Abstract
Pain originating from the hip may be referred to the groin and anterior thigh. We investigated sensory dorsal root ganglion neurons innervating the hip and the inguinal skin in rats using retrograde neurotransport and immunohistochemistry. A retrograde neurotracer Fluoro-Gold™ was injected into the left hip or inguinal skin of rats. Seven days later, we harvested bilateral dorsal root ganglions and counted the number of Fluoro-Gold™-labeled neurons positive for calcitonin gene-related peptide, a marker of nerve growth factor-dependent neurons, or isolectin B4, a marker of glial cell line-derived neurotrophic factor-dependent neurons. In the hip group, Fluoro-Gold™-labeled neurons were distributed throughout the left dorsal root ganglions from T13 to L5, primarily at L1, L2, L3, and L4, and the percentage of calcitonin gene-related peptide-positive neurons was higher than that of isolectin B4-binding neurons. In the inguinal skin group, Fluoro-Gold™-labeled neurons were distributed throughout the left dorsal root ganglions from T13 to L3, primarily at L1, L2, and L3, and the percentage of isolectin B4-binding neurons was higher than that of calcitonin gene-related peptide-positive neurons. These data suggest the sensory innervation pattern and characteristics of the sensory nerve of the rat hip are different from those of inguinal skin.
Introduction
Pain originating from the hip is thought to occur mostly in the groin and anterior thigh [7]. However, referred pain may occur in the buttock, thigh, groin, leg, foot, and knee [10]. It is not clear why the symptoms and pain, along with inflammatory or degenerative processes, in the area of the hip are so varied. Birnbaum et al. [5] reported the hip is innervated by the obturator, femoral sciatic, and superior gluteal nerves. However, the precise sensory innervation pattern and characteristics of the sensory nerve are unknown.
Previously, we reported dorsal root ganglion (DRG) neurons innervating the hip were distributed on multiple levels (L1-L4) [13]. However, the difference in level of innervation between hip and inguinal skin has not been clarified and it may be useful in the diagnosis of related pain when evaluating a patient with a potential hip disorder. Nociceptive information from the hip is transmitted to multilevel DRG neurons and nociceptive information from inguinal skin is transmitted to DRG neurons only in a few levels. Moreover, DRG neurons that innervate the hip and inguinal skin may overlap.
Nociceptive information is transmitted to the dorsal horn of the spinal cord by classically defined small dark cells in the DRG and these small DRG neurons are further divided into nerve growth factor (NGF)-sensitive neurons and glial cell line-derived neurotrophic factor (GDNF)-sensitive neurons [20]. NGF-sensitive neurons express the high-affinity NGF receptor tyrosine kinase A (TrkA) [2] whereas the GDNF-neurons express the GDNF receptor [12, 19, 20]. NGF and GDNF in these neurons regulate the expression of various pain-related molecules, including substance P, calcitonin gene-related peptide (CGRP), the P2X3 receptor, and vanilloid receptor 1, thereby regulating pain perception [14, 15]. The two neuron types can be distinguished by immunoreactivity (IR) for CGRP or isolectin B4 (IB4) binding [20]. Previous studies have raised the possibility anti-NGF and anti-GDNF have analgesic effects on pathologic pain states [4, 6, 9, 11, 16, 21]. However, these studies targeted neuropathic pain or pain from cutaneous tissue. Previously, we reported hip pain was transmitted mainly by CGRP-IR neurons [13]. Others reported medial ankle skin pain was transmitted mainly by IB4-binding neurons [3]. However, the differences in characteristics of DRG neurons between hip and inguinal skin have not been clarified.
We hypothesized DRG neurons that innervate the hip are different from DRG neurons that innervate the inguinal skin and can be distinguished by expression of CGRP and IB-4. Expression of CGRP implies a more significant involvement of neurogenic inflammation compared with nonpeptidergic IB4-binding neurons. We also hypothesized differences in modality of pain between the characteristics of DRG neurons innervating the hip and inguinal skin are illustrated by their populations of CGRP-IR and IB4-binding. Furthermore, NGF-sensitive neurons distinguished by immunoreactivity for CGRP are one of the key neurons involved in hip pain and GDNF-sensitive neurons distinguished by IB4-binding are one of the key neurons involved in inguinal skin pain.
Materials and Methods
We used 20 male Sprague-Dawley rats weighing 250 to 300 g divided into two groups. The rats were anesthetized with sodium pentobarbital (40 mg/kg intraperitoneally) and treated aseptically throughout the experiments. Using a 26-gauge needle, we injected 30 μL 1% Fluoro-Gold™ (FG) solution (Fluorochrome, Denver, CO) by intracapsular injection into the left hip (hip group, n = 10) or intracutaneous injection into the left inguinal skin (inguinal skin group, n = 10) of each rat. For the hip group, we used a posterior approach to the left hip by making the incision in line with the posterior border of the femur, then dividing the gluteus maximus muscle and the short external rotator muscle in the middle to expose the hip capsule. To test the first hypothesis, we determined the distribution of DRG neurons innervating the hip and inguinal skin using a retrograde tracing method. To test the second hypothesis, we determined the differences of the number of CGRP-labeled and IB4-binding DRG neurons innervating the hip and inguinal skin using retrograde neurotransport, lectin-binding affinity, and immunohistochemistry. To test the third hypothesis, we determined the differences in characteristics of DRG neurons innervating the hip and the inguinal skin by comparing the mean percentage of CGRP-IR neurons with the mean percentage of IB4-binding neurons. Protocols for all animal procedures in these experiments followed the 1996 revision of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (http://www.nap.edu/readingroom/books/labrats/) and were approved by the ethics committee of Chiba University, Japan.
Seven days after FG injection, we anesthetized the rats with sodium pentobarbital (40 mg/kg intraperitoneally) and perfused them transcardially with 0.9% saline, followed by 500 mL 4% paraformaldehyde in phosphate buffer (0.1 mol/L, pH 7.4). Bilateral DRGs from T12 to L6 were resected and the specimens immersed in 4% paraformaldehyde overnight at 4°C. After changing to 0.01 mol/L phosphate-buffered saline (PBS) containing 20% sucrose for 20 hours at 4°C, the DRGs were sectioned on a cryostat at 10-μm thickness and mounted on poly-L-lysine-coated slides.
Endogenous tissue peroxidase activity was quenched by soaking DRG sections for 30 minutes in 0.3% hydrogen peroxide solution in 0.01 mol/L PBS. Sections then were incubated overnight at 4°C in a blocking solution of 0.01 mol/L PBS containing 0.3% Triton® X-100 (Sigma-Aldrich Corp., St. Louis, MO) and 3% skim milk. To test for IB4 binding and CGRP expression, sections were incubated overnight at 4°C in biotin-labeled IB4 (Chemicon, Temecula, CA) or rabbit antibody to CGRP (Chemicon), which were diluted 1:1000 in blocking solution. We then incubated the sections with Alexa Fluor® 594-conjugated goat anti-rabbit (Alexa Fluor® 594 is similar to Texas Red) for CGRP-IR (Molecular Probes Inc, Eugene, OR) or Alexa Fluor® 488-conjugated streptavidin conjugate for IB4 binding (Molecular Probes), both diluted 1:1000 in blocking solution. After each incubation, the sections were rinsed three times in 0.01 mol/L PBS.
Approximately 10 to 15 sections from each DRG were examined by the authors (TN, SO, SY, KT, YH) in a blinded fashion using a fluorescent microscope (Nikon Corp, Tokyo, Japan). We photographed each section and five observers judged the cells independently. A positive cell was defined as one that more than three of five observers judged to be positive.
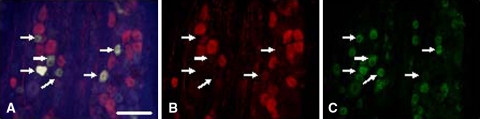
To determine the distribution of DRG neurons innervating the hip and inguinal skin, Fluoro-Gold was injected as a tracer into rats. In the hip group, FG-labeled DRG neurons, in which FG was transported from the hip, were present in the left DRGs from T13 to L5. FG-labeled neurons were detected using an ultraviolet-1A filter (365-nm wavelength for excitation and 420-nm wavelength for emission), and each labeled neuron was examined to determine whether it was positive for CGRP or IB4-binding glycoprotein with a fluorescein isothiocyanate filter (465-nm wavelength for excitation and 505-nm wavelength for emission). FG-labeled neurons (Fig. 1A), and double-labeled FG- and CGRP-IR (Fig. 1B) or IB4-binding (Fig. 1C) neurons, were counted. We counted only FG-labeled neurons with a clearly apparent nucleus to avoid counting the same cell twice in adjacent sections. FG-labeled neurons were then investigated for CGRP-IR and IB4-binding to distinguish NGF-sensitive neurons (CGRP-IR positive) from GDNF-sensitive neurons (IB4-binding).
Fig. 1A–C.
Fluor-Gold labeling and CGRP-IR or IB4-binding of neurons in the rat inguinal skin are shown. (A) Neurons (arrows) indicate FG-labeled neurons innervating the inguinal skin. Scale bar = 100 μm (Stain, FG-labeled; original magnification, x100). (B) Neurons (arrows) do not bind CGRP-IR (Stain, double, CGRP and Alexa Fluor® 594; original magnification, x100). (C) Conversely, neurons (arrows) show IB4 binding (Stain, double stain, IB4 and Alexa Fluor® 488; original magnification, x100).
We determined differences in the number of FG-labeled DRG neurons in the DRGs from T12 to L6 using the one-factor ANOVA followed by the multiple-comparison Tukey-Kramer test. We compared the numbers of FG-labeled and IB4-binding and CGRP-IR neurons in DRGs from T12 to L6 using the Mann-Whitney U test. The mean percentages of CGRP-IR or IB4-binding neurons relative to the total number of DRG neurons labeled by FG injection of either the hip or inguinal skin were compared using the Mann-Whitney U test. Analysis was performed using Statcel2 software (OMS, Saitama, Japan).
Results
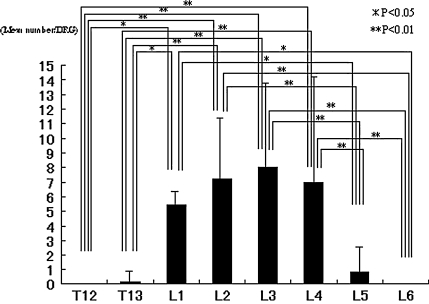
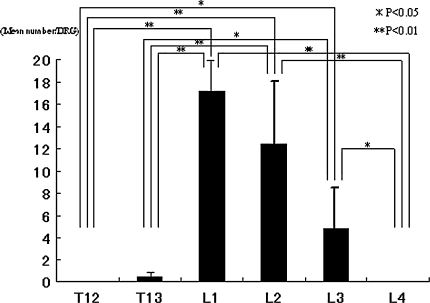
More DRGs innervated the hip than DRGs that innervated inguinal skin. Moreover, DRGs that innervated the hip and inguinal skin overlapped in L1, L2, and L3. We identified 287 FG-labeled neurons in the 10 rats with the injected hips, with 16 to 45 labeled neurons per rat. No labeled neurons were observed at T12 or L6 in the left DRGs or in the contralateral DRGs from T12 to L6. The mean number of FG-labeled neurons differed (p = 6.6 × 10−9) between the DRGs with the largest in L3. The mean number of FG-labeled neurons in L1, L2, L3, and L4 was higher than the mean number of labeled neurons in T12, T13, L5, and L6 (Fig. 2). In the inguinal skin group, FG-labeled DRG neurons, in which FG was transported from inguinal skin, were present in the left DRGs from T13 to L3. We found 348 FG-labeled neurons in the 10 rats examined, with 25 to 58 labeled neurons per rat. No labeled neurons were observed at T12, L4, L5, or L6 in the left DRGs or in the contralateral DRGs from T12 to L6. The mean number of FG-labeled neurons differed (p = 4.19 × 10−10) between each DRG. The largest mean number of labeled neurons was seen in L1. The mean number of FG-labeled neurons in L1, L2, and L3 was higher than the mean number of labeled neurons in T12, T13, and L4 (Fig. 3).
Fig. 2.
The graph shows the distribution of the mean number of FG-labeled DRG neurons innervating the hip. There is a difference in the number of FG-labeled neurons in each DRG (p = 6.6 × 10−9). In post hoc testing the numbers of FG-labeled neurons in L1, L2, L3, and L4 were higher than the numbers of labeled neurons in T12, T13, L5, and L6.
Fig. 3.
Distribution of the mean number of FG-labeled DRG neurons innervating inguinal skin is shown. There is a difference in the number of FG-labeled neurons in each DRG (p = 4.19 × 10−10). In post hoc testing the largest number of labeled neurons was in L1, and the numbers of FG-labeled neurons in L1, L2, and L3 were higher than the numbers of labeled neurons in T12, T13, and L4.
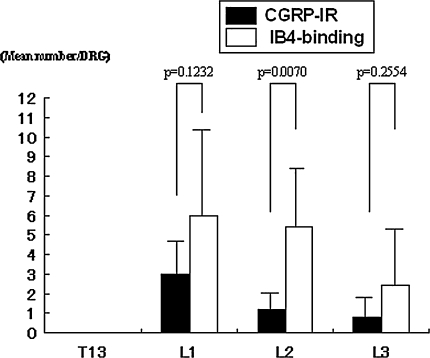
There were more CGRP-IR neurons in DRG neurons innervating the hip than in DRG neurons innervating the inguinal skin. In contrast, we found more IB4-binding neurons in DRG neurons innervating the inguinal skin than in DRG neurons innervating the hip. In the hip group, FG-labeled CGRP-IR neurons were present in the left DRGs from L1 through L5. There were few FG-labeled IB4-binding neurons present in the left DRGs from L2 through L4. Of the FG-labeled neurons, the mean number of CGRP-IR neurons was 2.0 ± 1.3 (mean ± standard error) in L1, 1.8 ± 1.7 in L2, 2.7 ± 1.7 in L3, 3.2 ± 3.2 in L4, and 0.4 ± 1.2 in L5. The mean number of IB4-binding neurons was 0.2 ± 0.4 in L2, 0.3 ± 0.5 in L3, and 0.2 ± 0.6 in L4. The mean number of FG-labeled CGRP-positive neurons was higher than the mean number of FG-labeled neurons binding IB4 in L1 (p = 0.0005), L2 (p = 0.0124), L3 (p = 0.0001), and L4 (p = 0.007) (Fig. 4). In the inguinal skin group, FG-labeled CGRP-IR and IB4-binding neurons were present in the left DRGs from L1 through L3. Of the FG-labeled neurons, the mean number of CGRP-IR neurons was 3.0 ± 1.8 in L1, 1.2 ± 0.8 in L2, and 0.8 ± 1.0 in L3. The mean number of IB4-binding neurons was 6.0 ± 4.4 in L1, 5.4 ± 3.0 in L2, and 2.4 ± 2.9 in L3. The mean number of FG-labeled neurons binding IB4 was higher (p = 0.007) than the mean number of FG-labeled CGRP-positive neurons in L2 (Fig. 5).
Fig. 4.
A comparison of the CGRP-IR and IB4-binding neurons innervating the hip is shown. In the hip group, the number of FG-labeled CGRP-positive neurons was higher than the numbers of FG-labeled neurons binding IB4 in L1 (p = 0.0005), L2 (p = 0.0124), L3 (p = 0.0001), and L4 (p = 0.0070).
Fig. 5.
A comparison of the CGRP-IR and IB4-binding neurons innervating inguinal skin is shown. In the inguinal skin group, the number of FG-labeled neurons binding IB4 was higher than the number of FG-labeled CGRP-positive neurons in L2 (p = 0.0070).
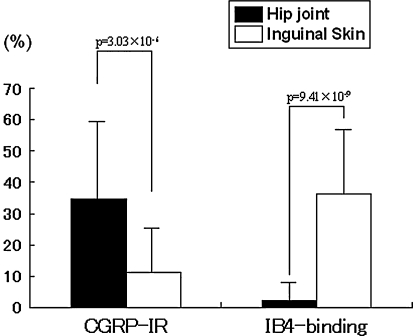
The mean percentage of CGRP-IR neurons innervating the hip relative to the total number of neurons was higher (p = 3.03 × 10−6) than the mean percentage innervating the inguinal skin. In contrast, the mean percentage of IB4-binding neurons innervating the inguinal skin relative to the total number of neurons was higher (p = 9.41 × 10−9) than the mean percentage innervating the hip. In the hip group, of the total number of FG-labeled neurons, 34.7% ± 25.2% were CGRP-IR and 2.2% ± 5.7% were IB4 binding. In the inguinal group, of the total number of FG-labeled neurons, 11.2% ± 12.7% were CGRP-IR and 36.1% ± 20.5% were IB4 binding (Fig. 6).
Fig. 6.
The percentage of CGRP-IR neurons innervating the hip relative to the total number of neurons was higher than the mean percentage innervating inguinal skin (p = 3.03 × 10−6). In contrast, the percentage of IB4-binding neurons innervating inguinal skin relative to the total number of neurons was higher than the mean percentage innervating the hip (p = 9.41 × 10−9).
Discussion
In comparing hip and inguinal skin, we addressed the following hypotheses: (1) Nociceptive information from the hip is transmitted to multilevel DRG neurons and nociceptive information from the inguinal skin is transmitted to DRG neurons only in a few levels; (2) DRG neurons that innervated the hip are different from DRG neurons that innervated the inguinal skin as each expresses CGRP or IB − 4, respectively; and (3) Differences in modality of pain are seen because NGF-sensitive neurons (CGRP-positive) are key neurons involved in hip pain and GDNF-sensitive neurons (IB4-binding) are key neurons involved in inguinal skin pain.
There are some limitations to our study. First, the FG solution was distributed throughout the entire hip including the capsule, synovial lining, bone, and cartilage. Therefore, sensory innervation of specific tissues in the hip could not be identified and there may be different sensory innervation patterns. Further experimentation is needed to clarify the sensory innervation pattern. Second, all measurements were made by five observers so the interobserver variability potentially is limited. Third, we used rats for all examinations. The rat thoracic spinal cord is divided into 13 segments and the lumbar spinal cord is divided into six segments. Although the number of segments in the human and rat differ slightly, the form of segmentation and structure of the spinal cord are similar in rats and humans.
A few reports have described the anatomic sensory innervation [5] and pain referral pattern [10] of the hip. However, the precise sensory innervation pattern of the sensory nerve in the hip and inguinal skin is not clear. Using the retrograde neurotracer FG, we examined the distribution of DRG neurons innervating the hip and inguinal skin. Our data suggest the rat hip is innervated from the ipsilateral T13 to L5 DRGs and rat inguinal skin is innervated from the ipsilateral T13 to L3 DRGs. Most of the DRG neurons innervating the rat hip were distributed in L1, L2, L3, and L4, and those innervating the inguinal skin in L1, L2, and L3. These results support findings from one anatomic human study. Birnbaum et al. [5] reported the anteromedial area in the hip is innervated by the articular branch of the obturator nerve and the anterior portion is innervated by the articular branch of the femoral nerve in the anterior hip capsule, and these areas reportedly are innervated by nerves derived from levels L2 to L4. They also reported the posterior hip capsule is innervated by the articular branch of the sciatic nerve and the posteromedial area is innervated by the superior gluteal nerve, and these nerves were derived from levels L4 to S1. More DRGs innervated the hip than DRGs that innervated inguinal skin. Moreover, DRGs that innervate the hip and inguinal skin overlapped in L1, L2, and L3. These results may explain why pain originating from the hip can be felt widely in the thigh or knee and the hip area.
Several reports have described the classification of DRG neurons [2, 12, 17, 20]. DRG neurons can be distinguished by immunoreactivity for CGRP and IB4-binding. In the current study, we performed immunocytochemistry to study FG-labeled DRG neurons in the hip and inguinal skin and observed higher numbers of CGRP-IR neurons in DRG neurons innervating the hip than in DRG neurons innervating inguinal skin. In contrast, we observed higher numbers of IB4-binding neurons in DRG neurons innervating inguinal skin than in DRG neurons innervating the hip. Nociceptive information normally is transmitted by small DRG neurons to the dorsal horn of the spinal cord. These small neurons may be subclassified into two groups. The first group, comprising peptide-containing neurons, contains CGRP [2, 20]. CGRP-expressing neurons terminate in lamina I and the outer layer of lamina II in the spinal dorsal horn [12]. The second group, comprising nonpeptide-expressing neurons, lacks peptides but expresses IB4-binding glycoprotein [20]. DRG neurons binding IB4 terminate at the inner part of lamina II in the spinal dorsal horn [12]. The peptidergic subpopulation is considered particularly important in inflammatory conditions, whereas the IB4-binding subpopulation is involved in neuropathic states [20]. In addition, IB4-binding neurons have been implicated in the nociception of acute pain. When IB4-binding neurons are selectively destroyed by toxins, animals show behavioral signs of decreased sensitivity to acute pain [17]. IB4-binding neurons may mediate several pain states originating from nerve injury, including acute and chronic pain. Thus, taking into consideration the results of previous studies along with the current study, CGRP-IR neurons may play a major role in hip pain sensation through peptidergic DRG neurons involved with sensation of inflammatory pain.
Several reports have described the proportion of CGRP-IR neurons and IB-4 binding neurons [1, 3, 8] and the correlation between the expression of CGRP and the NGF receptor, and between the expression of IB4-binding glycoprotein and the GDNF receptor. In our study, relative to the total number of FG-labeled DRG neurons, the percentage of CGRP-IR neurons innervating the hip was higher than the percentage innervating inguinal skin. In contrast, the percentage of IB4-binding neurons innervating inguinal skin was higher than the percentage innervating the hip. Others have indicated the proportion of IB4-binding neurons innervating the skin (43%) was higher than the proportion innervating the bladder (29%) [3]. Muscle afferent neurons exhibit 22% CGRP-IR and 5% IB4 binding. However, cutaneous afferent neurons exhibit 26% CGRP-IR and 44% IB4 binding [1]. IB4-binding neurons are not present in the rat knee [8]. Taking into consideration these studies and the current results, it seems likely organs located in deep layers of the body such as the joints are innervated by fewer IB4-binding DRG neurons.
These data suggest nociceptive information from the hip is transmitted mainly by NGF-sensitive neurons and not by GDNF-sensitive neurons. However, this conclusion should be interpreted cautiously because we have not examined the expression of NGF and GDNF receptors. GDNF has analgesic effects in neuropathic pain states [6], whereas NGF produces thermal and mechanical hyperalgesia [18]. There is a strong correlation between the expression of CGRP and the NGF receptor [2] and between the expression of IB4-binding glycoprotein and the GDNF receptor [12]. Thus, sensitivity to NGF may be higher in DRG neurons innervating the hip than in DRG neurons innervating inguinal skin, whereas sensitivity to GDNF may be higher in DRG neurons innervating inguinal skin than in DRG neurons innervating the hip.
Our results lead us to believe that NGF is one of the key molecules involved in hip pain. Numerous studies have suggested neutralizing NGF using anti-NGF or a TrkA-IgG fusion protein can prevent hyperalgesia induced by inflammation and nerve injury [4, 9, 11, 16, 21]. Unfortunately, treatment with neutralizing NGF is limited because it has no effect on GDNF-sensitive neurons. However, our results reveal there are few GDNF-sensitive neurons innervating the hip. Therefore, neutralizing NGF may be more effective in relieving hip pain than pain in other areas.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Ambalavanar R, Moritani M, Haines A, Hilton T, Dessem D. Chemical phenotypes of muscle and cutaneous afferent neurons in the rat trigeminal ganglion. J Comp Neurol. 2003;460:167–179. [DOI] [PubMed]
- 2.Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci. 1995;7:1484–1494. [DOI] [PMC free article] [PubMed]
- 3.Bennett DL, Dmietrieva N, Priestley JV, Clary D, McMahon SB. trkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neurosci Lett. 1996;206:33–36. [DOI] [PubMed]
- 4.Bennett DL, Koltzenburg M, Priestley JV, Shelton DL, McMahon SB. Endogenous nerve growth factor regulates the sensitivity of nociceptors in the adult rat. Eur J Neurosci. 1998;10:1282–1291. [DOI] [PubMed]
- 5.Birnbaum K, Prescher A, Hessler S, Heller KD. The sensory innervation of the hip joint: an anatomical study. Surg Radiol Anat. 1997;19:371–375. [DOI] [PubMed]
- 6.Boucher TJ, Okuse K, Bennett DL, Munson JB, Wood JN, McMahon SB. Potent analgesic effects of GDNF in neuropathic pain states. Science. 2000;290:124–127. [DOI] [PubMed]
- 7.Greene WB. Hip and thigh—an overview. In: Essential of Musculoskeletal Care. 2nd Ed. Rosemont, IL: Academy of Orthopaedic Surgeons; 2001:292–293.
- 8.Ivanavicius SP, Blake DR, Chessell IP, Mapp PI. Isolectin B4 binding neurons are not present in the rat knee joint. Neuroscience. 2004;128:555–560. [DOI] [PubMed]
- 9.Koltzenburg M, Bennett DL, Shelton DL, McMahon SB. Neutralization of endogenous NGF prevents the sensitization of nociceptors supplying inflamed skin. Eur J Neurosci. 1999;11:1698–1704. [DOI] [PubMed]
- 10.Lesher JM, Dreyfuss P, Hager N, Kaplan M, Furman M. Hip joint pain referral patterns: a descriptive study. Pain Med. 2008;9:22–25. [DOI] [PubMed]
- 11.McMahon SB, Bennett DL, Priestley JV, Shelton DL. The biological effects of endogenous nerve growth factor on adult sensory neurons revealed by a trkA-IgG fusion molecule. Nat Med. 1995;1:774–780. [DOI] [PubMed]
- 12.Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. [DOI] [PubMed]
- 13.Nakajima T, Ohtori S, Inoue G, Koshi T, Yamamoto S, Nakamura J, Takahashi K, Harada Y. The characteristics of dorsal-root ganglia and sensory innervation of the hip in rats. J Bone Joint Surg Br. 2008;90:254–257. [DOI] [PubMed]
- 14.Priestley JV, Michael GJ, Averill S, Liu M, Willmott N. Regulation of nociceptive neurons by nerve growth factor and glial cell line derived neurotrophic factor. Can J Physiol Pharmacol. 2002;80:495–505. [DOI] [PubMed]
- 15.Ramer MS, Bradbury EJ, McMahon SB. Nerve growth factor induces P2X(3) expression in sensory neurons. J Neurochem. 2001;77:864–875. [DOI] [PubMed]
- 16.Ro LS, Chen ST, Tang LM, Jacobs JM. Effect of NGF and anti-NGF on neuropathic pain in rats following chronic constriction injury of the sciatic nerve. Pain. 1999;79:265–274. [DOI] [PubMed]
- 17.Rucker HK, Holloway JA. Viscerosomatic convergence onto spinothalamic tract neurons in the cat. Brain Res. 1982;243:155–157. [DOI] [PubMed]
- 18.Safieh-Garabedian B, Poole S, Allchorne A, Winter J, Woolf CJ. Contribution of interleukin–1 beta to the inflammation-induced increase in nerve growth factor levels and inflammatory hyperalgesia. Br J Pharmacol. 1995;115:1265–1275. [DOI] [PMC free article] [PubMed]
- 19.Silverman JD, Kruger L. Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: relation of lectin reactivity to peptide and enzyme markers. J Neurocytol. 1990;19:789–801. [DOI] [PubMed]
- 20.Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632. [DOI] [PubMed]
- 21.Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62:327–331. [DOI] [PubMed]