Abstract
The hTRPC [human TRPC (canonical transient receptor potential)] family of non-selective cation channels is proposed to mediate calcium influx across the plasma membrane via PLC (phospholipase C)-coupled receptors. Heterologously expressed hTRPC3 and hTRPC7 have been localized at the cell surface; however, a large intracellular component has also been noted but not characterized. In the present study, we have investigated the intracellular pool in COS-7 cells and have shown co-localization with markers for both the TGN (trans-Golgi network) and the cis-Golgi cisternae by immunofluorescence microscopy. Addition of BFA (Brefeldin A) to cells expressing hTRPC3 or hTRPC7 resulted in the redistribution of the Golgi component to the endoplasmic reticulum, indicating that this pool is present in both the Golgi stack and the TGN. Expression of either TRPC3 or TRPC7, but not TRPC1 or the cell surface marker CD8, resulted in a 2–4-fold increase in secreted alkaline phosphatase in the extracellular medium. Based on these results, we propose that an additional function of these members of the hTRPC family may be to enhance secretion either by affecting transport through the Golgi stack or by increasing fusion at the plasma membrane.
Keywords: calcium, cation channel trafficking, transient receptor potential (TRP), canonical TRP (TRPC), secretory pathway, trans-Golgi network
Abbreviations: BFA, Brefeldin A; DAG, diacylglycerol; DMEM, Dulbecco's modified Eagle's medium; dTRP, Drosophila TRP; EGFP, enhanced green fluorescent protein; ER, endoplasmic reticulum; FCS, foetal calf serum; HEK-293 cell, human embryonic kidney cell; HRP, horseradish peroxidase; IP3, inositol trisphosphate; NSF, N-ethylmaleimide-sensitive factor; PLC, phospholipase C; SEAP, a secreted form of alkaline phosphatase; SNAP, NSF-attachment protein; TGN, trans-Golgi network; TNFR, tumour necrosis factor receptor; TRP, transient receptor potential; TRPC, canonical TRP; hTRPC, human TRPC; TRPM8, melastatin TRP8; TRPML, mucolipin TRP
INTRODUCTION
The TRP (transient receptor potential) protein superfamily consists of calcium-permeable non-selective cation channels first described in a Drosophila phototransduction mutant associated with a defect in light-induced calcium entry [1]. The number of mammalian homologues of dTRP (Drosophila TRP) that have been identified over the past decade have expanded into a superfamily, which has been further divided into seven subfamilies (reviewed in [2]). Except for the lack of a voltage sensor in S4, the structure of all of the TRP channels resembles that of voltage-gated ion channels; it includes six predicted transmembrane segments with N-terminal and C-terminal cytoplasmic tails and a re-entrant pore loop between S5 and S6. In addition, analogously to Kv channels, TRPs most likely form tetrameric structures.
In contrast with other ion channel families, the TRP family members are predominantly grouped according to sequence homology and are diverse in terms of modes of activation, ion selectivity, tissue distribution and function both between and within each subfamily. TRPCs (canonical TRPs; also known as ‘classical’ TRPs) are most closely related to dTRP and are also subdivided into a further four groups based on sequence similarity: TRPC1 and TRPC2 (a pseudogene in humans) in their own categories, TRPC4 and TRPC5 forming the third grouping and TRPC3, TRPC6 and TRPC7 forming the fourth subfamily. All of the TRPC channels open in response to activation of PLC (phospholipase C), which leads to the production of IP3 (inositol trisphosphate) and DAG (diacylglycerol). The issue of whether (or which) TRPC family members are activated by IP3 and subsequent depletion of intracellular stores or an increase in DAG remains controversial [3]. Alternatively, or in combination with the mechanisms described above, activation of calcium influx through TRP channels also occurs by insertion of a vesicular pool into the plasma membrane [4–6].
Not only are there differences in tissue distribution, function and activation within TRP families, it is also becoming clear that there are differences in the subcellular distribution of these channels as well. Localization studies in conjunction with electrophysiological experiments have demonstrated the presence of both heterologously expressed and endogenous TRP proteins at the plasma membrane as predicted by a role in calcium influx [7]. However, it is apparent that large intracellular pools of TRP proteins also exist. As would be predicted from the insertional activation model, a number of studies report localization of TRP channels including the Caenorhabditis elegans TRP, TRP-3 [8], TRPC3 [6], TRPV5 (vanilloid TRP5) and TRPV6 [9], TRPC5 [5] and TRPV1 [10] to vesicular pools, which then fuse with the plasma membrane after stimulation.
More surprisingly, TRPs have been implicated in endosome/lysosome function after the gene responsible for the neurogenetic disorder mucolipidosis type IV was cloned and found to encode the group 2 TRP, TRPML (mucolipin TRP; mucolipin-1) [11]. TRPML and its homologue in C. elegans, CUP-5, have both been localized to late endosomes/lysosomes and have been proposed to have a role either in lysosome biogenesis [11] and/or in regulation of lysosomes [12]. Another subcellular compartment in which several types of calcium channels reside is the ER (endoplasmic reticulum). Both folding and assembly of channels occur in this compartment and, consequently, it has been difficult to establish whether TRP channels actually function in this organelle or are merely being assembled and transported; a recent report suggests that the cold/menthol-sensitive TRPM8 (melastatin TRP8) channel is not only resident in the ER, but has a role as an ER calcium-release channel [13]. Three distinct locations, namely the ER, plasma membrane and Golgi complex, have been reported for another group 2 TRP, TRPP2 (polycystin-2), mutations in which cause polycystic kidney disease (reviewed in [14]). Thus, in addition to their function as calcium influx channels at the plasma membrane, an increasing number of TRP channels have been demonstrated to act intracellularly in a variety of different organelles.
In the present study, we present a detailed analysis of the subcellular localization of the intracellular pools of hTRPC1, hTRPC3 and hTRPC7 and propose a novel role for both hTRPC3 and hTRPC7 in enhancing constitutive secretion.
MATERIALS AND METHODS
Antibodies and reagents
The following antibodies were used in immunofluorescence microscopy at a 1:300 dilution unless otherwise specified: anti-Myc monoclonal antibody (9E10) (a gift from Professor J. Paul Luzio, University of Cambridge), anti-FLAG monoclonal antibody M2 (Sigma, Poole, Dorset, U.K.), rabbit polyclonal Gpp130 and mouse anti-mannosidase II (Covance), and sheep anti-human TGN46 (Serotec). Goat anti-rabbit IgG–Alexa Fluor® 594 and goat anti-mouse IgG–Alexa Fluor® 488 were purchased from Molecular Probes. Anti-Myc monoclonal antibody, clone 4A6, was purchased from Upstate Biotechnology and used at a dilution of 1:200 for immunoblotting. The polyclonal rabbit anti-hTRPC7 antibody has been described previously [15]. Specific antibodies were purified by affinity chromatography on Sepharose 4B coupled with the peptide, concentrated and stored at −20 °C prior to use. The antibody was used at a dilution of 1:100 for immunofluorescence and 1:1000 for immunoblotting. All other reagents were obtained from Sigma unless otherwise specified.
Constructs
Myc–hTRPC3 in expression vector pcDNA3 and FLAG–hTRPC1β in pcDNA3.1 were gifts from Dr Craig Montell (Johns Hopkins University, Baltimore, MD, U.S.A.) [16]. The full-length cDNA-coding sequence for hTRPC7 (GenBank® accession number AJ272034) was subcloned from pCR-Blunt into the HindIII and KpnI sites of the pFLAG-CMV vector (Sigma) to produce an N-terminal epitope-tagged version of the polypeptide.
ΔpMep4-hTRPC3 was generated by the insertion of a 700 nt KpnI/XhoI fragment from the 5′-end of pcDNA3-hTRPC3 into the ΔpMep4 mammalian expression vector [17]. The 3′ XhoI fragment of hTRPC3 was then cloned into the ΔpMep4 XhoI site. Products containing this insert in the correct orientation were determined by restriction mapping and sequencing. The pcDNA3-EGFPhTRPC3 vector was constructed by inserting a KpnI fragment containing EGFP (enhanced green fluorescent protein) into the KpnI site of pcDNA3-hTRPC3 to create a cDNA encoding an N-terminally EGFP-tagged hTRPC3.
Cells and transfections
African green monkey kidney cells (COS-7) were grown in DMEM (Dulbecco's modified Eagle's medium; Sigma) containing 10% (v/v) FCS (foetal calf serum) and 100 units/ml penicillin–streptomycin. Transfections were performed using the GeneJuice® transfection reagent (Novagen) using 1 μg of construct DNA except for co-transfections wherein 0.5 μg of each plasmid was used. Further manipulations of the cells were carried out 24 h post-transfection unless otherwise specified. For the generation of stable cell lines, COS-7 cells were transfected with the inducible expression vector ΔpMep4-hTRPC3 and stable cell lines were isolated after selection in 50 μg/ml hygromycin B (Sigma) for approx. 2 weeks. Three clones were chosen for further study based on differential levels of expression under uninduced versus induced conditions. For inducible expression, 50 μM ZnCl2 was added to the medium and cells were incubated for a further 24–48 h prior to processing for immunocytochemistry or immunoblotting. The rat pituitary cell line, GH4C1, was a gift from Professor Priscilla Dannies (Department of Pharmacology, Yale University) and was grown in a 1:1 (v/v) mixture of DMEM and Ham's F-10 medium supplemented with 15% (v/v) horse serum.
Protein extraction and immunoblotting
Confluent cells were harvested by scraping on ice into lysis buffer consisting of 100 mM Tris/HCl (pH 8.0), 1 mM MgCl2, 0.1 mM PMSF and a protease inhibitor cocktail (Complete Mini, EDTA-free; Roche) (buffer A) and frozen at −80 °C until use. Frozen cells were thawed on ice, homogenized in a Dounce homogenizer and diluted in 0.25 M sucrose, 10 mM Tris/Hepes (pH 7.4) containing protease inhibitors. The suspension was centrifuged at 3000 g for 15 min at 4 °C and the supernatant was then centrifuged at 50000 g for 30 min. The pellet was resuspended in buffer A and protein determinations were conducted using the Bio-Rad protein assay. Crude membrane pellet (20 μg) was resolved on an 8% (w/v) SDS Tris/glycine gel and the gel was transferred to nitrocellulose. Monoclonal anti-Myc 4A6 (Upstate Biotechnology; 1:1000) was used to detect hTRPC3 in COS-hTRPC3-1 cells and polyclonal anti-TRPC7 for detection in GH4C1 cells (1:1000), followed by anti-mouse IgG–HRP (horseradish peroxidase) or anti-rabbit IgG–HRP (Amersham Biosciences, Little Chalfont, Buckinghamshire, U.K.) respectively. Detection was carried out with ECL® Western Blotting Detection reagent (Amersham). Densitometry was conducted using the Epi Chemi II Darkroom and the captured luminescence was imaged using LABWORKS software (UVP Lab Products, Cambridge, U.K.).
SEAP (a secreted form of alkaline phosphatase) assays
COS-7 cells and clonal COS-7 cells stably expressing N-terminal Myc-tagged human TRPC3 were transiently transfected with pSEAP2-Control mammalian expression vector (BD Biosciences Clontech, Palo Alto, CA, U.S.A.) using GeneJuice® Transfection Reagent (Novagen, La Jolla, CA, U.S.A.) for 24 h, followed by induction with 50 μM ZnCl2 for a further 24 h. Samples of the medium were harvested at 48 and 72 h post-transfection, and the level of the heat-stable secreted placental alkaline phosphatase in 15 μl aliquots was determined using the BD Great EscAPe™ SEAP chemiluminescent assay (BD Biosciences Clontech). Assays were conducted in transparent 96-well flat-bottomed microtitre plates, and luminescence was detected for 10 s integrals using a FARcyte™ plate luminometer (Amersham Biosciences). COS-7 cells were also transiently co-transfected with pSEAP2-Control mammalian expression vector and secondary recombinant mammalian expression plasmids. Extracellular medium was harvested and assayed as described above. Immunofluorescence microscopy was performed on the pSEAP2-Control transfected cells to ensure that the transfection efficiency was constant between the different sample groups.
Immunocytochemistry
Indirect immunofluorescence microscopy was performed as described previously [18]. Briefly, cells were washed with PBS and fixed for 20 min at room temperature (24 °C) using 2% (w/v) paraformaldehyde and permeabilized for 5 min with methanol at −20 °C or 0.1% (v/v) Triton X-100 at room temperature, blocked with 10% FCS in PBS for 20 min, followed by addition of primary antibody at the dilutions indicated above. After washing, secondary antibody was added and the cells were incubated for 20 min, followed by washing and mounting with Mowiol (Calbiochem). When required, cultured cells were incubated with 5 μg/ml BFA (Brefeldin A) for 2 h at 37 °C as previously described [18].
Imaging
Cells were visualized using either a Zeiss LSM 510 confocal microscope (Oberkochen), with a Plan-Apochromat ×63 1.4 NA oil immersion objective lens or a Nikon TE2000 fluorescence microscope equipped with a Hamamatsu ER-ORCA CCD camera (charge-coupled-device camera) and a Sutter Lambda 10-2 controller and filter wheel. In the latter case, images were captured using PerkinElmer Ultraview software. The final images were processed using Adobe Photoshop 5.0 (Adobe Systems, Mountain View, CA, U.S.A.).
Statistical analysis
Results are presented as means±S.E.M. with the number of experiments indicated in the Figure captions.
RESULTS
Localization of transiently expressed hTRPC1, hTRPC3 and hTRPC7 in COS-7 cells
A number of different approaches including immunocytochemistry have been used to analyse the expression and distribution of TRPC proteins in various tissues as well as heterologously expressing cell lines. Although expression at the plasma membrane, in vesicles and in the ER have been documented for many of the TRP family members, several reports allude to a large intracellular pool of hTRPC3 that also appears to co-localize with the Golgi complex [19–21]; this observation, however, has not been characterized further. In order to determine the exact localization of the intracellular pool of hTRPC3, we first investigated the localization pattern of N-terminally tagged constructs of hTRPC1, hTRPC3 and hTRPC7 in COS-7 cells. We used this cell line since the flattened morphology and large size enable clear identification of intracellular compartments and structures.
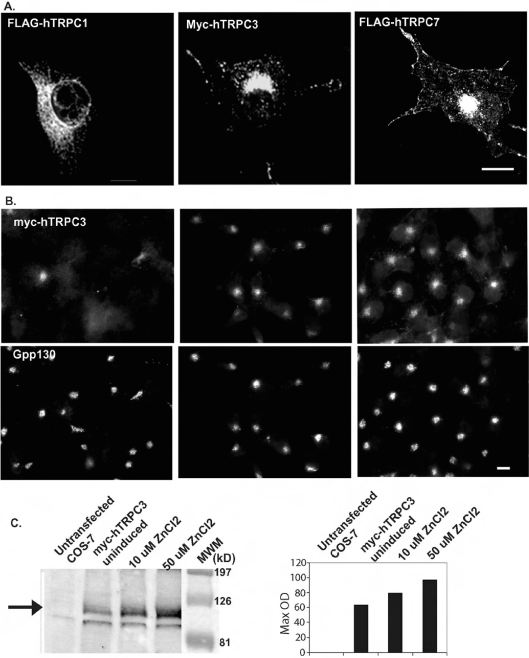
We transiently transfected COS-7 cells with a full-length construct of hTRPC3 containing an N-terminal Myc epitope (Myc–hTRPC3) [16]. As shown in Figure 1(A) (middle image), overexpressed Myc–hTRPC3 is concentrated in a perinuclear location as well as in punctate structures throughout the periphery and at the plasma membrane. A similar distribution was seen for FLAG–hTRPC7 (Figure 1A, last image); however, a more distinctive plasma-membrane localization was also observed. In contrast with the results obtained with hTRPC3 and hTRPC7, our results indicate that FLAG–hTRPC1β [16,22] localizes predominantly to the ER in COS-7 cells (Figure 1A, first image).
Figure 1. Expression of FLAG–hTRPC1, Myc–hTRPC3 and FLAG–hTRPC7 in COS-7 cells.
(A) COS-7 cells transiently expressing FLAG–hTRPC1, Myc–hTRPC3 or FLAG–hTRPC7 were processed for immunofluorescence microscopy using antibodies to the epitope tags. (B) A clonal cell line expressing Myc–hTRPC3 downstream of the metallothionein promoter (COS-hTRPC3-1) was grown for 48 h in the absence (uninduced) or presence of 10 or 50 μM ZnCl2 to induce increasing levels of expression. Cells were fixed and processed for immunofluorescence microscopy using a monoclonal antibody to the Myc epitope tag. (C) Untransfected COS-7 and COS-hTRPC3-1 cells were treated as in (B) and processed for immunoblotting. The immunoblot obtained using a monoclonal anti-Myc antibody is shown on the left. The filled arrow indicates the position of hTRPC3, which is absent in the untransfected cells. Increases in expression levels were quantified and are expressed as arbitrary units in the right-hand panel. Scale bars, 10 μm.
In order to determine whether the large intracellular pool of hTRPC3 is due to overexpression and consequent saturation within the exocytic pathway resulting in the Golgi-like distribution, COS-7 cells were stably transfected with Myc–hTRPC3 under the regulation of the inducible metallothionein promoter contained in the expression vector ΔpMEP4 (COS-hTRPC3-1). Even under uninduced conditions, some expression was detected by both immunofluorescence and immunoblotting (Figures 1B and 1C). However, at these low levels of expression, the distribution of hTRPC3 in these cells is similar to that after transient transfection with little or no detectable signal apparent at the plasma membrane and most of the signal appearing intracellularly (Figure 1B). With increasing expression levels, the concentration in the perinuclear area and in punctate structures increases without a concomitant increase at the cell surface. These results suggest that the perinuclear localization is not simply due to overexpression resulting in an increased concentration in the secretory pathway.
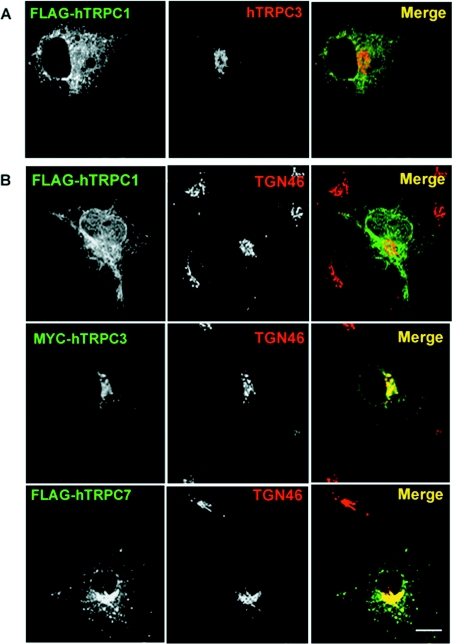
In order to confirm the distribution data described above, we also conducted co-transfection experiments with hTRPC1β and hTRPC3. Based on previous immunoprecipitation results [23], hTRPC1 and hTRPC3 would not be expected to interact, and indeed the localization of hTRPC1 did not appear to alter on co-transfection with hTRPC3 [7]; the intracellular distribution of hTRPC3, however, was not investigated in these experiments. In contrast, recent reports based on immunoprecipitation results suggest that TRPC1 and TRPC3 will heteromultimerize [24]. Results presented in Figure 2(A) demonstrate that COS-7 cells co-transfected with hTRPC1β and hTRPC3 exhibited expression patterns for both proteins that resembled the pattern in singly transfected cells. Minimal co-localization was apparent and the intracellular pool of hTRPC3 remained unaffected.
Figure 2. Co-expression of TRPC1 and TRPC3 does not alter their subcellular distribution.
(A) COS-7 cells were co-transfected with both FLAG–hTRPC1 and Myc–hTRPC3 constructs. Distribution of the tagged proteins was determined using a monoclonal anti-FLAG antibody and a rabbit polyclonal antiserum to TRPC3. Co-expression of the two constructs did not result in redistribution of either isoform. (B) hTRPC3 and hTRPC7, but not hTRPC1, co-localize with TGN46. Double-label immunofluorescence studies were performed on COS-7 cells transiently transfected with hTRPC1 (top panel) using a monoclonal antibody to the FLAG epitope tag and a sheep polyclonal antibody to a marker of the TGN, TGN46. Minimal overlap is seen as represented by a lack of yellow in the merged image. Cells transiently expressing hTRPC3 (middle panel) and hTRPC7 (bottom panel) were processed for immunofluorescence microscopy using a mouse anti-Myc epitope antibody, 9E10 (hTRPC3), or monoclonal anti-FLAG (hTRPC7) and sheep anti-TGN46 (middle panels). Overlap between hTRPC3 and both TGN46 and Gpp130 is shown in yellow in the merged images. Scale bar, 10 μm.
The intracellular pool of hTRPC3 and hTRPC7 localizes to both the TGN (trans-Golgi network) and the Golgi stack
One model for TRP activation includes an insertion into the plasma membrane from an intracellular pool. There is a growing body of evidence to support this model (reviewed in [25]); however, although there is evidence for rapid insertion of TRPCs from a sub-plasmalemmal pool using TIRF (total internal reflection fluorescence) microscopy [5], the possibility of contributions by other intracellular pools to this process has not been investigated. Recycling from the TGN through the plasma membrane has been well characterized for a number of proteins including TGN38 [26] and furin [27]. Precedence for transport of receptors from the TGN to the cell surface on stimulation has also been demonstrated for two members of the TNFR (tumour necrosis factor receptor) family, TNFR1 and Fas [28]. It was therefore of interest to determine whether the perinuclear localization of hTRPC3 and hTRPC7 corresponded to the TGN using double-immunofluorescence labelling of transiently transfected COS-7 cells with a marker for the TGN, TGN46 [29]. For comparison, cells were also transfected with hTRPC1β and processed for immunofluorescence using a monoclonal anti-FLAG antibody and sheep anti-TGN46. Limited, if any, co-localization was detected for hTRPC1 and TGN46 (Figure 2B, top panel); however, a high degree of co-localization was seen between both hTRPC3 and hTRPC7 and TGN46 in the perinuclear region (Figure 2B, middle and lower panels). These results along with the co-transfection experiment support the conclusion that these TRPC3/7 and TRPC1 isoforms reside in separate intracellular compartments.
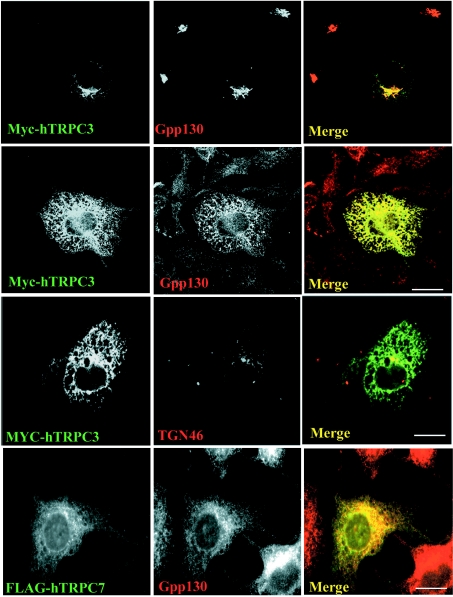
Although most of the intracellular pools of hTRPC3 and hTRPC7 co-localized with TGN46, the co-localization was not complete. The possibility that some of the hTRPC3 concentrates in the Golgi stack was approached initially by conducting double labelling with hTRPC3 and a marker for the cis-Golgi, Gpp130 [30]. As for TGN46, there was also extensive co-localization of hTRPC3 (Figure 3, top panel) with Gpp130. This approach, however, does not provide definitive means for the dissection of the TGN from the Golgi stack. BFA is a fungal metabolite that inhibits ARFs (ADP-ribosylation factors) and consequently coat formation; the consequence of this inhibition is the disassembly and redistribution of the Golgi complex. In contrast with the TGN, which collapses to a small dot around the MTOC (microtubule organizing centre), BFA causes the Golgi stacks to be redistributed into the ER in COS-7 cells [18]. Surprisingly, after BFA treatment, hTRPC3 and hTRPC7 clearly redistribute with Gpp130 into the ER (Figure 3, panels 2 and 4), whereas TGN46 appeared in the classic ‘BFA dot’ (Figure 3, panel 3). This indicates that hTRPC3 localizes not only to the TGN but also to the Golgi stacks.
Figure 3. hTRPC3 and hTRPC7 localize to the Golgi stack.
COS cells transiently transfected with Myc–hTRPC3 or FLAG–hTRPC7 were treated with (bottom three panels) or without (top panel; control) 5 μg/ml BFA for 2 h, fixed and processed for immunofluorescence microscopy using monoclonal antibodies to either the Myc or FLAG epitopes respectively and either rabbit anti-Gpp130 or sheep anti-TGN46 as indicated. Overlap between the redistributed Myc–hTRPC3 and Gpp130 is shown in yellow in the merged image. Scale bar, 10 μm.
Endogenous TRPC7 localizes to the Golgi complex in a rat pituitary cell line, GH4C1
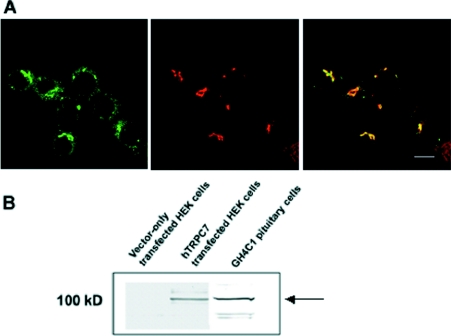
A key question concerning possible effects of overexpression in transfected cells on subcellular distribution prompted us to examine various cell lines to see if we could detect endogenous protein using immunofluorescence microscopy. Unfortunately, none of the cell lines tested gave an unequivocal signal with either a commercially available or our own polyclonal anti-hTRPC3 antibody. However, as TRPC7 appears to have a similar distribution to TRPC3, and TRPC7 mRNA levels have been reported to be highest in the pituitary [31], we used a polyclonal anti-TRPC7 antibody raised to the extreme C-terminus [15] to assess distribution in a rat pituitary cell line, GH4C1. Consistent with the perinuclear concentration in COS-7 cells expressing FLAG–hTRPC7, the endogenous protein was detected in a Golgi-like area close to the nucleus (Figure 4A). Double-labelling experiments with the cis-Golgi marker α-mannosidase II indicate a high degree of co-localization although not complete overlap. Immunoblotting of whole cell lysate revealed a positive signal at approx. 100 kDa, corresponding to the same band detected in HEK-293 cells (human embryonic kidney cells) overexpressing hTRPC7 that is not detected in untransfected cells (Figure 4B). This supports the finding that the signal detected by immunofluorescence was indeed TRPC7.
Figure 4. Endogenous TRPC7 co-localizes with a marker for the cis-Golgi, mannosidase II, in a rat pituitary cell line, GH4C1.
(A) GH4C1 cells were fixed and processed for immunocytochemistry using a polyclonal rabbit anti-hTRPC7 antibody detected by a goat anti-rabbit Alexa Fluor® 488 (green) and a monoclonal anti-mannosidase II antibody detected by a goat anti-mouse Alexa Fluor® 594 (red). Overlap is depicted by yellow (merge). (B) Immunoblot demonstrating the specificity of the polyclonal rabbit antisera raised to TRPC7. Whole cell lysate from either untransfected (negative control) or hTRPC7-transfected HEK-293 cells (positive control) and GH4C1 cells were subjected to SDS/PAGE and then immunoblotted using the affinity-purified anti-TRPC7 serum. A band of approx. 100 kDa is detected in both the hTRPC7-transfected HEK-293 cells and the GH4C1 cells but is absent in the untransfected controls. Scale bar, 10 μm.
Expression of either hTRPC3 or hTRPC7, but not hTRPC1, enhances constitutive secretion
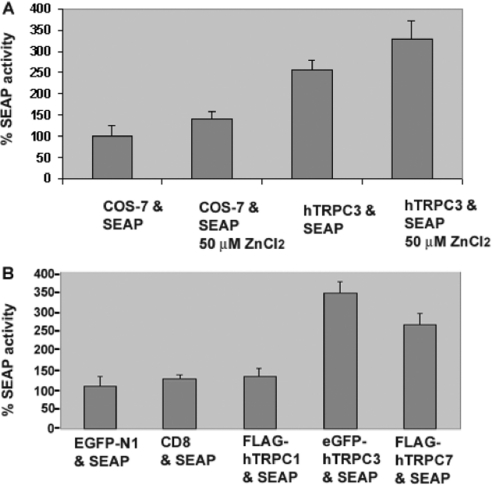
As a central organelle of the secretory pathway, one of the major functions of the Golgi complex is in sorting and modifying proteins destined for the plasma membrane, endosomal compartments or specialized secretory granules. These processes are dependent on both the formation of vesicles and the fusion of these vesicles with a target membrane. Several studies implicate a role for luminal calcium in organellar fusion events, particularly endosome/lysosome fusion [32]. We therefore reasoned that one possible consequence of having a calcium channel in the Golgi complex might be to increase vesicle fusion and therefore constitutive secretion. In order to test this hypothesis, COS-7 control or COS-hTRPC3-1 cells were transfected with a plasmid encoding a SEAP [33]. The newly synthesized enzyme is translocated into the lumen of the ER, transported constitutively through the Golgi complex and then targeted for secretion at the plasma membrane. The amount of alkaline phosphatase present in the cell culture medium of untransfected control, untransfected control under inducing conditions (50 μM ZnCl2) and uninduced or induced COS-hTRPC3 was measured 48 h after transfection. As indicated in Figure 1(C), hTRPC3 is expressed at low levels in this cell line even in the absence of induction. The 2-fold increase in secretion of the enzyme over control even in uninduced cells suggests that the presence of hTRPC3 has a significant effect on transport through the secretory pathway. A 3-fold increase was observed after induction of COS-hTRPC3 cells compared with control cells treated with 50 μM ZnCl2; there was also a significant increase when compared with uninduced cells (Figure 5A).
Figure 5. hTRPC3 and hTRPC7, but not hTRPC1, increase constitutive secretion.
(A) Control untransfected and COS-hTRPC3-1 cells were transfected with a construct that expresses a soluble secreted form of alkaline phosphatase. At 24 h post-transfection, they were cultured in the presence or absence of 50 μM ZnCl2 for a further 48 h and assayed in triplicate for SEAP activity. The results are presented as a percentage of enzyme activity (luminescence) in cells expressing SEAP alone. The results are means±S.E.M. for three independent experiments. (B) COS-7 cells were co-transfected with the SEAP vector and EGFP-N1, CD8, FLAG–hTRPC1, EGFP–TRPC3 or FLAG–hTRPC7 and grown 48 h prior to assaying for SEAP activity. Values are presented as a percentage of the total enzyme activity (luminescence) in the cells expressing EGFP. The results are means±S.E.M. for three independent experiments.
To examine whether this was specific for hTRPC3, we conducted similar experiments using hTRPC1, hTRPC7 and a resident cell surface protein that normally traffics to the plasma membrane through the Golgi complex, CD8. In the absence of stable cell lines expressing these proteins, it was necessary to carry out these studies on transiently transfected cells. In our hands, co-transfections often result in cells not only co-expressing but also expressing either one or the other protein. Hence, for all experiments, the number of cells expressing both the protein of interest and SEAP was determined by immunofluorescence to ensure comparable levels of both co-transfected and singly transfected cells (results not shown). Expression of each construct was compared with the expression of EGFP-N1 to control for effects of transfection alone. As can be seen in Figure 5(B), expression of neither CD8 nor hTRPC1 had significant effects on the amount of SEAP in the medium; however, the expression of both hTRPC3 and its closely related family member, hTRPC7, increased constitutive secretion by at least 2.5-fold, and almost 3.5-fold in the case of hTRPC3. These findings suggest that not only are hTRPC3 and hTRPC7 expressed in the Golgi complex, but they also have a significant effect on a major function of this organelle, constitutive secretion.
DISCUSSION
Despite an increasing number of studies focusing on hTRPC3 function and localization, an in-depth analysis of its subcellular distribution has not previously been conducted. The initial hypothesis that TRPCs function as store-operated calcium channels predicts a plasma-membrane localization for the TRPC family. However, a large intracellular pool of hTRPC3 is apparent in many immunofluorescence micrographs [19–21,34]. Most dramatically, in immunofluorescence studies on the expression of TRPC isoforms in mouse skeletal-muscle fibres, endogenous TRPC1, TRPC4 and TRPC6 were clearly localized to the plasma membrane; however, TRPC3 in particular was found almost exclusively intracellularly in perinuclear structures reminiscent of the Golgi [21]. We have characterized the distribution of hTRPC3 using double-label immunofluorescence microscopy in COS-7 cells and demonstrate that: (i) a major pool of hTRPC3 co-localizes with markers for both the TGN and the Golgi stack, (ii) it differs from hTRPC1, which is also found predominantly intracellularly but remains associated with the ER, and (iii) a large proportion of the perinuclear pool redistributes after BFA treatment, suggesting that it localizes throughout the Golgi stack. A similar distribution pattern is observed for hTRPC7; considering the close sequence homology of these subfamily members and their ability to interact as demonstrated by co-immunoprecipitation experiments, this is not surprising [23,35]. Although previous studies include evidence for TRPC1 on the plasma membrane, our results show a completely intracellular distribution which is consistent with results from subcellular fractionation of endogenous TRPC1 in platelets [36] and sperm [19] and overexpressed TRPC1 in HEK-293 cells [7]. Potential ER exit motifs are present within the amino acid sequence of TRPC3/7 but are lacking in TRPC1. Putative ER retention signals are also present in TRPC1; these are now being investigated to determine whether the difference in trafficking between these subfamily members is due to specific targeting signals.
The best-characterized trafficking routes for proteins recycling through the plasma membrane back to an intracellular store is to and from the TGN or from the TGN through the plasma membrane to recycling endosomes or specialized pools such as for the glucose transporter, GLUT4, or neurotransmitters. Hence, our first hypothesis as to a possible function for the TGN/Golgi-localized pool of hTRPC3 was as a ‘readily releasable’ storage pool. A number of studies have previously demonstrated translocation of a variety of TRP family members from an intracellular pool to the plasma membrane including a C. elegans TRPC homologue, TRP-3 [8], TRPV1 [10], TRPC3 [37] and TRPC5 [5]. Most of these studies postulate that the translocation occurs from a vesicular pool close to the plasma membrane. Our finding that most of the intracellular pool was localized in a perinuclear localization overlapping the Golgi stack was therefore surprising. An intriguing possibility, however, is that the translocatable pool is at least partially Golgi-derived. Singh et al. [6] clearly demonstrate a decrease in the agonist-stimulated plasma membrane localization of TRPC3 after treatment of cells for 30–60 min with BFA, a compound that disrupts the Golgi stack and intra-Golgi transport but does not inhibit post-Golgi transport to the cell surface [38]. Since the pool of TRPC3 that was being translocated was reported to be a sub-plasmalemmal vesicular pool, BFA should not have inhibited this translocation step, but it would inhibit translocation from the Golgi stack. Precedence for transport occurring along this trafficking route in response to external stimuli comes from studies on the stimulation of Fas from the Golgi to the cell surface, which occurs on stimulation by p53 [39]. Although this could be one possibility for our finding that a large pool of TRPC3 resides in the Golgi stack, it raised the question as to whether TRPC3 may have functions in the Golgi in addition to, or instead of, acting as a calcium entry channel at the plasma membrane.
A variety of Ca2+-dependent functions occur within the Golgi, which include post-translational modifications of secretory proteins [40], formation of COPI (coatamer protein I) coats on Golgi–ER transport vesicles [41] and both anterograde and retrograde vesicular transport through and to the Golgi complex [42,43]. In addition to having a role in coat formation, release of luminal calcium from vesicles has also been suggested to play a role in vesicle fusion as shown for insulin-containing secretory granules with the plasma membrane [44]. Thus we hypothesized that an alternative function for TRPC3 could be mediating vesicle fusion, either between the ER and the cis-Golgi, transport from the trans-Golgi/TGN and/or increasing fusion at the plasma membrane. Indeed, TRPC3 has already been shown to interact by yeast two hybrid with VAMP2 (vesicle-associated membrane protein 2), NSF (N-ethylmaleimide-sensitive factor) and α-SNAP (α-NSF-attachment protein) [6], which normally reside in vesicles and play a role in vesicle fusion. TRPM7 has also recently been localized to synaptic vesicle membranes where it forms complexes with SNAREs (SNAP receptors) and their associated proteins, e.g. snapin, as well as the scaffolding protein synapsin [45]. Synapsin has been postulated to anchor vesicles in a ‘readily releasable’ pool ready to refill the docked pool after a round of synaptic vesicle release. Therefore an additional role of TRP channels may, as previously postulated, be to act as scaffolding proteins (reviewed in [46]).
Increasing the level of hTRPC3 expression produced a concomitant increase in the amount of constitutive secretion of alkaline phosphatase. One possible mechanism could simply be that an increase in calcium-permeable channels at the cell surface results in an increase in cytosolic calcium levels, which may increase secretion non-specifically. Since the levels of intracellular calcium are acutely regulated by uptake into calcium-storing organelles or extrusion via calcium pumps, this may not be the most likely scenario. Also, an increase in cytosolic calcium levels is most often triggered by activation of a cell surface receptor; in this case, there is no stimulation involved. As our induction system is based on activation of the metallothionein promoter, the use of heavy metals such as zinc and cadmium is required to induce expression; consequently, there is also a possibility that they may have some effect on channel function. There are minimal accounts of effects of either cadmium or zinc on TRPC channels specifically, although both permeability and inhibition have been reported for ‘non-selective calcium entry channels’ and for dTRP (reviewed in [47]). However, the only report of inhibition for dTRP expressed in a Xenopus oocyte was at a concentration of 1 mM and we routinely do not go above 50 μM for induction. In addition, our results indicate that secretion of alkaline phosphatase was increased to a similar extent above control after expression of GFP–TRPC3 and FLAG-tagged TRPC7, which are constitutively expressed and do not require the addition of heavy metals. Another possible mechanism for increasing secretion is that there is an increase in the number of Golgi transport vesicles being produced or an increase in the number of fusion events at the plasma membrane. This does not appear to be a general consequence of having a TRP channel resident within the secretory pathway as TRPC1, which we find localized to the ER, has no effect on constitutive secretion.
To probe the calcium dependence of different transport steps within the cell, Chen et al. [43] demonstrated that the anterograde transport of VSV-G (vesicular-stomatitis-virus glycoprotein) protein from the Golgi was shown to be blocked by calcium chelation using the membrane-permeant calcium chelator, BAPTA/AM [bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid tetrakis(acetoxymethyl ester)]. Since BAPTA [bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid] has been found to be more effective than EGTA at inhibiting many of the organellar fusion and transport events within the cell, most likely due to a much faster rate of calcium binding, it has been proposed that calcium transients or gradients are more important than steady-state calcium levels. This would also suggest the necessity for either a vesicular or organellar calcium release channel that could mediate rapid but localized increases in calcium; TRPC3 could fulfil this function. Since a number of agents that affect the ability of TRPC3 to mediate calcium entry at the plasma membrane are also integral to the Golgi complex, e.g. DAG, PLCγ and PLD, an intriguing possibility is that these agents may affect channel function at both locations. It has recently been shown that DAG levels in the Golgi are critical for Golgi secretory function [48]. DAG also activates mitochondrial cationic channels and elicits the release of sequestered calcium in this organelle [49], so it is possible that this may also occur in the Golgi mediated via TRP channels. Indeed, release of Golgi calcium in response to menthol, an activator of TRPM8, has recently been reported, but surprisingly this response could also be elicited in cells without endogenous TRPM8, suggesting that other TRP channels may be responsible for this release from Golgi stores [50]. TRPC3/7 could be ideal candidates.
Acknowledgments
We thank Dr Craig Montell for the gift of the hTRPC1 and hTRPC3 constructs and Professor J. Paul Luzio for kindly supplying the 9E10 ascites, helpful discussions and much appreciated support. This work was supported by the Wellcome Trust, grant number 069934.
References
- 1.Hardie R. C., Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- 2.Montell C. Drosophila TRP channels. Eur. J. Physiol. 2005;451:19–28. doi: 10.1007/s00424-005-1426-2. [DOI] [PubMed] [Google Scholar]
- 3.Putney J. W. Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here) Cell Calcium. 2007;42:103–110. doi: 10.1016/j.ceca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cayouette S., Lussier M. P., Mathieu E. L., Bousquet S. M., Boulay G. Exocytotic insertion of TRPC6 channels into the plasma membrane upon Gq protein-coupled receptor activation. J. Biol. Chem. 2004;279:7241–7246. doi: 10.1074/jbc.M312042200. [DOI] [PubMed] [Google Scholar]
- 5.Bezzerides V. J., Ramsey I. S., Kotecha S., Greka A., Clapham D. E. Rapid vesicular translocation and insertion of TRP channels. Nat. Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- 6.Singh B. B., Lockwich T. P., Bandyopadhyay B. C., Liu X., Bollimuntha S., Brazer S. C., Combs C., Das S., Leenders A. G., Sheng Z. H., et al. VAMP2-dependent exocytosis regulates plasma membrane insertion of TRPC3 channels and contributes to agonist-stimulated Ca2+ influx. Mol. Cell. 2004;15:635–646. doi: 10.1016/j.molcel.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Hofmann T., Schaefer M., Schultz G., Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc. Natl. Acad. Sci. U.S.A. 2002;99:7461–7466. doi: 10.1073/pnas.102596199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X.-Z. S., Sternberg P. W. A C. elegans sperm TRP protein required for sperm-egg interactions during fertilization. Cell. 2003;114:285–297. doi: 10.1016/s0092-8674(03)00565-8. [DOI] [PubMed] [Google Scholar]
- 9.Hoenderop J. G., Voets T., Hoefs S., Weidema F., Prenen J., Nilius B., Bindels R. J. Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J. 2003;22:776–785. doi: 10.1093/emboj/cdg080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morenilla-Palao C., Planells-Cases R., Garcia-Sanz N., Ferrer-Montiel A. Regulated exocytosis contributes to protein kinase C potentiation of vanilloid receptor activity. J. Biol. Chem. 2004;279:25665–25672. doi: 10.1074/jbc.M311515200. [DOI] [PubMed] [Google Scholar]
- 11.Piper R. C., Luzio J. P. CUPpling calcium to lysosomal biogenesis. Trends Cell Biol. 2004;14:471–473. doi: 10.1016/j.tcb.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Soyombo A. A., Tjon-Kon-Sang S., Rbaibi Y., Bashllari E., Bisceglia J., Muallem S., Kiselyov K. TRP-ML1 regulates lysosomal pH and lysosomal lipid hydrolytic activity. J. Biol. Chem. 2005;281:7294–7301. doi: 10.1074/jbc.M508211200. [DOI] [PubMed] [Google Scholar]
- 13.Thebault S., Lemonnier L., Bidaux G., Flourakis M., Bavencoffe A., Gordienko D., Roudbaraki M., Delcourt P., Panchin Y., Shuba Y., et al. Novel role of cold/menthol-sensitive TRPM8 in the activation of store-operated channels in LNCaP human prostate cancer epithelial cells. J. Biol. Chem. 2005;280:39423–39435. doi: 10.1074/jbc.M503544200. [DOI] [PubMed] [Google Scholar]
- 14.Kottgen M., Walz G. Subcellular localization and trafficking of polycystins. Pflugers Arch. 2005;451:286–293. doi: 10.1007/s00424-005-1417-3. [DOI] [PubMed] [Google Scholar]
- 15.Sekaran S., Lall G. S., Ralphs K. L., Wolstenholme A. J., Lucas R. J., Foster R. G., Hankins M. W. 2-Aminoethoxydiphenylborane is an acute inhibitor of directly photosensitive retinal ganglion cell activity in vitro and in vivo. J. Neurosci. 2007;27:3981–3986. doi: 10.1523/JNEUROSCI.4716-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu X. Z., Li H. S., Guggino W. B., Montell C. Coassembly of TRP and TRPL produces a distinct store-operated conductance. Cell. 1997;89:1155–116417. doi: 10.1016/s0092-8674(00)80302-5. [DOI] [PubMed] [Google Scholar]
- 17.Roquemore E. P., Banting G. Efficient trafficking of TGN38 from the endosome to the trans-Golgi network requires a free hydroxyl group at position 331 in the cytosolic domain. Mol. Biol. Cell. 1998;9:2125–2144. doi: 10.1091/mbc.9.8.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reaves B., Banting G. Perturbation of the morphology of the trans-Golgi network following Brefeldin A treatment: redistribution of a TGN-specific integral membrane protein, TGN38. J. Cell Biol. 1992;116:85–94. doi: 10.1083/jcb.116.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trevino C. L., Serrano C. J., Beltran C., Felix R., Darszon A. Identification of mouse trp homologs and lipid rafts from spermatogenic cells and sperm. FEBS Lett. 2001;509:119–125. doi: 10.1016/s0014-5793(01)03134-9. [DOI] [PubMed] [Google Scholar]
- 20.McKay R. R., Szymeczek-Seay C. L., Lievremont J. P., Bird G. S., Zitt C., Jungling E., Luckhoff A., Putney J. W., Jr. Cloning and expression of the human transient receptor potential 4 (TRP4) gene: localization and functional expression of human TRP4 and TRP3. Biochem. J. 2000;351:735–746. [PMC free article] [PubMed] [Google Scholar]
- 21.Vandebrouck C., Martin D., Colson-Van Schoor M., Debaix H., Gailly P. Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J. Cell Biol. 2002;158:1089–1096. doi: 10.1083/jcb.200203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wes P. D., Chevesich J., Jeromin A., Rosenberg C., Stetten G., Montell C. TRPC1, a human homolog of a Drosophila store-operated channel. Proc. Natl. Acad. Sci. U.S.A. 1995;92:9652–9656. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goel M., Sinkins W. G., Schilling W. P. Selective association of TRPC channel subunits in rat brain synaptosomes. J. Biol. Chem. 2002;277:48303–48310. doi: 10.1074/jbc.M207882200. [DOI] [PubMed] [Google Scholar]
- 24.Yuan J. P., Zeng W., Huang G. N., Worley P. F., Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat. Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cayouette S., Boulay G. Intracellular trafficking of TRP channels. Cell Calcium. 2007;42:225–232. doi: 10.1016/j.ceca.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Reaves B., Banting G. Perturbation of the morphology of the trans-Golgi network following Brefeldin A treatment: redistribution of a TGN-specific integral membrane protein, TGN38. J. Cell Biol. 1992;116:85–94. doi: 10.1083/jcb.116.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol. 2002;10:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Storey H., Stewart A., Vandenabeele P., Luzio J. The p55 tumour necrosis factor receptor TNFR1 contains a trans-Golgi network localization signal in the C-terminal region of its cytoplasmic tail. Biochem. J. 2002;366:15–22. doi: 10.1042/BJ20020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ponnambalam S., Girotti M., Yaspo M., Owen C., Perry A. C., Suganuma T., Nillson T., Fired M., Banting G., Warren G. Primate homologues of rat TGN38: primary structure, expression and function. J. Cell Sci. 1996;109:675–685. doi: 10.1242/jcs.109.3.675. [DOI] [PubMed] [Google Scholar]
- 30.Linstedt A. D., Mehta A., Suhan J., Reggio H., Hauri H. Sequence and overexpression of GPP130/GIMPc: evidence for saturable pH-sensitive targeting of a type II early Golgi membrane protein. Mol. Biol. Cell. 1997;8:1073–1087. doi: 10.1091/mbc.8.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riccio A., Medhurst A. D., Mattei C., Kelsell R. E., Calver A. R., Randall A. D., Benham C. D., Pangalos M. N. mRNA distribution analysis of human TRPC family in CNS and peripheral tissues. Mol. Brain Res. 2002;109:95–104. doi: 10.1016/s0169-328x(02)00527-2. [DOI] [PubMed] [Google Scholar]
- 32.Pryor P. R., Mullock B. M., Bright N. A., Gray S. R., Luzio J. P. The role of intraorganellar Ca2+ in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J. Cell Biol. 2000;149:1053–1062. doi: 10.1083/jcb.149.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Towler M. C., Prescott A. R., James J., Lucocq J. M., Ponnambalam S. The manganese cation disrupts membrane dynamics along the secretory pathway. Exp. Cell Res. 2000;259:167–179. doi: 10.1006/excr.2000.4958. [DOI] [PubMed] [Google Scholar]
- 34.Wang W., O'Connell B., Dykeman R., Sakai T., Delporte C., Swaim W., Zhu X., Birnbaumer L., Ambudkar I. S. Cloning of Trp1beta isoform from rat brain: immunodetection and localization of the endogenous Trp1 protein. Am. J. Physiol. 1999;276:C969–C979. doi: 10.1152/ajpcell.1999.276.4.C969. [DOI] [PubMed] [Google Scholar]
- 35.Vannier B., Zhu X., Brown D., Birnbaumer L. The membrane topology of human transient receptor potential 3 as inferred from glycosylation-scanning mutagenesis and epitope immunocytochemistry. J. Biol. Chem. 1998;273:8675–8679. doi: 10.1074/jbc.273.15.8675. [DOI] [PubMed] [Google Scholar]
- 36.Hassock S. R., Zhu M. X., Trost C., Flockerzi V., Authi K. S. Expression and role of TRPC proteins in human platelets: evidence that TRPC6 forms the store-independent calcium entry channel. Blood. 2002;100:2801–2811. doi: 10.1182/blood-2002-03-0723. [DOI] [PubMed] [Google Scholar]
- 37.Singh B. B., Zheng C., Liu X., Lockwich T., Liao D., Zhu M. X., Birnbaumer L., Ambudkar I. S. Trp1-dependent enhancement of salivary gland fluid secretion: role of store-operated calcium entry. FASEB J. 2001;15:1652–1654. doi: 10.1096/fj.00-0749fje. [DOI] [PubMed] [Google Scholar]
- 38.Klausner R., Donaldson J., Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure J. Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett M., Macdonald K., Chan S. W., Luzio J. P., Simari R., Weissberg P. Cell surface trafficking of Fas: a rapid mechanism of p53-mediated apoptosis. Science. 1998;282:290–293. doi: 10.1126/science.282.5387.290. [DOI] [PubMed] [Google Scholar]
- 40.Austin C. D., Shields D. Prosomatostatin processing in permeabilized cells. Calcium is required for prohormone cleavage but not formation of nascent secretory vesicles. J. Biol. Chem. 1996;271:1194–1199. doi: 10.1074/jbc.271.2.1194. [DOI] [PubMed] [Google Scholar]
- 41.Ahluwalia J. P., Topp J. D., Weirather K., Zimmerman M., Stamnes M. A role for calcium in stabilizing transport vesicle coats. J. Biol. Chem. 2001;276:34148–34155. doi: 10.1074/jbc.M105398200. [DOI] [PubMed] [Google Scholar]
- 42.Porat A., Elazar Z. Regulation of intra-Golgi membrane transport by calcium. J. Biol. Chem. 2000;275:29233–29237. doi: 10.1074/jbc.M005316200. [DOI] [PubMed] [Google Scholar]
- 43.Chen J. L., Ahluwalia J. P., Stamnes M. Selective effects of calcium chelators on anterograde and retrograde protein transport in the cell. J. Biol. Chem. 2002;277:35682–35687. doi: 10.1074/jbc.M204157200. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell K. J., Pinton P., Varadi A., Tacchetti C., Ainscow E. K., Pozzan T., Rizzuto R., Rutter G. A. Dense core secretory vesicles revealed as a dynamic Ca2+ store in neuroendocrine cells with a vesicle-associated membrane protein aequorin chimaera. J. Cell Biol. 2001;155:41–51. doi: 10.1083/jcb.200103145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krapivinsky G., Mochida S., Krapivinsky L., Cibulsky S. M., Clapham D. E. The TRPM7 ion channel functions in cholinergic synaptic vesicles and affects transmitter release. Neuron. 2006;52:485–496. doi: 10.1016/j.neuron.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 46.Venkatachalam K., Montell C. TRP channels. Ann. Rev. Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mathie A., Sutton G. L., Clarke C. E., Veale E. L. Zinc and copper: pharmacological probes and endogenous modulators of neuronal excitability. Pharmacol. Ther. 2006;111:567–583. doi: 10.1016/j.pharmthera.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 48.Litvak V., Dahan N., Ramachandran S., Sabanay H., Lev S. Maintenance of the diacylglycerol level in the Golgi apparatus by the Nir2 protein is critical for Golgi secretory function. Nat. Cell Biol. 2005;7:225–234. doi: 10.1038/ncb1221. [DOI] [PubMed] [Google Scholar]
- 49.Chinopoulos C., Starkov A. A., Grigoriev S., Dejean L. M., Kinnally K. W., Liu X., Ambudkar I. S., Fiskum G. Diacylglycerols activate mitochondrial cationic channel(s) and release sequestered Ca2+ J. Bioenerg. Biomembr. 2005;37:237–247. doi: 10.1007/s10863-005-6634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahieu F., Owsianik G., Verbert L., Janssens A., De Smedt H., Nilius B., Voets T. TRPM8-independent menthol-induced Ca2+ release from endoplasmic reticulum and Golgi. J. Biol. Chem. 2007;282:3325–3336. doi: 10.1074/jbc.M605213200. [DOI] [PubMed] [Google Scholar]