Abstract
Women with benign proliferative breast disease are at increased risk of subsequent breast cancer. Estrogens and progesterone exert proliferative effects on mammary epithelium and combined hormone replacement therapy has been associated with increased breast cancer risk. We tested the effect of conjugated equine estrogen plus progestin on risk of benign proliferative breast disease in the Women's Health Initiative (WHI) randomized controlled trial. In the WHI trial of estrogen plus progestin, 16608 postmenopausal women were randomly assigned either to 0.625 mg/d of conjugated equine estrogen plus 2.5 mg/d of medroxyprogesterone acetate or to placebo. Baseline and annual breast exams and mammograms were required. The trial was terminated early (average follow-up, 5.5 years). We identified women who had had a biopsy for benign breast disease and subjected histologic sections from the biopsies to standardized review. Overall, 178 incident cases of benign proliferative breast disease were ascertained in the estrogen plus progestin group and 99 in the placebo group. Use of estrogen plus progestin was associated with a 74% increase in risk of benign proliferative breast disease (hazard ratio 1.74, 95% CI 1.35-2.25). For benign proliferative breast disease without atypia the hazard ratio was 2.00 (95% CI 1.50-2.66), while for atypical hyperplasia it was 0.76 (95% CI 0.38-1.52). Risk varied little by levels of baseline characteristics. The results of this study suggest that use of estrogen plus progestin may increase the risk of benign proliferative breast disease.
Keywords: estrogen, progestin, benign proliferative breast disease
Introduction
Benign proliferative breast disease is an established breast cancer risk factor (1, 2), and experimental and epidemiologic evidence supports the notion that it has malignant potential and represents a lesion on the pathway to invasive breast cancer (2-4). Recently, with the increase in use of mammography and with the emergence of core needle biopsy as a replacement for open surgical biopsy, the threshold for breast biopsy has been lowered, thereby facilitating the diagnosis of benign proliferative breast disease.
In the Women's Health Initiative randomized trial of estrogen plus progestin, there was an increase in breast cancer risk that emerged after several years of use of hormone therapy (5, 6). Given the proliferative effect of estrogens and progesterone on breast epithelial cells (7-9), the effect of combined hormone therapy on breast cancer risk might be mediated, in part, through detrimental effects on breast epithelium resulting in an increase in risk of benign proliferative breast disease. However, the influence of estrogen plus progestin on benign proliferative breast disease has not been reported previously in a randomized clinical trial setting. Therefore, we used the WHI trial to test the effect of estrogen plus progestin on risk of benign proliferative breast disease.
Material and methods
Study population
The WHI estrogen plus progestin trial has been described elsewhere (5). In brief, 16608 postmenopausal women aged 50-79 years at initial screening, with an intact uterus, who were likely to reside in the study area for 3 years, and who provided written informed consent, were enrolled between 1993 and 1998 at 40 clinical centers. Exclusions were based on competing risk, safety (e.g., prior breast cancer), and adherence and retention considerations. Women using postmenopausal hormones at screening were required to have a 3-month washout period before baseline evaluation. All participants were taught breast self-examination (reinforced during the trial). They also had a baseline mammogram and clinical breast examination; abnormal findings suggestive of breast cancer required that breast cancer be ruled out before the participant entered the trial. The WHI and the ancillary study reported here (which included all 40 clinical centers) were approved by institutional review boards at all participating institutions.
Study regimens, random assignment, and blinding
Participants were assigned randomly to receive either one daily tablet containing 0.625 mg of conjugated equine estrogen plus 2.5 mg of medroxyprogesterone acetate (Prempro®, Wyeth Ayerst, Philadelphia, PA) or an identical-looking placebo. Randomization involved use of a randomized permuted block algorithm, stratified by clinic and age. All medication bottles had a unique bar code to allow blinded dispensing.
Data collection
Comprehensive information on breast cancer risk factors was obtained at baseline in the parent trial by interview (for lifetime hormone use) and by self-report (for other covariates) using standardized questionnaires. The variables of interest included age, race/ethnicity, family history of breast cancer, body mass index, prior breast disease, age at menarche, age at first full-term pregnancy, parity, age at menopause, oral contraceptive use, postmenopausal hormone use, and mammographic screening history.
Follow-up
Participants were contacted after 6 weeks to assess symptoms and reinforce adherence, and were assessed for clinical outcomes at 6-monthly clinic visits, at which time their study pills for the next six months were dispensed. At their annual visits, participants were required to have a clinical breast examination and a pelvic examination, and the findings from their required annual mammogram were reviewed; study medications were withheld if the breast exams were not performed, but participants continued to be followed. Study medication was discontinued for several reasons, including use of non-study hormones, and development of breast cancer, endometrial pathology, or deep vein thrombosis. The trial was terminated early because breast cancer risk was increased and a summary measure of the overall balance of risks and benefits (based on monitored outcomes) suggested that risks exceeded benefits (5).
Ascertainment of outcome
The outcome of interest for the present study was histologically-confirmed incident benign proliferative breast disease with or without atypia (see “Histology”). Clinical events including breast cancers and breast biopsies for non-cancerous lesions were initially identified from self-administered questionnaires completed every 6 months. Breast cancers were confirmed by local and central adjudicators who reviewed medical records and pathology reports and who were blinded both to treatment assignment and to symptoms due to study medications. For the present study, women who reported breast biopsies which were free of cancer were identified and clinical centers were sent lists of potentially eligible subjects quarterly. Clinic staff contacted participants to obtain written informed consent to solicit the histologic sections resulting from the biopsies. To investigate the possibility that breast biopsies were missed by using this approach, the charts of 100 randomly selected participants who did not report breast biopsy were reviewed at one center and none was found to have unreported biopsies.
Histology
Hematoxylin and eosin-stained histologic sections were reviewed by the study pathologist (D.L.P.) who was blinded to the randomization assignment. The benign lesions were classified using well-established criteria as non-proliferative lesions, proliferative lesions without atypia (further classified according to whether they were mild, moderate, or florid in extent), or atypical (ductal/lobular) hyperplasia (1, 10, 11).
Statistical analysis
Incidence rates of benign proliferative breast disease in the estrogen plus progestin and placebo groups were compared based on the intention-to-treat principle using time-to-event analyses. The time to benign proliferative breast disease was defined as the number of days from the date of random assignment to the date of the first diagnostic biopsy after random assignment that showed benign proliferative breast disease. For those who did not develop the end-point of interest, follow-up time was censored at the date of last documented contact, diagnosis of breast cancer, mastectomy, death, or July 8, 2002 (the date of termination of the main WHI trial), whichever came first. Women who developed a non-proliferative benign breast lesion continued to be followed up because they remained at risk of developing a subsequent proliferative lesion. Event rates over time were summarized using cumulative hazard plots. The intervention effect was summarized using hazard ratios (HRs) and 95% confidence intervals (CIs) estimated from Cox proportional hazards models (12), with stratification by age, prior breast biopsies, and randomization to the WHI Dietary Modification and Calcium/Vitamin D supplementation trials (13). Stratification was time-dependent in the case of the Calcium/Vitamin D supplementation trial. The primary test of the association between estrogen plus progestin and risk of benign proliferative breast disease was that estimated from the model with the stratification variables specified above. The association was then further evaluated in various sensitivity analyses that were designed to assess the robustness of the main result. Specifically, we assessed the impact on the HR for the estrogen plus progestin effect of: 1) an “as treated” analysis (in which events were attributed to actual hormone use during the trial), 2) separate analyses that excluded (a) cases that arose during the first year of follow-up, (b) women who had used hormone therapy prior to trial commencement, (c) women who had had a breast biopsy prior to enrollment, and (d) women who were unblinded and reassigned from unopposed estrogen to estrogen plus progestin, 3) separate analyses that adjusted for (a) cumulative duration of hormone therapy prior to trial commencement, (b) frequency of protocol-mandated annual mammograms, and (c) frequency of clinical breast exams (in the latter two analyses, frequency of mammograms and clinical breast exams were treated as time-dependent covariates in the Cox models), and 4) an analysis that stratified by mammogram results. Interaction was investigated by including product terms between treatment assignment and indicator variables for the subsets of interest in Cox proportional hazards models stratified by age, prior breast biopsies, and randomization to the Dietary Modification and Calcium/vitamin D trials, and was assessed by testing the equality of the product term coefficients. The proportional hazards assumption, which was tested by fitting models containing a product term between the intervention and follow-up time and assessing the coefficient of the product term for statistical significance, was shown not to be violated. Annualized event rates were calculated for comparisons of absolute disease rates. All statistical tests were two-sided and results were considered statistically significant when two-sided p-values were ≤ 0.05.
Results
The randomization groups differed little at baseline with respect to age, ethnicity, breast cancer risk factors, participation in other WHI trials, and intake of energy, selected nutrients, and vitamins (Table 1).
Table 1.
Baseline Characteristics of Participants in the Women's Health Initiative Estrogen plus Progestin trial*
| No. (%) of Participants | ||
|---|---|---|
| Estrogen plus progestin
(n=8506) |
Placebo
(n=8102) |
|
| Age, years† | 63.2 (7.1) | 63.3 (7.1) |
| Race/ethnicity(%) | ||
| White | 7141(83.95) | 6805 (83.99) |
| Black | 548 (6.46) | 574 (7.10) |
| Hispanic | 471(5.54) | 415 (5.12) |
| American Indian | 25 (0.29) | 30 (0.37) |
| Asian/Pacific Islander | 194 (2.29) | 169 (2.09) |
| Other | 109 (1.28) | 87 (1.07) |
| Unknown | 18 (0.21) | 22 (0.27) |
| Family history of breast cancer (%)‡ | 1286 (15.12) | 1175 (14.50) |
| Gail model 5-y risk>1.75 (%) | 2841 (33.40) | 2682 (33.10) |
| Body mass index†§ | 28.5 (6.3) | 28.6 (6.5) |
| Prior breast biopsy(%) | ||
| No | 6340 (74.54) | 6278 (77.49) |
| 1 Biopsy | 956 (11.24) | 973 (12.01) |
| ≥2 Biopsies | 291 (3.42) | 288 (3.55) |
| Unknown | 919 (10.80) | 563 (6.95) |
| Age (years) at menarche (%) | ||
| ≤ 10 | 502 (5.90) | 525 (6.48) |
| 11 – 14 | 7040 (82.77) | 6670 (82.33) |
| ≥ 15 | 943 (11.09) | 870 (10.74) |
| Unknown | 21 (0.25) | 37 (0.46) |
| Age (years) at first full-term pregnancy (%) | ||
| Never had term pregnancy | 201 (2.36) | 199 (2.46) |
| < 20 | 1122 (13.19) | 1114 (13.75) |
| 20-29 | 4985 (58.61) | 4685 (57.83) |
| ≥ 30 | 723 (8.50)) | 621 (7.66) |
| Unknown | 1475 (17.34) | 1483 (18.30) |
| Parity (%) | ||
| Never | 856 (10.06) | 832 (10.27) |
| 1 | 690 (8.11) | 661 (8.16) |
| 2 | 1908 (22.43) | 1708 (21.08) |
| 3 | 2020 (23.75) | 1952 (24.09) |
| 4+ | 2991 (35.16) | 2912 (35.94) |
| Unknown | 41(0.48) | 37 (0.46) |
| Age (years) at natural menopause† | 50 (4.8) | 50 (4.7) |
| Oral contraceptive use | ||
| Ever used (%) | 3695 (43.44) | 3447 (42.55) |
| Duration of use, y† | 5.5 (5.3) | 5.7 (5.5) |
| Postmenopausal hormone use | ||
| Estrogen alone | ||
| Ever used (%) | 903 (10.62) | 865 (10.68) |
| Duration of use, y† | 3.5 (4.6) | 3.5 (5.5) |
| Estrogen plus progestin | ||
| Ever used (%) | 1516 (17.82) | 1396 (17.23) |
| Duration of use, y† | 3.9 (4.2) | 3.7 (4.2) |
| Mammography screening within 2y (%) | 5801 (68.02) | 5585 (68.93) |
| Enrolment in WHI Dietary Modification trial (%) | ||
| No | 6077 (71.44) | 5873 (72.49) |
| Dietary Modification | 972 (11.43) | 925 (11.42) |
| Placebo | 1457 (17.13) | 1304 (16.09) |
| Enrolment in WHI calcium plus vitamin D supplementation trial (%) | ||
| No | 3463 (40.71) | 3232 (39.89) |
| Calcium plus vitamin D group | 2508 (29.49) | 2475 (30.55) |
| Control group | 2535 (29.50) | 2395 (29.56) |
| Total daily energy intake, kcal† | 1663.8 (750.8) | 1657.8 (752.2) |
| Total daily fat intake, g† | 63.2 (37.6) | 62.8 (38.1) |
| Total daily calcium intake (supplements plus diet), mg† | 1110.0 (683.5) | 1114.3 (695.7) |
| Total daily vitamin D intake (supplements plus diet), mcg† | 8.8 (6.9) | 8.7 (6.8) |
Percentages may not sum to 100% because of rounding error.
Mean (standard deviation)
First-degree female relative
Calculated as weight in kilograms divided by the square of height in meters.
Data on follow-up, adherence, and unblinding were reported elsewhere (5). In brief, at the termination of the trial, vital status was known for 96.5% of subjects; 42% of women in the intervention group and 38% of those in the placebo group stopped taking study drugs for at least some time; and clinic gynecologists were unblinded to treatment assignment for 3444 (40.5%) women in the intervention group and 548 (6.8%) in the placebo group.
During follow-up (average duration, 5.5 years), we identified 982 potentially eligible biopsies that had been performed for benign breast disease. The eligibility of 11 biopsies could not be determined due to lack of consent, hospital refusal, and other reasons. Of the 971 biopsies confirmed to be eligible, consent was obtained for review of 969 histologic sections, of which 952 were obtained. Of the sections reviewed, 285 were from biopsies that occurred outside the period of the trial and 42 had no breast tissue. The remaining 625 sections were from 585 women. Of these women, 8 were censored (so that the corresponding section was excluded from consideration), one had no pathological diagnosis, 299 had a non-proliferative lesion (this category included cysts, fibrosis, apocrine metaplasia, fibroadenoma, radial scar, and micropapillomas), and 277 had an incident benign proliferative lesion.
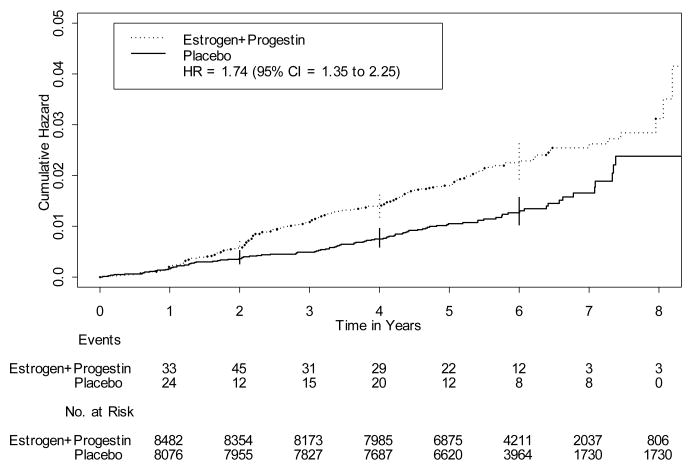
Overall, 178 cases of benign proliferative breast disease occurred in the intervention group and 99 occurred in the placebo group. Use of estrogen plus progestin was associated with a 74% increase in the risk of benign proliferative breast disease (HR = 1.74, 95% CI = 1.35 to 2.25) (Table 2). When examined by histologic sub-category, risk of benign proliferative breast disease was increased 2-fold for those who developed benign disease without atypia (HR = 2.00, 95% CI = 1.50 to 2.66) but was not increased for those who developed atypical hyperplasia (HR = 0.76, 95% CI = 0.38 to 1.52). However, the latter analysis was based on only 16 cases in the estrogen plus progestin group and 20 cases in the placebo group, and when these outcomes were combined with moderately extensive or florid proliferative disease without atypia, the hazard ratio was 1.43 (95% CI = 1.04 to 1.96). The Kaplan-Meier estimates of the overall cumulative hazard of benign proliferative breast disease show that the difference between the estrogen plus progestin and placebo groups emerged during the second year of follow-up and persisted through to the end of the follow-up period (Fig. 1). Risk of non-proliferative benign breast disease was increased 50% in association with estrogen plus progestin (HR = 1.53, 95% CI = 1.19 to 1.97).
Table 2.
Risk of Benign Proliferative Breast Disease in Association with Estrogen plus Progestin, Overall and by the Presence/Absence of Atypia
| No. of Cases
(Annualized %) |
||||
|---|---|---|---|---|
| Benign proliferative breast disease | Estrogen plus progestin
(N=8506) |
Placebo
(N=8102) |
Hazard Ratio
(95% CI)* |
P-Value† |
| All cases | 178 (0.38) | 99 (0.22) | 1.74 (1.35, 2.25) | <0.0001 |
| Benign proliferative breast disease without atypia | 162 (0.34) | 79 (0.18) | 2.00 (1.50, 2.66) | <0.0001 |
| Atypical hyperplasia | 16 (0.03) | 20 (0.05) | 0.76 (0.38, 1.52) | 0.44 |
Abbreviation: CI, confidence interval.
Proportional hazards model stratified by age, prior breast disease, and treatment assignment in the DM and Calcium plus Vitamin D supplement trials.
Figure 1.
Kaplan-Meier estimates of the cumulative hazard of benign proliferative breast disease in association with estrogen plus progestin, as compared with placebo (vertical lines are 95% confidence intervals (CI) at selected time points).
Risk of benign proliferative breast disease varied significantly by Gail model 5-year risk of breast cancer (pheterogeneity=0.01), being increased more than 2-fold in those in the upper and lower tertile levels of baseline Gail model 5-year risk of breast cancer, but unaltered in those in the middle tertile level (data not shown). For women who had used hormone therapy prior to randomization, the HR (95%CI) for the association between estrogen plus progestin and risk of benign proliferative breast disease was 2.58 (1.50 to 4.44), whereas for those who had not used hormone therapy prior to randomization it was 1.53 (1.13 to 2.06); the test for heterogeneity in the estimates of effect between these strata was not statistically significant (p=0.099). Risk of benign proliferative breast disease did not vary by levels of any of the other baseline demographic or dietary variables, or by levels of breast cancer risk factors (data not shown).
The overall estrogen plus progestin effect was robust to various sensitivity analyses. In an “as treated” analysis, in which events were attributed to actual hormone use (allowing for a 6-month lag), the HR for all forms of benign proliferative breast disease combined was 2.15 (95% CI = 1.58 to 2.94). The estrogen plus progestin effect was essentially unchanged by exclusion of the first year of follow-up (HR = 1.80, 95% CI = 1.37 to 2.38), but was reduced by about 10% by exclusion of women who had previously used hormone replacement therapy (HR = 1.52, 85% CI = 1.13 to 2.06) and increased slightly after exclusion of women with a prior breast biopsy (HR = 1.93, 95% CI = 1.17 to 3.20). Early in the trial, participants with a uterus were randomized either to estrogen alone or to estrogen plus progestin. However, after use of unopposed estrogen in those with a uterus was shown to be contraindicated (14), the 331 women who had been assigned to estrogen alone were unblinded and reassigned to estrogen plus progestin. After exclusion of these women, the risk of benign proliferative breast disease was unchanged (HR = 1.74, 95% CI = 1.34 to 2.27). The estrogen plus progestin effect was unchanged by adjustment for cumulative duration of use of hormone replacement therapy prior to randomization (HR = 1.74, 95% CI = 1.34 to 2.26),
Participants had protocol-mandated annual clinical breast exams and mammograms. On average, about 88% of both the estrogen plus progestin group and the placebo group had these exams annually (6). The effect of estrogen plus progestin was essentially unchanged after adjustment for the frequency of mammograms (HR = 1.73, 95% CI = 1.34 to 2.24) or clinical breast exams (HR = 1.75, 95% CI = 1.35 to 2.26). The effect of the intervention was reduced by about 10% after stratification by whether or not subjects had had at least one abnormal mammogram during follow up (HR = 1.55, 95% CI = 1.17 to 2.06), but did not differ significantly between strata defined by those who had at least one abnormal mammogram (HR = 1.20, 95% CI = 0.73 to 1.99) and those who did not have an abnormal mammogram (HR = 1.68, 95% CI = 1.20 to 2.34)(p for heterogeneity=0.28).
Discussion
Benign proliferative breast disease is important because it is associated with increased risk of subsequent breast cancer (1). In the randomized, double-blind, placebo-controlled trial reported here, use of 0.625 mg/day of conjugated equine estrogen and 2.5mg/day of medroxyprogesterone acetate was associated with a 74% increase in risk of benign proliferative breast disease. An “as treated” analysis that took into account actual hormone use suggested that the estrogen plus progestin effect might be slightly stronger. Although the increase in risk was confined essentially to those who developed proliferative lesions without atypia, only a small number of cases of atypical hyperplasia were observed during follow-up. Risk varied significantly by Gail model score, but not in a score-dependent manner, and given that many subgroup analyses were performed, this may represent a chance finding.
We are not aware of any previous randomized controlled trials that have examined the effect of estrogen plus progestin on the risk of benign proliferative breast disease (or of benign breast disease in general). Although use of exogenous hormones has been associated with increased risk of benign breast disease in several observational epidemiologic studies, these findings relate largely to use of conjugated estrogens alone (15). With respect to studies that have focused specifically on benign proliferative breast disease as the outcome of interest, two case-control studies showed no association (16, 17), whereas the one prospective study to date observed an increase in risk in association with relatively long-term use of exogenous hormones (primarily conjugated estrogens) (RR>8 years vs. never =1.70, 95% CI=1.06 to 2.72) (15). Of these latter three studies, the one study that provided an estimate for risk in association with combined hormone therapy showed no association (odds ratio = 1.02, 95% CI =0.75 to 1.39)(17).
In addition to these findings, a recent report from the other WHI hormone trial showed that administration of conjugated equine estrogen was associated with a two-fold increase in risk of benign proliferative breast disease (18). Although the magnitude of this effect exceeded that observed in the study reported here, the confidence intervals for the two estimates overlap substantially. Furthermore, the differences between the two study populations in terms of their baseline characteristics, event rates, length of intervention, and follow-up time dictate that caution be used in making direct comparison of these results (19). Nevertheless, the observation that both interventions increased the risk of benign proliferative breast disease whereas only estrogen plus progestin increased the risk of breast cancer (indeed, conjugated equine estrogen alone was associated with a statistically non-significant decrease in risk of breast cancer (hazard ratio=0.77, 95%CI=0.59 to 1.01)) is consistent with a possible role of progestins in breast cancer development.
Mammographic (breast) density refers to the relative amount and configuration of breast tissue as it appears on a mammogram, with fat appearing dark (radiolucent) and epithelial and stromal tissues appearing light (radiodense)(20). It has been postulated that variation between individuals in the extent of mammographic density is likely to reflect inter-individual variation in the number of epithelial and stromal cells in the breast, and therefore variation in the number of cells that are susceptible to the effects of mutagens (21). Women with dense tissue in at least 75% of the breast have been shown to have a 4-to 6-fold increase in the risk of subsequent breast cancer (compared to the risk for those with low density)(22). A relatively high mammographic density has also been associated with a nine-fold increase in risk of atypical hyperplasia and a twelve-fold increase in risk of hyperplasia without atypia (23). Mammographic density can be modified (24) and factors that modulate density might do so by changing the size of the population of cells (epithelial and stromal) that are potential targets for events that induce mutations. In this regard, combined estrogen-progestin preparations have been shown to increase mammographic density (25, 26) and abnormal mammograms (6), and we propose that these changes result from effects on mammary epithelial (and stromal) cells that simultaneously increase the occurrence of the histologic abnormalities that are the focus of the present report. Indeed, estrogens and progestins increase mammary epithelial proliferation rates (27), and continuous combined hormone replacement therapy preparations inhibit the sloughing of mammary epithelium that normally occurs after progesterone withdrawal with cyclic regimens (28). It is of note that breast cancers have been reported to occur predominantly in mammographically dense areas of the breast (29).
The strengths of this study include the large study population; the randomized, double blind, placebo-controlled study design; comprehensive breast cancer risk factor assessment; annual mammography and breast exams; and central pathology review, with successful retrieval of histologic sections from a high proportion of the biopsies reported.
As discussed elsewhere (5), this trial had several limitations, including the fact that it tested only one drug regimen; could not distinguish between the effects of conjugated equine estrogen and progestin; may have underestimated effects due to the higher rate of discontinuation in the active treatment arm and crossover to active treatment in the placebo group; and had decreased precision of effect estimates due to the fact that it was stopped early. With respect to the present study, potentially there are two additional limitations. Firstly, it is possible that there might have been differential ascertainment of benign proliferative breast disease in the two randomization groups. Specifically, it is possible that symptoms and signs induced by the use of estrogen plus progestin might have increased the likelihood of detection and subsequent biopsy of breast lesions in the intervention group resulting in more complete ascertainment of the outcome in this group than in the placebo group. Indeed, the clinic gynecologists of women in the intervention group were more likely to be unblinded to treatment assignment, and women in the intervention group had more abnormal mammograms and breast biopsies than those in the placebo group (6, 30). However, all participants were taught breast self-examination, and they had annual clinical breast exams and mammograms. Furthermore, compliance with the annual exams was extremely high and essentially the same in the two randomization groups, and adjustment for the frequency of mammograms and clinical breast exams did not change the corresponding estimates of effect for estrogen plus progestin substantially. The effect of estrogen plus progestin was somewhat weaker in those with at least one abnormal mammogram, but given the strong relationship between mammographic density and benign proliferative breast disease it is difficult to disentangle the effect of the intervention on these two outcomes. Nevertheless, the possibility of differential ascertainment cannot be excluded given that estrogen plus progestin was associated with increased risk of both proliferative and non-proliferative forms of benign breast disease. (Given that (appropriately) breast biopsies were not performed on all participants, it is conceivable that there was some under-ascertainment of the outcome in both groups.) Secondly, the outcome (benign proliferative breast disease) might have been misclassified. However, any such misclassification is likely to have been non-differential and therefore to have biased the effect estimates towards the null (31).
In conclusion, although differential ascertainment of outcome in the intervention and placebo groups cannot be excluded as a potential explanation for the findings reported here, the results of this study raise the possibility that use of estrogen plus progestin increases the risk of benign proliferative breast disease.
Acknowledgments
The authors thank the WHI investigators and staff for their outstanding dedication and commitment. A list of key investigators involved in this research follows. A full listing of WHI investigators can be found at the following website: http://www.whi.org.
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Elizabeth Nabel, Jacques Rossouw, Shari Ludlam, Linda Pottern, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg, Ruth E. Patterson, Anne McTiernan; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings.
Clinical Centers: (Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Jennifer Hays; (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn Manson; (Brown University, Providence, RI) Annlouise R. Assaf; (Emory University, Atlanta, GA) Lawrence Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Judith Hsia; (Los Angeles Biomedical Research Institute at Harbor- UCLA Medical Center, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Evelyn Whitlock; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush Medical Center, Chicago, IL) Henry Black; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis; (University of Arizona, Tucson/Phoenix, AZ) Tamsen Bassford; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Howard Judd; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O'Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Denise Bonds; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Susan Hendrix.
Grant support: The WHI program is funded by the National Heart, Lung and Blood Institute, U.S. Department of Health and Human Services. The present study was supported by National Cancer Institute RO1 CA 077290-07 (Thomas E. Rohan).
Footnotes
Financial disclosures: Dr. Smoller has received an honorarium from the Gerson Lehrman Council of HealthCare Advisors. Dr. McTiernan has consulted for Novartis, Proctor&Gamble, and Zymogenetics (her husband owns stock in and consults for Merck). Dr. Page has advised attorneys on HRT and has been paid for expert testimony.
Role of the sponsor: The WHI data were collected by the participating institutions. The WHI Paper and Proposals committee and NHLBI reviewed and approved the report before submission.
Disclaimer: The findings and conclusions in this report are those of the authors and not necessarily those of the agency.
References
- 1.Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229–37. doi: 10.1056/NEJMoa044383. [DOI] [PubMed] [Google Scholar]
- 2.Santen RJ, Mansel R. Benign breast disorders. N Engl J Med. 2005;353:275–85. doi: 10.1056/NEJMra035692. [DOI] [PubMed] [Google Scholar]
- 3.Wang DY, Fentiman I. Epidemiology and endocrinology of benign breast disease. Breast Cancer Res Treat. 1985;6:5–36. doi: 10.1007/BF01806008. [DOI] [PubMed] [Google Scholar]
- 4.Lakhani SR. The transition from hyperplasia to invasive carcinoma of the breast. J Pathol. 1999;187:272–8. doi: 10.1002/(SICI)1096-9896(199902)187:3<272::AID-PATH265>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 6.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial. JAMA. 2003;289:3243–53. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 7.Yager JD, Davidson NE. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–82. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 8.Russo J, Russo IH. The role of estrogen in the initiation of breast cancer. J Steroid Biochem Mol Biol. 2006;102:89–96. doi: 10.1016/j.jsbmb.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conner P. Breast response to menopausal hormone therapy--aspects on proliferation, apoptosis and mammographic density. Ann Med. 2007;39:28–41. doi: 10.1080/07853890601039842. [DOI] [PubMed] [Google Scholar]
- 10.Page DL, Schuyler PA, Dupont WD, et al. Atypical lobular hyperplasia as a unilateral predictor of breast cancer risk: a retrospective cohort study. Lancet. 2003;361:125–9. doi: 10.1016/S0140-6736(03)12230-1. [DOI] [PubMed] [Google Scholar]
- 11.Page DL, Rogers LW. Combined histologic and cytologic criteria for the diagnosis of mammary atypical ductal hyperplasia. Hum Pathol. 1992;23:1095–7. doi: 10.1016/0046-8177(92)90026-y. [DOI] [PubMed] [Google Scholar]
- 12.Cox DR. Regression models and life tables (with discussion) J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 13.Hays J, Hunt JR, Hubbell FA, et al. The Women's Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 14.The Writing Group for the PEPI Trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA. 1996;275:370–5. doi: 10.1001/jama.1996.03530290040035. [DOI] [PubMed] [Google Scholar]
- 15.Rohan TE, Miller AB. Hormone replacement therapy and risk of benign proliferative epithelial disorders of the breast. Eur J Cancer Prev. 1999;8:123–30. doi: 10.1097/00008469-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Berkowitz GS, Kelsey JL, LiVolsi VA, et al. Exogenous hormone use and fibrocystic breast disease by histopathologic component. Int J Cancer. 1984;34:443–9. doi: 10.1002/ijc.2910340403. [DOI] [PubMed] [Google Scholar]
- 17.Friedenreich C, Bryant H, Alexander F, et al. Risk factors for benign proliferative breast disease. Int J Epidemiol. 2000;29:637–44. doi: 10.1093/ije/29.4.637. [DOI] [PubMed] [Google Scholar]
- 18.Rohan TE, Negassa A, Chlebowski RT, et al. Conjugated equine estrogen and risk of benign proliferative breast disease: a randomized, controlled trial. J Natl Cancer Inst. 2008;100:563–71. doi: 10.1093/jnci/djn075. [DOI] [PubMed] [Google Scholar]
- 19.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 20.Boyd NF, Lockwood GA, Byng JW, Tritchler DL, Yaffe MJ. Mammographic densities and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 1998;7:1133–44. [PubMed] [Google Scholar]
- 21.Boyd NF, Lockwood GA, Martin LJ, et al. Mammographic density as a marker of susceptibility to breast cancer: a hypothesis. IARC Sci Publ. 2001;154:163–9. [PubMed] [Google Scholar]
- 22.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356:227–36. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 23.Boyd NF, Jensen HM, Cooke G, Han HL. Relationship between mammographic and histological risk factors for breast cancer. J Natl Cancer Inst. 1992;84:1170–9. doi: 10.1093/jnci/84.15.1170. [DOI] [PubMed] [Google Scholar]
- 24.Boyd NF, Greenberg C, Lockwood G, et al. Effects at two years of a low-fat, high-carbohydrate diet on radiologic features of the breast: results from a randomized trial. Canadian Diet and Breast Cancer Prevention Study Group. J Natl Cancer Inst. 1997;89:488–96. doi: 10.1093/jnci/89.7.488. [DOI] [PubMed] [Google Scholar]
- 25.Greendale GA, Reboussin BA, Sie A, et al. Effects of estrogen and estrogen-progestin on mammographic parenchymal density. Postmenopausal Estrogen/Progestin Interventions (PEPI) Investigators. Ann Intern Med. 1999;130:262–9. doi: 10.7326/0003-4819-130-4_part_1-199902160-00003. [DOI] [PubMed] [Google Scholar]
- 26.McTiernan A, Martin CF, Peck JD, et al. Estrogen-plus-progestin use and mammographic density in postmenopausal women: women's health initiative randomized trial. J Natl Cancer Inst. 2005;97:1366–76. doi: 10.1093/jnci/dji279. [DOI] [PubMed] [Google Scholar]
- 27.Haslam SZ, Osuch JR, Raafat AM, Hofseth LJ. Postmenopausal hormone replacement therapy: effects on normal mammary gland in humans and in a mouse postmenopausal model. J Mammary Gland Biol Neoplasia. 2002;7:93–105. doi: 10.1023/a:1015726608146. [DOI] [PubMed] [Google Scholar]
- 28.Campagnoli C, Clavel-Chapelon F, Kaaks R, Peris C, Berrino F. Progestins and progesterone in hormone replacement therapy and the risk of breast cancer. J Steroid Biochem Mol Biol. 2005;96:95–108. doi: 10.1016/j.jsbmb.2005.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ursin G, Hovanessian-Larsen L, Parisky YR, Pike MC, Wu AH. Greatly increased occurrence of breast cancers in areas of mammographically dense tissue. Breast Cancer Res. 2005;7:R605–8. doi: 10.1186/bcr1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chlebowski R, Anderson G, Pettinger M, et al. Estrogen plus progestin and breast cancer detection by means of mammography and breast biopsy. Arch Intern Med. 2008;168:370–7. doi: 10.1001/archinternmed.2007.123. [DOI] [PubMed] [Google Scholar]
- 31.Rothman KJ. Modern Epidemiology. Boston: Little, Brown & Company; 1986. p. 106. [Google Scholar]