F2 offspring from Mnf+/−:dystrophin+/− intercrosses, only two double mutants (n = 11) survived the weaning period, and those animals died before 3 months of age. Because of this complete neonatal lethality, we could not assess muscle histology in older animals of the Mnf−/−:mdx genotype. Animals heterozygous for the Mnf-null allele and lacking dystrophin (Mnf+/−:mdx) are viable into adulthood, but are smaller in size than Mnf+/+:mdx mice at all ages. Even haploinsufficiency for MNF in the mdx background greatly exacerbates the myopathic phenotype. Mnf+/−:mdx mice have evidence of persistent and extensive myonecrosis up to 6 months of age (Fig. 7). By comparison, muscles of Mnf+/+:mdx animals exhibit only minimal evidence of necrosis after 3 months of age with little or no fibrosis (Fig. 7).
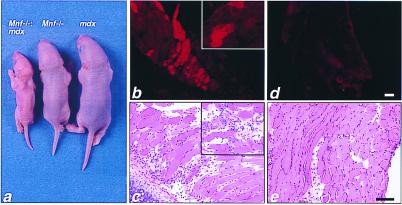
Figure 6.
F2 progeny of the Mnf null and mdx intercross. (a) Four-day-old littermates including the Mnf-mdx double knockout (Left), Mnf null (Middle), and mdx (Right). (b–e) Two-week-old Mnf-mdx double knockout and mdx littermates were injected with Evans blue dye, which accumulates in cells with damaged membranes (19–23) (n = 3 for each group). (b) Extensive dye uptake in the double mutant chest wall skeletal muscle and diaphragm (Inset) is apparent when using fluorescent microscopy (to identify Evan's blue dye positive myofibers) with an absence of uptake in the chest wall of mdx mice at this age (d). (c) Adjacent sections of chest wall musculature and diaphragm (Inset) were stained with hematoxylin and eosin. Note evidence of necrosis and inflammation in the double mutant chest wall/diaphragm (Inset) skeletal muscle (c) compared with a lack of inflammation associated with the chest wall/diaphragm of the 2-week-old mdx littermates (e). (Bar = 100 μ.)
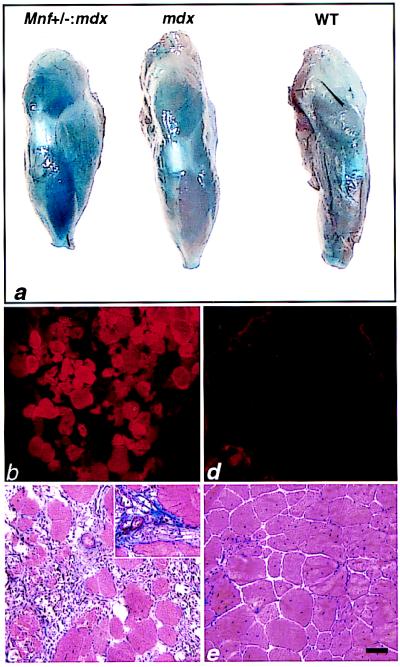
Figure 7.
Increased myonecrosis in the tibialis anterior (TA) muscle of Mnf+/−:mdx mice compared with mdx mice. (a) Macroscopic evaluation of Evans blue staining after i.v. dye injection into 6-month-old Mnf+/−:mdx, mdx, and wild-type mice. Note extensive blue dye incorporation in the double mutant TA compared with the mdx or wild-type TA (n = 6 mice in each group). (b and c) Fluorescent microscopy of cryosectioned TA muscles revealing extensive dye-filled myofibers in damaged myofibers of the Mnf+/−:mdx mouse (b) compared with the mdx skeletal muscle (d). (c) Adjacent sections of double mutant TA stained with Masson trichrome reveals extensive ongoing myonecrosis and fibrosis (Inset). (e) Adjacent sections of mdx TA stained with Masson trichrome reveals an absence of inflammation and a relative absence of fibrosis at 6 months of age. (Bar = 100 μ.)
Discussion
MNF Proteins Are Required for Proper Functioning of Myogenic Stem Cells.
Targeted disruption of the Mnf gene reveals a critical role for proteins encoded by this gene during muscle regeneration in the adult mouse. MNF proteins are not essential for correct patterning of skeletal muscle groups during embryonic and fetal development, nor for establishing the satellite cell population within adult muscle tissues. Rather, MNFs appear to be required for correct temporal orchestration of molecular and cellular events necessary for muscle repair. In the absence of MNF proteins, satellite cell function is perturbed with respect to the timing of expression of myogenic determination genes and genes that control cell cycle progression. These defects were observed both in the analysis of myoblasts cultured from neonatal animals and regenerating muscles from intact adult mice. As a consequence of this dysregulation, muscle repair becomes ineffective.
The conclusion that MNF proteins are important for correct function of satellite cells is supported by multiple lines of evidence presented here. First, growth retardation in Mnf null mice may reflect a defect in satellite cell function, because satellite cells are thought to be required to maintain muscle mass, even in the absence of overt muscle injury. The satellite cell defect may not, however, account entirely for the slow growth phenotype of Mnf−/− animals. Although immunoreactive MNF proteins are detectable selectively in satellite cells of adult animals, mRNA encoding MNF is expressed more broadly, such that low levels of MNF protein expression may be pertinent to cell regulation in other cell types. Moreover, MNF mRNA and protein are expressed during embryonic and fetal life in the developing heart and brain, as well as in myogenic precursor cells within the myotome of the somite. Although no morphological defects are observed in cardiovascular or neural structures of adult mice lacking MNF, the frequency at which Mnf−/− offspring of heterozygote intercrosses are observed (6% of 186 live births) suggests a significant rate of fetal death in the absence of MNF, in addition to the adult phenotype described here.
Direct evidence for a satellite cell defect in mice without MNF is provided by their response to muscle injury produced by injection of cardiotoxin. The regenerative response of wild-type mice to this standardized form of muscle damage is orderly and predictable, as assessed by histological or biochemical criteria. The activation of satellite cells is evident histologically as a pronounced cellular proliferation, accompanied by induction of MyoD within the first 6 h. The phase of cellular proliferation is limited to approximately 48 h, after which morphological (small myofibers with centralized nuclei) and biochemical (increased expression of myogenin) hallmarks of muscle regeneration are evident. By 10 days, muscle repair in wild-type animals is virtually complete. In the absence of MNF, satellite cells retain an ability to proliferate in response to muscle injury, but the subsequent events that characterize the regenerative process in wild-type animals are delayed and ineffective.
The conclusion that MNF regulates satellite cell function also is based on the profound exacerbation of skeletal myopathy in mdx mice that are deficient in MNF. The mdx mouse is one of several murine models that have been developed previously for the study of muscular dystrophy resulting from defects in dystrophin and related proteins (27, 28). Although the mdx mouse has provided some important insights into the pathobiology of DMD in humans, the myopathy in these animals is milder than in humans, and does not restrict their lifespan (19–23, 29). The mdx mouse typically manifests myofiber degeneration and necrosis at weaning (3 weeks of age) that peaks at 4–6 weeks (19, 22, 23). With the exception of certain specific muscle groups (notably diaphragm), skeletal muscles of older animals exhibit only minor histological evidence of ongoing myofiber damage (19, 22, 30). The mdx mouse adapts to muscle degeneration with an expansion of the satellite cell population and muscle hypertrophy, thereby avoiding the compromised muscle function that afflicts humans who lack dystrophin (19–23, 29). In addition to the spontaneous mdx mutation, myopathic mouse models include genetically engineered strains with knockouts of MyoD or utrophin crossed into the mdx background (12, 22, 23, 25, 26). Each of these models exhibits features that resemble DMD in humans, but the myopathic phenotype we observed in mdx mice lacking MNF is by far the most severe. In addition, mdx animals with haploinsufficiency at the Mnf locus show evidence of skeletal myopathy that more closely resembles humans with DMD than the relatively mild phenotype of the mdx mouse in the absence of Mnf mutations.
Our results should be compared with those reported by Megeney et al. (12) who examined the consequences of MyoD deficiency on muscle regeneration. After crush injury to skeletal muscles of adult MyoD−/− animals, muscle repair was delayed, and mice lacking both MyoD and dystrophin (mdx:MyoD−/− double mutants) exhibited myopathic histology and premature death at approximately 1 year of age. These previous findings support the notion that the dysregulation of MyoD expression we observed in Mnf−/−:mdx animals is pertinent to satellite cell dysfunction in our model. However, the more severe phenotype of Mnf−/−mdx mice, with death occurring before 3 months of age, indicates that additional abnormalities resulting from a deficiency of MNF are important for satellite cell function.
Forkhead/Winged Helix Proteins in Stem Cell Biology and Tissue Repair.
Several members of the forkhead/winged helix family of transcription factors are known to exert important regulatory functions during development in the control of cell proliferation and differentiation, and tissue morphogenesis (17, 31–33). With respect to stem cells and/or tissue repair in particular, a mammalian forkhead/winged helix protein termed Genesis is expressed selectively in embryonic stem cells (34), and a protein related to HNF-3 (35) has been identified in regenerating hepatocytes. Our current data concerning MNF, however, provide direct evidence for a specific role for members of this extended gene family in the regulation of stem cell function.
The signature motif that identifies members of the forkhead/winged helix family of proteins represents a sequence-specific DNA binding domain, and several of these proteins are known to function as transcription factors. For the most part, however, specific target genes that are regulated by these proteins and account for their effects on developmental decisions have not been identified. In the case of MNF proteins, we have determined that the MNF-β isoform functions as a transcriptional repressor in conjunction with corepressor proteins of the mSin3 family (36), but the specific target genes pertinent to its cellular functions are unknown. The biochemical function of MNF-α remains uncharacterized, but it includes a latent transcriptional activation domain (16), suggesting that under certain conditions (as a result of posttranslational modification and/or binding of cofactors) it may induce transcription of target genes. Because MNF-β is expressed in quiescent satellite cells that are blocked both for cell cycle entry and terminal differentiation, an appealing concept is that MNF-β may function to repress transcription of downstream genes that serve to promote one or both of these processes. Upon satellite cell activation after muscle injury, MNF-β is down-regulated, whereas MNF-α becomes more abundant. This pattern is reversed again as muscle regeneration proceeds and the quiescent myogenic stem cell population is re-established. Because of this reciprocating pattern of expression, and because the two MNF isoforms share an identical DNA binding domain, it is attractive to speculate that MNF-α activates the same set of target genes that is repressed by MNF-β. In this manner, MNF could serve to coordinate the timing of critical cellular events required for muscle repair and satellite cell renewal. This notion is supported by evidence for dysregulated expression of cell cycle mediators and myogenic determination genes in Mnf−/− cells, but we cannot yet identify the direct targets for the actions of MNFs.
A Novel Murine Model of Muscular Dystrophy and Satellite Cell Dysfunction.
The analysis of mice deficient in both MNF and dystrophin not only supports a functional role for MNF transcription factors in regulating biologic events associated with muscle regeneration, but establishes a unique strain of mice that more closely resembles human DMD than the mdx model. These animals should prove useful for future studies to advance the understanding of the role of satellite cell dysfunction in the pathobiology of muscular dystrophies and for testing the potential efficacy of new therapeutic measures.
Acknowledgments
We thank John Shelton for assistance with the photographic prints that appear in this manuscript and Drs. Kevin P. Campbell, Eric Olson, Louis Kunkel, and Michael Rudnicki for helpful discussions throughout the course of this study. This work was supported by grants from the Muscular Dystrophy Association, American Heart Association Texas Affiliate, National Institutes of Health (AR40849, HL54794, and HL06296), and the D.W. Reynolds Foundation.
Abbreviations
- MNF
myocyte nuclear factor
- DMD
Duchenne's muscular dystrophy
- RT
reverse transcription
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.100501197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.100501197
References
- 1.Gussoni E, Blau H M, Kunkel L M. Nat Med. 1997;3:970–977. doi: 10.1038/nm0997-970. [DOI] [PubMed] [Google Scholar]
- 2.Engel A G, Yamamoto M, Fischbeck K H. In: Myology. Engel A G, Franzini-Armstrong C, editors. New York: McGraw–Hill; 1994. pp. 1130–1188. [Google Scholar]
- 3.Monaco A, Neve R, Colletti-Feener C, Bertelson C, Kurnit D, Kunkel L M. Nature (London) 1986;323:646–650. doi: 10.1038/323646a0. [DOI] [PubMed] [Google Scholar]
- 4.Burghes A, Logan C, Hu X, Belfall B, Worton R, Ray P. Nature (London) 1987;328:434–437. doi: 10.1038/328434a0. [DOI] [PubMed] [Google Scholar]
- 5.Hoffman E P, Brown R H, Kunkel L M. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 6.Bell C D, Conen P E. J Neurol Sci. 1968;7:529–544. doi: 10.1016/0022-510x(68)90058-0. [DOI] [PubMed] [Google Scholar]
- 7.Schultz E. Dev Biol. 1996;175:84–94. doi: 10.1006/dbio.1996.0097. [DOI] [PubMed] [Google Scholar]
- 8.Feldman J L, Stockdale F E. Dev Biol. 1992;153:217–226. doi: 10.1016/0012-1606(92)90107-r. [DOI] [PubMed] [Google Scholar]
- 9.Weissman I L. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
- 10.Darr K C, Schultz E. Am J Physiol. 1989;67:1827–1834. doi: 10.1152/jappl.1989.67.5.1827. [DOI] [PubMed] [Google Scholar]
- 11.Schultz E. Dev Biol. 1996;175:84–94. doi: 10.1006/dbio.1996.0097. [DOI] [PubMed] [Google Scholar]
- 12.Megeney L A, Kablar B, Garrett K, Anderson J E, Rudnicki M A. Genes Dev. 1996;10:1173–1183. doi: 10.1101/gad.10.10.1173. [DOI] [PubMed] [Google Scholar]
- 13.Cornelison D D W, Wold B J. Dev Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- 14.Cossu G, Tajbakhsh S, Buckingham M. Trends Genet. 1996;12:218–223. doi: 10.1016/0168-9525(96)10025-1. [DOI] [PubMed] [Google Scholar]
- 15.Garry D J, Yang Q, Bassel-Duby R, Williams R S. Dev Biol. 1997;188:280–294. doi: 10.1006/dbio.1997.8657. [DOI] [PubMed] [Google Scholar]
- 16.Yang Q, Bassel-Duby R, Williams R S. Mol Cell Biol. 1997;17:5236–5243. doi: 10.1128/mcb.17.9.5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai E, Clark K L, Burley S K, Darnell J E., Jr Proc Natl Acad Sci USA. 1993;90:10421–10423. doi: 10.1073/pnas.90.22.10421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amalfitano A, Chamberlain J S. Muscle Nerve. 1996;19:1549–1553. doi: 10.1002/(SICI)1097-4598(199612)19:12<1549::AID-MUS4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 19.Straub V, Rafael J A, Chamberlain J S, Campbell K P. J Cell Biol. 1997;139:375–385. doi: 10.1083/jcb.139.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimario J S, Uzman A, Strohman R C. Dev Biol. 1991;148:314–321. doi: 10.1016/0012-1606(91)90340-9. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda R, Nishikawa A, Tanaka H. J Biochem. 1995;118:959–964. doi: 10.1093/jb/118.5.959. [DOI] [PubMed] [Google Scholar]
- 22.Straub V, Venzke D, Lee J, Leveille C, Campbell K. Am J Pathol. 1998;153:1623–1630. doi: 10.1016/s0002-9440(10)65751-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grady R M, Teng H B, Nichol M C, Cunningham J C, Wilkinson R S, Sanes J R. Cell. 1997;90:729–738. doi: 10.1016/s0092-8674(00)80533-4. [DOI] [PubMed] [Google Scholar]
- 24.Pavlath G K, Rando T A, Blau H M. J Cell Biol. 1994;127:1923–1932. doi: 10.1083/jcb.127.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bulfield G, Siller W G, Wight P A L, Moore K J. Proc Natl Acad Sci USA. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sicinski P, Geng Y, Rydercook A S, Barnard E A, Darlison M G, Barnard P J. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 27.Duclos F, Straub V, Moore S A, Venzke D P, Hrstka R F, Crosbie R H, Durbeej M, Lebakken C S, Ettinger A J, van der Meulen J, et al. J Cell Biol. 1998;142:1461–1471. doi: 10.1083/jcb.142.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deconinck A E, Rafael J A, Skinner J A, Brown S C, Potter A C, Metzinger L, Watt D J, Dickson J G, Tinsley J M, Davies K E. Cell. 1997;90:717–727. doi: 10.1016/s0092-8674(00)80532-2. [DOI] [PubMed] [Google Scholar]
- 29.McGeachie J K, Grounds M D, Partridge T A, Morgan J E. J Neurol Sci. 1993;119:169–179. doi: 10.1016/0022-510x(93)90130-q. [DOI] [PubMed] [Google Scholar]
- 30.Carnwath J W, Shotton D M. J Neurol Sci. 1987;80:39–54. doi: 10.1016/0022-510x(87)90219-x. [DOI] [PubMed] [Google Scholar]
- 31.Xuan S, Baptista C A, Balas G, Tao W, Soares V C, Lai E. Neuron. 1995;14:1141–1152. doi: 10.1016/0896-6273(95)90262-7. [DOI] [PubMed] [Google Scholar]
- 32.Winnier G E, Hargett L, Hogan L M. Genes Dev. 1997;11:926–940. doi: 10.1101/gad.11.7.926. [DOI] [PubMed] [Google Scholar]
- 33.Freyaldenhoven B, Freyaldenhoven M, Iacovoni J, Vogt P K. Cancer Res. 1997;57:123–129. [PubMed] [Google Scholar]
- 34.Sutton J, Costa R, Klug M, Field L, Xu D, Largaespada D, Fletcher C, Jenkins N, Copeland N, Klemsz M, Hromas R. J Biol Chem. 1996;271:23126–23133. doi: 10.1074/jbc.271.38.23126. [DOI] [PubMed] [Google Scholar]
- 35.Ye H, Kelly T, Samadani U, Lim L, Rubio S, Overdier D, Roebuck K, Costa R. Mol Cell Biol. 1997;17:1626–1641. doi: 10.1128/mcb.17.3.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Q, Kong Y, Rothermel B, Garry D J, Bassel-Duby R, Williams R S. Biochem J. 2000;345:335–343. [PMC free article] [PubMed] [Google Scholar]