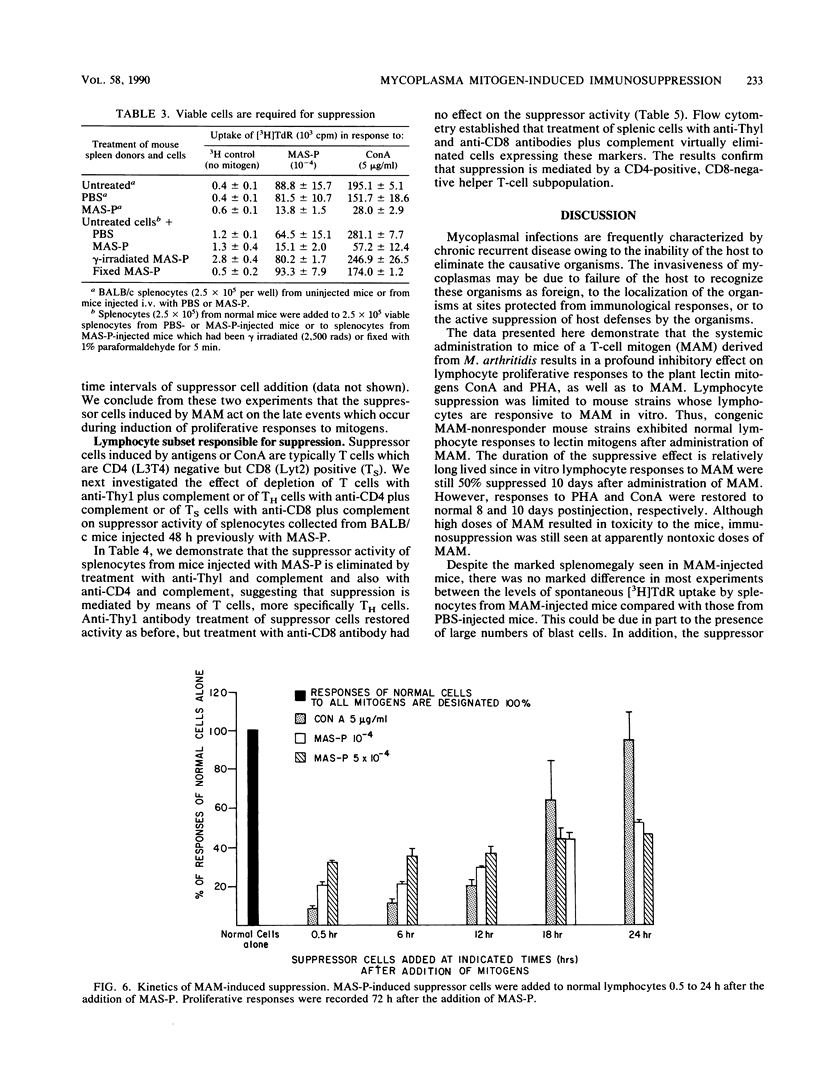
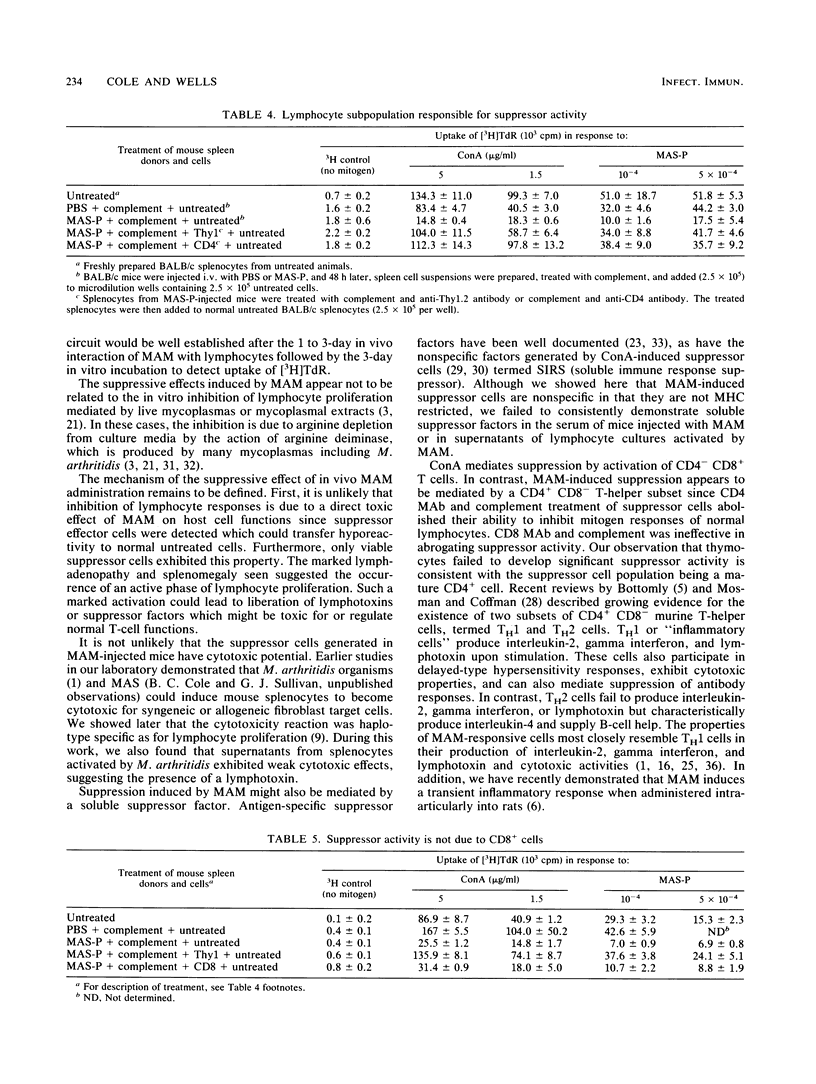
Abstract
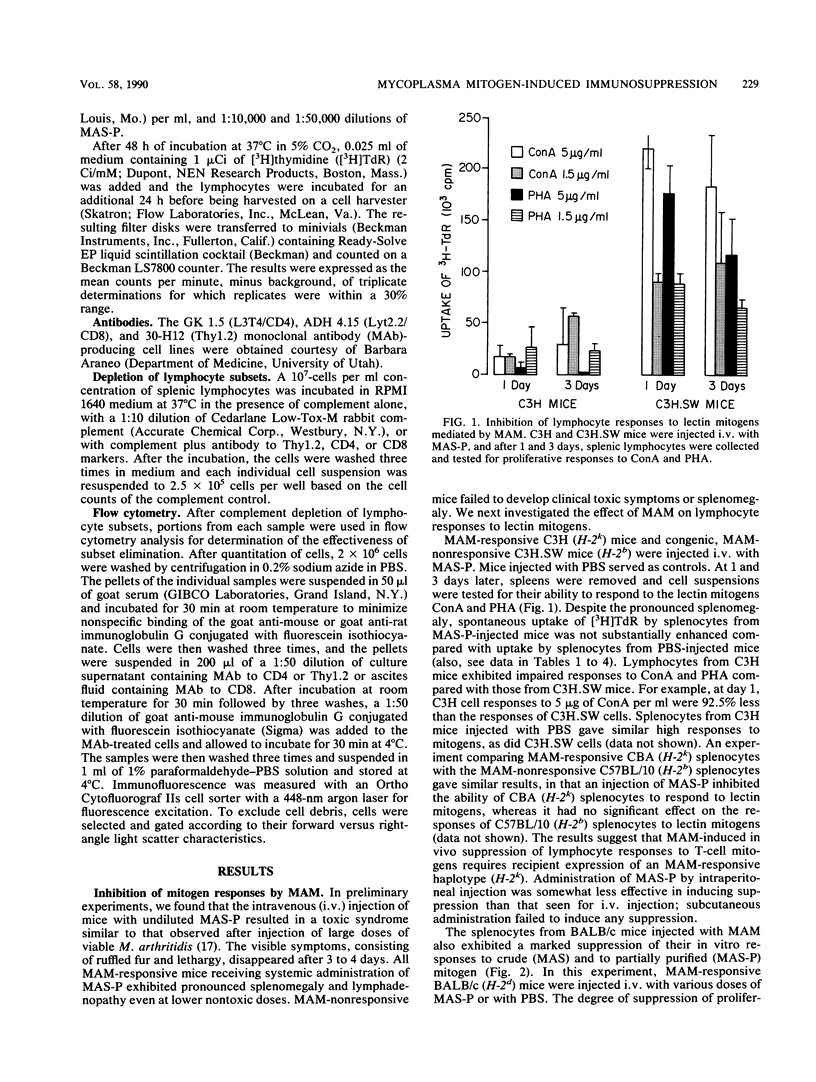
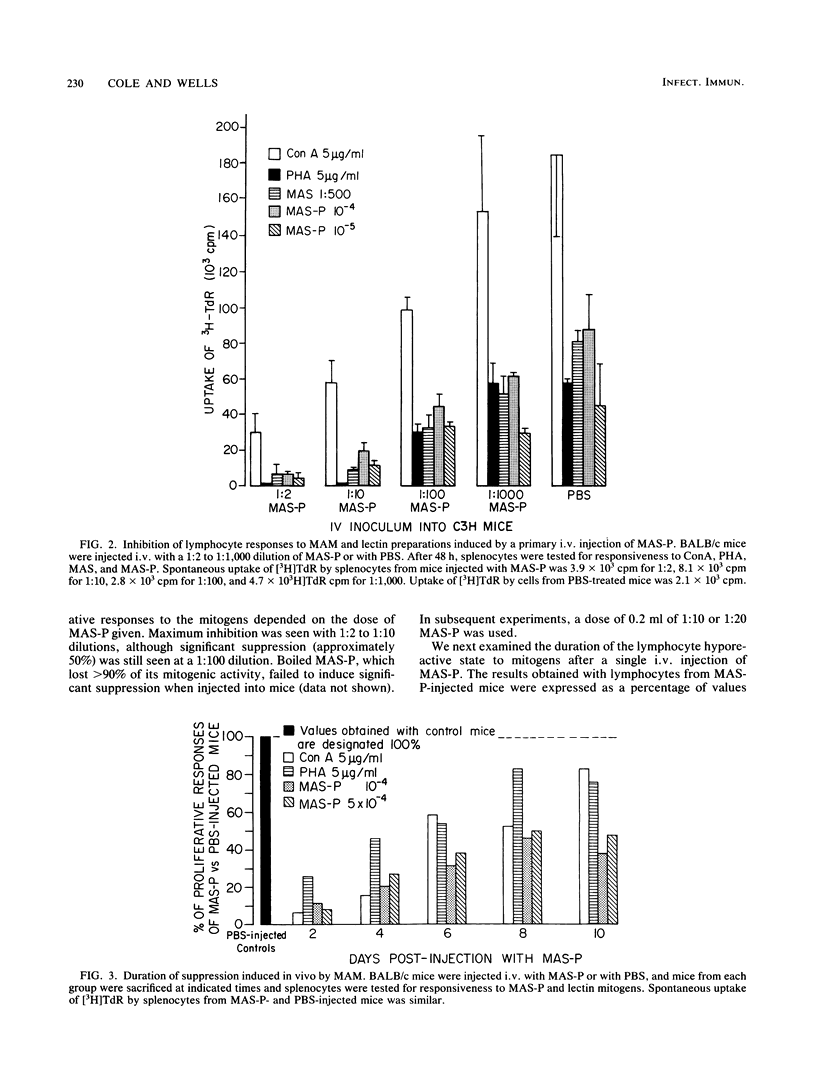
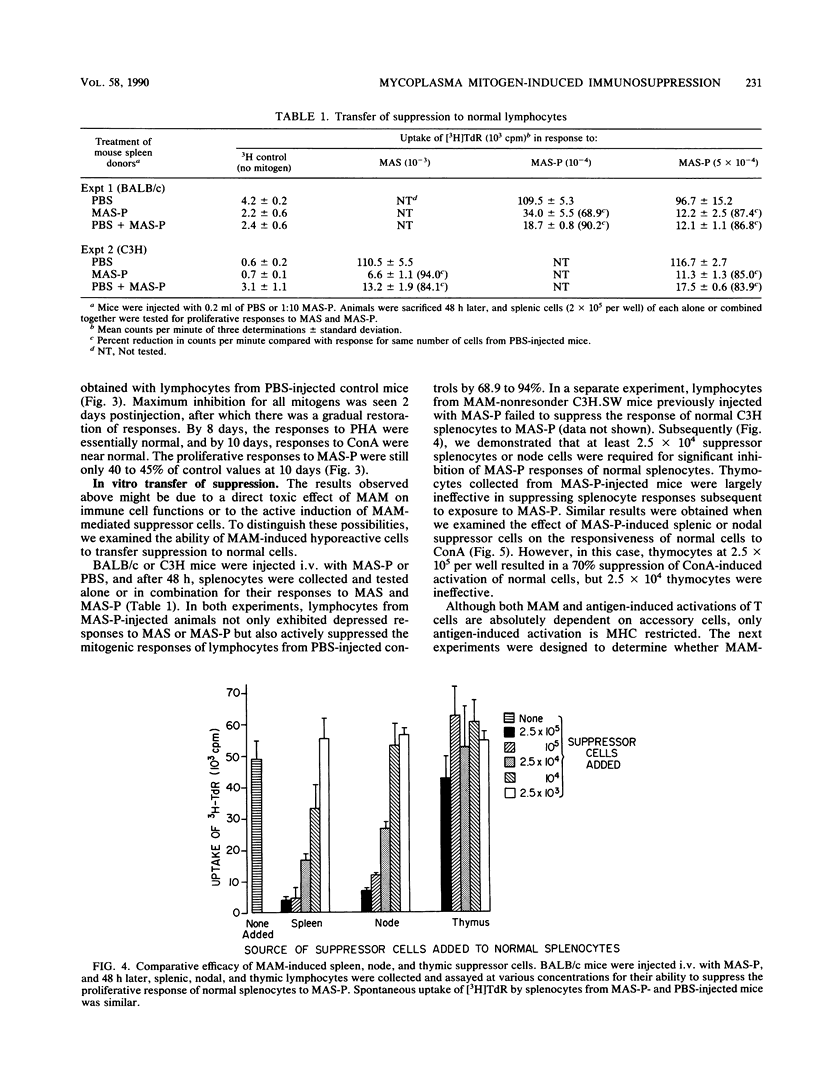
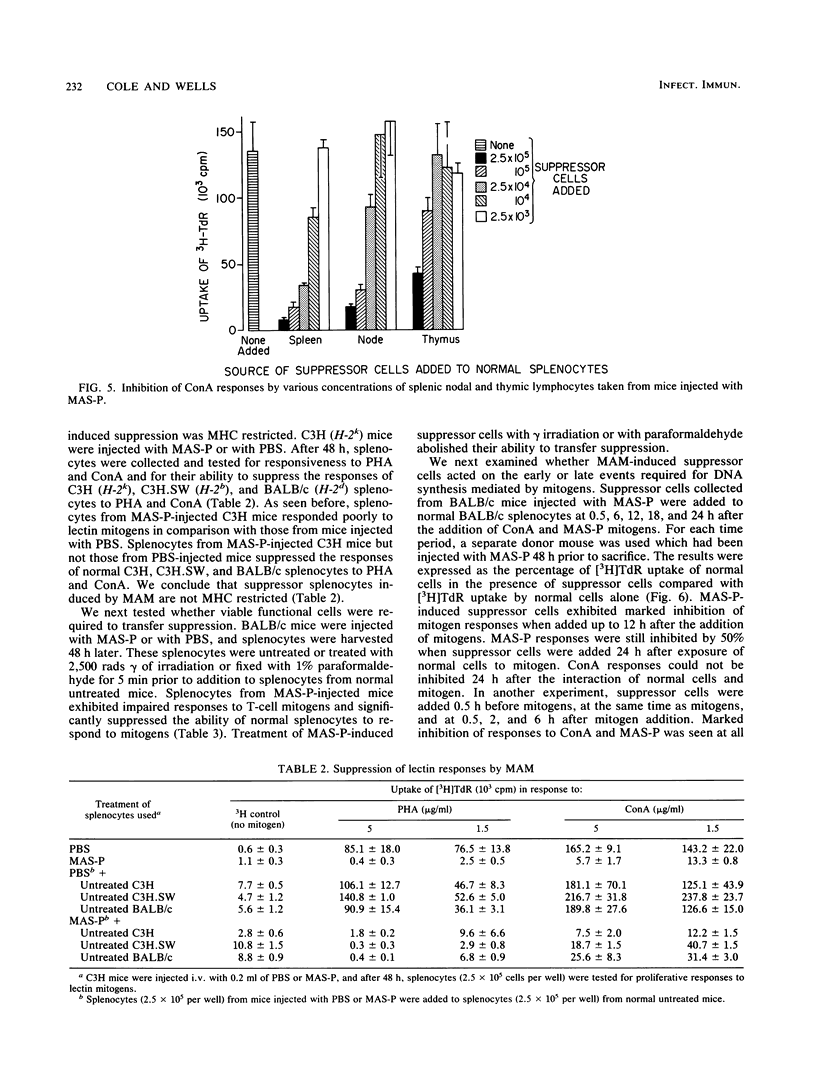
We have previously shown that Mycoplasma arthritidis produces a soluble T-cell mitogen (MAM) which is active for most mouse strains that express the alpha chain of the I-E molecule (E alpha) encoded within the murine major histocompatibility complex. The lymphocytes from mice injected intravenously with the MAM exhibited a marked decrease in their ability to respond in vitro to MAM, to phytohemagglutinin, or to concanavalin A T-cell mitogens. Suppression could only be induced in MAM-responsive mouse strains and was most marked 1 to 4 days postinjection. Splenic and node cells and, to a lesser extent, thymic cells from MAM-injected mice could inhibit the ability of lymphocytes from normal mice to respond to MAM and lectin mitogens. A minimum of 2.5 x 10(4) viable cells was required for significant transfer of suppression, and no major histocompatibility complex restrictions were seen. Unlike concanavalin A-induced suppressor cells, which consist of a CD4-, CD8+ T-cell subset, suppressor cells induced by MAM were due to a CD4+ CD8- subset. We hypothesize that MAM may play a role in M. arthritidis-mediated disease by both its inflammatory and immunosuppressive properties.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge K. E., Cole B. C., Ward J. R. Mycoplasma-dependent activation of normal lymphocytes: induction of a lymphocyte-mediated cytotoxicity for allogeneic and syngeneic mouse target cells. Infect Immun. 1977 Nov;18(2):377–385. doi: 10.1128/iai.18.2.377-385.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin C. L., Cole B. C., Sullivan G. J., Washburn L. R., Wiley B. B. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. V. A small basic protein from culture supernatants is a potent T cell mitogen. J Immunol. 1986 Sep 1;137(5):1581–1589. [PubMed] [Google Scholar]
- Barile M. F., Leventhal B. G. Possible mechanism for Mycoplasma inhibition of lymphocyte transformation induced by phytohaemagglutinin. Nature. 1968 Aug 17;219(5155):750–752. doi: 10.1038/219751a0. [DOI] [PubMed] [Google Scholar]
- Bekoff M. C., Cole B. C., Grey H. M. Studies on the mechanism of stimulation of T cells by the Mycoplasma arthritidis-derived mitogen. Role of class II IE molecules. J Immunol. 1987 Nov 15;139(10):3189–3194. [PubMed] [Google Scholar]
- Bottomly K. A functional dichotomy in CD4+ T lymphocytes. Immunol Today. 1988 Sep;9(9):268–274. doi: 10.1016/0167-5699(88)91308-4. [DOI] [PubMed] [Google Scholar]
- Cannon G. W., Cole B. C., Ward J. R., Smith J. L., Eichwald E. J. Arthritogenic effects of Mycoplasma arthritidis T cell mitogen in rats. J Rheumatol. 1988;15(5):735–741. [PubMed] [Google Scholar]
- Cole B. C., Cahill J. F., Wiley B. B., Ward J. R. Immunological responses of the rat to Mycoplasma arthritidis. J Bacteriol. 1969 Jun;98(3):930–937. doi: 10.1128/jb.98.3.930-937.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Daynes R. A., Ward J. R. Mycoplasma-mediated activation of normal mouse lymphocytes: induction of cytotoxic lymphocytes and lymphocyte transformation by Mycoplasma arthritidis are under Ir gene control. J Immunol. 1981 Mar;126(3):922–927. [PubMed] [Google Scholar]
- Cole B. C., Daynes R. A., Ward J. R. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. I. Transformation is associated with an H-2-linked gene that maps to the I-E/I-C subregion. J Immunol. 1981 Nov;127(5):1931–1936. [PubMed] [Google Scholar]
- Cole B. C., Daynes R. A., Ward J. R. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. III. Ir gene control of lymphocyte transformation correlates with binding of the mitogen to specific Ia-bearing cells. J Immunol. 1982 Oct;129(4):1352–1359. [PubMed] [Google Scholar]
- Cole B. C., Kartchner D. R., Wells D. J. Stimulation of mouse lymphocytes by a mitogen derived from Mycoplasma arthritidis. VII. Responsiveness is associated with expression of a product(s) of the V beta 8 gene family present on the T cell receptor alpha/beta for antigen. J Immunol. 1989 Jun 15;142(12):4131–4137. [PubMed] [Google Scholar]
- Cole B. C., Overall J. C., Jr, Lombardi P. S., Glasgow L. A. Mycoplasma-mediated hyporeactivity to various interferon inducers. Infect Immun. 1975 Dec;12(6):1349–1354. doi: 10.1128/iai.12.6.1349-1354.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Piepkorn M. W., Wright E. C. Influence of genes of the major histocompatibility complex on ulcerative dermal necrosis induced in mice by Mycoplasma arthritidis. J Invest Dermatol. 1985 Oct;85(4):357–361. doi: 10.1111/1523-1747.ep12276973. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Thorpe R. N., Hassell L. A., Washburn L. R., Ward J. R. Toxicity but not arthritogenicity of Mycoplasma arthritidis for mice associates with the haplotype expressed at the major histocompatibility complex. Infect Immun. 1983 Sep;41(3):1010–1015. doi: 10.1128/iai.41.3.1010-1015.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Thorpe R. N. I-E/I-C region-associated induction of murine gamma interferon by a haplotype-restricted polyclonal T-cell mitogen derived from Mycoplasma arthritidis. Infect Immun. 1984 Jan;43(1):302–307. doi: 10.1128/iai.43.1.302-307.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Thorpe R. N. Induction of human gamma interferons by a mitogen derived form Mycoplasma arthritidis and by Phytohemagglutinin: differential inhibition with monoclonal anti-HLA.DR antibodies. J Immunol. 1983 Nov;131(5):2392–2396. [PubMed] [Google Scholar]
- Cole B. C., Ward J. R. Interaction of Mycoplasma arthritidis and other mycoplasmas with murine peritoneal macrophages. Infect Immun. 1973 May;7(5):691–699. doi: 10.1128/iai.7.5.691-699.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Ward J. R., Jones R. S., Cahill J. F. Chronic proliferative arthritis of mice induced by Mycoplasma arthritidis. I. Induction of disease and histopathological characteristics. Infect Immun. 1971 Oct;4(4):344–355. doi: 10.1128/iai.4.4.344-355.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copperman R., Morton H. E. Reversible inhibition of mitosis in lymphocyte cultures by non-viable Mycoplasma. Proc Soc Exp Biol Med. 1966 Dec;123(3):790–795. doi: 10.3181/00379727-123-31605. [DOI] [PubMed] [Google Scholar]
- Daynes R. A., Novak J. M., Cole B. C. Comparison of the cellular requirements for human T cell transformation by a soluble mitogen derived from Mycoplasma arthritidis and concanavalin A. J Immunol. 1982 Sep;129(3):936–938. [PubMed] [Google Scholar]
- Feldmann M., Erb P., Kontiainen S., Todd I., Woody J. N. Comparison of antigen-specific I-region-associated cell interaction factors. Ann N Y Acad Sci. 1979;332:591–604. doi: 10.1111/j.1749-6632.1979.tb47153.x. [DOI] [PubMed] [Google Scholar]
- Kaklamanis E., Pavlatos M. The immunosuppressive effect of mycoplasma infection. I. Effect on the humoral and cellular response. Immunology. 1972 Apr;22(4):695–702. [PMC free article] [PubMed] [Google Scholar]
- Kirchner H., Nicklas W., Giebler D., Keyssner K., Berger R., Storch E. Induction of interferon gamma in mouse spleen cells by culture supernatants of mycoplasma arthritidis. J Interferon Res. 1984 Summer;4(3):389–397. doi: 10.1089/jir.1984.4.389. [DOI] [PubMed] [Google Scholar]
- Lynch D. H., Cole B. C., Bluestone J. A., Hodes R. J. Cross-reactive recognition by antigen-specific, major histocompatibility complex-restricted T cells of a mitogen derived from Mycoplasma arthritidis is clonally expressed and I-E restricted. Eur J Immunol. 1986 Jul;16(7):747–751. doi: 10.1002/eji.1830160706. [DOI] [PubMed] [Google Scholar]
- Matthes M., Schrezenmeier H., Homfeld J., Fleischer S., Malissen B., Kirchner H., Fleischer B. Clonal analysis of human T cell activation by the Mycoplasma arthritidis mitogen (MAS). Eur J Immunol. 1988 Nov;18(11):1733–1737. doi: 10.1002/eji.1830181112. [DOI] [PubMed] [Google Scholar]
- Pierce C. W., Kapp J. A. Regulation of immune responses by suppressor T cells. Tohoku J Exp Med. 1976;5:91–143. [PubMed] [Google Scholar]
- Rich R. R., Pierce C. W. Biological expressions of lymphocyte activation. 3. Suppression of plaque-forming cell responses in vitro by supernatant fluids from concanavalin A-activated spleen cell cultures. J Immunol. 1974 Apr;112(4):1360–1368. [PubMed] [Google Scholar]
- SCHIMKE R. T., BARILE M. F. Arginine breakdown in mammalian cell culture contaminated with pleuropneumonia-like organisms (PPLO). Exp Cell Res. 1963 May;30:593–596. doi: 10.1016/0014-4827(63)90337-9. [DOI] [PubMed] [Google Scholar]
- Simberkoff M. S., Thorbecke G. J., Thomas L. Studies of PPLO infection. V. Inhibition of lymphocyte mitosis and antibody formation by mycoplasmal extracts. J Exp Med. 1969 Jun 1;129(6):1163–1181. doi: 10.1084/jem.129.6.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Taniguchi M., David C. S. Suppressive and enhancing T-cell factors as I-region gene products: properties and the subregion assignment. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):119–127. doi: 10.1101/sqb.1977.041.01.016. [DOI] [PubMed] [Google Scholar]
- Yowell R. L., Cole B. C., Daynes R. A. Utilization of T cell hybridomas to establish that a soluble factor derived from Mycoplasma arthritidis is truly a genetically restricted polyclonal T cell activator. J Immunol. 1983 Aug;131(2):543–545. [PubMed] [Google Scholar]


