Abstract
Natural killer (NK) cells are well recognized for their ability to provide a first line of defence against viral pathogens and they are increasingly being implicated in immune responses against certain bacterial and parasitic infections. Reciprocally, viruses have devised numerous strategies to evade the activation of NK cells and have influenced the evolution of NK-cell receptors and their ligands. NK cells contribute to host defence by their ability to rapidly secrete cytokines and chemokines, as well as to directly kill infected host cells. In addition to their participation in the immediate innate immune response against infection, interactions between NK cells and dendritic cells shape the nature of the subsequent adaptive immune response to pathogens.
Natural killer (NK) cells contribute to the innate immune defence against tumours and microbial pathogens. They sense their environment by using a sophisticated repertoire of receptors that allows them to distinguish between normal cells and transformed cells or cells infected with intracellular pathogens1 (BOX 1). NK cells are most abundant in the blood, liver and spleen, but they are also present in lymph nodes, and they can migrate into inflamed or tumour-bearing tissues and organs. 'Resting' mature NK cells constitutively express transcripts for certain cytokines, such as interferon-γ (IFNγ)2, and they contain pre-formed cytolytic mediators (granzymes and perforin), stored in intracellular granules3. Despite already being armed for attack, NK cells require activation by type I interferons (that is, IFNα or IFNβ) or pro-inflammatory cytokines, such as interleukin-15 (IL-15), IL-12 and IL-18, before becoming fully functional effector cells that can provide optimal host defence against infections. In many situations, dendritic cells (DCs) are probably the main source of the type I IFN and IL-12 that is necessary for NK-cell activation, and in turn, IFNγ produced by NK cells can affect the maturation and effector functions of DCs, as well as other leukocytes, including macrophages, granulocytes and other lymphocytes that are responding to the infection. In some cases, NK cells can directly recognize and respond to a cell infected with a virus, but cognate recognition of the infected cell is not absolutely required for NK cells to participate in a response because they can be activated as bystander cells simply by exposure to IFNs and cytokines in their environment. For example, culture of NK cells with IL-12 and IL-18 (or other combinations of cytokines with IL-12) is sufficient to initiate secretion of IFNγ, without requiring any deliberate engagement of the activating receptors expressed by NK cells that detect alterations in the cell surface of the infected or transformed cells. In addition, because NK cells express CD 16, an activating Fc receptor for IgG (FcγR), they can attack virus-infected cells that are coated with IgG, with specificity being contributed by the antibody. Therefore, the relative contribution of and effector mechanisms involved in an NK-cell response to a given pathogen can vary considerably. Finally, some viruses have devised means to evade detection by NK cells, which emphasizes the importance of NK cells in host defence.
Although NK cells were initially identified in the 1970s on the basis of their ability to kill tumour cells, soon thereafter, activated NK cells were also detected in virus-infected hosts. Studies from several laboratories showed that NK cells isolated after infection with any of several different viruses had increased cytolytic activity in vitro against tumour-cell targets, and that viral infection can result in NK-cell proliferation and recruitment to the infected tissues and organs (reviewed in REFS 4,5). In many cases, the increased NK-cell-mediated killing was attributed to activation of the NK cells by the production of type I IFNs induced in the host by viral infection, because it was known that NK cells could be directly stimulated by exposure to IFNα IFNβ. Although type I IFNs are not mitogenic for NK cells, they do induce the production of IL-15 (REF. 6), which is a potent growth factor for NK cells, in addition to augmenting the cytotoxicity and cytokine production of NK cells. Hence, the induction of type I IFNs and IL-15 by viral infection could well account for the presence of activated NK cells in virus-infected hosts. Perhaps the first clear evidence indicating cognate recognition of a viral pathogen by NK cells that is important to host protection emerged from studies of mouse cytomegalovirus (MCMV). The MCMV model system has greatly advanced our understanding at a molecular level of how NK cells respond directly, and indirectly, to viral infection and it has revealed a remarkable relationship between the evolution of the host and a persistent virus.
Box 1 NK cells in immunity to microbial pathogens other than viruses
Although natural killer (NK) cells have been studied most extensively in terms of viral and tumour immunity, there is an increasing awareness that NK cells are activated, and in some situations potentially have a protective role, in immune responses to certain microorganisms, including Legionella pneumophila109, Mycobacterium tuberculosis 110–112, Shigella flexneri113, Borrelia burgdorferi114, Brugia malayi115, Leishmania major116,117, Toxoplasma gondii118,119, Cryptococcus neoformans120, Trypanosoma cruzi121, Plasmodium yoelii122 and Plasmodium falciparum (reviewed in REF. 123). As yet, there is no clear evidence for direct, cognate recognition of bacteria, fungi or parasites by NK cells. However, because certain bacterial infections have been shown to induce the expression of NKG2D (NK group 2, member D) ligands, this provides a possible mechanism for NK-cell activation. In addition, the activation of myeloid cells by pathogen-encoded Toll-like-receptor ligands results in the production of pro-inflammatory cytokines, such as interleukin-12 (IL-12) and IL-18, which are potent inducers of interferon-γ (IFNγ) production by NK cells. The rapid secretion of IFNγ by NK cells at the site of infection, causing the activation of macrophages and dendritic cells, might bean important component of the immune response in many of these infections. Animal models and population-based studies of infected humans will be crucial to unveil how NK cells have evolved to respond to these diverse infections.
In this Review, I highlight recent advances in our understanding of the receptors and effector mechanisms used by NK cells to control certain viral infections in humans and mice, and the molecular basis for viral evasion of NK-cell-mediated protection.
NK cells in immunity to cytomegaloviruses
Ly49-receptor-dependent resistance to MCMV
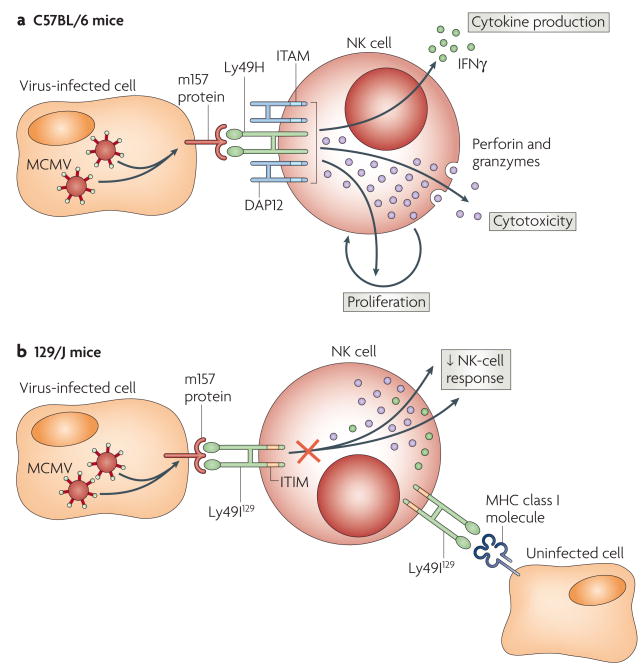
CMVs are herpesviruses, which comprise a family of large double-stranded DNA viruses that commonly infect, among others, humans and mice. Early studies showed that infection with MCMV activates mouse NK cells in vivo7. But more importantly, NK cells are required for the efficient control of MCMV replication in C57BL/6 mice8, and this resistance to infection is conferred by a single dominant genetic locus in the NK-cell complex (NKC) on mouse chromosome 6 (REF. 9). Subsequent studies showed that the activating DAP12-associated receptor Ly49H10,11 is responsible for resistance to MCMV in C57BL/6 mice12–15 and that Ly49H directly binds to a viral glycoprotein, ml57, which is expressed on the surface of MCMV-infected cells soon after infection16–17. Although Ly49H+ NK cells are required for efficient control of MCMV replication, essentially all NK cells (including Ly49H− NK cells from C57BL/6 mice and NK cells from MCMV-sensitive strains of mice lacking the Ly49h gene) become activated after infection with MCMV, probably as a consequence of the cytokine and type I IFN secretion that is induced by the viral infection. However, unlike the NK cells from MCMV-sensitive mouse strains or the Ly49H− NK cells from C57BL/6 mice, Ly49H+ NK cells proliferate and have more persistent activation after infection18. Whether the proliferation of Ly49H+ NK cells after MCMV infection is important for resolution of the disease has not been addressed. It is possible that the protection conferred by Ly49H+ NK cells is mainly due to their more efficient cytolytic activity and cytokine production. Passage of MCMV through resistant C57BL/6 mice results in mutation of the gene encoding m157 (REFS 19,20), and sequencing of MCMV isolated from wild mice revealed frequent loss-of-function mutations in m157 (REF 19), which shows that there is a selective pressure against the expression of m157 in MCMV-infected cells.
These findings raised the question of why MCMV would possess a gene that confers a selective disadvantage to its survival. This might be explained, in part, by the finding that m157 binds not only to the activating Ly49H receptor, but also to inhibitory Ly49 receptors in certain mouse strains16 (FIG. 1). For example, m157 binds the inhibitory receptor Ly49I in MCMV-susceptible 129/J mice, thereby potentially conferring a selective advantage to the virus at the population level. Although Ly49I is expressed by only a small subset of NK cells in 129/J mice, this receptor might be expressed by a greater proportion of NK cells, and thereby have a greater impact on MCMV infection, in other mouse strains. An important distinction is that Ly49I binds to MHC class I ligands, in addition to ml57, where as Ly49H does not bind to any host MHC class I molecules. The crystal structure of ml57 has revealed that this viral protein has significant homology to MHC class I molecules21, which indicates that the virus 'stole' a mouse MHC class I gene to engage inhibitory Ly49 receptors and dampen the NK-cell-mediated response against MCMV, thereby providing a selective advantage. The affinity of m157 for the inhibitory Ly49I receptor is higher than the affinity of its MHC class I ligand21, which is consistent with the concept that the viral protein evolved to suppress NK-cell responses against MCMV-infected cells that had down-regulated the host MHC class I molecules (presumably to evade cytotoxic T cells)22. The extracellular domains of the inhibitory Ly49I and activating Ly49H receptors are similar, and based on mathematical models, it seems that the activating Ly49 receptors evolved more recently that the inhibitory Ly49 receptors23. Diversification of the Ly49 receptor family, with the creation of activating receptors, might well have been driven by selective pressures imposed by viral pathogens, such as MCMV
Figure 1. Activating and inhibitory NK-cell receptors for MCMV.
a. Natural killer (NK) cells from C57BL/6 mice express the DAPl2-associated activating Ly49H receptor, which initiates cell-mediated cytotoxicity (through perforin and granzymes), cytokine production (such as interferon-γ (IFNγ)) and proliferation when these NK cells encounter mouse cytomegalovirus (MCMV)-infected cells that express the viral protein m157 on their cell surface, b. NK cells in certain other strains of mice, such as 129/J mice, have inhibitory receptors such as Ly49l129, which binds to m157 on the cell surface of MCMV-infected cells and dampens the NK-cell response. Ly49l129 binds both host MHC class I ligands and MCMV m157, whereas Ly49H binds only m157 and not host MHC class I molecules. ITAM, immunoreceptor tyrosine-based activation motif; HIM, immunoreceptor tyrosine-based inhibitory motif.
Some mouse strains that lack the Ly49h gene also resist MCMV infection through NK-cell-mediated protection. For example, Ma/My mice, which lack Ly49H, have NK-cell-dependent resistance to MCMV infection24. Genetic analysis showed that genes in the NKC and the H2k haplotype are necessary for resistance. Another DAP12-associated receptor, Ly49P, expressed by Ma/My mice was shown to respond only to MCMV-infected cells expressing H2-Dk, and recognition of these cells was blocked by an H2-Dk-specific monoclonal antibody. These findings surprisingly imply a form of H2-mediated restriction of NK-cell recognition, and indicate that Ly49P either recognizes a peptide induced by MCMV infection of the host cells or a viral protein that associates with H2-Dk. Additional genes in the NKC confer NK-cell-mediated protection against MCMV in wild mice25, and multiple genetic loci are involved in resistance to MCMV in NZW mice26, although the receptors that are involved have not been identified.
Evasion of NKG2D-mediated host protection by MCMV
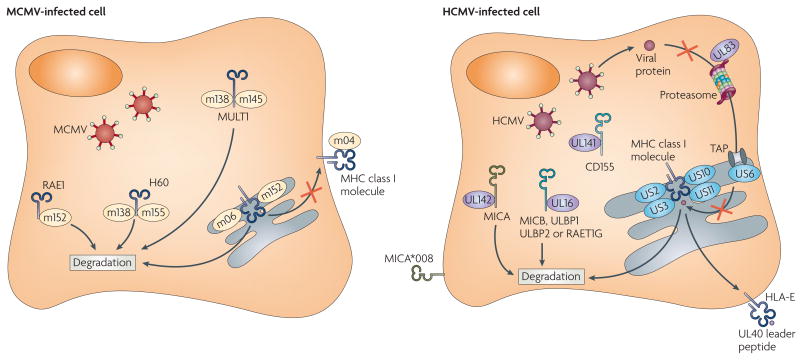
The selective pressures exerted on MCMV have also been shown by studies exploring the role of the activating NKG2D (NK group 2, member D) receptor in viral immunity. The NKG2D receptor, which is expressed by almost all mouse NK cells, recognizes a family of MHC-class-I-related proteins, namely RAE1 (retinoic acid early transcript 1), MULT1 (murine UL16-binding protein (ULBP)-like transcript 1) and H60 (REF. 1). Remarkably, MCMV has devoted considerable resources to blocking the expression of these NKG2D ligands on the surface of infected cells: the viral protein m152 inhibits expression of RAE1 (REF. 27); m155 and m138 downregulate expression of H60 (REFS 28,29); and MULT1 is targeted by m145 and m138 (REFS 29,30) (FIG. 2). In MCMV-infected cells, the genes encoding these NKG2D ligands are transcribed, but the viral proteins interact with and cause the degradation of the NKG2D ligand proteins, thereby circumventing detection by NKG2D on NK cells. MCMV has seemingly blocked all attempts by the NKG2D pathway to exert control on infection by this virus, although given the polymorphic nature of the genes encoding NKG2D ligands, additional alleles or loci might exist in some mouse strains that have not yet been evaded by the virus. Interestingly, some of these viral proteins are multi-functional: m152 downregulates both MHC class I molecules and RAE1 (REF. 27), whereas m138 inhibits the expression of both H60 and MULT1, as well as functioning as a viral Fc receptor29.
Figure 2. HCMV and MCMV proteins affecting NK-cell-mediated recognition of virus-infected cells.
The virus-encoded proteins m04, m06 and m152 inhibit MHC class I expression on the surface of mouse cytomegalovirus (MCMV)-infected cells by a complex process that differs depending on the MHC class I alleles present in the host22. m04 can be expressed on the cell surface of MCMV-infected cells in a complex with MHC class I molecules; however, how this influences the recognition of MHC class I molecules by receptors on natural killer (NK) cells or T cells is unknown. Human cytomegalovirus (HCMV) also blocks the expression of MHC class I molecules in infected cells in an allele-specific manner (reviewed in REF. 65). The HCMV proteins US2, US3, US10 and US11 interact with the MHC class I heavy chains on their own or with the heavy chains complexed with β2-microglobulin, ultimately resulting in their degradation, whereas US6 blocks TAP (transporter associated with antigen processing) function and UL83 inhibits protein entry into the proteasome. UL40 provides a leader peptide that binds to HLA-E allowing its expression on the surface of HCMV-infected cells, presumably for interactions with the inhibitory CD94-NKG2A(NK group 2, member A) receptor on NK cells. Both MCMV and HCMV inhibit expression of the NKG2D ligands in infected cells. The MCMV-encoded m152 protein targets RAE1 (retinoicacid early transcript 1), as well as MHC class I molecules, for degradation; m145 and m138 cooperate to prevent MULTl (murine UL16-binding protein (ULBP)-like transcript 1) expression; and m138 and m155 cause degradation of H60. In humans, the HCMV-encoded UL16 protein inhibits expression of MICB (MHC-class-I-polypeptide-related sequence B), ULBP1, ULBP2 and RAET1G (retinoic-acid early transcript 1C), whereas UL142 prevents expression of MICA. Certain allelesof MICA, such as the common allele MICA*008, are resistant to downregulation by HMCV because of a truncation of the cytoplasmic domain. CD155, a ligand for the activating NK-cell receptors DNAM1 (DNAX adhesion molecule 1)and CD96, is targeted by UL141.
NK-cell-mediated protection against MCMV
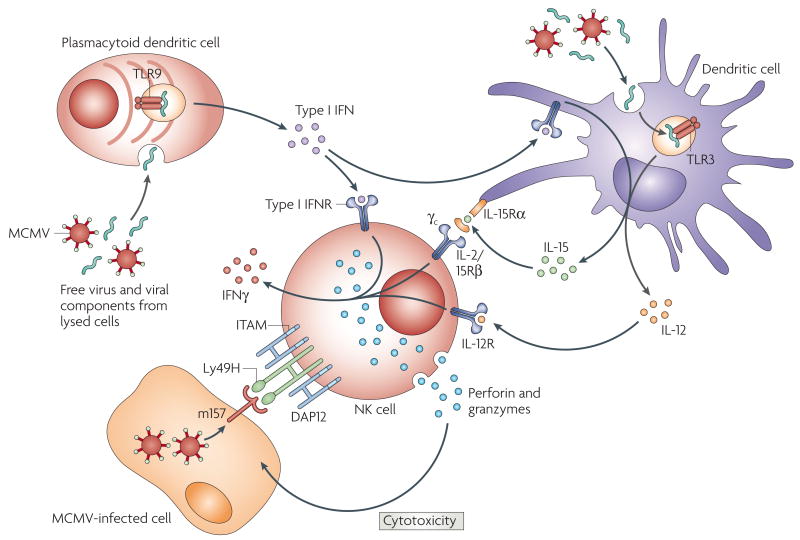
After infection with MCMV, NK cells are activated and are mobilized to sites of infection, such as the liver. This recruitment of NK cells in C57BL/6 mice requires virus-induced production of IFNα, which induces the expression of CC-chemokine ligand 2 (CCL2; also known as MCP1) by resident macrophages to recruit CC-chemokine receptor 2 (CCR2)-bearing macrophages into the liver, which in turn produce CCL3 (also known as MIP1α) that attracts NK cells to the site of infection31–32. The importance of type I IFN in resistance to MCMV infection has been shown by studies using mice deficient in Toll-like receptor 2 (TLR2), TLR3 or TLR9 or their downstream signalling adaptors TRIP (TIR (Toll/IL-1-receptor)-domain-containing adaptor protein inducing IFNβ) and MyD88, which are required for efficient IFN induction33–34. Plasmacytoid dendritic cells (pDCs; also known as interferon-producing cells (IPCs)) and DCs are the main source of the type I IFN that is required for NK-cell activation and recruitment35 (FIG. 3). Type I IFNs induce DCs to produce IL-15 (REF. 36), an important NK-cell growth and differentiation factor that is required for NK-cell-mediated control of MCMV. Moreover, MCMV infection induces IL-12 and IL-18 secretion by DCs, and these cytokines augment the production of IFNγ by NK cells37–38. Reciprocally, activated Ly49H+ NK cells in C57BL/6 mice protect DCs from infection and destruction by MCMV37 (FIG. 3). Studies using perforin-or IFNγ-deficient NK cells have shown that both of these effector molecules are required for the efficient control of MCMV replication in infected mice39–40. NK cells themselves express certain TLRs — for example, activated NK cells express TLR3 and TLR9 (REFS 41–44) — but it is not known whether intrinsic TLR-dependent activation of NK cells during viral infection, rather than TLR-mediated DC activation, is important in disease control.
Figure 3. Interactions between DCs and NK cells during MCMV infection.
Infection with mouse cytomegalovirus (MCMV) triggers the production of type I interferons(IFNs) by plasmacytoid dendritic cells (pDCs; also known as interferon-producing cells (IPCs)) through Toll-like receptor 9 (TLR9) and the production of cytokines, such as interleukin-12 (IL-12), by DCs through TLR3, as well as type I IFN-induced production of IL-15 by DCs. IL-15 is presented to natural killer (NK) cells by the IL-15 receptor α-chain (IL-15Rα) on the cell surface of DCs, resulting in NK-cell activation. IL-12, in combination with IL-15 and potentially other pro-inflammatory cytokines, induces the secretion of IFNγ by NK cells. About half of the NK cells in C57BL/6 mice express the DAP12-associated Ly49H receptor and recognize the MCMV-encoded m157 glycoprotein that is present on the cell surface of MCMV-infected cells. Interactions between Ly49H on the NK cells and m157 on the infected cells cause extensive proliferation of the Ly49H+ NK cells, as well as cell-mediated cytotoxicity (due to the directional release of perforin and granzymes) and cytokine production. IFNγ produced by activated NK cells amplifies the maturation and effector functions of DCs and other cells in the microenvironment. ITAM, immunoreceptor tyrosine-based activation motif.
NK cells provide early control of MCMV replication, but they can also influence the ensuing virus-specific T-cell-mediated response. Robbins and colleagues45 have reported that in congenic BALB/c mice with the protective C57BL/6 Ly49h gene, NK cells decrease the viral load, thereby decreasing the production of type I IFNs by pDCs, which is immunosuppressive to the host. As a result of the decreased type I IFN production by pDCs, cytotoxic T lymphocytes (CTLs) are generated more rapidly. However, consistent with prior observations46, NK cells ultimately restrain the expansion and production of IFNγ by virus-specific CD4+ and CD8+ T cells in MCMV-infected C57BL/6 hosts. In other words, although NK cells seem to augment the generation of CTLs early during the immune response to MCMV infection, NK cells also, by as-yet-undefined mechanisms, ultimately restrain the clonal expansion of virus-specific CD4+ and CD8+ T cells later after infection. However, interpretation of these experiments involving the depletion of NK cells or type I IFNs is complicated because these treatments markedly alter the viral load, which itself might also significantly affect the T-cell response.
NK-cell recognition of human CMV
Functional Ly49 genes do not exist in humans, and so far there is no evidence that their counterparts, the KIR (killer-cell immunoglobulin-like receptor) genes, encode receptors analogous to Ly49H or Ly49P that can directly recognize human CMV-infected cells. Nonetheless, a human patient selectively lacking NK cells, but with normal B and T cells, was found to suffer life-threatening illness after infection with HCMV47. There are intriguing hints that NK cells that express the DAP12-associated CD94-NKG2C receptor complex preferentially proliferate after co-culture with HCMV-infected cells48. Both the activating CD94-NKG2C receptor complex and the highly related inhibitory CD94-NKG2A receptor complex recognize HLA-E as their ligand49, although these functionally diverse receptors might discriminate between different peptides presented by HLA-E50, potentially allowing the selective activation of CD94-NKG2C-bearing NK cells to mediate immunity against HCMV. The leader peptide derived from the HCMV protein UL40 can assemble into the peptide-binding groove of HLA-E and when this UL40 peptide is overexpressed with HLA-E it can engage the inhibitory CD94-NKG2A receptor complex and suppress NK-cell activation51–53. Further studies are required to determine how the CD94-NKG2A and CD94-NKG2C receptor complexes potentially regulate the immune response to HCMV
CMVs are exquisitely species specific, and each CMV has evolved and adapted to its own mammalian host. Of note, the CMV genes that are involved in immune evasion have evolved independently and are tailored to the host species. For example, the proteins encoded by HCMV that are responsible for downregulating MHC class I expression in humans have no structural homology to the MCMV proteins that disrupt mouse MHC class I expression (reviewed in REF. 54). However, similar to MCMV, HCMV has developed strategies to evade the human NKG2D receptor, and has done so by evolving unique genes to prevent expression of the human NKG2D ligands, MICA (MHC-class-I-polypeptide-related sequence A), MICB and ULBP (UL16-binding protein) (FIG. 2). HCMV UL16 was first shown to target MICB, ULBP1, ULBP2 and RAET1G (retinoic acid early transcript 1G) for degradation55–56, whereas more recently, UL142 has been shown to block the expression of proteins encoded by some, but not all, alleles of MICA57. Another potential mechanism for the disruption of NKG2D-ligand expression was indicated by the finding that a microRNA encoded by HCMV miR-UL112, selectively targets MICB for downregulation in infected cells58. The extraordinary polymorphism of the genes encoding MICA (61 alleles) and MICB (30 alleles) (see the HLA Informatics Group website) might reflect the host's attempt to counter the viral immune-evasion strategies that thwart NKG2D-mediated immune responses. In this regard, a commonly expressed allele of MICA, MICA*008, has a premature stop codon that truncates the cytoplasmic domain and renders it resistant to downregulation by HCMV59, thereby possibly providing an advantage to humans possessing this allele.
HCMV encodes other proteins that affect NK-cell activation. UL18. an HCMV-encoded MHC class I protein that binds peptides and associates with β2-microglobulin, binds with high affinity to the inhibitory receptor ILT2 (immunoglobulin-like transcript 2; also known as LIR1, CD85j and LILRB1)60. ILT2, which is expressed by a subset of NK cells and by all monocytes, recognizes an epitope in the α3 domain of MHC class I molecules that is conserved in most human HLA-A, HLA-B, HLA-C, HLA-E, HLA-F and HLA-G proteins61. Presumably, UL18 engages the inhibitory ILT2 receptor and suppresses immune responses against HCMV-infected cells that have downregulated their expression of MHC class I proteins. However, it has proved essentially impossible to detect UL18 on the surface of HCMV-infected cells to validate this speculation62.
The UL141 protein encoded by HCMV specifically targets and prevents the expression of CD155, a cell-surface glycoprotein that functions as a ligand for the activating NK-cell receptors DNAM1 (DNAX adhesion molecule 1; also known as CD226) and CD96 (REF. 63) (FIG. 2). Interestingly, CD155 functions as a poliovirus receptor, but whether NK cells are involved in protection against poliovirus has not been addressed. The HCMV structural protein pp65 has been reported to interact with the activating NKp30 receptor on NK cells and dissociate the receptor from its CD3ζ signalling adaptor protein, thereby preventing NK cells from recognizing and attacking HCMV-infected cells64. However, a mechanism to explain how pp65 might gain access to the transmembrane regions of NK cells is lacking, and the finding is as yet unconfirmed.
Downregulation of MHC class I molecules by CMVs
CMVs have invested a considerable amount of their genomes to ensure that the expression of host MHC class I proteins is disrupted; MCMV proteins m04, m06 and ml52 block the expression of certain MHC class I gene products in infected mouse cells22, and the HCMV proteins US2, US3, US6, US10, US11 and UL83 disrupt human MHC class I expression by numerous strategies, including inhibiting the generation of peptides and disrupting the intracellular trafficking and assembly of the MHC class I heavy chains (reviewed in REF 65) (FIG. 2). According to the missing-self hypothesis66, the downregulation of MHC class I expression on virus-infected cells should render them more susceptible to attack by NK cells. Surprisingly, in vitro experiments to address this issue haven't convincingly supported this assumption. Many of the studies testing the ability of human NK cells to kill HCMV-infected cells have used allogeneic fibroblasts as targets, thereby greatly complicating interpretation of the results. In one of the few studies using autologous fibroblasts as targets and a mutant HCMV strain that lacked most of the proteins (US2-US11) that downregulate HLA class I expression, there was little evidence that down-regulation of MHC class I expression had a significant impact on the ability of human NK cells to recognize and respond to the HCMV-infected cells67. Similarly, whether the decreased expression of MHC class I molecules owing to the actions of m04, m06 and m152 has a significant impact on the ability of mouse NK cells to control infection with MCMV has not been addressed (although this is complicated by the ability of m152 to downregulate expression of both MHC class I molecules and the NKG2D ligand RAE1). So, the jury is still out on whether the modulation of MHC class I expression by CMVs is important in NK-cell-mediated control of these viruses.
Immunoevasion of NK cells by rat CMV
Recent studies have also identified a viral protein encoded by rat CMV that functions as a virulence factor by engaging an inhibitory NKR-P1B receptor on rat NK cells68, documenting yet another mechanism devised by CMVs to avoid NK-cell-mediated host protection. Interestingly, polymorphic variants of rat NKR-P1B have evolved that do not bind to this viral decoy, again indicating that the virus might drive evolution of this NK-cell receptor family. Collectively, the existence of numerous proteins in mouse, human and rat CMV that modulate the cellular ligands of the activating and inhibitory NK-cell receptors strongly supports the importance of NK cells in host defence against this pathogen.
NK cells in immunity to other herpesviruses
NK cells also participate in immune responses against other herpesviruses, although their role in these has been less well characterized than for CMVs. The first human patient who was found to selectively lack NK cells suffered from severe infections not only with HCMV, but also with varicella zoster virus (VZV) and herpes simplex virus (HSV)47. Although there is extensive older literature documenting the activation of NK cells during HSV-1 infection (reviewed in REF. 4), it remains unclear whether there is cognate recognition of the HSV-1-infected cells by NK cells or whether the effects of HSV-1 on NK cells are an indirect result of the high-level production of type I IFN and other cytokines that is induced by HSV infection. There are no reports of any viral proteins encoded by HSV-1 or HSV-2 that preferentially modulate or function as decoys for ligands of known NK-cell receptors. Although it has been controversial whether NK cells control the replication of HSV-1 in mice, genetic resistance to HSV-1 infection in mice has been mapped to a locus on mouse chromosome 6 near the NKC region69, but this locus is distinct from that of the Ly49h gene that is responsible for resistance to MCMV70. Recently, another NK-cell-deficient paediatric patient died of VZV infection, which indicates that NK cells have a crucial role in protection against this pathogen71. A more detailed evaluation of the interaction between NK cells and VZV-infected cells is warranted.
Epstein-Barr virus (EBV), another human herpesvirus, transforms B cells, causing B-cell lymphomas in immunosuppressed patients. EBV-transformed B-cell lines are relatively resistant to NK-cell-mediated attack, mainly owing to their high level of expression of MHC class I molecules. However, a recent study showed that when latent EBV was reactivated in transformed B cells, they became susceptible to NK-cell-mediated lysis, which was partially inhibited by blocking the activating NKG2D and DNAM1 receptors on NK cells72. In addition, a patient with a selective immunodeficiency of NK cells, caused by a mutant gene mapping to chromosome 8, has been reported to have an EBV-associated lymphoproliferative disorder73. Epidemiological studies have indicated a protective effect for the activating KIR2DS1 and KIR3DS1 genes in patients with EBV-associated Hodgkin’s lymphoma74, although there is as yet no direct evidence for the involvement of these receptors in the recognition of EBV-transformed cells.
NK cells in immunity to poxviruses
Other than MCMV the clearest evidence for an essential protective role of NK cells in viral infection comes from studies of mouse-pox (ectromelia) virus — a member of the orthopoxvirus genus, which includes variola virus (the causative agent of smallpox) and vaccinia virus. In mousepox-resistant mouse strains, such as C57BL/6 mice, the depletion of NK cells results in massive viraemia and death75. Similar to resistance to MCMV NK-cell-mediated resistance to mousepox virus was mapped to the NKC on chromosome 6 by experiments in which resistant C57BL/6 mice were crossed with susceptible DBA/2J mice, although other genes were also implicated76. Host protection requires the concerted action of NK cells, T cells and antibodies77–78, with type I and II IFNs and perform being crucial components of the response75–79–80. Recent studies have shown that the activating NKG2D receptor of NK cells is responsible, in part, for the resistance of C57BL/6 mice to infection with mouse-pox virus81. Mousepox virus induces the expression of NKG2D ligands in infected tissues and treatment of infected mice with a neutralizing NKG2D-specific antibody results in increased viral titres and mortality in C57BL/6 mice. The depletion of NK cells results in higher mortality than does blocking NKG2D, which indicates that other NK-cell pathways are involved in the protective response.
Human NK cells lyse vaccinia-virus-infected cells and this can be partially blocked by antibodies specific for NKp30, NKp44 and NKp46 (REF. 82). On infected cells, vaccinia virus seems to cause downregulation of HLA-E, but not of other MHC class I proteins, thereby causing the preferential lysis of these target cells by NK cells that express the inhibitory CD94-NKG2A receptor, which recognizes HLA-E83. Although it is not a natural pathogen of mice, higher litres of vaccinia virus were detected in the spleen and liver of infected mice that had been depleted of NK cells with an NKl.l-specific monoclonal antibody compared with controls8. Interestingly, the depletion of NK cells resulted in higher vaccinia virus litres in C57BL/6, but not C3H mice8; however, the Ly49H+ NK cells that are responsible for the control of MCMV are not required for control of vaccinia virus13–18.
Monkeypox and cowpox viruses (but not vaccinia or variola viruses) encode soluble proteins that function as NKG2D receptor antagonists84. So, whereas MCMV and HCMV have evolved the strategy of retaining and degrading NKG2D ligands inside infected cells, monkeypox and cowpox viruses have devised another mechanism to evade NKG2D-mediated host immunity: they mask the NKG2D receptor on NK cells and T cells before they have the opportunity to interact with infected cells. It therefore seems that avoiding NKG2D is a common theme in viral evasion.
NK cells in immunity to other viruses
Influenza virus
As in other infections, NK cells become activated in influenza-virus-infected hosts as a consequence of IFN and cytokine production by DCs and possibly by other cell types. In humans, NK cells are activated and produce IFNγ when co-cultured with autologous DCs infected with influenza A virus, and this NK-cell activation is blocked in the presence of neutralizing antibodies specific for NKG2D and NKp46 (REF. 85). The activation of NK cells by influenza-A-virus-infected DCs also depends on the secretion of IFNα and IL-12 by the DCs, which promotes NK-cell-mediated cytotoxicity and IFNγ production, respectively. Previous studies have indicated that NKp46 binds to influenza virus haemag-glutinin86 to allow NK-cell-mediated recognition of influenza-virus-infected cells. NK cells accumulate in the lungs of mice infected with influenza virus, and although NK-cell migration to the lungs was normal in NKp46-deficient mice, these animals suffered higher mortality than their control counterparts87.
Hepatitis viruses
Epidemiological studies have shown an intriguing relationship between the highly polymorphic HLA and KIR genes and the resolution of hepatitis C virus (HCV) infection88. Previous studies reported that many of the inhibitory KIR proteins expressed by NK cells bind to HLA-C proteins on the surface of target cells and thereby prevent killing of these targets. HLA-C proteins have been divided into two categories, group 1 and group 2, on the basis of a dimorphic polymorphism in the al domain of the HLA-C heavy chain. These group 1 and group 2 HLA-C proteins are specifically recognized by different members of the KIR family (reviewed in REF. 89). Khakoo and colleagues observed that the ability to clear HCV infections is associated with individuals possessing the KIR2DL3 and HLA-C group 1 alleles88. Although functional studies to precisely detail the mechanism have not yet been reported, these investigators speculate that an individual expressing an inhibitory KIR with low affinity for its ligands (as is the case with KIR2DL3 and HLA-C) might mount a stronger attack against viral infection, thereby resolving the disease. That HCV might be trying to evade NK cells is also indicated by the finding that E2, the main HCV envelope protein, binds to CD81 on the surface of human NK cells and suppresses NK-cell cytokine production, proliferation and cytotoxicity90–91. The difficulties in culturing HCV and the lack of an animal model for this disease hamper mechanistic studies to further dissect this potentially important interaction between NK cells and HCV-infected cells.
In transgenic mouse models of human hepatitis B virus (HBV) infection92–93, a population of non-classical CD1d-restricted NKT cells are required to initiate the liver pathology. Blocking NKG2D can efficiently prevent acute hepatitis in this system94–95. In this mouse model of HBV, NK cells alone are unable to cause hepatitis, but they might interact with activated NKT cells to influence the disease course. Unlike CMV, HBV has a small genome and is probably unable to avoid its NKG2D-dependent recognition and activation of lymphocytes. Instead, HBV antigens can lead to the upregulation of RAE1 expression on infected hepatocytes and thereby affect NKG2D expression on NKT cells, their subsequent cytokine production and the resulting hepatopathology95.
HIV-1
As for HCV, KIR and HLA polymorphisms have been reported to significantly influence the progression to AIDS in HIV-infected individuals96. Previous studies had shown that HIV-infected individuals expressing HLA-B alleles with the Bw4 epitope have delayed onset of AIDS97; however, protection is significantly increased when the individual also possesses certain alleles of the KIR3DL1 locus96. Different alleles of the KIR3DL1 gene either encode inhibitory KIR3DL1 receptors that have immunoreceptor tyrosine-based inhibitory motifs (ITIMs) that suppress NK-cell activation when encountering target cells expressing an HLA-Bw4 ligand98 or encode an activating receptor, designated KIR3DS1, that lacks ITIMs and transmits activating signals through its association with the immunoreceptor tyrosine-based activation motif (ITAM)-bearingDAP12 adaptor99–100. Possession of either an inhibitory KIR3DL1 receptor or the activating KIR3DS1 receptor, together with an HLA-Bw4 allele encoding an isoleucine residue at position 80 (HLA-Bw4-I80) significantly slows progression to AIDS96. HIV-infected individuals with this compound KIR3DS1 plus HLA-Bw4-I80 haplotype are protected against opportunistic infection, but not AIDS-associated malignancies101.
The inhibitory KIR3DL1 molecules clearly recognize HLA-Bw4 proteins98–102, but attempts to show direct interactions between KIR3DS1 and HLA-Bw4 proteins have failed99–103. Although evidence for the direct binding of KIR3DS1 to HLA-Bw4 was lacking, Alter and colleagues have reported that KIR3DS1+ NK cells, but not KIR3DS1 - NK cells, suppress HIV replication in HLA-Bw4-infected cells in vitro104. However, how the inhibitory KIR3DL1 confers protection is counterintuitive. Remarkably, the KIR allele shown to be most protective, KIR3DL1*004 (REF. 96), encodes a protein that cannot be expressed on the cell surface of NK cells and is probably degraded intracellularly105. Clearly, this is a puzzle that when resolved will greatly alter the current paradigms of NK-cell recognition.
Interestingly, the Nef (negative factor) protein encoded by HIV has been shown to preferentially downregulate the expression of HLA-A and HLA-B, but not HLA-C106. Potentially, this provides a mechanism for HIV-infected cells to avoid recognition by HLA-A- and HLA-B-restricted HlV-specific CTLs, but to engage the inhibitory KIR expressed by NK cells in the infected individual. A recent study has shown that Nef might also downregulate expression of NKG2D ligands107, which indicates another potential evasion mechanism.
Concluding remarks
Studies of humans and mice have clearly shown that there is a robust activation of NK cells during viral infections. Lessons from MCMV have surprisingly shown that the activation of NK cells alone is insufficient to control viral replication or mediate resolution of the infection. For example, NK cells in BALB/c mice are clearly activated by MCMV infection, as shown by the upregulation of expression of activation markers on these NK cells and by their robust production of IFNγ; nonetheless, the depletion of NK cells in BALB/c mice has much less impact on viral litres than it does in C57BL/6 mice. Only when the Ly49h gene was introduced into BALB/c mice were the NK cells able to substantially control MCMV infection15. These striking findings clearly indicate that it is necessary to distinguish between a non-productive NK-cell response and a productive NK-cell response in different viral infections to understand whether NK cells are helpful, neutral or even potentially detrimental to the host. An example of the last situation comes from studies of infection with Listeria monocytogenes, an intracellular bacterial infection, in which depletion of NK cells increases the ability of the host to control the infection108.
The importance of NK cells in viral infection is perhaps best supported by the fact that several unrelated viral pathogens have evolved strategies to counter recognition by NK cells. Similar to the numerous examples of viruses manipulating expression of MHC class I molecules in infected host cells, presumably to avoid recognition by cytotoxic T lymphocytes, the NKG2D pathway seems to be another favourite target for viral evasion of NK cells, as viral proteins that disrupt the host's NKG2D ligands have already been discovered in the herpesvirus and poxvirus families. New insights into the relevance of other NK-cell receptors will probably emerge from studies of other viral virulence factors that result in evasion of NK-cell-mediated immunity.
Perhaps the most intriguing revelation is that viruses seem to be driving the evolution of the mammalian genes encoding the NK-cell receptors or their ligands. This is best exemplified by the apparent conversion of the inhibitory receptors encoded by Ly49i, which recognize the MHC-class-I-like m157 glycoprotein of MCMV into the activating receptors encoded by Ly49h, thereby conferring NK-cell-mediated resistance to MCMV in C57BL/6 mice. The remarkable diversity in the KIR gene family and hints from epidemiological studies in HIV- and HCV-infected individuals indicate that viruses might be shaping, at the population level, human evolution. Achieving a balance of these inhibitory and activating NK-cell receptors in the human population might allow for survival of the host, but also the viruses.
Acknowledgments
I thank R. Welsh, L. Sigal, M. Fang, E. Mocarski, S. Vilarinho and members of my laboratory for helpful discussion. I am an American Cancer Society Research Professor supported by grants from the National Institutes of Health, USA.
- Granzymes
Proteolytic enzymes that are present in the cytoplasmic granules of cytotoxic T lymphocytes and natural killer cells. Granzymes activate caspases in the target cells, and this causes apoptosis.
- Perforin
A protein with similarity to the ninth component of complement. It is present in the cytoplasmic granules of cytotoxic T lymphocytes and natural killer cells. Perforin subunits assemble into a pore-forming structure that causes membrane damage in the target cell.
- Ma/My mice
A strain of inbred mice that is relatively resistant to infection with mouse cytomegalovirus MCMV). These mice express the activating NK-cell receptor Ly49P, which recognizes MCMV-infected cells that express H2-Dk.
- Plasmacytoid dendritic cells
(pDCs). Also known as interferon-producing cells. A type of dendritic cell that is specialized for the production of type I interferons after stimulation by viruses.
- HLA-E
A human MHC class I molecule that is composed of the HLA-E heavy chain, β2-microglobulin. and a peptide that is often derived from the leader peptide: of other MHC class polypeptides or from certain microbial pathogens.
- NKT cells
(Natural killer T cells). A subset of T cells expressing an invariant αβ T-cell receptor (TCR) that can recognize the lipid α-galactoceramide bound to CD 1 d. Another population referred to as non-classical or type II NKT cells also recognize CD 1 d-associated antigens, but are unable to bind α-galactoceramide and do not express the invariant αβ TCR.
- Bw4 epitope
The proteins encoded by the HLA-B gene (~960 alleles) are divided into two groups, designated Bw4 and Bw6 based on a serologically defined epitope at amino-acid residues 77–83 in the α domain of the HLA-B heavy chain. The Bw4 epitope is also present in a small subset of HLA-A proteins. The Bw4 epitope is recognized by the inhibitory natural-killer-cell receptor KIR3DL1.
- Immunoreceptor tyrosine-based inhibitory motif (ITIM).
A sequence motif, defined as Ile/Val/Leu/Ser-x-Tyr-x-x-Leu/Val (where x denotes any amino acid) present in the cytoplasmic domain of inhibitory receptors. When the tyrosine residue is phosphorylated, ITIMs recruit lipid or tyrosine phosphatases. such as SHP1, SHP2 or SHIP which mediate the inhibitory function of these receptors.
References
- 1.Lanier LL. NK cell recognition. Anna Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 2.Stetson DB, et al. Constitutive cytokine mRNAs mark natural killer (NK) and NKT cells poised for rapid effector function. J Exp Meci. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fehniger TA, et al. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 2007;26:798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Welsh RM, Vargas-Cortes M. In: The Natural Killer Cell. Lewis CE, McGee JOD, editors. IRL Press; Oxford. UK: 1992. pp. 108–150. [Google Scholar]
- 5.Lodoen MB, Lanier LL. Viral modulation of NK cell immunity. Nature Rev Microbiol. 2005;3:59–69. doi: 10.1038/nrmicro1066. [DOI] [PubMed] [Google Scholar]
- 6.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CDS+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 7.Quinnan GV, Manischewitz JE. The role of natural killer cells and antibody-dependent cell-mediated cytotoxicity during murine cytomegalovirus infection. J Exp Meci. 1979;150:1549–1554. doi: 10.1084/jem.150.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukowski JF, Woda BA, Habu S, Okumura K, Welsh RM. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis in vivo. Jlmmunol. 1983;131:1531–1538. [PubMed] [Google Scholar]
- 9.Scalzo AA, et al. The effect of the Cmv-1 resistance gene, which is linked to the natural killer cell gene complex, is mediated by natural killer cells. Jlmmunol. 1992;149:581–589. [PubMed] [Google Scholar]
- 10.Smith KM, Wu J, Bakker ABH, Phillips JH, Lanier LL. Cutting edge: Ly49D and Ly49H associate with mouse DAP 12 and form activating receptors. Jlmmunol. 1998;161:7–10. [PubMed] [Google Scholar]
- 11.Sjolin H, et al. Pivotal role of KARAP/DAP12 adaptor molecule in the natural killer cell-mediated resistance to murine cytomegalovirus infection. J Exp Med. 2002;195:825–834. doi: 10.1084/jem.20011427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown MG, et al. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 2001;292:934–937. doi: 10.1126/science.1060042. [DOI] [PubMed] [Google Scholar]
- 13.Daniels KA, et al. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J Exp Med. 2001;194:29–44. doi: 10.1084/jem.194.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HS, et al. Susceptibility to mouse cytomegalovirus is associated with depletion of an activating natural killer cell receptor of the C-type lectin superfamily. Nature Genetics. 2001;28:42–45. doi: 10.1038/ng0501-42. [DOI] [PubMed] [Google Scholar]
- 15.Lee SH, et al. Transgenic expression of the activating natural killer receptor Ly49H confers resistance to cytomegalovirus in genetically susceptible mice. J Exp Med. 2003;197:515–526. doi: 10.1084/jem.20021713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 17.Smith HR, et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc NatlAcad Sci USA. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. References 16 and 17 show that the activating NK-cell receptor Ly49H directly recognizes the MCMV-encoded m157 protein on the surface of MCMV-infected cells, which was the first demonstration of specific cognate recognition of a viral pathogen by NK cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dokun AO, et al. Specific and nonspecific NK cell activation during virus infection. Nature Immnunol. 2001;2:951–956. doi: 10.1038/ni714. [DOI] [PubMed] [Google Scholar]
- 19.Voigt V, et al. Murine cytomegalovirus m 157 mutation and variation leads to immune evasion of natural killer cells. Proc NatlAcad Sci USA. 2003;100:13483–13488. doi: 10.1073/pnas.2233572100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.French AR, et al. Escape of mutant double-stranded DNA virus from innate immune control. Immunity. 2004;20:747–756. doi: 10.1016/j.immuni.2004.05.006. References 19 and 20 show that selective pressures by NK cells expressing the activating Ly49H receptor force mutation of the gene encoding m 157 in MCMV. [DOI] [PubMed] [Google Scholar]
- 21.Adams EJ, et al. Structural elucidation of the m 157 mouse cytomegalovirus ligand for Ly49 natural killer cell receptors. Proc NatlAcad Sci USA. 2007;104:10128–10133. doi: 10.1073/pnas.0703735104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagner M, Gutermann A, Podlech J, Reddehase MJ, Koszinowski UH. Major histocompatibility complex class I allele-specific cooperative and competitive interactions between immune evasion proteins of cytomegalovirus. J Exp Med. 2002;196:805–816. doi: 10.1084/jem.20020811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abi-Rached L, Parham P. Natural selection drives recurrent formation of activating killer cell immunoglobulin-like receptor and Ly49 from inhibitory homologues. J Exp Med. 2005;201:1319–1332. doi: 10.1084/jem.20042558. This is a report on the computational analysis of the sequences of human, primate and mouse NK-cell receptors, which shows that the inhibitory receptors are evolutionarily older than the related activating receptors, and indicates that strong selective pressures, probably mediated by microbial pathogens, are driving the evolution of these mammalian genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desrosiers MP, et al. Epistasis between mouse Klra and major histocompatibility complex class I loci is associated with a new mechanism of natural killer cell-mediated innate resistance to cytomegalovirus infection. Nature Genet. 2005;37:593–599. doi: 10.1038/ng1564. This study shows that resistance to MCMV in Ma/My mice requires genes in both the NKC and MHC class I regions. The activating Ly49P NK-cell receptor from Ma/My mice can only recognize MCMV-infected cells that express H2-Dk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adam SG, et al. Cmv4, a new locus linked to the NK cell gene complex, controls innate resistance to cytomegalovirus in wild-derived mice. J Immunol. 2006;176:5478–5485. doi: 10.4049/jimmunol.176.9.5478. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez M, Sabastian P, Clark P, Brown MG. Cmv 1 -independent antiviral role of NK cells revealed in murine cytomegalovirus-infected New Zealand white mice.J. Immunol. 2004;173:6312–6318. doi: 10.4049/jimmunol.173.10.6312. [DOI] [PubMed] [Google Scholar]
- 27.Lodoen M, et al. NKG2D-mediated natural killer cell protection against cytomegalovirus is impaired by viral gp40 modulation of retinoic acid early inducible 1 gene molecules. J Exp Med. 2003;197:1245–1253. doi: 10.1084/jem.20021973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lodoen M, et al. The cytomegalovirus m 155 gene product subverts NK cell antiviral protection by disruption of H60-NKG2D interactions. J Exp Med. 2004;200:1075–1081. doi: 10.1084/jem.20040583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lenac T, et al. The herpesviral Fc receptor fcr-1 down-regulates the NKG2D ligands MULT-1 and H60. J Exp Med. 2006;203:1843–1850. doi: 10.1084/jem.20060514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krmpotic A, et al. NK cell activation through the NKG2D ligand MULT-1 is selectively prevented by the glycoprotein encoded by mouse cytomegalovirus gene m145. J Exp Med. 2005;201:211–220. doi: 10.1084/jem.20041617. References 27–30 show that MCMV has evolved several genes to prevent the expression of the host proteins RAE1, MULT1 and H60 in MCMV-infected cells, and that these viral proteins function as virulence factors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salazar-Mather TP, Orange JS, Biron CA. Early murine cytomegalovirus (MCMV) infection induces liver natural killer (NK) cell inflammation and protection through macrophage inflammatory protein lα (MIP-1α)-dependent pathways. J. Exp Med. 1998;187:1–14. doi: 10.1084/jem.187.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hokeness KL, Kuziel WA, Biron CA, Salazar-Mather TP. Monocyte chemoattractant protein-1 and CCR2 interactions are required for IFN-α/β-induced inflammatory responses and antiviral defense in liver. J Immunol. 2005;174:1549–1556. doi: 10.4049/jimmunol.174.3.1549. [DOI] [PubMed] [Google Scholar]
- 33.Tabeta K, et al. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc Natl Acad Sci USA. 2004;101:3516–3521. doi: 10.1073/pnas.0400525101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szomolanyi-Tsuda E, Liang X, Welsh RM, Kurt-Jones EA, Finberg RW. Role for TLR2 in NK cell-mediated control of murine cytomegalovirus in vivo. J Virol. 2006;80:4286–4291. doi: 10.1128/JVI.80.9.4286-4291.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krug A, et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity. 2007;26:503–517. doi: 10.1016/j.immuni.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nature Immunol. 2003;4:175–181. doi: 10.1038/ni880. This study describes reciprocal and mutually beneficial interactions between NK cells and DCs during infection with MCMV. [DOI] [PubMed] [Google Scholar]
- 38.Dalod M, et al. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon α/β. J Exp Med. 2003;197:885–898. doi: 10.1084/jem.20021522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tay CH, Welsh RM. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J Virol. 1997;71:267–275. doi: 10.1128/jvi.71.1.267-275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loh J, Chu DT, O'Guin AK, Yokoyama WM, Virgin HW. Natural killer cells utilize both perforin and γ interferon to regulate murine cytomegalovirus infection in the spleen and liver. J Virol. 2005;79:661–667. doi: 10.1128/JVI.79.1.661-667.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sivori S, et al. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci USA. 2004;101:10116–10121. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt KN, et al. APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. J Immunol. 2004;172:138–143. doi: 10.4049/jimmunol.172.1.138. [DOI] [PubMed] [Google Scholar]
- 43.Girart MV, Fuertes MB, Domaica CL, Rossi LE, Zwirner NW. Engagement of TLR3, TLR7, and NKG2D regulate IFN-γ secretion but not NKG2D-mediated cytotoxicity by human NK cells stimulated with suboptimal doses of IL-12. J. Immunol. 2007;179:3472–3479. doi: 10.4049/jimmunol.179.6.3472. [DOI] [PubMed] [Google Scholar]
- 44.Zhang SY, et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 45.Robbins SH, et al. Natural killer cells promote early CD8 T cell responses against cytomegalovirus. PLoS Pathog. 2007;3:e123. doi: 10.1371/journal.ppat.0030123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Su HC, et al. NK cell functions restrain T cell responses during viral infections. Eur: J Immunol. 2001;31:3048–3055. doi: 10.1002/1521-4141(2001010)31:10<3048::aid-immu3048>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 47.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. W Eng J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 48.Guma M, et al. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood. 2005;107:3624–3631. doi: 10.1182/blood-2005-09-3682. [DOI] [PubMed] [Google Scholar]
- 49.Braud VM, et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B, and C. Nature. 1998;391:795–798. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 50.Llano M, et al. HLA-E-bound peptides influence recognition by inhibitory and triggering CD94/NKG2 receptors: preferential response to an HLA-G-derived nonamer. Eur J Immunol. 1998;28:2854–2863. doi: 10.1002/(SICI)1521-4141(199809)28:09<2854::AID-IMMU2854>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 51.Tomasec P, et al. Surface expression of HLA-E-, an inhibitor of natural killer cells, enhanced by human cytomegalovirus gpUL40. Science. 2000;287:1031–1033. doi: 10.1126/science.287.5455.1031. [DOI] [PubMed] [Google Scholar]
- 52.Ulbrecht M, et al. Cutting edge: the human cytomegalovirus UL40 gene product contains a ligand for HLA-E and prevents NK cell-mediated lysis. J Immunol. 2000;164:5019–5022. doi: 10.4049/jimmunol.164.10.5019. [DOI] [PubMed] [Google Scholar]
- 53.Wang EC, et al. UL40-mediated NK evasion during productive infection with human cytomegalovirus. Proc Natl Acad Sci USA. 2002;103:2292–2297. doi: 10.1073/pnas.112680099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pinto AK, Hill AB. Viral interference with antigen presentation to CDS+ T cells: lessons from cytomegalovirus. Viral Immunol. 2005;18:434–444. doi: 10.1089/vim.2005.18.434. [DOI] [PubMed] [Google Scholar]
- 55.Cosman D, et al. ULBPs, novel MHC class I-related molecules, bind to CMV glycoprotein UL16 and stimulate NK cytotoxicity through the NKG2D receptor. Immunity. 2001;14:123–133. doi: 10.1016/s1074-7613(01)00095-4. [DOI] [PubMed] [Google Scholar]
- 56.Bacon L, et al. Two Human ULBP/RAET1 molecules with transmembrane regions are ligands for NKG2D.J. Immunol. 2004;173:1078–1084. doi: 10.4049/jimmunol.173.2.1078. [DOI] [PubMed] [Google Scholar]
- 57.Chalupny NJ, Rein-Weston A, Dosch S, Cosman D. Down-regulation of the NKG2D ligand MICA by the human cytomegalovirus glycoprotein UL142. Biochem Biophys Res Commun. 2006;346:175–181. doi: 10.1016/j.bbrc.2006.05.092. [DOI] [PubMed] [Google Scholar]
- 58.Stern-Ginossar N, et al. Host immune system gene targeting by a viral miRNA. Science. 2007;317:376–381. doi: 10.1126/science.1140956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zou Y, Bresnahan W, Taylor RT, Stastny P. Effect of human cytomegalovirus on expression of MHC class I-related chains A. J Immunol. 2005;174:3098–3104. doi: 10.4049/jimmunol.174.5.3098. [DOI] [PubMed] [Google Scholar]
- 60.Cosman D, et al. A novel immunoglobulin superfamily receptor for cellular and viral MHC class I molecules. Immunity. 1997;7:273–282. doi: 10.1016/s1074-7613(00)80529-4. [DOI] [PubMed] [Google Scholar]
- 61.Chapman TL, Heikeman AP, Bjorkman PJ. The inhibitory receptor LIR-1 uses a common binding interaction to recognize class I MHC molecules and the viral homolog UL18. Immunity. 1999;11:603–613. doi: 10.1016/s1074-7613(00)80135-1. [DOI] [PubMed] [Google Scholar]
- 62.Leong CC, et al. Modulation of natural killer cell cytotoxicity in human cytomegalovirus infection: the role of endogenous class I MHC and a viral class I homolog. J Exp Med. 1998;187:1681–1687. doi: 10.1084/jem.187.10.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tomasec P, et al. Downregulation of natural killer cell-activating ligand CD 155 by human cytomegalovirus UL141. Nature Immunol. 2005;6:181–188. doi: 10.1038/ni1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arnon TI, et al. Inhibition of the NKp30 activating receptor by pp65 of human cytomegalovirus. Nature Immunol. 2005;6:515–523. doi: 10.1038/ni1190. [DOI] [PubMed] [Google Scholar]
- 65.Lin A, Xu H, Yan W. Modulation of HLA expression in human cytomegalovirus immune evasion. Cell Mol Immunol. 2007;4:91–98. [PubMed] [Google Scholar]
- 66.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defense strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 67.Carr WH, Little AM, Mocarski E, Parham P. NK cell-mediated lysis of autologous HCMV-infected skin fibroblasts is highly variable among NK cell clones and polyclonal NK cell lines. Clin Immunol. 2002;105:126–140. doi: 10.1006/clim.2002.5273. [DOI] [PubMed] [Google Scholar]
- 68.Voigt S, et al. Cytomegalovirus evasion of innate immunity by subversion of the NKR-P1 B:Clr-b missing-self axis. Immunity. 2007;26:617–627. doi: 10.1016/j.immuni.2007.03.013. This study shows that rat CMV encodes a viral protein related to the C-type lectin family that engages the inhibitory NKR-P 1B receptor in strains of rats that are susceptible to CMV infection. [DOI] [PubMed] [Google Scholar]
- 69.Pereira RA, Scalzo A, Simmons A. Cutting edge: a NK complex-linked locus governs acute versus latent herpes simplex virus infection of neurons. J Immunol. 2001;166:5869–5873. doi: 10.4049/jimmunol.166.10.5869. [DOI] [PubMed] [Google Scholar]
- 70.Lundberg P, et al. A locus on mouse chromosome 6 that determines resistance to herpes simplex virus also influences reactivation, while an unlinked locus augments resistance of female mice. J Virol. 2003;77:11661–11673. doi: 10.1128/JVI.77.21.11661-11673.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Etzioni A, et al. Fatal varicella associated with selective natural killer cell deficiency. J Pediatr. 2005;146:423–425. doi: 10.1016/j.jpeds.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 72.Pappworth IY, Wang EC, Rowe M. The switch from latent to productive infection in Epstein-Barr virus-infected B cells is associated with sensitization to NK cell killing. J Virol. 2007;81:474–482. doi: 10.1128/JVI.01777-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Eidenschenk C, et al. A novel primary immunodeficiency with specific natural-killer cell deficiency maps to the centromeric region of chromosome 8. Am J Hum Genet. 2006;78:721–727. doi: 10.1086/503269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Besson C, et al. Association of killer cell immunoglobulin-like receptor genes with Hodgkin's lymphoma in a familial study. PLoS ONE. 2007;2:e406. doi: 10.1371/journal.pone.0000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jacoby RO, Bhatt PN, Brownstein DG. Evidence that NK cells and interferon are required for genetic resistance to lethal infection with ectromelia virus. Arch Virol. 1989;108:49–58. doi: 10.1007/BF01313742. [DOI] [PubMed] [Google Scholar]
- 76.Delano ML, Brownstein DG. Innate resistance to lethal mousepox is genetically linked to the NK gene complex on chromosome 6 and correlates with early restriction of virus-replication by cells with an NK phenotype. J Virol. 1995;69:5875–5877. doi: 10.1128/jvi.69.9.5875-5877.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chaudhri G, Panchanathan V, Bluethmann H, Karupiah G. Obligatory requirement for antibody in recovery from a primary poxvirus infection. J Virol. 2006;80:6339–6344. doi: 10.1128/JVI.00116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fang M, Sigal LJ. Antibodies and CDS+ T cells are complementary and essential for natural resistance to a highly lethal cytopathic virus. J Immunol. 2005;175:6829–6836. doi: 10.4049/jimmunol.175.10.6829. [DOI] [PubMed] [Google Scholar]
- 79.Karupiah G, et al. Inhibition of viral replication by interferon-γ-induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 80.Panchanathan V, Chaudhri G, Karupiah G. Interferon function is not required for recovery from a secondary poxvirus infection. Proc Natl Acad Sci USA. 2005;102:12921–12926. doi: 10.1073/pnas.0505180102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fang M, Lanier LL, Sigal LJ. A role for NKG2D in NK cell-mediated resistance to poxvirus disease. PLoS Pathogen. 2008;8:e30. doi: 10.1371/journal.ppat.0040030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chisholm SE, Reyburn HT. Recognition of vaccinia virus-infected cells by human natural killer cells depends on natural cytotoxicity receptors. J Virol. 2006;80:2225–2233. doi: 10.1128/JVI.80.5.2225-2233.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brooks CR, Elliott T, Parham P, Khakoo SI. The inhibitory receptor NKG2A determines lysis of vaccinia virus-infected autologous targets by NK cells. J Immunol. 2006;176:1141–1147. doi: 10.4049/jimmunol.176.2.1141. [DOI] [PubMed] [Google Scholar]
- 84.Campbell JA, Trossman DS, Yokoyama WM, Carayannopoulos LN. Zoonotic orthopoxviruses encode a high-affinity antagonist of NKG2D. J Exp Med. 2007;204:1311–1317. doi: 10.1084/jem.20062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Draghi M, et al. NKp46 and NKG2 D recognition of infected dendritic cells is necessary for NK cell activation in the human response to influenza infection. J Immunol. 2007;178:2688–2698. doi: 10.4049/jimmunol.178.5.2688. [DOI] [PubMed] [Google Scholar]
- 86.Mandelboim O, et al. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature. 2001;409:1055–1060. doi: 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 87.Gazit R, et al. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr 1. Nature Immunol. 2006;7:517–523. doi: 10.1038/ni1322. [DOI] [PubMed] [Google Scholar]
- 88.Khakoo SI, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–874. doi: 10.1126/science.1097670. This study provides intriguing hints that the resolution of HCV infection in humans involves cooperative interactions between certain HLA-C molecules and inhibitory KIRs. [DOI] [PubMed] [Google Scholar]
- 89.Vilches C, Parham P. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Anna Rev Immunol. 2002;20:217–251. doi: 10.1146/annurev.immunol.20.092501.134942. [DOI] [PubMed] [Google Scholar]
- 90.Crotta S, et al. Inhibition of natural killer cells through engagement of CD8 1 by the major hepatitis C virus envelope protein. J Exp Med. 2002;195:35–41. doi: 10.1084/jem.20011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tseng CT, Klimpel GR. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J Exp Med. 2002;195:43–49. doi: 10.1084/jem.20011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kakimi K, Guidotti LG, Koezuka Y, Chisari EV. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J Exp Med. 2000;192:921–930. doi: 10.1084/jem.192.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Baron JL, et al. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity. 2002;16:583–594. doi: 10.1016/s1074-7613(02)00305-9. [DOI] [PubMed] [Google Scholar]
- 94.Chen Y, et al. Increased susceptibility to liver injury in hepatitis B virus transgenic mice involves NKG2D-ligand interaction and natural killer cells. Hepatology. 2007;46:706–715. doi: 10.1002/hep.21872. [DOI] [PubMed] [Google Scholar]
- 95.Vilarinho S, Ogasawara K, Nishimura S, Lanier LL, Baron JL. Blockade of NKG2D on NKT cells prevents hepatitis and the acute immune response to hepatitis B virus. Proc Natl Acad Sci USA. 2007;104:18187–18192. doi: 10.1073/pnas.0708968104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martin MP, et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nature Genet. 2007;39:733–740. doi: 10.1038/ng2035. A population-based study that implicates HLA-B and certain KIRs in protection against progression to AIDS in HIV-infected individuals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Flores-Villanueva PO, et al. Control of HIV-1 viremia and protection from AIDS are associated with HLA-Bw4 homozygosity. Proc Natl Acad Sci USA. 2001;98:5140–5145. doi: 10.1073/pnas.071548198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Litwin V, Gumperz J, Parham P, Phillips JH, Lanier LL. NKB1: An NK cell receptor involved in the recognition of polymorphic HLA-B molecules. J Exp Med. 1994;180:537–543. doi: 10.1084/jem.180.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Carr WH, et al. Cutting edge: KIR3DS1, a gene implicated in resistance to progression to AIDS, encodes a DAP 12-associated receptor expressed on NK cells that triggers NK cell activation. J Immunol. 2007;178:647–651. doi: 10.4049/jimmunol.178.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Trundley A, Frebel H, Jones D, Chang C, Trowsdale J. Allelic expression patterns of KIR3DS1 and 3DL1 using the Z27 and DX9 antibodies. Em J Immunol. 2007;37:780–787. doi: 10.1002/eji.200636773. [DOI] [PubMed] [Google Scholar]
- 101.Qi Y, et al. KIR/HLA Pleiotropism: protection against both HIV and opportunistic infections. PLoS Pathog. 2006;2:e79. doi: 10.1371/journal.ppat.0020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gumperz JE, Litwin V, Phillips JH, Lanier LL, Parham P. The Bw4 public epitope of HLA-B molecules confers reactivity with NK cell clones that express NKB 1, a putative HLA receptor. J Exp Med. 1995;181:1133–1144. doi: 10.1084/jem.181.3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gillespie GM, et al. Lack of KIR3DS1 binding to MHC class I Bw4 tetramers in complex with CD8+ T cell epitopes. AIDS Res Hum Retroviruses. 2007;23:451–455. doi: 10.1089/aid.2006.0165. [DOI] [PubMed] [Google Scholar]
- 104.Alter G, et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med. 2007;204:3027–3036. doi: 10.1084/jem.20070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pando MJ, Gardiner CM, Gleimer M, McQueen KL, Parham P. The protein made from a common allele of KIR3DL1 (3DL1 *004) is poorly expressed at cell surfaces due to substitution at positions 86 in Ig domain 0 and 182 in Ig domain 1. J Immunol. 2003;171:6640–6649. doi: 10.4049/jimmunol.171.12.6640. [DOI] [PubMed] [Google Scholar]
- 106.Cohen GB, et al. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 107.Cerboni C, et al. Human immunodeficiency virus 1 Nef protein downmodulates the ligands of the activating receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J. Gen Virol. 2007;88:242–250. doi: 10.1099/vir.0.82125-0. [DOI] [PubMed] [Google Scholar]
- 108.Teixeira HC, Kaufmann SHE. Role of NK1.1 + cells in experimental listeriosis: NK1.1 + cells are early IFN-γ producers but impair resistance to Listeria rnonocytogenes infection. J Immunol. 1994;152:1873–1882. [PubMed] [Google Scholar]
- 109.Sporri R, Joller N, Albers U, Hilbi H, Oxenius A. MyD88-dependent IFN-γ production by NK cells is key for control of Legioriella prieurnophila infection. J Immunol. 2006;176:6162–6171. doi: 10.4049/jimmunol.176.10.6162. [DOI] [PubMed] [Google Scholar]
- 110.Junqueira-Kipnis AP, et al. NK cells respond to pulmonary infection with Mycobacterium tuberculosis, but play a minimal role in protection. J Immunol. 2003;171:6039–6045. doi: 10.4049/jimmunol.171.11.6039. [DOI] [PubMed] [Google Scholar]
- 111.Feng CG, et al. NK Cell-Derived IFN-γ differentially regulates innate resistance and neutrophil response in T cell-deficient hosts infected with Mycobacterium tuberculosis. J Immunol. 2006;177:7086–7093. doi: 10.4049/jimmunol.177.10.7086. [DOI] [PubMed] [Google Scholar]
- 112.Vankayalapati R, et al. Role of NK cell-activating receptors and their ligands in the lysis of mononuclear phagocytes infected with an intracellular bacterium. JImmunol. 2005;175:4611–4617. doi: 10.4049/jimmunol.175.7.4611. [DOI] [PubMed] [Google Scholar]
- 113.Le-Barillec K, et al. Roles for T and NK cells in the innate immune response to Shigella flexneri. J Immunol. 2005;175:1735–1740. doi: 10.4049/jimmunol.175.3.1735. [DOI] [PubMed] [Google Scholar]
- 114.Brown CR, Reiner SL. Activation of natural killer cells in arthritis-susceptible but not arthritis-resistant mouse strains following Borrelia burgdorferi infection. Infect Irnrnun. 1998;66:5208–5214. doi: 10.1128/iai.66.11.5208-5214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Babu S, Porte P, Klei TR, Shultz LD, Rajan T. V Host NK cells required for the growth of the human filarial parasite Brugia rnalayi in mice. J Immunol. 1998;161:1428–1432. [PubMed] [Google Scholar]
- 116.Laouar Y, Sutterwala ES, Gorelik L, Flavell RA. Transforming growth factor-p controls T helper type 1 cell development through regulation of natural killer cell interferon-γ. Nature Immunol. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 117.Schleicher U, et al. NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs. J Exp Med. 2007;204:893–906. doi: 10.1084/jem.20061293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sher A, Oswald IP, Hieny S, Gazzinelli RT. Toxoplasma gondii induces a T-independent IFN-γ response in natural killer cells that requires both adherent accessory cells and tumor necrosis factor-α. J Immunol. 1993;150:3982–3989. [PubMed] [Google Scholar]
- 119.Hunter CA, Subauste CS, Van Cleave VH, Remington JS. Production of γ interferon by natural killer cells from Toxoplasma gondii/infected SCID mice: regulation by interleukin-10, interleukin- 12, and tumor necrosis factor a. Infect Irnrnun. 1994;62:2818–2824. doi: 10.1128/iai.62.7.2818-2824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ma LL, et al. NK cells use perforin rather than granulysin for anticryptococcal activity. J Immunol. 2004;173:3357–3365. doi: 10.4049/jimmunol.173.5.3357. [DOI] [PubMed] [Google Scholar]
- 121.Duthie MS, Kahn SJ. NK cell activation and protection occur independently of natural killer T cells during Trupariosorna cruzi infection. Int Immunol. 2005;17:607–613. doi: 10.1093/intimm/dxh239. [DOI] [PubMed] [Google Scholar]
- 122.Roland J, et al. NK cell responses to Plasrnodiurn infection and control of intrahepatic parasite development. J Immunol. 2006;177:1229–1239. doi: 10.4049/jimmunol.177.2.1229. [DOI] [PubMed] [Google Scholar]
- 123.Hansen DS, D'Ombrain MC, Schofield L. The role of leukocytes bearing Natural Killer Complex receptors and Killer Immunoglobulin-like Receptors in the immunology of malaria. Curr: Opin Immunol. 2007;19:416–423. doi: 10.1016/j.coi.2007.07.011. [DOI] [PubMed] [Google Scholar]