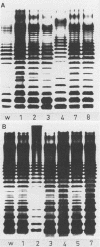
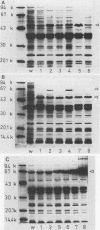
Abstract
Serum resistance is a major virulence factor of gram-negative bacteria, and K-1 polysaccharide has been shown to contribute to serum resistance in selected strains. To obtain further information about the role of K-1 in serum resistance and to find out whether loss of the ability to produce K-1 can induce loss of serum resistance, we studied the serum resistance of mutants derived from completely serum-resistant, K-1-positive blood culture isolates of Escherichia coli by selection for resistance to infection with K-1 specific bacteriophages. The amounts of K-1 polysaccharide produced by wild-type strains and mutants were measured, and outer membrane protein and lipopolysaccharide (LPS) patterns were analyzed. In each group of mutants, several highly serum-sensitive strains were found. All mutant strains expressed less K-1 than did the corresponding wild-type strains. Mutants that became highly serum sensitive always had less K-1 than did mutants with less-pronounced changes of serum resistance. A few mutants derived from different wild-type strains showed increased expression of outer membrane proteins with molecular weights of about 46,000 and 67,000. All of the wild-type strains examined had smooth-type LPS, and only two mutants had altered LPS structures; alterations of mutants in outer membrane proteins and LPS could not be correlated with alterations of serum resistance. The results indicate that for K-1-positive blood culture strains of E. coli, K-1 expression is a prerequisite for serum resistance, and loss of ability to synthesize K-1 leads to loss of serum resistance.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRY G. T., ABBOTT V., TSAI T. Relationship of colominic acid (poly N-acetylneuraminic acid) to bacteria which contain neuraminic acid. J Gen Microbiol. 1962 Oct;29:335–352. doi: 10.1099/00221287-29-2-335. [DOI] [PubMed] [Google Scholar]
- Björkstén B., Bortolussi R., Gothefors L., Quie P. G. Interaction of E. coli strains with human serum: lack of relationship to K1 antigen. J Pediatr. 1976 Dec;89(6):892–897. doi: 10.1016/s0022-3476(76)80592-6. [DOI] [PubMed] [Google Scholar]
- Bortolussi R., Ferrieri P., Björkstén B., Quie P. G. Capsular K1 polysaccharide of Escherichia coli: relationship to virulence in newborn rats and resistance to phagocytosis. Infect Immun. 1979 Jul;25(1):293–298. doi: 10.1128/iai.25.1.293-298.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulnois G. J., Roberts I. S., Hodge R., Hardy K. R., Jann K. B., Timmis K. N. Analysis of the K1 capsule biosynthesis genes of Escherichia coli: definition of three functional regions for capsule production. Mol Gen Genet. 1987 Jun;208(1-2):242–246. doi: 10.1007/BF00330449. [DOI] [PubMed] [Google Scholar]
- Fearon D. T. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1971–1975. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., Joiner K., Leive L. Serum-resistant mutants of Escherichia coli O111 contain increased lipopolysaccharide, lack an O antigen-containing capsule, and cover more of their lipid A core with O antigen. J Bacteriol. 1984 Sep;159(3):877–882. doi: 10.1128/jb.159.3.877-882.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harriman G. R., Podack E. R., Braude A. I., Corbeil L. C., Esser A. F., Curd J. G. Activation of complement by serum-resistant Neisseria gonorrhoeae. Assembly of the membrane attack complex without subsequent cell death. J Exp Med. 1982 Oct 1;156(4):1235–1249. doi: 10.1084/jem.156.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. J., Glynn A. A. The virulence for mice of strains of Escherichia coli related to the effects of K antigens on their resistance to phagocytosis and killing by complement. Immunology. 1971 May;20(5):767–777. [PMC free article] [PubMed] [Google Scholar]
- Jarvis G. A., Vedros N. A. Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect Immun. 1987 Jan;55(1):174–180. doi: 10.1128/iai.55.1.174-180.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner K. A. Complement evasion by bacteria and parasites. Annu Rev Microbiol. 1988;42:201–230. doi: 10.1146/annurev.mi.42.100188.001221. [DOI] [PubMed] [Google Scholar]
- Joiner K. A., Hammer C. H., Brown E. J., Cole R. J., Frank M. M. Studies on the mechanism of bacterial resistance to complement-mediated killing. I. Terminal complement components are deposited and released from Salmonella minnesota S218 without causing bacterial death. J Exp Med. 1982 Mar 1;155(3):797–808. doi: 10.1084/jem.155.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Kubens B. S., Wettstein M., Opferkuch W. Two different mechanisms of serum resistance in Escherichia coli. Microb Pathog. 1988 Nov;5(5):371–379. doi: 10.1016/0882-4010(88)90037-x. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- McCabe W. R., Kaijser B., Olling S., Uwaydah M., Hanson L. A. Escherichia coli in bacteremia: K and O antigens and serum sensitivity of strains from adults and neonates. J Infect Dis. 1978 Jul;138(1):33–41. doi: 10.1093/infdis/138.1.33. [DOI] [PubMed] [Google Scholar]
- Moll A., Manning P. A., Timmis K. N. Plasmid-determined resistance to serum bactericidal activity: a major outer membrane protein, the traT gene product, is responsible for plasmid-specified serum resistance in Escherichia coli. Infect Immun. 1980 May;28(2):359–367. doi: 10.1128/iai.28.2.359-367.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkonen S., Häyrinen J., Finne J. Polyacrylamide gel electrophoresis of the capsular polysaccharides of Escherichia coli K1 and other bacteria. J Bacteriol. 1988 Jun;170(6):2646–2653. doi: 10.1128/jb.170.6.2646-2653.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt J. K-1 antigen of Escherichia coli: epidemiology and serum sensitivity of pathogenic strains. Infect Immun. 1978 Oct;22(1):219–224. doi: 10.1128/iai.22.1.219-224.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluschke G., Mayden J., Achtman M., Levine R. P. Role of the capsule and the O antigen in resistance of O18:K1 Escherichia coli to complement-mediated killing. Infect Immun. 1983 Dec;42(3):907–913. doi: 10.1128/iai.42.3.907-913.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. B., McCracken G. H., Jr, Gotschlich E. C., Orskov F., Orskov I., Hanson L. A. Escherichia coli K1 capsular polysaccharide associated with neonatal meningitis. N Engl J Med. 1974 May 30;290(22):1216–1220. doi: 10.1056/NEJM197405302902202. [DOI] [PubMed] [Google Scholar]
- Sarff L. D., McCracken G. H., Schiffer M. S., Glode M. P., Robbins J. B., Orskov I., Orskov F. Epidemiology of Escherichia coli K1 in healthy and diseased newborns. Lancet. 1975 May 17;1(7916):1099–1104. doi: 10.1016/s0140-6736(75)92496-4. [DOI] [PubMed] [Google Scholar]
- Silver R. P., Finn C. W., Vann W. F., Aaronson W., Schneerson R., Kretschmer P. J., Garon C. F. Molecular cloning of the K1 capsular polysaccharide genes of E. coli. Nature. 1981 Feb 19;289(5799):696–698. doi: 10.1038/289696b0. [DOI] [PubMed] [Google Scholar]
- Suerbaum S., Leying H., Kroll H. P., Gmeiner J., Opferkuch W. Influence of beta-lactam antibiotics and ciprofloxacin on cell envelope of Escherichia coli. Antimicrob Agents Chemother. 1987 Jul;31(7):1106–1110. doi: 10.1128/aac.31.7.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983 Mar;47(1):46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. W., Parton R. A protein factor associated with serum resistance in Escherichia coli. J Med Microbiol. 1977 May;10(2):225–232. doi: 10.1099/00222615-10-2-225. [DOI] [PubMed] [Google Scholar]
- Taylor P. W., Robinson M. K. Determinants that increase the serum resistance of Escherichia coli. Infect Immun. 1980 Jul;29(1):278–280. doi: 10.1128/iai.29.1.278-280.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis K. N., Boulnois G. J., Bitter-Suermann D., Cabello F. C. Surface components of Escherichia coli that mediate resistance to the bactericidal activities of serum and phagocytes. Curr Top Microbiol Immunol. 1985;118:197–218. doi: 10.1007/978-3-642-70586-1_11. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Vermeulen C., Cross A., Byrne W. R., Zollinger W. Quantitative relationship between capsular content and killing of K1-encapsulated Escherichia coli. Infect Immun. 1988 Oct;56(10):2723–2730. doi: 10.1128/iai.56.10.2723-2730.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]