SUMMARY
In mammals, the supporting cell lineage in an embryonic gonad communicates the sex-determining decision to various sexually dimorphic cell types in the developing embryo, including the germ cells. However the molecular nature of the sex-determining signals that pass from the supporting cells to the germ cells is not well understood. We have identified a conserved transmembrane protein, Sdmg1, due to its male-specific expression in mouse embryonic gonads. Sdmg1 is expressed in the Sertoli cells of embryonic testes from 12.5 dpc, and in granulosa cells of growing follicles in adult ovaries. In Sertoli cells, Sdmg1 is localised to endosomes, and knock-down of Sdmg1 in Sertoli cell lines causes mis-localisation of the secretory SNARE Stx2 and defects in membrane trafficking. Upregulation of Sdmg1 appears to be part of a larger programme of changes to membrane trafficking pathways in embryonic Sertoli cells, and perturbing secretion in male embryonic gonads in organ culture causes male-to-female germ cell sex reversal. These data suggest that changes that occur in the cell biology of embryonic Sertoli cells may facilitate the communication of male sex-determining decisions to the germ cells during embryonic development.
Keywords: mouse, primordial germ cells, Sertoli cell, membrane trafficking, Sdmg1
INTRODUCTION
In mice, the embryo's decision to develop as male or female depends on its sex chromosome constitution: XY embryos become male, and XX embryos become female. This decision is triggered by the presence or absence of the dominant male-determining Sry gene located on the Y chromosome (Koopman et al., 1991; Lovell-Badge and Robertson, 1990). Sry is expressed in the supporting cells of the developing gonad, a sexually dimorphic cell lineage that can differentiate into Sertoli cells in the testis or granulosa cells in the ovary (Albrecht and Eicher, 2001; Wilhelm et al., 2005). Sry expression in the supporting cells induces their differentiation into male Sertoli cells, which then induce other cell types in the gonad to develop as male (Palmer and Burgoyne, 1991). This results in the gonad differentiating into a testis, which is then thought to masculinise the rest of the embryo (Jost, 1947). As the direct action of Sry is restricted to the supporting cell lineage during embryogenesis (Palmer and Burgoyne, 1991), the nascent Sertoli cells must communicate their male sex-determining decision to various additional cell types in the developing embryo (Ross and Capel, 2005).
One of the cell lineages with which the nascent Sertoli cells must communicate is the germline. In male embryos, germ cells respond to the male gonadal environment around 12.5 days post coitum (dpc), committing them to differentiate along the male spermatogenic pathway, and inhibiting oogenesis and the initiation of meiosis (Adams and McLaren, 2002; McLaren and Southee, 1997). However, the molecular nature of the germ cell sex-determining signals is not currently understood. Sexually dimorphic development of the germ cells could be brought about by a meiosis-inducing substance in the embryonic ovary, a meiosis-preventing substance in the embryonic testis, or both (McLaren, 1984). Studies demonstrating that germ cells can initiate meiosis in a variety of ectopic locations and culture conditions (Chuma and Nakatsuji, 2001; Farini et al., 2005; McLaren and Southee, 1997; Zamboni and Upadhyay, 1983) suggest that should a meiosis-inducing substance exist, its expression cannot be restricted to the embryonic ovary. In contrast, little is known about whether a male meiosis-preventing substance exists, or what its molecular identity might be.
It has recently been proposed that retinoic acid produced by the mesonephros or adrenal gland diffuses into the embryonic gonad where it acts as a meiosis-inducing substance in the embryonic ovary, but is metabolised by the Sertoli cell-derived Cyp26b1 enzyme to prevent meiosis in the developing testis (Bowles et al., 2006; Koubova et al., 2006). However, the observation that ectopic germ cells present in the mesonephros of male embryos, which contains abundant levels of retinoic acid (Bowles et al., 2006), do not usually initiate meiosis (McLaren, 1984) is not consistent with this model. Thus additional signalling molecules are required to account for the differences in germ cell behaviour between male and female embryos.
In this study we characterise a novel conserved transmembrane protein that is expressed in embryonic Sertoli cells at the time of germ cell masculinisation and is required for normal membrane trafficking in Sertoli cell lines. We also describe changes that occur in the secretory pathway in embryonic Sertoli cells, and demonstrate that perturbing secretion in male embryonic gonads induces male-to-female germ cell sex reversal.
MATERIALS AND METHODS
Mice
Outbred MF1 or CD1 mice were naturally mated, with noon on the day the vaginal plug was found termed 0.5 dpc. Embryos older than 12.5 dpc were sexed by gonad morphology, 11.5 dpc embryos were sexed by PCR for Ube1X and Ube1Y (Chuma and Nakatsuji, 2001).
Molecular Biology
Standard molecular biology manipulations were performed essentially as described (Sambrook and Russell, 2001). RNA was prepared using TRI Reagent (Sigma-Aldrich, Gillingham, UK), and oligo dT-primed cDNA synthesised using Superscript III (Invitrogen, Paisley, UK). Primers for Sdmg1 were 5′-AGTGAATGACCAGCCAGGCTGCC-3′ and 5′-CCCCTACAGGTCCTCTGAGGGAATC-3′. Primers for Gapdh were 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′. Transmembrane helix predictions were performed using TMHMM (Krogh et al., 2001).
In Situ Hybridisation
Digoxigenin-labelled RNA probes were generated against nucleotides 26 – 531 of Sdmg1 by in vitro transcription (Roche Applied Science, Burgess Hill, UK). In situ hybridisation on 7 μm wax sections of Bouin's-fixed tissue was performed as described (Meehan et al., 2000).
Immunostaining
For immunofluorescence on cultured cells, cells were grown on coverslips then fixed with 3.7% formaldehyde in PBS. For cryosections, tissue was fixed with 3.7% formaldehyde in PBS, embedded in OCT compound (VWR, Lutterworth, UK) and cryosectioned at 10 μm. After fixation, samples were blocked (PBS, 1-10% serum, 0.1% Tween-20), and incubated with primary then secondary antibodies diluted in blocking solution. Images were acquired on a MRC1024 Confocal Microscope (Bio-Rad, Hercules, California, USA), or Axioplan II fluorescence microscope (Carl Zeiss, Welwyn Garden City, UK) equipped with a Coolsnap digital camera (Photometrics, Tucson, Arizona, USA). Preimplantation embryos were fixed with 3.7% formaldehyde in PBS and processed as whole mounts (Arney et al., 2002).
For immunohistochemistry on 7 μm wax sections, tissue was fixed with Bouin's solution (Sigma-Aldrich) at room temperature or with 4% paraformaldehyde in PBS at 4°C. Antigen retrieval was performed by boiling for 20 minutes in 0.1 M citrate buffer pH 6 in a microwave oven. Sections were then blocked, incubated with primary antibodies, and bound antibodies visualised using an Envision HRP-linked detection kit (Dako, Ely, UK) followed by counterstaining with haematoxylin.
Antibodies
Amino acids 352-449 of Sdmg1 were fused to a glutathione-S-transferase (GST) affinity tag (GE Healthcare, Little Chalfont, UK) and the fusion protein expressed in E. coli, purified, and used to raise antibodies in rabbits (Abcam, Cambridge, UK). Serum was cross-adsorbed to GST coupled to Affigel (Bio-Rad), then anti-Sdmg1 antibodies were affinity-purified using Affigel-GST-Sdmg1(352-449), and eluted with 0.1 M glycine pH 2.1. Sources and dilutions of primary antibodies are listed in Supplementary Table S1. Secondary antibodies were used as directed by the suppliers (Invitrogen). DNA was stained with 2 μM TOTO-3 (Invitrogen) or 2 μg/mL DAPI.
Topology of Sdmg1
The Sdmg1 open reading frame was cloned into pEYFP-N1 (Clontech) to fuse YFP to the C-terminus of Sdmg1 via a GAGADPPVAT polypeptide linker, and a haemagglutinin (HA) epitope (amino acids MVYPYDVPDYAEF) was then fused to the N-terminus. NIH3T3 cells were transiently transfected with pHA-Sdmg1-YFP using Lipofectamine 2000 (Invitrogen). Immunofluorescence on non-permeabilised cells was performed by incubating coverslips with PBS containing 10% goat serum and primary antibodies at 4°C for 1 hour, followed by fixation, blocking and secondary antibody incubation as described above.
Electron Microscopy and Immuno-Electron Microscopy
For electron microscopy (EM), 13.5 dpc embryonic gonads were fixed with 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M cacodylate buffer pH 7.2 at 4°C, post-fixed in 1% osmium tetroxide, stained with 0.5% uranyl acetate and embedded in Araldite CY212 resin. 70 nm ultrathin sections were post-stained with uranyl acetate and lead citrate.
For immunoEM, 13.5 dpc embryonic gonads or differentiating SK11 Sertoli cells were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer pH 7.2, embedded in 10% gelatin, and infused with 1.7 M sucrose/15% polyvinyl pyrrolidone. 80 nm ultrathin frozen sections were collected with 1% methylcellulose in 1.65 M sucrose and labeled with 10 μg/mL anti-Sdmg1antibodies, followed by 10 nm gold-protein A (Slot and Geuze, 1983).
Generation of Knock-Down Cell Lines
Complementary oligonucleotides to generate shRNA against nucleotides 1368-1388 of Sdmg1, or against GFP (nucleotides 1321-1341 of pEGFP-N1, accession number U55762) were cloned into pSilencer-1.0-U6 (Ambion). A neomycin expression cassette (Zheng et al., 1999) was inserted into the pSilencer-shRNA constructs. Complementary oligonucleotides to generate miRNA against nucleotides 428-448 of Sdmg1 were cloned into pcDNA6.2-GW/EmGFP-miR (Invitrogen). The resulting plasmids, along with the pcDNA6.2-GW/EmGFP-miR negative control plasmid (Invitrogen), were linearised and transfected into SK11 Sertoli cells using Lipofectamine 2000. Stably transfected cells were selected with blasticidin or Geneticin (Invitrogen) and independent clones isolated by limiting dilution.
Urogenital Ridge Cultures and Aggregations
Urogenital ridges were cultured on agar blocks as described (McLaren and Southee, 1997). Brefeldin A (Sigma-Aldrich) was used at 1 μg/ml; retinoic acid (Sigma Aldrich) at 0.7 μM; ketoconazole (Sigma-Aldrich) at 0.7 μM or 40μM; and control cultures used equivalent volumes of solvent. Urogenital ridges were cultured for 4 or 6 days then fixed with Bouin's solution and analysed by haematoxylin and eosin staining of wax sections, or by immunohistochemistry for Amh, or Mvh and Sycp3 on wax sections.
For aggregations, 11.5 dpc female urogenital ridges and SK11 cell lines were trypsinised to single cell suspensions. 4 × 105 urogenital ridge cells were mixed with 1 × 105 SK11 cells, then aggregated and cultured on agar blocks as described (McLaren and Southee, 1997). Aggregates were cultured for 4 days then fixed in Bouin's solution and analysed by histology.
RESULTS
Identification and Cloning of Sdmg1
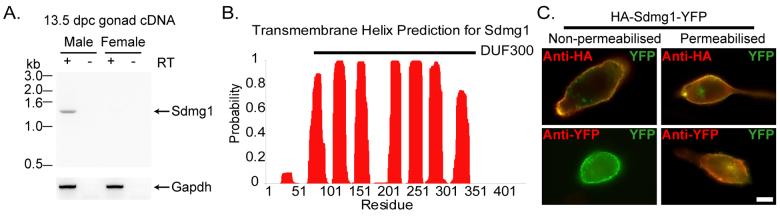
In order to investigate the molecular basis of germ cell sex determination we sought to identify genes that are differentially expressed between male and female gonads during germ cell sex determination (Adams and McLaren, 2002). During a molecular screen for sex-specific genes expressed by germ cells at 12.5 dpc we identified a 241 bp male-specific cDNA fragment by representational difference analysis (Adams and McLaren, 2002) that we named Sdmg1 (Sexually Dimorphic, expressed in Male Gonads 1; Tmem184a – Mouse Genome Informatics). In silico analysis of ESTs extended this fragment into a 1,453 bp putative full-length clone containing a 1350 bp open reading frame encoding a 49 kD protein (Supplementary Figure S1). The integrity and male-specific expression of Sdmg1 was confirmed by RT-PCR on 13.5 dpc male gonads (Figure 1A), and sequencing of the RT-PCR product.
Figure 1. Sdmg1 is a Transmembrane Protein Expressed in Male Embryonic Gonads.
A. RT-PCR showing male-specific expression of Sdmg1 in 13.5 dpc gonads. RT-PCR for Gapdh, and RT-PCR without reverse transcriptase (RT) are shown as controls. B. Transmembrane helix prediction for Sdmg1. The DUF300 domain is indicated with a black bar. C. Topology of Sdmg1. NIH3T3 cells were transiently transfected with HA-Sdmg1-YFP (green) and immunostained with anti-HA or anti-YFP antibodies (red). Antibody accessibility indicates that the N-terminal HA epitope is extracellular and the C-terminal YFP epitope is intracellular. Scale bar 10 μm.
Sdmg1 is a member of a family of largely uncharacterised proteins possessing a DUF300 (Domain of Unknown Function 300) protein domain that has been conserved throughout eukaryotic evolution (Supplementary Figure S2). A single DUF300 family member is present in yeast and nematode genomes, whereas vertebrates each have three DUF300-containing proteins: Tmem34, C22orf5 and Sdmg1 (Supplementary Figure S2). Sdmg1 is predicted to have seven transmembrane domains (Figure 1B), a cytosolic C-terminus and a lumenal/extracellular N-terminus. We confirmed the topology of Sdmg1 by assaying the accessiblity of antibodies to N-terminal and C-terminal epitope tags in transiently transfected NIH3T3 cells (Figure 1C). Sdmg1 does not possess an N-terminal signal peptide, but has a potential C-terminal dileucine targeting motif (439-EKRMLI-444) implicated in endosome/lysosome targeting of transmembrane proteins (Bonifacino and Traub, 2003). Thus Sdmg1 is an evolutionally conserved transmembrane protein that has a sexually dimorphic expression pattern during embryonic gonad development.
Expression Profile of Sdmg1
We confirmed that Sdmg1 is expressed in a sexually dimorphic manner during the period of germ cell sex determination by immunostaining (Figure 2A) and whole-mount in situ hybridisation (Supplementary Figure S3) on embryonic gonads. Although we could not detect Sdmg1 expression in male or female gonads at 11.5 dpc, Sdmg1 mRNA and protein are both present in the testis cords of male gonads at 12.5 and 13.5 dpc, and were not detected in female gonads at these stages (Figure 2A, Supplementary Figure S3). Within the embryonic testis cords, Sdmg1 protein is present in punctate structures in the cytoplasm of Sertoli cells, and is not present in germ cells (Figure 2B). In situ hybridisation on testes from We/We mutant embryos that lack germ cells (Lyon et al., 1996) also suggests that Sdmg1 is expressed by the Sertoli cells in the embryonic testis cords (Supplementary Figure S3).
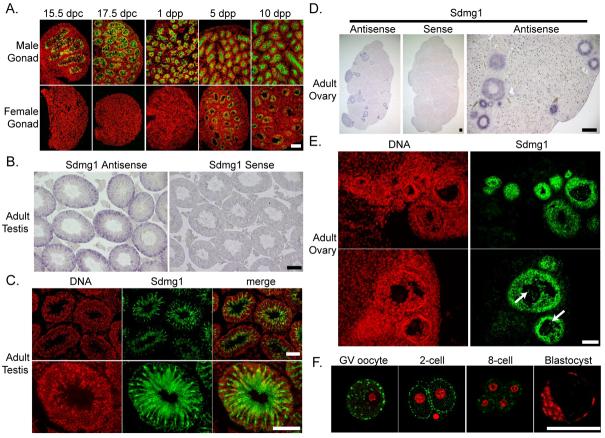
Figure 2. Expression of Sdmg1 During Embryonic Gonad Development.
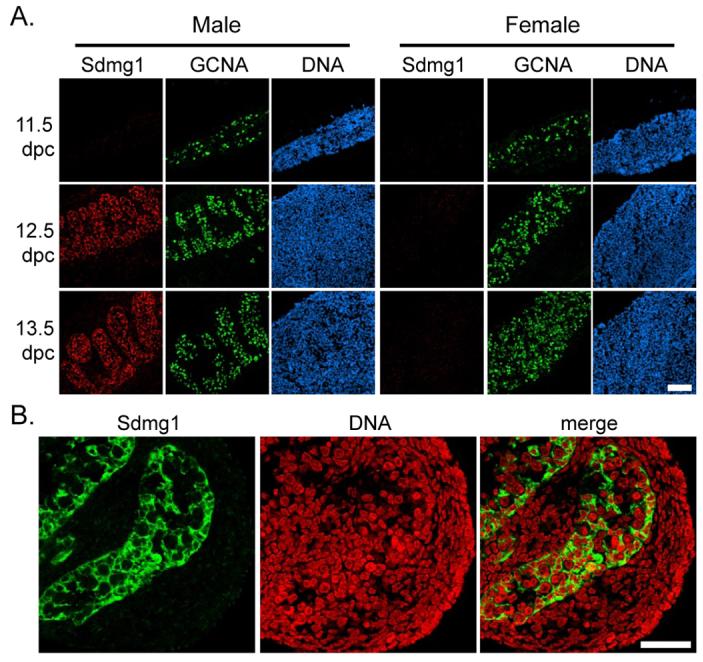
A. Immunostaining for Sdmg1 (red) showing male-specific expression in the gonads from 12.5 dpc. Germ cells are labelled with anti-GCNA antibody (green), DNA is shown in blue. Scale bar 100 μm. B. Immunostaining in 13.5 dpc testes showing punctate staining for Sdmg1 (green) in Sertoli cell cytoplasm. DNA is shown in red. Scale bar 50 μm.
We continued to characterise Sdmg1 expression during gonad development in embryos and adults. Sdmg1 is expressed in Sertoli cells throughout late embryogenesis and early post-natal development (Figure 3A). In adult testes, Sdmg1 mRNA and protein is present in the Sertoli cells (Figure 3B,C), with the protein localised in the apical cytoplasm (Figure 3C). Sdmg1 is not present in female gonads during embryogenesis but its expression is upregulated in granulosa cells, the female counterpart of Sertoli cells, in growing follicles a few days after birth (Figure 3A). In the adult ovary, Sdmg1 mRNA and protein is expressed in granulosa cells in growing follicles (Figure 3D,E). The upregulation of Sdmg1 expression in granulosa cells a few days after birth is coincident with the onset of folliculogenesis and is when granulosa cells first start to influence oocyte growth and differentiation (McLaren, 2000). Thus Sdmg1 is expressed in both Sertoli cells and granulosa cells at the stage when each of these supporting cells starts to communicate with germ cells and regulate germ cell differentiation.
Figure 3. Expression of Sdmg1 During Gametogenesis.
A. Sdmg1 is expressed in the testis cords in from 15.5 dpc to 10 dpp, and in ovarian follicles from 5 dpp. Sdmg1 immunostaining is shown in green, DNA in red. B. In situ hybridisation showing Sdmg1 mRNA expression in Sertoli cells in adult testis (antisense probe, purple precipitate). C. Immunostaining of adult testis cryosections showing Sdmg1 (green) in Sertoli cell cytoplasm. D. In situ hybridisation showing Sdmg1 mRNA expression in granulosa cells in follicles in adult ovaries. E. Immunostaining of adult ovary cryosections showing Sdmg1 (green) in the granulosa cells of follicles. Some Sdmg1 is also present in the oocyte cytoplasm (arrows). F. Immunostaining for Sdmg1 (green) in germinal vesicle (GV) stage oocytes and pre-implantation embryos. Scale bars 100 μm.
Although we did not detect any Sdmg1 protein in male germ cells (Figure 2B, 3C), some Sdmg1 is present in discrete punctate cytoplasmic structures in female germ cells in growing follicles in adult ovaries (Figure 3E, arrows). Sdmg1 is also present in punctate cytoplasmic structures in germinal vesicle stage oocytes, and in pre-implantation embryos (Figure 3F). The punctate anti-Sdmg1 staining is localised to the cortical region of 2-cell stage embryos, and is not present in blastocysts (Figure 3F). This may represent a maternal contribution of Sdmg1 protein to pre-implantation embryos.
Sdmg1 is Associated With Endosomes in the SK11 Sertoli Cell Line
In adult mice, Sdmg1 does not have a widespread expression profile: multiple tissue Northern and Western blotting showed that Sdmg1 expression was restricted to testes (Supplementary Figure S4A,B) and that Sdmg1 is N-glycosylated (Supplementary Figure S4C). However, analysis of EST databases suggests that Sdmg1 is expressed in pancreas, salivary gland and mammary gland, secretory exocrine tissues that were not represented on the multiple tissue blots. Immunostaining confirmed that Sdmg1 is expressed in these secretory tissues and localised to secretory granules (D.B. and I.R.A., unpublished), suggesting that Sdmg1 may play a role in membrane trafficking. To investigate whether Sdmg1 functions in membrane trafficking in Sertoli cells, we used the SK11 Sertoli cell line derived from 10 days post partum (dpp) mice carrying a temperature-sensitive allele of the SV40 T antigen (Walther et al., 1996). SK11 Sertoli cells are maintained in an undifferentiated proliferating state at 33°C, but differentiate to form large, flattened cells at 39.5°C (Walther et al., 1996). Immunostaining revealed that Sdmg1 is localised to the perinuclear region in undifferentiated SK11 cells (Figure 4A). In differentiating SK11 cells, peripheral clusters of punctate Sdmg1 staining are often present in addition to the perinuclear staining (Figure 4B). These peripheral clusters of Sdmg1 staining varied in number and size, but were always present in actin-poor regions of the cell (Figure 4C).
Figure 4. Sdmg1 is Localised to Endosomes in Sertoli Cells.
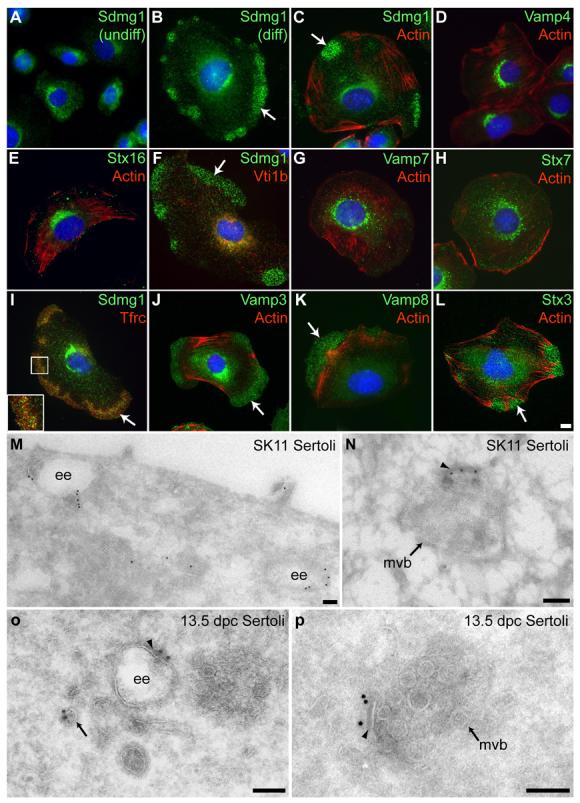
A. Immunostaining shows Sdmg1 is perinuclear in undifferentiated (undiff.) SK11 Sertoli cells grown at 33°C. B. Sdmg1 is present in perinuclear and peripheral clusters (arrow) in differentiating (diff.) SK11 Sertoli cells grown at 39.5°C for 1 day. C. The peripheral clusters (arrows) of Sdmg1 staining are localised to actin-poor parts of the cell. DH. Trans-Golgi network markers Vamp4 and Stx16, and endosomal markers Vti1b, Vamp7 and Stx7 all have a perinuclear distribution in differentiating SK11 Sertoli cells. I-L. The recycling endosome marker Tfrc, and the Vamp3, Vamp8 and Stx3 SNAREs associated with secretory granules all exhibit peripheral clusters of punctate staining (arrows) in differentiating SK11 Sertoli cells. DNA is shown in blue, scale bar 10 μm. M-P. ImmunoEM for Sdmg1 (10 nm gold particles) in differentiating SK11 Sertoli cells (M,N) and 13.5 dpc Sertoli cells (O,P). Sdmg1 is localised to the limiting membrane of early endosomes (ee) and multivesicular bodies (mvb), and is often associated with an electron-dense region of the limiting membrane (arrowhead). Sdmg1 is also present on tubulo-vesicular membranes (M, arrow in P). Scale bar 100 nm.
Sdmg1 contains a potential endosomal targeting sequence (Supplementary Figure S1). We investigated whether the perinuclear and peripheral clusters of Sdmg1 staining might represent different classes of endosome (Perret et al., 2005) by immunostaining for SNAREs (soluble N-ethylmaleimide-sensitive factor attachment protein receptors, Chen and Scheller, 2001; Jahn and Scheller, 2006) and other membrane trafficking markers. SNAREs marking the trans-Golgi network (Vamp4, Stx16) and some endosomal SNAREs and markers (Vti1B, Stx7, Vamp7, EEA1) were mainly perinuclear in differentiated SK11 cells, and were not present in peripheral Sdmg1-like clusters (Figure 4D-H, D.B. and I.R.A., unpublished). Other endosomal SNAREs and markers (Tfrc, Vamp3, Vamp8) were present in peripheral Sdmg1-like clusters in differentiated SK11 cells (Figure 4I-K). Vamp3 and Vamp8 are both components of pancreatic secretory granules (Gaisano et al., 1996b; Wang et al., 2004), and although the basolateral recycling endosome marker Tfrc (transferrin receptor) partially co-localises with peripheral Sdmg1 clusters, the peripheral Sdmg1 puncta appeared to be distinct from the peripheral Tfrc puncta (Figure 4I, inset). Stx3, which is present in pancreatic secretory granules, and on the plasma membrane in other cell types (Band and Kuismanen, 2005; Gaisano et al., 1996a), was also localised to peripheral Sdmg1-like punctate clusters in differentiating SK11 cells (Figure 4L). Although a number of secretory granule SNAREs localise to peripheral punctate clusters in differentiating SK11 cells, we have been unable to detect any secretory granules in these cells by EM (D.A.S. and I.R.A., unpublished). However, endosomes are present in both perinuclear and peripheral regions of differentiating SK11 cells (D.A.S. and I.R.A., unpublished).
To investigate the subcellular localisation of Sdmg1 at the ultrastructural level we performed immunoEM on differentiating SK11 cells. By immunoEM, Sdmg1 was localised to the limiting membrane of endosomes at various maturation stages (Figure 4M,N). Sdmg1 was found on electron-lucent structures likely to be early endosomes (Figure 4M), and on multivesicular bodies (Figure 4N). Sdmg1 was also associated with tubulo-vesicular membranes (Figure 4M). The localisation of the C-terminal anti-Sdmg1 antibodies to the cytosolic face of membranes by immunoEM is consistent with the Sdmg1 topology determined earlier. ImmunoEM for Sdmg1 on 13.5 dpc embryonic testes confirmed that Sdmg1 also localises to endosomes, multivesicular bodies and tubulo-vesicular membranes in embryonic Sertoli cells (Figure 4O,P). Sdmg1 was often localised to an electron-dense stretch of the cytosolic surface of endosomal membrane (Figure 4N-P arrowheads). Clathrin and Stx7 have both been localised to this region of endosomal membranes, which may be where sorting occurs (Prekeris et al., 1999; Sachse et al., 2002).
Thus Sdmg1 appears to be associated with two distinct endosomal populations in differentiating SK11 Sertoli cells: perinuclear endosomes that contain the endosomal markers Vamp7, Stx7 and EEA1; and peripheral endosomes containing Vamp8, Vamp3 and Stx3 SNAREs that localise to secretory granules in pancreatic acinar cells. In polarised cell types that lack secretory granules, Vamp8 localises to apical endosomes (Steegmaier et al., 2000). One interpretation of this data is that the peripheral endosomes in differentiating SK11 Sertoli cells are apical endosomes, and the perinuclear endosomes are common to both apical and basolateral pathways.
Knock-Down of Sdmg1 Impairs Membrane Trafficking in SK11 Sertoli Cell Lines
In order to investigate whether Sdmg1 is required for membrane trafficking we generated stable Sdmg1 knock-down SK11 Sertoli cell lines. The Sdmg1 knock-down lines are viable but grow at a slower rate than the wild-type cells (D.B.and I.R.A, unpublished). Immunostaining confirmed that the SK11 cell lines carrying anti-Sdmg1 shRNAs or miRNAs have reduced levels of Sdmg1 protein (Figure 5A-F).
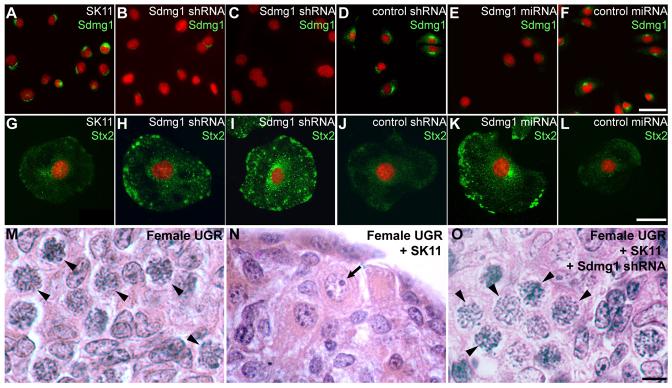
Figure 5. Sdmg1 is Required for Membrane Trafficking and Secretion in SK11 Sertoli Cells.
A-F. Immunostaining of undifferentiated SK11 Sertoli cell lines shows that lines stably transfected with Sdmg1 knock-down constructs (Sdmg1 shRNA and Sdmg1 miRNA) have reduced levels of Sdmg1 protein. G-L. Immunostaining of SK11 Sertoli cells after 2 days of differentiation shows that Stx2 accumulates intracellularly in lines stably transfected with Sdmg1 knock-down constructs. DNA is shown in red. Scale bars 50 μm. M-O. Histology of 11.5 dpc female urogenital ridge tissue cultured after aggregation with or without SK11 Sertoli cell lines. Most germ cells develop into female meiotic oocytes after aggregation without SK11 cells (M, arrowheads), into male prospermatogonia (N, arrow) after aggregation with SK11 cells, and into female meiotic oocytes after aggregation with Sdmg1 knock-down SK11 cells (O, arrowheads). Scale bar 10 μm.
We assayed membrane trafficking in the differentiated Sdmg1 knock-down SK11 cell lines by immunofluorescence for various SNAREs. Vamp4, Vamp8, and Stx3 showed no obvious change in their subcellular localisation in differentiated Sdmg1 knock-down SK11 cells (D.B. and I.R.A., unpublished). However, the plasma membrane SNARE Stx2, which is an apical plasma membrane SNARE for secretory granule fusion in pancreatic acinar cells (Hansen et al., 1999), was mis-localised in Sdmg1 knock-down cells. In the parental SK11 Sertoli cell line, and the shRNA/miRNA negative control cell lines, Stx2 is distributed between the plasma membrane and perinuclear region, but in Sdmg1 knock-down cell lines Stx2 accumulates in intracellular structures (Figure 5G-L). Thus Sdmg1 is required for the normal subcellular distribution of Stx2.
As Sdmg1 is required to localise the secretory SNARE Stx2, we next investigated whether Sdmg1 has a role in secretion. Although Sdmg1 is localised to endosomes, secretion through endosomal intermediates is a major secretory route in some polarised cell types (Ang et al., 2004; Lock and Stow, 2005). However, as we do not know the identity of any secretory cargo trafficking through Sdmg1-positive endosomes, we set up a bioassay for SK11 cell secretion.
Sertoli cells signal to and masculinise germ cells during embryonic development, therefore we aggregated SK11 cells with 11.5 dpc female urogenital ridge tissue and assayed whether SK11 Sertoli cells could masculinise female germ cells. As expected, most germ cells from the re-aggregated female urogenital ridge tissue cultured without SK11 cells differentiated into meiotic oocytes (Figure 5M, 95% of germ cells develop as female, n=109), consistent with previous experiments (Adams and McLaren, 2002). However, when SK11 Sertoli cells were aggregated with this tissue, few meiotic oocytes were found in the aggregates, and male prospermatogonia were present (Figure 5N, 75% of germ cells develop as male, n=102). The ability of SK11 Sertoli cells to influence female germ cell development appears to depend on Sdmg1 as knock-down of Sdmg1 impaired the ability of SK11 Sertoli cells to influence female germ cell development (Figure 5O, 96% of germ cells develop as female, n=103). shRNA negative control SK11 Sertoli cells behaved in a similar manner to the parental SK11 Sertoli cells in this aggregate system (D.B. and I.R.A., unpublished).
The effect that SK11 Sertoli cells have on female germ cell development could be due to SK11 Sertoli cells directly or indirectly masculinising developing germ cells, or causing the degeneration of female meiotic germ cells. The relatively low number of surviving germ cells in the aggregates containing SK11 Sertoli cells (D.B. and I.R.A., unpublished) indicates that degeneration of female meiotic oocytes probably contributes to this effect. The observation that knock-down of Sdmg1 impairs the ability of SK11 Sertoli cells to masculinise germ cells is consistent with knock-down of Sdmg1 perturbing membrane trafficking, and presumably secretion of signalling molecules.
Embryonic Sertoli Cells Are Specialised Secretory Cells
As Sdmg1 is expressed in embryonic Sertoli cells, and is associated with secretory granules in secretory exocrine tissues (D.B. and I.R.A, unpublished), we sought to determine whether embryonic Sertoli cells contain secretory granules. Secretory granules have previously been reported in embryonic rat Sertoli cells (Hatier and Grignon, 1980). We observed secretory granules in mouse embryonic Sertoli cells in 13.5 dpc testes by EM (Figure 6A-C). We also found large prominent secretory granules in male germ cells at this stage (Figure 6D,E). Germ cells in 13.5 dpc ovaries contained similar secretory granules to those seen in male germ cells at 13.5 dpc (D.A.S. and I.R.A., unpublished). We were unable to determine if supporting cells in 13.5 dpc ovaries contained secretory granules, as these cells do not have a readily recognisable morphology at this stage.
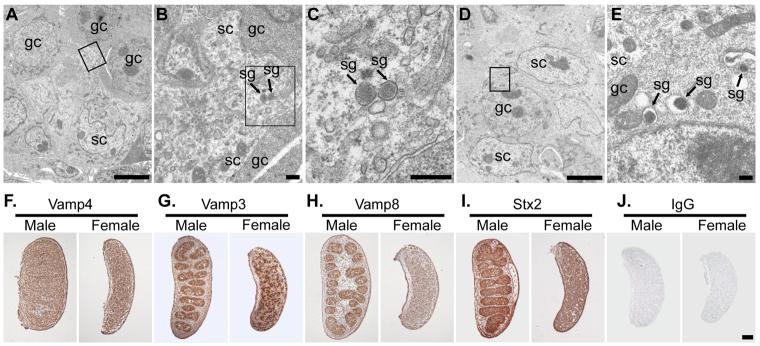
Figure 6. Embryonic Sertoli Cells Are Specialised Secretory Cells.
EM of 13.5 dpc testes showing secretory granules in Sertoli cells and germ cells (A-E). A,D. Low magnification image showing arrangement of germ cells (gc) and Sertoli cells (sc). B,C,E. Higher magnification of boxed regions in A, B and D respectively. Secretory granules (sg) are indicated. Scale bars 5 μm in A,D; 0.1 μm in B,C,E. F-I. Immunohistochemistry for SNAREs (brown precipitate) in 13.5 dpc ovaries and testes. F. Vamp4 (trans-Golgi network) is ubiquitously expressed. G. Vamp3 (secretory granules, endosomes) is abundant in a subset of cells in male and female gonads. H, I.Vamp8 (secretory granules, endosomes) and Stx2 (apical plasma membrane) are abundant in testis cords. J. Non-specific IgG controls show no significant staining. Scale bar 100 μm.
We then investigated whether there is a widespread upregulation of components of the regulated secretory pathway in embryonic Sertoli cells at 13.5 dpc (Figure 6F-J). The trans-Golgi network SNARE Vamp4 is ubiquitously expressed throughout male and female gonads (Figure 6F). However the secretory granule SNARE Vamp3 is highly expressed in testis cords at 13.5 dpc, and in cells distributed throughout the ovary at 13.5 dpc (Figure 6G). This staining pattern resembles germ cells, and may reflect the prominent secretory granules that we observed in this cell type. Immunostaining for the secretory granule SNARE Vamp8 showed that this protein is upregulated in the testis cords of 13.5 dpc male gonads compared to females (Figure 6H). Stx2, an apical plasma membrane SNARE involved in pancreatic secretory granule fusion, is also upregulated in the testis cords of 13.5 dpc male gonads (Figure 6I). Both Vamp8 and Stx2 are present in the Sertoli cell cytoplasm in the testis cords (D.B. and I.R.A, unpublished). Thus, embryonic Sertoli cells upregulate expression of the secretory granule SNARE Vamp8, and possess secretory granules, suggesting that embryonic Sertoli cells are a secretory cell type.
Perturbation of Secretion in Male Embryonic Gonads Causes Germline Sex Reversal
The differences that we have described in the membrane trafficking pathway between male and female supporting cells in embryonic gonads may reflect a requirement for the embryonic Sertoli cells to secrete masculinising factors to various cell types during testis development, including the germ cells. Therefore we tested whether membrane trafficking is important for germ cell sex determination by culturing urogenital ridges with brefeldin A. Brefeldin A blocks recruitment of coat proteins onto intracellular membranes, and is a potent inhibitor of secretion and post-Golgi trafficking (Klausner et al., 1992). Although prolonged treatment of mouse embryonic gonads with brefeldin A for longer than 24 hours resulted in toxicity, shorter exposure times of around 6 hours did not appear to be toxic to the developing tissue (D.B. and I.R.A, unpublished), or to mouse pre-implantation embryos (Ogawa et al., 1997).
Germ cells in 11.5 dpc female urogenital ridges treated with brefeldin A differentiated into meiotic oocytes in a manner comparable to control female urogenital ridges (Figure 7A-F). Meiotic oocytes were detected by their distinctive histological appearance (Figure 7A,D, arrowheads), and confirmed by immunostaining for the synaptonemal complex protein Sycp3 (Figure 7C,F, arrowheads). As Sycp3 RNA and protein can be detected in both male and female germ cells in mid-late gestation embryos (Chuma and Nakatsuji, 2001; Di Carlo et al., 2000), assembly of this protein into thread-like synaptonemal complex structures is a more robust marker for meiosis than Sycp3 gene expression (Novak et al., 2006). Almost all of the Mvh-positive germ cells in female urogenital ridges cultured with or without brefeldin A were in meiosis demonstrating that transient treatment of 11.5 dpc female urogenital ridges with brefeldin A does not influence female germ cell development.
Figure 7. Perturbing Secretion Induces Male-to-Female Germ Cell Sex Reversal in Embryonic Gonad Cultures.
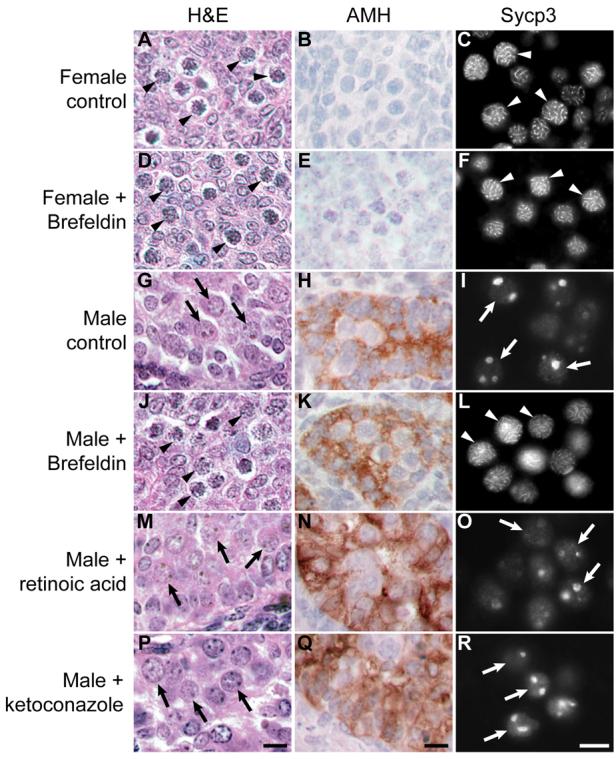
11.5 dpc urogenital ridges were transiently treated with or without brefeldin A to block secretion, or were cultured continuously with retinoic acid or ketoconazole. Urogenital ridges were cultured for 4 days and analysed by histology (H&E) to assess germ cell development, and immunostained for anti-Mullerian Hormone (AMH, brown precipitate) to assess Sertoli cell development. Urogenital ridges were also cultured for 6 days and immunostained for Sycp3. Sycp3 labels the thread-like synaptonemal complex in meiotic oocytes, and is present as large aggregates in prospermatogonia. Examples of meiotic oocytes are labelled with arrowheads; examples of prospermatogonia are labelled with arrows. Scale bars 10 μm.
In contrast, transient treatment of 11.5 dpc male urogenital ridges with brefeldin A induced germ cell sex reversal in culture (Figure 7G-L). In control male cultures, germ cells differentiated into prospermatogonia (Figure 7G, arrows). These prospermatogonia express Sycp3, but Sycp3 does not assemble into thread-like synaptonemal complex, and is present in nuclear aggregates (Figure 7I, arrows), consistent with previous reports (Chuma and Nakatsuji, 2001; Di Carlo et al., 2000). However, when 11.5 dpc male urogenital ridges were transiently treated with brefeldin A for 6 hours, many of the germ cells differentiated into meiotic oocytes containing thread-like synaptonemal complex structures (Figure 7J,L, arrowheads). Brefeldin A did not appear to cause gross defects in Sertoli cell differentiation (Figure 7K). The meiotic oocytes tended to be present in one pole of the cultured male gonad, with the ‘sex-reversed’ area of the cultured gonad comprising around one quarter of the entire gonad. This data suggests that germ cell differentiation along the male pathway requires secretion in the developing testis.
One possible interpretation of our data is that brefeldin A interferes with Cyp26b1-mediated metabolism of retinoic acid (Bowles et al., 2006; Koubova et al., 2006). We tested this possibility by comparing the behaviour of male gonads treated with brefeldin A, exogenous retinoic acid, and the Cyp26 inhibitor ketoconazole (Bowles et al., 2006; Koubova et al., 2006). In contrast to the germ cell sex-reversal seen with transient brefeldin A-treatment, continuous culture of 11.5 dpc male urogenital ridges in exogenous retinoic acid did not cause the appearance of significant numbers of meiotic germ cells. Typically around 1% of the Mvh-positive cells had meiotic Sycp3 staining, but the vast majority of the germ cells developed as male (Figure 7M-O, arrows). Treatment of 11.5 dpc male urogenital ridges with 40 μM ketoconazole (Bowles et al., 2006) resulted in widespread cell death and a failure of tissue development (D.B. and I.R.A. unpublished). Treatment of 11.5 dpc male urogenital ridges with 0.7 μM ketoconazole (Koubova et al., 2006) resulted in better gonad development, but the majority of the germ cells developed as male (Figure 7P-R, arrows), and only a small number of germ cells (around 1%) were meiotic. We confirmed that retinoic acid or ketoconazole treatment was sufficient to induce expression of Stra8 in male gonads as reported previously (Bowles et al., 2006; Koubova et al., 2006) (Supplementary Figure S5). Therefore although manipulation of retinoic acid levels or Cyp26 activity can induce inappropriate gene expression in male gonads, these treatments do not appear to induce meiosis or change germ cell fate.
DISCUSSION
Sdmg1 Functions in Membrane Trafficking
This study describes the identification and characterisation of a novel transmembrane protein associated with membrane trafficking. Knock-down of Sdmg1 in SK11 Sertoli cells shows that Sdmg1 is required for correct trafficking of the plasma membrane SNARE Stx2. However, we cannot distinguish whether the intracellular accumulation of Stx2 is a primary consequence of Sdmg1 knock-down, or if this is a secondary effect of perturbing some other aspect of post-Golgi trafficking or Stx2 expression. Stx2 is localised to the apical plasma membrane in pancreatic acinar cells (Gaisano et al., 1996a), and actively cycles between the plasma membrane and endosomes in cell culture (Band and Kuismanen, 2005). In Sdmg1 knock-down cells, the intracellular accumulations of Stx2 could be caused by Stx2 becoming trapped in an endosome compartment, or by Stx2 being mis-sorted when passing through endosomes. As the secretory granule/endosome marker Vamp8 does not accumulate like Stx2 in Sdmg1 knock-down cells, it is perhaps more likely that Stx2 is being mis-sorted when passing through endosomes in Sdmg1 knock-down cells. The impaired ability of Sdmg1 knock-down SK11 Sertoli cells to influence the development of female germ cells in aggregate culture is also consistent with Sdmg1 knock-down disrupting normal membrane trafficking in the SK11 Sertoli cells. However, further work is required to elucidate the molecular basis of the Stx2 mis-localisation and impaired masculinising ability of the Sdmg1 knock-down Sertoli cell lines.
The data in this manuscript suggests that Sdmg1 has a function in endosomes, but it is not clear what the biochemical function of Sdmg1 is. The transmembrane nature of Sdmg1 could potentially allow this protein to co-ordinate sorting of intra-lumenal cargo with lipid microdomains or cytosolic membrane trafficking proteins, or could allow Sdmg1 to form a channel to transport molecules into, or out of, endosomes to influence the lumenal environment. An alternative possibility is that Sdmg1 could facilitate transport of some proteins through the membrane trafficking system. Many developmental signalling molecules, including Hedgehogs, Wnts and EGF receptor ligands, are modified during secretion, or require accessory factors for their secretion (Coudreuse and Korswagen, 2007; Nusse, 2003; Urban et al., 2001). Secretion of Hedgehog and Wnt signals have been proposed to occur through specialised secretory pathways and/or endosomal intermediates (Coudreuse and Korswagen, 2007) By analogy, it is possible that Sdmg1 is part of a complex that modifies or interacts with trafficking cargo or signalling molecules in some way. Biochemical characterisation of Sdmg1 and its interacting partners will be required to test some of these hypotheses.
Membrane Trafficking in Embryonic Sertoli Cells
One of the main functions of the embryonic Sertoli cells is to communicate the male sex determining decision to various sexually dimorphic cell types in the urogenital system, therefore it would seem reasonable that Sertoli cells may have to alter or upregulate their membrane trafficking pathway to facilitate this. Furthermore, nascent Sertoli cells undergo a mesenchymal to epithelial transition, and presumably a concomitant cell polarisation, during formation of the testis cords. The polarisation of the Sertoli cells, and the resulting need to sort cargo to apical and basolateral destinations, may also underly some of the changes in the membrane trafficking pathway that we have observed in the embryonic Sertoli cells.
The SNAREs Vamp8 and Stx2 are both upregulated in male embryonic gonads. In the adult testis, Vamp8 is not expressed in the Sertoli cells, but Stx2 is (D.B. and I.R.A., unpublished). The expression of Vamp8 in embryonic but not adult Sertoli cells correlates with the transient presence of secretory granules in rat Sertoli cells (Hatier and Grignon, 1980). However, surviving Vamp8 knock-out mice are fertile (Wang et al., 2004), demonstrating that Vamp8 expression is not essential for Sertoli cell function. In contrast, Stx2 knock-out mice are male sterile due to defective spermatogenesis (Wang et al., 2006). Thus Stx2 may be important for Sertoli cell function in adult mice, but the reported phenotype does not suggest a problem during embryonic gonadogenesis (Wang et al., 2006). The high levels of similarity between different SNARE proteins may allow some compensation for the loss of Vamp8 or Stx2 function in embryonic Sertoli cells. A more detailed examination of gonad development and Sertoli cell function in single and multiple SNARE knock-out mice might prove to be informative.
Microarray analysis of gene expression has been used to identify differences in gene expression between male and female embryonic gonads on a genome-wide scale, and Sdmg1 is present in the male-upregulated datasets in these studies (Beverdam and Koopman, 2006; Nef et al., 2005). Other components of the regulated secretion machinery such as Cadps (Rupnik et al., 2000) and Rab3b (Schluter et al., 2002) are also present in the male-upregulated datasets (Nef et al., 2005). During the course of our study, Sdmg1 mRNA expression was reported in embryonic and adult Sertoli cells, and Sdmg1 was proposed to have predicted kinase activity (Svingen et al., 2007). We have been unable to identify any sequence similarity between Sdmg1 and any protein kinase. However, the human C22orf5 DUF300-containing protein was isolated in a screen for cDNAs that activate MAP kinases (Matsuda et al., 2003). Although our study has focussed on Sdmg1 rather than C22orf5, there is growing evidence that localisation of activate MAP kinases to signalling endosomes is important for regulating signal transduction cascades (von Zastrow and Sorkin, 2007).
Interestingly, the active phosphorylated form of Erk2 localises to peripheral punctate clusters, like Sdmg1, in differentiating SK11 Sertoli cells (D.B and I.R.A, unpublished). Further research is needed to investigate any functional relationship between Sdmg1 expression and the MAP kinase pathway in Sertoli cells.
Germ Cell Sex Determination
The molecular mechanisms involved in regulating germline sex determination during mouse embryonic development remain largely uncharacterised. The differences between germ cell behaviour in male and female gonads is a consequence of germ cells responding to different gonadal environments, but it is not clear which factors are responsible for this. If a female meiosis-inducing substance exists, its expression must be fairly widespread (Chuma and Nakatsuji, 2001; Farini et al., 2005; McLaren and Southee, 1997; Zamboni and Upadhyay, 1983). In contrast, the observation that germ cells can develop as male in the testis interstitium, or even in the adjacent mesonephros, suggests that a diffusible meiosis-preventing substance exists in the embryonic testis (McLaren, 1984; McLaren, 2003).
Retinoic acid has recently been proposed to be a meiosis-inducing substance (Bowles et al., 2006; Koubova et al., 2006). The widespread distribution of retinoic acid would fit with germ cells being able to initiate meiosis in a variety of ectopic conditions, but experiments demonstrating that exogenous retinoic acid can induce expression of meiotic markers in cultures of 12.5 dpc male gonads (Bowles et al., 2006; Koubova et al., 2006) contradict experiments showing that germ cells isolated from 12.5dpc male gonads are unable to initiate meiosis when re-aggregated with female gonadal tissue (Adams and McLaren, 2002). Furthermore, culture of rat male embryonic gonads in exogenous retinoic acid did not result in the initiation of meiosis, but did induce cell death and a reduction in the number of Sertoli cells and germ cells (Li and Kim, 2004).
The retinoic acid model is largely based on changes in gene expression that are detected when embryonic gonads are cultured with activators or inhibitors of retinoic acid or Cyp26b1 (Bowles et al., 2006; Koubova et al., 2006). However, the published histology of the cultured gonads (Bowles et al., 2006) does not support this model: female gonads cultured with retinoic acid receptor anatagonists do not appear to contain prospermatogonia; and male gonads cultured with Cyp26b1 inhibitor do not appear to contain meiotic oocytes, which can usually be distinguished by their characteristic condensed thread-like chromatin reflecting chromosome synapsis during meiosis, but rather appear to contain pyknotic nuclei, a feature of dying cells characterised by condensation of the nucleus to a small solid structureless mass. These data can be reconciled in a model where retinoic acid may be directly or indirectly influencing germ cell gene expression, but is not acting as a trigger for germ cell sex determination or the initiation of meiosis. Thus additional signalling molecules are likely to be important for regulating the differences between germ cell development in male and female gonads.
Our experiments suggest that a brefeldin A-sensitive meiosis-preventing substance exists in the male embryonic gonad. Furthermore, as embryonic Sertoli cells upregulate Sdmg1 and Vamp8, and contain secretory granules, any or all of these parts of the membrane trafficking pathway may be involved in facilitating secretion of a meiosis-preventing substance out of the embryonic Sertoli cells. Indeed, differences between membrane trafficking pathways in male and female embryonic supporting cells could generate sex-specific activities from molecules expressed in supporting cells of both sexes. It will be of interest to identify which molecules traffic through Sdmg1 compartments in embryonic Sertoli cells, and whether these molecules are involved in germ cell sex determination in vivo.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the Lister Institute of Preventive Medicine (I.R.A), the Medical Research Council (D.B., I.R.A., A.A.P.) and the Wellcome Trust (D.A.S.) for financial support. I.R.A is a Lister Research Fellow. A.A.P is a MRC Career Development Fellow. We thank George Enders (University of Kansas Medical Center, Kansas, USA) for anti-GCNA antibody, Scottie Robinson (CIMR, Cambridge) for help and advice, and Nick Hastie, Howard Cooke and Rupert Ollinger (MRC HGU, Edinburgh) for critical reading of the manuscript.
REFERENCES
- Adams IR, McLaren A. Sexually dimorphic development of mouse primordial germ cells: switching from oogenesis to spermatogenesis. Development. 2002;129:1155–1164. doi: 10.1242/dev.129.5.1155. [DOI] [PubMed] [Google Scholar]
- Albrecht K, Eicher E. Evidence that Sry is expressed in pre-Sertoli cells and Sertoli and granulosa cells have a common precursor. Dev. Biol. 2001;240:92–107. doi: 10.1006/dbio.2001.0438. [DOI] [PubMed] [Google Scholar]
- Ang AL, Taguchi T, Francis S, Folsch H, Murrells LJ, Pypaert M, Warren G, Mellman I. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J. Cell Biol. 2004;167:531–543. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arney KL, Bao S, Bannister AJ, Kouzarides T, Surani MA. Histone methylation defines epigenetic asymmetry in the mouse zygote. Int J Dev Biol. 2002;46:317–20. [PubMed] [Google Scholar]
- Band AM, Kuismanen E. Localization of plasma membrane t-SNAREs syntaxin 2 and 3 in intracellular compartments. BMC Cell Biology. 2005;6:26. doi: 10.1186/1471-2121-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverdam A, Koopman P. Expression profiling of purified mouse gonadal somatic cells during the critical time window of sex determination reveals novel candidate genes for human sexual dysgenesis syndromes. Human Molecular Genetics. 2006;15:417–431. doi: 10.1093/hmg/ddi463. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annual Review of Biochemistry. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, et al. Retinoid Signaling Determines Germ Cell Fate in Mice. Science. 2006;312:596–600. doi: 10.1126/science.1125691. [DOI] [PubMed] [Google Scholar]
- Chen YA, Scheller RH. SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol. 2001;2:98–106. doi: 10.1038/35052017. [DOI] [PubMed] [Google Scholar]
- Chuma S, Nakatsuji N. Autonomous transition into meiosis of mouse fetal germ cells in vitro and its inhibition by gp130-mediated signaling. Developmental Biology. 2001;229:468–479. doi: 10.1006/dbio.2000.9989. [DOI] [PubMed] [Google Scholar]
- Coudreuse D, Korswagen HC. The making of Wnt: new insights into Wnt maturation, sorting and secretion. Development. 2007;134:3–12. doi: 10.1242/dev.02699. [DOI] [PubMed] [Google Scholar]
- Di Carlo AD, Travia G, De Felici M. The meiotic specific synaptonemal complex protein SCP3 is expressed by female and male primordial germ cells of the mouse embryo. International Journal of Developmental Biology. 2000;44:241–244. [PubMed] [Google Scholar]
- Farini D, Scaldaferri ML, Iona S, La Sala G, De Felici M. Growth factors sustain primordial germ cell survival, proliferation and entering into meiosis in the absence of somatic cells. Developmental Biology. 2005;285:49–56. doi: 10.1016/j.ydbio.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Gaisano HY, Ghai M, Malkus PN, Sheu L, Bouquillon A, Bennett MK, Trimble WS. Distinct cellular locations of the syntaxin family of proteins in rat pancreatic acinar cells. Mol. Biol. Cell. 1996a;7:2019–2027. doi: 10.1091/mbc.7.12.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaisano HY, Sheu L, Grondin G, Ghai M, Bouquillon A, Lowe A, Beaudoin A, Trimble WS. The vesicle-associated membrane protein family of proteins in rat pancreatic and parotid acinar cells. Gastroenterology. 1996b;111:1661–1669. doi: 10.1016/s0016-5085(96)70030-6. [DOI] [PubMed] [Google Scholar]
- Hansen NJ, Antonin W, Edwardson JM. Identification of SNAREs Involved in Regulated Exocytosis in the Pancreatic Acinar Cell. Journal of Biological Chemistry. 1999;274:22871–22876. doi: 10.1074/jbc.274.32.22871. [DOI] [PubMed] [Google Scholar]
- Hatier R, Grignon G. Ultrastructural study of Sertoli cells in rat seminiferous tubules during intrauterine life and the postnatal period. Anatomy and Embryology. 1980;160:11–27. doi: 10.1007/BF00315646. [DOI] [PubMed] [Google Scholar]
- Jahn R, Scheller RH. SNAREs - engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Jost A. Recherches sur la differenciation sexuelle de l'embryon de lapin. Arch. Anat. Microsc. Morphol. Exp. 1947;36:271–315. [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC. Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proceedings of the National Academy of Sciences. 2006;103:2474–2479. doi: 10.1073/pnas.0510813103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A, Larsson B, von Heijne G, Sonnhammer ELL. Predicting transmembrane protein topology with a hidden markov model: application to complete genomes. Journal of Molecular Biology. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- Li H, Kim KH. Retinoic Acid Inhibits Rat XY Gonad Development by Blocking Mesonephric Cell Migration and Decreasing the Number of Gonocytes. Biology of Reproduction. 2004;70:687–693. doi: 10.1095/biolreprod.103.023135. [DOI] [PubMed] [Google Scholar]
- Lock JG, Stow JL. Rab11 in Recycling Endosomes Regulates the Sorting and Basolateral Transport of E-Cadherin. Mol. Biol. Cell. 2005;16:1744–1755. doi: 10.1091/mbc.E04-10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell-Badge R, Robertson E. XY female mice resulting from a heritable mutation in the murine primary testis determining gene, Tdy. Development. 1990;109:635–646. doi: 10.1242/dev.109.3.635. [DOI] [PubMed] [Google Scholar]
- Lyon MF, Rastan S, Brown SDM. Genetic variants and strains of the laboratory mouse. 3rd edition Oxford, UK: Oxford University Press; 1996. [Google Scholar]
- Matsuda A, Suzuki Y, Honda G, Muramatsu S, Matsuzaki O, Nagano Y, Doi T, Shimotohno K, Harada T, Nishida E, et al. Large-scale identification and characterization of human genes that activate NF-kappaB and MAPK signaling pathways. Oncogene. 2003;22:3307–3318. doi: 10.1038/sj.onc.1206406. [DOI] [PubMed] [Google Scholar]
- McLaren A. Meiosis and differentiation of mouse germ cells. In: Evans CW, Dickinson HG, editors. Controlling Events in Meiosis. 38th Symposium of the Society for Experimental Biology; Cambridge, UK: Company of Biologists; 1984. pp. 7–23. [PubMed] [Google Scholar]
- McLaren A. Germ and somatic cell lineages in the developing gonad. Molecular and Cellular Endocrinology. 2000;163:3–9. doi: 10.1016/s0303-7207(99)00234-8. [DOI] [PubMed] [Google Scholar]
- McLaren A, Southee D. Entry of mouse embryonic germ cells into meiosis. Developmental Biology. 1997;187:107–113. doi: 10.1006/dbio.1997.8584. [DOI] [PubMed] [Google Scholar]
- McLaren A. Primordial germ cells in the mouse. Developmental Biology. 2003;262:1–15. doi: 10.1016/s0012-1606(03)00214-8. [DOI] [PubMed] [Google Scholar]
- Meehan T, Schlatt S, O'Bryan MK, de Kretser DM, Loveland KL. Regulation of germ cell and Sertoli cell development by activin, follistatin and FSH. Developmental Biology. 2000;220:225–237. doi: 10.1006/dbio.2000.9625. [DOI] [PubMed] [Google Scholar]
- Nef S, Schaad O, Stallings NR, Cederroth CR, Pitetti JL, Schaer G, Malki S, Dubois-Dauphin M, Boizet-Bonhoure B, Descombes P, et al. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Developmental Biology. 2005;287:361–377. doi: 10.1016/j.ydbio.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Novak I, Lightfoot DA, Wang H, Eriksson A, Mahdy E, Hoog C. Mouse Embryonic Stem Cells Form Follicle-Like Ovarian Structures but Do Not Progress Through Meiosis. Stem Cells. 2006;24:1931–1936. doi: 10.1634/stemcells.2005-0520. [DOI] [PubMed] [Google Scholar]
- Nusse R. Wnts and Hedgehogs: lipid-modified proteins and similarities in signaling mechanisms at the cell surface. Development. 2003;130:5297–5305. doi: 10.1242/dev.00821. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Mori T, Shimizu H. Effect of Brefeldin-A on the Development of Mouse 4-Cell Embryos In Vitro. Journal of Reproduction and Development. 1997;43:151–156. [Google Scholar]
- Palmer SJ, Burgoyne PS. In situ analysis of fetal, prepubertal and adult XX↔XY chimaeric mouse testes: Sertoli cells are predominantly, but not exclusively, XY. Development. 1991;112:265–268. doi: 10.1242/dev.112.1.265. [DOI] [PubMed] [Google Scholar]
- Perret E, Lakkaraju A, Deborde S, Schreiner R, Rodriguez-Boulan E. Evolving endosomes: how many varieties and why? Current Opinion in Cell Biology. 2005;17:423–434. doi: 10.1016/j.ceb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Prekeris R, Yang B, Oorschot V, Klumperman J, Scheller RH. Differential Roles of Syntaxin 7 and Syntaxin 8 in Endosomal Trafficking. Mol. Biol. Cell. 1999;10:3891–3908. doi: 10.1091/mbc.10.11.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AJ, Capel B. Signaling at the crossroads of gonad development. Trends in Endocrinology and Metabolism. 2005;16:19–25. doi: 10.1016/j.tem.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Rupnik M, Kreft M, Sikdar SK, Grilc S, Romih R, Zupancic G, Martin TFJ, Zorec R. Rapid regulated dense-core vesicle exocytosis requires the CAPS protein. Proceedings of the National Academy of Sciences. 2000;97:5627–5632. doi: 10.1073/pnas.090359097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachse M, Urbe S, Oorschot V, Strous GJ, Klumperman J. Bilayered Clathrin Coats on Endosomal Vacuoles Are Involved in Protein Sorting toward Lysosomes. Mol. Biol. Cell. 2002;13:1313–1328. doi: 10.1091/mbc.01-10-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular Cloning: a laboratory manual. New York: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Schluter OM, Khvotchev M, Jahn R, Sudhof TC. Localization Versus Function of Rab3 Proteins. Evidence for a common regulatory role in controlling fusion. Journal of Biological Chemistry. 2002;277:40919–40929. doi: 10.1074/jbc.M203704200. [DOI] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ. The use of protein A-colloidal gold (PAG) complexes as immunolabels in ultrathin frozen sections. In: Cuello AC, editor. Immunohistochemistry. Chichester, UK: John Wiley & Sons; 1983. pp. 323–346. [Google Scholar]
- Steegmaier M, Lee KC, Prekeris R, Scheller RH. SNARE Protein Trafficking in Polarized MDCK Cells. Traffic. 2000;1:553–560. doi: 10.1034/j.1600-0854.2000.010705.x. [DOI] [PubMed] [Google Scholar]
- Svingen T, Beverdam A, Bernard P, McClive P, Harley VR, Sinclair AH, Koopman P. Sex-specific expression of a novel gene Tmem184a during mouse testis differentiation. Reproduction. 2007;133:983–989. doi: 10.1530/REP-06-0379. [DOI] [PubMed] [Google Scholar]
- Urban S, Lee JR, Freeman M. Drosophila Rhomboid-1 Defines a Family of Putative Intramembrane Serine Proteases. Cell. 2001;107:173–182. doi: 10.1016/s0092-8674(01)00525-6. [DOI] [PubMed] [Google Scholar]
- von Zastrow M, Sorkin A. Signaling on the endocytic pathway. Current Opinion in Cell Biology. 2007;19:436–445. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther N, Jansen M, Ergun S, Kascheike B, Ivell R. Sertoli Cell Lines Established from H-2KbtsA58 Transgenic Mice Differentially Regulate the Expression of Cell-Specific Genes. Experimental Cell Research. 1996;225:411–421. doi: 10.1006/excr.1996.0192. [DOI] [PubMed] [Google Scholar]
- Wang CC, Ng CP, Lu L, Atlashkin V, Zhang W, Seet LF, Hong W. A Role of VAMP8/Endobrevin in Regulated Exocytosis of Pancreatic Acinar Cells. Developmental Cell. 2004;7:359–371. doi: 10.1016/j.devcel.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang L, Iordanov H, Swietlicki EA, Zheng Q, Jiang S, Tang Y, Levin MS, Rubin DC. Epimorphin−/− mice have increased intestinal growth, decreased susceptibility to dextran sodium sulfate colitis, and impaired spermatogenesis. J. Clin. Invest. 2006;116:1535–1546. doi: 10.1172/JCI25442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm D, Martinson F, Bradford S, Wilson MJ, Combes AN, Beverdam A, Bowles J, Mizusaki H, Koopman P. Sertoli cell differentiation is induced both cell-autonomously and through prostaglandin signaling during mammalian sex determination. Developmental Biology. 2005;287:111–124. doi: 10.1016/j.ydbio.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Zamboni L, Upadhyay S. Germ cell differentiation in mouse adrenal glands. Journal of Experimental Zoology. 1983;228:173–193. doi: 10.1002/jez.1402280204. [DOI] [PubMed] [Google Scholar]
- Zheng B, Mills AA, Bradley A. A system for rapid generation of coat color-tagged knockouts and defined chromosomal rearrangements in mice. Nucleic Acids Res. 1999;27:2354–60. doi: 10.1093/nar/27.11.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.