Abstract
Persistent firing is believed to be a crucial mechanism for memory function including working memory. Recent in vivo and in vitro findings suggest an involvement of metabotropic glutamate receptors (mGluRs) in persistent firing. Using whole-cell patch recording techniques in a rat entorhinal cortex (EC) slice preparation, we tested if EC layer III neurons display persistent firing due to mGluR-activation, independently from cholinergic activation. Stimulation of the angular bundle drove persistent firing in 90% of the cells in the absence of cholinergic agonist. The persistent firing was typically stable for more than 4.5 min at which point persistent firing was terminated by the experimenter. The average frequency of the persistent firing was 2.1 Hz, ranging from 0.4 to 5.5 Hz. This persistent firing was observed even in the presence of atropine (2 μM), suggesting that the persistent firing can occur independent of cholinergic activation. Furthermore, ionotropic glutamate and GABAergic synaptic blockers (2 mM kynurenic acid, 100 μM picrotoxin and 1 μM CGP55845) did not block the persistent firing. On the other hand, blockers of group I mGluRs (100 μM LY367385 and 20 μM MPEP) completely blocked or suppressed the persistent firing. An agonist of group I mGluRs (20 μM DHPG) greatly enhanced the persistent firing induced by current injection. These results indicate that persistent firing can be driven through group I mGluRs in entorhinal layer III neurons suggesting that glutamatergic synaptic input alone could enable post-synaptic neurons to hold input signals in the form of persistent firing.
Keywords: Learning and memory, Parahippocampal, Patch Clamp, Rat, Synaptic
Introduction
Persistent firing is observed in a variety of brain regions including the entorhinal cortex (EC; Frank & Brown, 2003; Major & Tank, 2004). Here, we define persistent firing as repetitive spiking activity of neurons that persists after termination of the triggering stimulus. Persistent firing in the EC is observed in rat in vitro preparations (Klink & Alonso, 1997; Egorov et al., 2002; Tahvildari et al., 2007), and appears during the delay period of delayed match to sample tasks in rats and primates in vivo (Otto & Eichenbaum, 1992; Suzuki et al., 1997). This neural activity might underlie blood flow changes observed with fMRI during delay periods of delayed matching tasks (Schon et al., 2004, 2005). Persistent firing in the EC is believed to be an important mechanism for working memory (Fransén et al., 2002, 2006; Hasselmo & Eichenbaum, 2005; Hasselmo & Stern, 2006).
Cholinergic activation plays an important role in the induction of persistent firing in the EC. In a rat in vitro preparation, brief current injections to the soma induce persistent firing only in the presence of cholinergic agonists such as carbachol in medial EC layer II (Klink & Alonso, 1997), layer V (Egorov et al., 2002) and lateral EC layer III (Tahvildari et al., 2007) neurons. In vitro studies have also shown that persistent firing can be induced independent of synaptic interactions and that the activation of a calcium-activated nonselective cationic current (ICAN) through the M1 muscarinic receptor gives the depolarizing drive for persistent firing (Egorov et al., 2002; Reboreda et al., 2006). Related to this, the changes in fMRI activation observed during delay periods are also reduced by the muscarinic cholinergic antagonist scopolamine in humans (Schon et al., 2005), and scopolamine has been shown to impair performance of delayed matching tasks (Penetar and McDonough, 1983; Robbins et al., 1997; Koller et al., 2003).
On the other hand, recent in vivo findings have shown an involvement of group I metabotropic glutamate receptors (mGluRs) in working memory (Naie & Manahan-Vaughan, 2004; Mikami et al., 2007; Hayashi et al., 2007). It has also been shown that group I mGluR can modulate ICAN and transient receptor potential-like (TRP) channels, which are strong candidates as molecular correlates for ICAN (Congar et al., 1997; Gee et al., 2003; Ene et al., 2007; Fowler et al., 2007). These findings suggest that persistent firing could also be driven through mGluR activation, possibly independently from cholinergic receptor activation. This may provide a novel perspective that glutamatergic synaptic input alone could induce persistent firing in the post-synaptic neuron. However, the contribution of mGluR to persistent firing has not been fully explored.
In this study, we tested if neurons from deep layer III of medial EC display persistent firing through group I mGluR activation in the absence of cholinergic agonists using whole-cell patch recording techniques in an EC slice preparation.
Materials and methods
Slice preparation
All experimental protocols were approved by the Institutional Animal Care and Use Committee at Boston University. Long-Evans rats (postnatal days 21 to 27; Charles River, Wilmington, MA) were deeply anesthetized with ketamine/xylazine (95 mg/Kg ketamine and 2.8 mg/Kg xylazine) through intraperitoneal injection and absences of both pedal and tail pinch reflex were confirmed. Ice-cold modified artificial cerebrospinal fluid (ACSF) containing (in mM) 110 choline chloride, 2.5 KCl, 1.25 NaH2PO4, 26 NaHCO3, 0.5 CaCl2, 7 MgCl2, 7 glucose, 3 pyruvic acid and 1 ascorbic acid (pH adjusted to 7.4 by saturation with 95% O2 - 5% CO2) was intracardially perfused. The brain was then removed from the cranium and placed in ice-cold modified ACSF. 350 μm-thick slices of the hippocampal-entorhinal region were cut near horizontally with a 30 degree offset (cutting more dorsal at more rostal regions) using a Vibroslicer (World Precision Instruments, Sarasota, FL, USA). Slices were transferred to a holding chamber, where they were kept submerged for over an hour at room temperature before recording. The holding chamber was filled with ACSF containing (in mM) 124 NaCl, 3 KCl, 1.25 NaH2PO4, 26 NaHCO3, 1.6 CaCl2, 1.8 MgSO4, 10 glucose (pH adjusted to 7.4 by saturation with 95% O2 - 5% CO2).
Electrophysiological recording
Slices were transferred to a submerged recording chamber and perfused with ACSF, maintaining the temperature in between 33 to 35 °C for recordings. Patch pipettes were fabricated from borosilicate glass capillaries by means of a P-87 horizontal puller (Sutter Instrument, Novato, CA, USA). Patch pipettes were filled with intracellular solution containing (in mM) 120 K-gluconate, 10 HEPES, 0.2 EGTA, 20 KCl, 2 MgCl, 7 phosphocreatine-diTris, 4 Na2ATP and 0.3 TrisGTP (pH adjusted to 7.3 with KOH). The intracellular solution also contained 0.1 % biocytin for the purpose of labeling. When filled with this solution, the patch pipettes had a resistance of 3–5 MΩ. Slices were visualized with an upright microscope (Zeiss Axioskop 2), equipped with a ×40 water-immersion objective lens, and a near-infrared charge-coupled device (CCD) camera (JAI CV-M50IR, San Jose, CA, USA). Locations of the cells in the slice were confirmed to be in the deep half of layer III of the medial EC by biocytin staining after recording in 78 % of the cells used. Tight seals (>1 GΩ) were formed on cell bodies and the membrane was ruptured with negative pressure. Current-clamp recordings were made with a Multi Clamp 700B amplifier (Axon Instruments, Foster City, CA, USA). Signals were lowpass filtered at 5 kHz or 10 kHz and sampled at 10 kHz or 20 kHz, respectively, using Clampex 9.0 software (Axon Instruments, Foster City, CA, USA). Liquid junction potential of 10 mV was not corrected.
Synaptic stimulation was given by a clustered bipolar electrode (Distance between poles: 40 μm, Overall tip diameter: 80 μm) placed in the angular bundle. Duration of the stimulation pulse was 200 μs and intensity was 8 mA. This stimulation produced EPSPs with a 5.0 mV peak amplitude on average in normal ACSF (without synaptic blockers) and the same stimulus intensity was used for all other conditions. Distance from the tip of the stimulation electrode to the recorded neuron (measured in 37 slices in which we could clearly see marks of stimulation electrode and biocytin filled layer III cells) was 580 ± 17 μm ranging from 430 to 870 μm.
Chemicals
Stock solutions of atropine (2 mM, in water), DHPG (10 mM, in water), CGP55845 (4 mM, in DMSO), MPEP (20 mM, in DMSO) and LY367385 (100 mM, in 1.1 eq. NaOH) were prepared and diluted more than a thousand times in the ACSF. Kynurenic acid and picrotoxin were directly dissolved in the ACSF. Chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) and Tocris Bioscience (Ellisville, MO, USA).
Data analysis
Clampfit 9.0 (Axon Instruments, Foster City, CA, USA) and Matlab (MathWorks, Natick, MA, USA) were used for data analysis. Input resistance was measured from the voltage deflection in response to a 30 pA hyperpolarizing current pulse injection at a membrane potential between −60 to −65 mV. To observe the EPSP induced by a single synaptic stimulation, the angular bundle was stimulated with single pulse at a membrane potential near the resting level (Fig. 2(c)).
The frequency of persistent firing was measured as the average firing frequency of the neuron during the period from 10 to 30 s after the termination of the angular bundle stimulation. The “number of spikes during stimulation” was measured as the number of spikes elicited by the angular bundle stimulation during the 2 s stimulation period. The “membrane potential during stimulation” was measured as the average membrane potential during the 2 s stimulation period. To estimate the relationship between the spikes during angular bundle stimulation and resultant persistent firing, the “efficiency of one spike” was obtained by dividing the “frequency of the persistent firing” by the “number of spikes during stimulation”.
The significance was evaluated using a one-way ANOVA (Fig. 2) and one-way repeated measures ANOVA (Figs. 3 and 5) followed by a Tukey post hoc test to compare more than three recording conditions. A paired t test was used to compare two recording conditions (Figs. 4 and 6). Significance level < 0.05 (ns: not significant, *: 0.01 ≤ P < 0.05, **: 0.001 ≤ P < 0.01, ***: P < 0.001) was used. Data are expressed as means ± SEM.
Results
Synaptic induction of persistent firing in normal ACSF without cholinergic activation
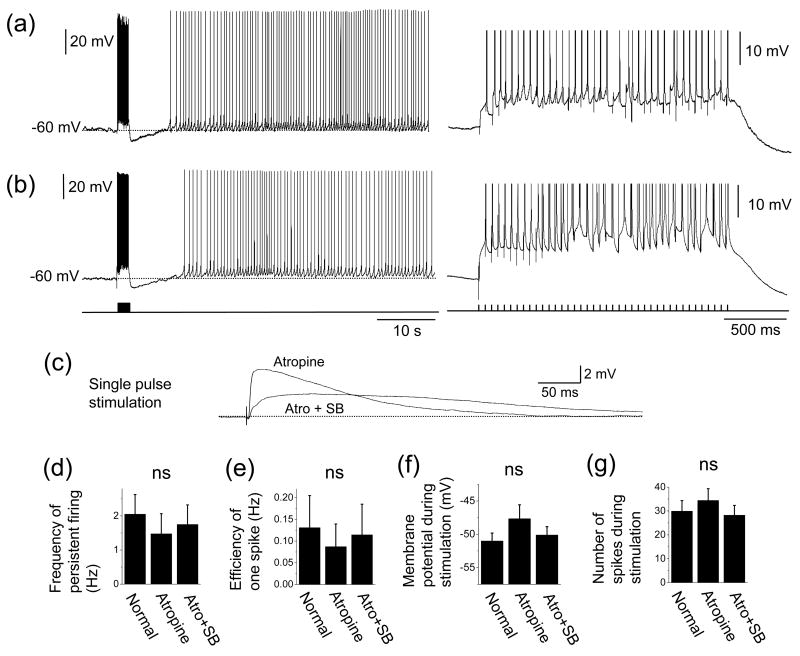
We obtained whole-cell recordings from principal cells with small to medium sized, pyramidal-shape soma in deep layer III. Average resting potential and membrane input resistance in normal ACSF were −66.6 ± 1.2 mV (n = 8) and 239.9 ± 13.0 MΩ (n = 10), respectively. Once a stable recording was obtained, the membrane potential was depolarized by a constant current injection to just below the firing threshold. In the following, we denote this potential “baseline” membrane potential. By always setting the baseline membrane potential just below the firing threshold, persistent firing could be studied and compared among different pharmacological conditions. After waiting at least 10 s to ensure that the baseline membrane potential was not drifting, the angular bundle was stimulated at 20 Hz for 2 s. Angular bundle stimulation induced a brief period of firing and this was followed by persistent firing in 91% (10 out of 11) of the cells in the absence of cholinergic agonist in normal ACSF (Fig. 1(a) and (b)). The frequency of the persistent firing was typically stable for more than 4.5 min at which point we terminated the persistent firing with a hyperpolarizing current injection. Average frequency of the persistent firing was 2.1 ± 0.6 Hz, ranging from 0.4 to 5.5 Hz (n = 10). Fig. 1(c) and (d) show magnification of trace in (a) during stimulation. Angular bundle stimulation caused EPSPs and action potentials on top of EPSPs.
Fig. 1.
Persistent firing in a physiological condition without cholinergic agonist. (a) Stimulation of the angular bundle (20 Hz, 2s) caused stable persistent firing. Bottom trace shows timing of stimulation. (b) Frequency of the persistent firing in (a). (c) Magnification of membrane potential trace during stimulation in (a). (d) Higher magnification of initial part of the stimulation in (c). A: stimulus artifact, E: EPSP, S: spike.
Synaptic induction of persistent firing in the presence of muscarinic cholinergic receptor antagonist and synaptic blockers
To eliminate the contribution of endogenous acetylcholine for muscarinic cholinergic receptor activation (Cole & Nicoll, 1983), we bath applied the muscarinic cholinergic receptor antagonist atropine (2 μM) in a different group of cells. Persistent firing was induced by the same angular bundle stimulation in 92% (12 out of 13) of the cells in the presence of atropine (Fig. 2(a)), suggesting that the persistent firing in these cells can be obtained independent of muscarinic cholinergic activation. The frequency of persistent firing with atropine (1.5 ± 0.6 Hz) was, on average, lower than that in the normal ACSF shown in the previous section (Fig. 1) but the difference was not significant (Fig. 2(d); ANOVA, F2,28 = 0.25, P = 0.78). The efficiency of one spike (see Methods) did not differ significantly from that in the normal ACSF (Fig. 2(e); ANOVA, F2,28 = 0.12, P = 0.87). These results indicate that muscarinic modulation was not providing the principal depolarization for persistent firing. Membrane potential during stimulation (Fig. 2(f); ANOVA, F2,28 = 1.2, P = 0.32) and number of spikes during stimulation (Fig. 2(g); ANOVA, F2,28 = 0.50, P = 0.61) did not differ as well, compared to those in the normal ACSF.
Fig. 2.
Persistent firing with cholinergic receptor antagonist and synaptic blockers. (a) Left: An example of persistent firing in cholinergic antagonist atropine (2 μM). Right: Magnification of trace during stimulation. (b) An example of persistent firing in ionotropic glutamate synaptic blocker and GABAergic synaptic blockers (2 mM kynurenic acid, 100 μM picrotoxin and 1 μM CGP55845) as well as atropine. (c) Response to single pulse synaptic stimulation. Atro: atropine. SB: synaptic blockers. (d) Frequency of persistent firing in normal ACSF (shown in Fig. 1), atropine, and atropine + synaptic blockers. (e) Efficiency of one spike. (f) Membrane potential during synaptic stimulation. (g) Number of spikes during synaptic stimulation. ns: not significant.
We further tested, in a different group of cells, contribution of ionotropic glutamate and GABAergic synaptic input to the persistent firing. We bath applied kynurenic acid (2 mM), picrotoxin (100 μM) and CGP55845 (1 μM) to suppres ionotropic glutamate receptors and GABAA and GABAB receptors, respectively, in addition to atropine (2 μM). When single shock stimulation was applied to the angular bundle, the fast EPSP peak was significantly reduced while the slow part was enhanced with these synaptic blockers (Fig. 2(c)).
Stimulation of the angular bundle at 20 Hz for 2 s drove persistent firing in 82% of cells (9 out of 11) in the presence of these synaptic blockers (Fig. 2(b)). The frequency of the persistent firing did not differ significantly from other conditions (Fig. 2(d); ANOVA, F2,28 = 0.25, P = 0.78). The efficiency of one spike (Fig. 2(e); ANOVA, F2,28 = 0.12, P = 0.87), membrane potential during stimulation (Fig. 2(f); ANOVA, F2,28 = 1.2, P = 0.32) and number of spikes during stimulation (Fig. 2(g); ANOVA, F2,28 = 0.50, P = 0.61) did not differ from the former two conditions. These results indicate that deep layer III neurons show persistent firing when they are synaptically stimulated independent of muscarinic activation and major ionotropic glutamate, GABAA and GABAB synaptic transmission. In all three conditions (normal ACSF, atropine, and atropine and synaptic blockers) post-synaptic response was contaminated with occasional anti-dromic spiking in three cells. Since we did not find a qualitative difference between these neurons and others, these neurons were included in our analysis. Firing frequency of persistent firing of these cells were 1.6 ± 0.5 Hz in normal ACSF, 2.9 ± 2.3 Hz in atropine and 2.2 ± 0.7 Hz in atropine and synaptic blockers.
Effect of group I mGluR blockers on persistent firing induced by synaptic stimulation
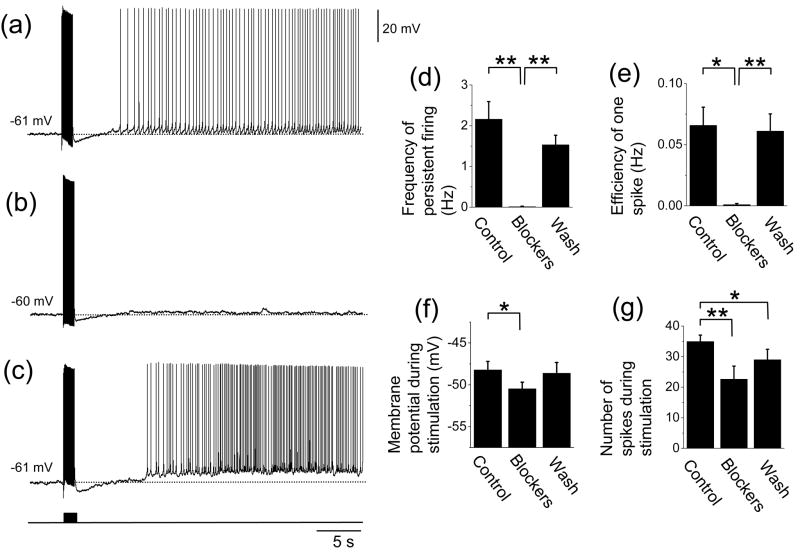
Next, to evaluate the contribution of mGluR activation, we chose cells that had clear persistent firing in the presence of ionotropic glutamate blocker and GABAergic synaptic blockers (2 mM kynurenic acid, 100 μM picrotoxin and 1 μM CGP55845) in addition to atropine (2 μM). The average frequency of the persistent firing was 2.2 ± 0.4 Hz in this condition (Fig. 3(a) and (d); n = 13). In the same group of cells, we further applied group I mGluR blockers LY367385 (100 μM) and MPEP (20 μM). Application of the group I mGluR blockers completely blocked the persistent firing in 92% (12 out of 13) of the cells (Fig. 3(b)). In the one remaining cell, persistent firing with a very low frequency (0.15 Hz) was induced. The average frequency of persistent firing (0.01 ± 0.01 Hz, n = 13), calculated as an average of the twelve neurons at 0 Hz and one neuron at 0.15 Hz, was significantly lower with the group I mGluR blockers (Fig. 3(d); ANOVA, F2,24 = 8.4, P < 0.01; Tukey test, P < 0.01). Although both membrane potential during stimulation (Fig. 3(f); ANOVA, F2,24 = 4.0, P < 0.05; Tukey test, P < 0.05) and number of spikes during stimulation (Fig. 3(g); ANOVA, F2,24 = 8.2, P < 0.01; Tukey test, P < 0.01) were significantly lower with the group I mGluR blockers, the efficiency of one spike was also significantly lower (Fig. 3(e); ANOVA, F2,24 = 6.5, P < 0.01; Tukey test, P < 0.05), suggesting that the decreased persistent firing was not caused simply by reduced depolarization or reduced spikes during stimulation.
Fig. 3.
Effect of mGluR blockers on persistent firing induced by synaptic stimulation. (a) – (c) Persistent activity in a representative cell in (a) control (with atropine, ionotropic glutamate synaptic blockers and GABAergic synaptic blockers), (b) group I mGluR blockers and (c) after wash out of the group I mGluR blockers. (d) Frequency of persistent firing. (e) Efficiency of one spike to cause persistent firing. (f) Membrane potential during stimulation. (g) Number of spikes during stimulation. Note significant decrease in the frequency of persistent firing and the efficiency of one spike. Significances from Tukey post-hoc test are shown. *: 0.01 ≤ P < 0.05, **: 0.001 ≤ P < 0.01, ***: P < 0.001. Differences between pairs without asterisks were not significant.
In the presence of group I mGluR blockers, input resistances of the cells decreased in nine cells and increased in four cells, resulting in an average decrease (Control: 265.4 ± 18.3 MΩ, n = 13; Blockers: 230.5 ± 19.4 MΩ, n = 13). While one of the cells in which persistent firing was not completely blocked showed decreased input resistance, persistent firing was blocked completely in all the four cells which showed increased input resistance, indicating that reduced input resistance was not the cause of the absence of the persistent firing.
After washout of the group I mGluR blockers, persistent firing could be induced by the angular bundle stimulation again, showing that the effect of the mGluR blockers was reversible (Fig 3(c)). These results suggest that persistent firing induced by angular bundle stimulation largely depends on the group I mGluR activation.
Baseline membrane potential at which synaptic stimulation was applied was chosen as the membrane potential just below threshold as in the previous section. In the presence of mGluR blockers, baseline potential was at a more depolarized level (Control: −60.6 ± 0.5 mV, Blockers: −57.1 ± 1.1 mV) probably due to higher firing threshold (Control: −46.6 ± 0.7 mV, Blockers: −44.9 ± 0.7 mV). In eight cells, we tested the synaptic stimulation from the same baseline membrane potential as in the case without mGluR blockers (control condition) (Control: −60.1 ± 0.6 mV, Blockers: −60.5 ± 0.7 mV). At this potential, none of these cells showed persistent firing in the presence of the mGluR blockers (Data not shown).
Persistent firing induced by current stimulation
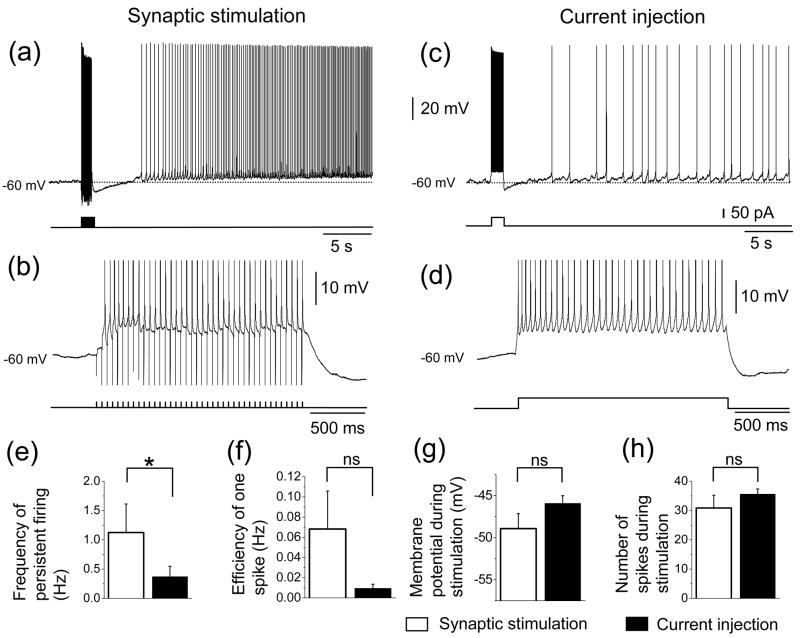
In the presence of atropine (1 or 2 μM), we compared persistent firing induced by angular bundle stimulation (synaptic stimulation; Fig. 4(a) and (b)) with persistent firing induced by brief (2 s) injections of current to the soma through the recording electrode (Fig. 4(c) and (d)). While angular bundle stimulation induced persistent firing in 93% (14 out of 15) of the cells, current injection induced persistent firing in 80 % (12 out of 15) of the same cells. These 12 cells were a sub group of the 14 cells that showed persistent firing with angular bundle stimulation. The frequency of the persistent firing induced by current injection was significantly lower than that induced by synaptic stimulation (Fig. 4(e); T-test, t14 = 2.2, P < 0.05). The efficiency of one spike was also lower, on average, with the current injection (Fig. 4(f)), but was not significantly different (T-test, t14 = 1.5, P = 0.15). The membrane potential during stimulation (Fig. 4(g); T-test, t14 = −1.5, P = 0.15) and the number of spikes elicited during stimulation (Fig. 4(h); T-test, t14 = −1.1, P = 0.29) were not significantly different in the two conditions. The baseline potentials from which persistent firing was induced, were not different between synaptic stimulation and current injection protocols (Current injection: −57.8 ± 0.8 mV, n = 15; Synaptic stimulation: −58.2 ± 0.7 mV; T-test, t14 = 0.77, P = 0.45; n = 15).
Fig. 4.
Comparison of persistent firing induced by synaptic stimulation and current injection. (a) Persistent firing induced by synaptic stimulation. (b) Magnification of trace during stimulation in (a). (c) Persistent firing induced by current injection in the same cell as in (a). (d) Magnification of trace during stimulation in (c). (e) Frequency of persistent firing (white = synaptic stimulation, black = current injection). (f) Efficiency of one spike. (g) Membrane potential during stimulation. (h) Number of spikes induced during stimulation. *: 0.01 ≤ P < 0.05.
Three neurons showed antidromic firing during angular bundle stimulation. These three neurons showed persistent firing with higher frequency (2.9 Hz with synaptic stimulation and 0.9 Hz with current injection) and higher efficiency of one spike (0.05 Hz with synaptic stimulation and 0.02 Hz with current injection) compared to the persistent firing induced by current injection.
These results suggest that current injection can also induce persistent firing. However, the frequency of persistent firing induced by current injection was lower than that induced by synaptic stimulation, presumably because of the lack of group I mGluR activation.
Persistent firing induced by current stimulation is insensitive to mGluR blockers
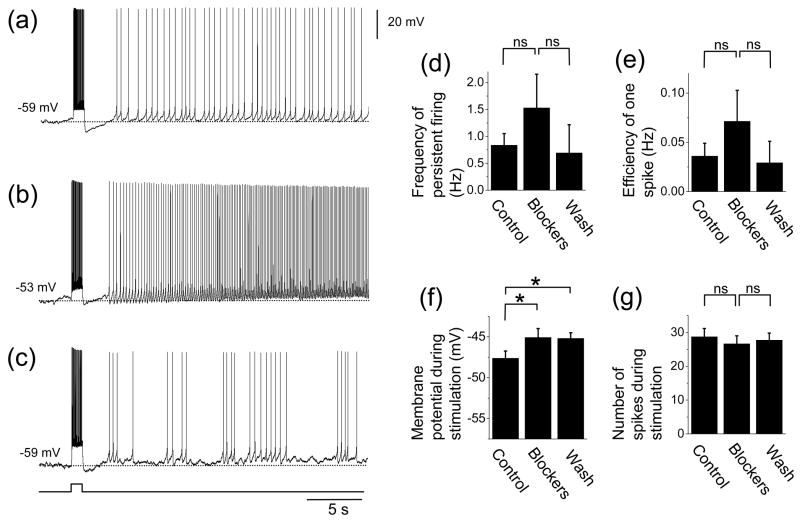
Effects of the group I mGluR blockers on persistent firing induced by current injection were investigated in 13 cells that showed persistent firing with current injection with the presence of atropine (2 μM) and synaptic blockers (2 mM kynurenic acid, 100 μM picrotoxin and 1 μM CGP55845) (Fig. 5(a)). 12 cells out of these 13 cells were the same cells as in the previous section where mGluR blockers were tested on the synaptically induced persistent firing (Fig. 3). With regard to the induction of persistent firing with current injection, application of group I mGluR blockers LY367385 (100 μM) and MPEP (20 μM) enhanced persistent firing in 54% (7 out of 13) of the cells while it suppressed persistent firing in 46% (6 out of 13) of the cells. As a result, group I mGluR blockers did not have a significant effect on the firing frequency of the persistent firing induced by current injection (Fig. 5(d); ANOVA, F2,24 = 2.3, P = 0.12) or efficiency of one spike (Fig. 5(e); ANOVA, F2,24 = 3.5, P < 0.05; Tukey test, P > 0.05, ns). The average increase in these parameters could be due to the higher baseline membrane potential used with the presence of mGluR blockers. ANOVA values for Fig. 5(f) and Fig. 5(g) were F2,24 = 6.0, P < 0.01 and F2,24 = 2.5, P = 0.11, respectively.
Fig. 5.
Effect of group I mGluR blockers on persistent firing induced by current injection. (a) - (c) Persistent activity in (a) control (with atropine, ionotropic glutamate synaptic blockers and GABAergic synaptic blockers), (b) group I mGluR blockers and (c) after wash out of the group I mGluR blockers. (d) Frequency of persistent firing. (e) Efficiency of one spike to cause persistent firing. (f) Membrane potential during stimulation. (g) Number of spikes during stimulation. Significances from Tukey post-hoc test are shown. *: 0.01 ≤ P < 0.05. Differences between pairs without asterisks were not significant.
As noted above, input resistances of the cells decreased in nine cells and increased in four cells during mGluR blockade, resulting in an average decrease. Persistent firing induced by current injection was blocked in all the four cells with increased input resistance, indicating that increased input resistance was not the cause for the spared persistent firing induced by current injection in mGluR blockers. These results indicate that the persistent firing induced with current injection is not necessarily dependent on the group I mGluR activation.
As in the earlier section with mGluR blockers and synaptic stimulations (Fig. 3), in the presence of mGluR blockers, a more depolarized baseline potential was chosen (Control: −60.2 ± 0.5 mV, Blockers: −56.4 ± 0.7 mV) due to higher firing threshold. However, in seven cells, we also tested current injection stimulation from the same membrane potential as in the control condition (Control: −59.7 ± 0.8 mV, Blockers: −59.9 ± 0.6 mV). With this more hyperpolarized baseline membrane potential, none of these cells showed persistent firing in the presence of the mGluR blockers (Data not shown).
Effect of group I mGluR agonist
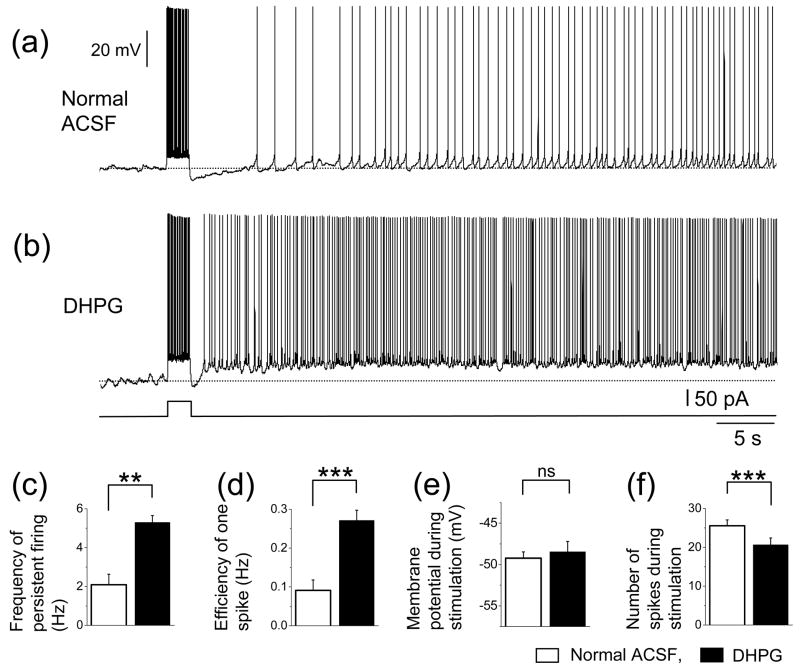
If group I mGluR activation contributes to the persistent firing induced by synaptic stimulation, application of an agonist of group I mGluRs could enhance the persistent firing without synaptic stimulation. We tested the effect of a group I mGluR agonist DHPG (5 μM) on the persistent firing induced by current injection. Persistent firing was induced by current injection in normal ACSF without atropine and synaptic blockers (Fig. 6(a)). Application of DHPG greatly enhanced persistent firing induced by current injection (Fig. 6(b)). The frequency of persistent firing(Fig. 6(c); T-test, t8 = −4.7, P < 0.01) and efficiency of one spike significantly increased (Fig. 6(d); T-test, t8 = −7.5, P < 0.001). While membrane potential during stimulation did not change significantly (Fig. 6(e); T-test, t8 = −0.84, P = 0.42), number of spikes during stimulation was reduced significantly (Fig. 6(f); T-test, t8 = 5.2, P < 0.001). This contributed to the increased measurement of the efficiency of one spike. These results strongly suggest that activation of group I mGluRs by agonist can strongly contribute to the persistent firing in the absence of synaptic stimulation.
Fig. 6.
Effect of group I mGluR agonist on persistent firing induced by current injection. (a) Persistent firing induced by current injection to the soma in normal ACSF. (b) Persistent firing induced by current injection to the soma in DHPG (5 μM). (c) Frequency of persistent firing (white = normal ACSF, black = DHPG). (d) Efficiency of one spike. (e) Membrane potential during stimulation. (f) Number of spikes during stimulation. **: 0.001 ≤ P < 0.01, ***: P < 0.001.
We further tested the effect of DHPG on persistent firing induced by synaptic stimulation. Persistent firing was induced by synaptic stimulation (20 Hz for 2 s) in normal ACSF without atropine and synaptic blockers in three cells. Application of DHPG clearly increased the frequency of persistent firing in all three cells (Normal ACSF: 2.2 ± 1.7 Hz; DHPG: 6.2 ± 2.2 Hz; Data not shown). These results indicate that group I mGluR activation also promotes persistent firing induced by synaptic stimulation in physiological conditions where ionotropic synaptic blockers are not present.
Graded persistent firing induced by synaptic stimulation
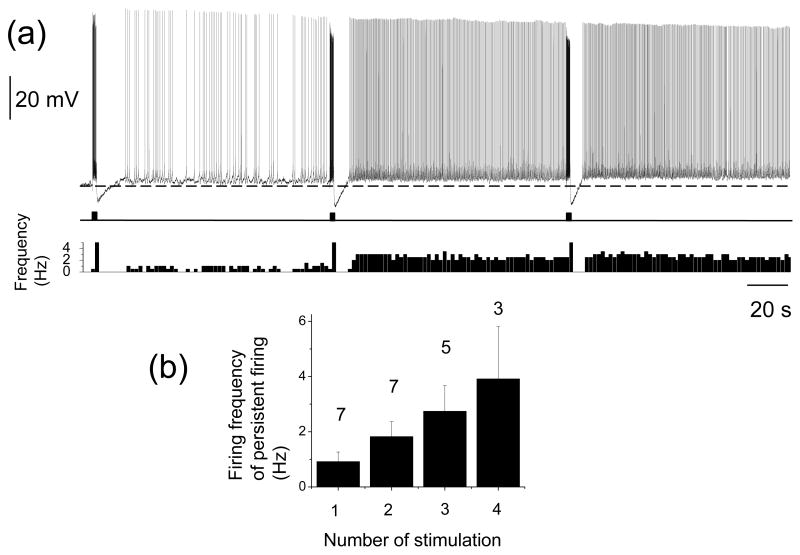
It has been shown that repeated application of current injection can raise the frequency of the persistent firing in a graded manner in EC layer V cells in the presence of a cholinergic receptor agonist (Egorov et al., 2002). Graded persistent firing is believed to be an important feature of persistent firing because this mechanism allows neurons to integrate synaptic input over time (Fransén et al., 2006). We tested if persistent firing induced by synaptic stimulation in the absence of cholinergic agonist has a graded nature. We applied 0.5 or 2 s of angular bundle stimulation at 20 Hz multiple times in the presence of atropine (2 μM). Each stimulation was separated by more than 30 s from the previous stimulation. Persistent firing was graded up at least two steps in 70 % (7 out of 10) of the cells tested (Fig. 7(a)). As summarized in the Fig. 7(b), frequency of the persistent firing graded up as a function of the number of applied synaptic stimulations. Similar graded persistent firing was observed also in the presence of synaptic blockers (2 mM kynurenic acid, 100 μM picrotoxin and 1 μM CGP55845) in addition to atropine (2 μM) in 78 % (7 out of 9) of cells tested (data not shown). These results suggest that layer III cells in the medial EC can show graded persistent firing independently from cholinergic activation.
Fig. 7.
Graded persistent firing induced by synaptic stimulation in cholinergic muscarinic receptor antagonist. (a) An example of graded persistent firing in the presence of atropine. (b) Change in firing frequency of persistent firing as the function of number of repeated stimulation pulses. Numbers on top of the bar graphs show number of cells.
Discussion
In the present study, we have shown that angular bundle synaptic stimulation can induce persistent firing in EC layer III neurons without cholinergic activation. Significant suppression of persistent firing by group I mGluR blockers suggests that group I mGluR activation contributes to this synaptically induced persistent firing. The group I mGluR agonist greatly enhanced persistent firing induced by current injection. These results indicate that group I mGluR activation contributes to persistent firing and that synaptic input to these neurons could be sufficient for the induction of the persistent firing in these neurons.
It has been shown that muscarinic receptor activation is important for the persistent firing in the EC (Egorov et al., 2002; Schon et al., 2005; Tahvildari et al., 2007). Our findings here suggest that persistent firing can be induced not only through muscarinic cholinergic receptor activation but also through group I mGluR activation. This view is supported by the intracellular mechanism for induction of persistent firing. Pharmacological studies have indicated that persistent firing in the EC layer V neurons is driven through ICAN and the molecular correlate for ICAN has been suggested to be a type of TRP channel (Al-Yahya et al., 2003; Reboreda et al., 2006). It has been shown that TRPC channels exist in the EC (von Bohlen und Halbach et al. 2005, Fowler et al., 2007) and TRP channels can be activated both through muscarinic receptor activation and through group I mGluR activation (Gee et al., 2003; Kim et al., 2003; Tozzi et al., 2003; Bengtson et al., 2004; Moran et al., 2004; Cayouette & Boulay, 2007; Fowler et al., 2007). Activation of ICAN or TRP channels through mGluRs is reported to induce long-lasting depolarization in many types of neurons. Induction of persistent firing by agonists of group I mGluRs has been shown in EC layer V neurons (Al-Yahya et al., 2003). In addition, mGluR agonists have been shown to cause enhancement of delayed after-depolarization in turtle motoneurons (Svirskis & Hounsgaard, 1998; Perrier et al., 1999), and in neurons from rat prefrontal cortex, lateral septum and subiculum (Fowler et al., 2007). Synaptic stimulation can produce small long-lasting depolarization through mGluR activation in prefrontal layer V neurons (Hagenston et al., 2007). These results support the idea that persistent firing can be induced either through muscarinic receptor activation or group I mGluR activation in the EC through activation of ICAN or TRP channels.
Persistent firing is believed to provide an important aspect of neural activity that underlies working memory (Fransén et al., 2002, 2006; Hasselmo & Eichenbaum, 2005; Hasselmo & Stern, 2006). While involvement of muscarinic receptor activation in working memory has been reported (McGaughy et al., 2005), other lines of study have shown an involvement of group I mGluR activation in working memory. Homayoun & Moghaddam (2006) have shown that periods of elevated firing were reduced for prefrontal cortex neurons when an mGluR5 blocker was given. Three different groups have shown impairment of working memory in rats after application of mGluR blockers (Naie & Manahan-Vaughan, 2004; Hayashi et al., 2007; Mikami et al., 2007). In particular, Mikami et al. (2007) have shown that working memory was impaired by application of (R,S)-1-Aminoindan-1,5-dicarboxylic acid (AIDA), an mGluR1 blocker, but this impairment was reduced when they additionally applied the acetylcholinesterase inhibitor donepezil. They further verified that this ameliorative effect of donepezil depended on muscarinic receptors. Therefore these in vivo results also support the idea that persistent firing can be supported either by activation of muscarinic receptor or group I mGluRs.
Persistent firing in the EC has been studied intensively using current injection to the soma (Egorov et al., 2002; Reboreda et al., 2007; Tahvildari et al., 2007). Synaptic stimulation is a more physiologically natural way to induce persistent firing. Induction of persistent firing through synaptic stimulation has been reported in the EC (Egorov et al., 2002; Tahvildari et al., 2007) and amygdala (Egorov et al., 2006) in in vitro preparations. Egorov et al. (2002) and Tahvildari et al. (2007) used the muscarinic cholinergic receptor agonist carbachol and Egorov et al. (2006) used the acetylcholinesterase inhibitor eserine to set modulatory conditions for induction of persistent firing. In contrast, we demonstrated that angular bundle stimulation alone was sufficient to induce persistent firing in normal ACSF without pharmacological treatment. For synaptic stimulation, we used a 20 Hz train with 2 s duration. This is a physiological firing pattern observed in hippocampal CA1 neurons, for example, during an odor-discrimination task (Eichenbaum et al., 1987). 20 Hz is also in a physiological firing frequency range of entorhinal neurons (Alonso et al., 1987, Hafting et al., 2005). The frequency of persistent firing induced by the synaptic stimulation in normal ACSF was 2.1 ± 0.6 Hz. This frequency is very similar to the firing frequencies of activity during delay period (typically 0.5 – 4 Hz) in rat entorhinal neurons recorded in vivo (Young et al., 1997). This suggests that persistent activity of the type observed in this study may underlie “delay” activity which maintains memory representations.
In this study, we showed that synaptic stimulation causes persistent firing with higher firing frequency than that induced by current injection (Fig. 4). Since the duration of stimulation and the number of spikes during stimulation were almost the same in the two stimulation paradigms, this difference is most probably attributed to the mGluR activation in the case of synaptic activation. With current injection, persistent firing might be induced because of the smaller amount of ICAN activated solely by the increase in intra-cellular calcium level (Gee et al., 2003) due to depolarization during stimulation. This could explain the fact that persistent firing induced by current injection was not consistently blocked by group I mGluR blockers (Fig. 5). With the somatic current injection, the dendritic depolarization and calcium level increase could be spread evenly covering a large dendritic area while the depolarization and calcium level increase could be limited to the stimulated branch with synaptic stimulation. In the presence of the mGluR blockers, current injection could cause more ICAN activation because of this broader spread of depolarization than in the case with synaptic stimulation, resulting in spared persistent firing with current injection but not with the synaptic stimulation.
Contrary to our observation that persistent firing is induced by current injection in the presence of atropine in the medial EC layer III neurons, current injection in the presence of atropine is reported not to induce persistent firing in the medial EC layer V cells (Egorov et al., 2002). In our study, we recorded from medial EC layer V neurons in addition to EC layer III neurons (data not shown). In line with previous finding (Egorov et al., 2002), neurons in layer V with larger soma usually did not show persistent firing either with synaptic stimulation or current injection. However, persistent firing was seen in some neurons with soma that have a small size and pyramidal shape, which is typical in layer III. With the application of DHPG, layer V cells showed persistent firing regardless of the size of the soma (data not shown). These observations suggest that a more effective synaptic stimulation that produces stronger activation of group I mGluRs might cause persistent firing in these layer V cells as well.
Integration by cationic currents has been shown to occur over long time intervals, lasting for tens of seconds to minutes (Batchelor et al. 1997, Wyart et al., 2005, Oestreich et al. 2006). Graded persistent firing in the EC might therefore be the source of activity in the hippocampus that varies slowly over behaviourally relevant time scales. It has been reported that neurons from medial EC layer V show graded persistent firing (Egorov et al., 2002) while neurons from lateral EC layer III do not show graded persistent firing (Tahvildari et al., 2007) with repeated current injection. Graded persistent firing has also been reported in lateral amygdala neurons (Egorov et al., 2006) with current injection and synaptic stimulation. However, graded persistent firing in the absence of cholinergic activation has not been explored. Our observation has shown that group I mGluR-induced persistent firing can also be graded (Fig. 7) indicating that the cellular integration of the input signal could also be done through glutamatergic synaptic input to medial EC layer III neurons.
The acetylcholine level in the brain is modulated depending on the animal’s behavioral state. It is believed that high acetylcholine levels enhance mechanisms for memory encoding whereas low acetylcholine levels shift entorhinal-hippocampal networks to dynamics appropriate for memory consolidation (Hasselmo, 1999). During memory consolidation, memories initially stored in the hippocampus are potentially consolidated in neocortical regions. The output from the hippocampus is conveyed mainly through subiculum and angular bundle to the entorhinal cortex (Naber et al., 2001). Therefore, we used the angular bundle as the stimulation site in this study. Anatomical connections from the subiculum to layer III of the entorhinal cortex have been reported (Kloosterman et al., 2003). Electrophysiological synaptic responses have been observed in neurons from superficial layers of the EC including layer III in response to angular bundle stimulation (Scharfman, 1996; Bear et al., 1995).
In vivo studies support the idea that mGluR mediated persistent firing is important for working memory function during active exploration which corresponds to the encoding stage (Naie & Manahan-Vaughan, 2004; Hayashi et al., 2007; Mikami et al., 2007). On the other hand, during the consolidation stage, decreases in acetylcholine levels may make the induction of muscarinic receptor mediated persistent firing more difficult. Our observations indicate that persistent firing could still be induced through group I mGluR activation in this stage. Therefore, mGluR mediated persistent firing could be an important neural activity not only for the memory encoding stage but also for the memory consolidation stage, being driven by brief synaptic input from the hippocampus or subiculum, for example by sharp-wave activity during slow-wave-sleep.
Acknowledgments
This work was supported by NIMH MH60013, MH61492, Silvio O. Conte Center grant NIMH MH71702, NIMH MH60450, NSF SLC SBE 0354378, NIDA DA16454 (part of the CRCNS program) and JSPS Postdoctoral Fellowship for Research Abroad.
References
- Alonso A, García-Austt E. Neuronal sources of theta rhythm in the entorhinal cortex of the rat. II Phase relations between unit discharges and theta field potentials. Exp Brain Res. 1987;67:502–509. doi: 10.1007/BF00247283. [DOI] [PubMed] [Google Scholar]
- Al-Yahya E, Hamel E, Kennedy TE, Alonso AA, Egorov AV. Persistent activity in entorhinal cortex neurons induced by muscarinic and metabotropic glutamate receptor activation and its dependent on TRP channels. Soc Neurosci Abstr. 2003 Program No. 377.5. [Google Scholar]
- Batchelor AM, Garthwaite J. Frequency detection and temporally dispersed synaptic signal association through a metabotropic glutamate receptor pathway. Nature. 1997;385:74–77. doi: 10.1038/385074a0. [DOI] [PubMed] [Google Scholar]
- Bear J, Fountain NB, Lothman EW. Responses of the superficial entorhinal cortex in vitro in slices from naive and chronically epileptic rats. J Neurophysiol. 1996;76:2928–2940. doi: 10.1152/jn.1996.76.5.2928. [DOI] [PubMed] [Google Scholar]
- Bengtson CP, Tozzi A, Bernardi G, Mercuri NB. Transient receptor potential-like channels mediate metabotropic glutamate receptor EPSCs in rat dopamine neurones. J Physiol. 2004;555:323–330. doi: 10.1113/jphysiol.2003.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayouette S, Boulay G. Intracellular trafficking of TRP channels. Cell Calcium. 2007;42:225–232. doi: 10.1016/j.ceca.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Cole AE, Nicoll RA. Acetylcholine mediates a slow synaptic potential in hippocampal pyramidal cells. Science. 1983;221:1299–1301. doi: 10.1126/science.6612345. [DOI] [PubMed] [Google Scholar]
- Congar P, Leinekugel X, Ben-Ari Y, Crepel V. A long-lasting calcium-activated nonselective cationic current is generated by synaptic stimulation or exogenous activation of group I metabotropic glutamate receptors in CA1 pyramidal neurons. J Neurosci. 1997;17:5366–5379. doi: 10.1523/JNEUROSCI.17-14-05366.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorov AV, Hamam BN, Fransén E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420:173–178. doi: 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- Egorov AV, Unsicker K, von Bohlen und Halbach O. Muscarinic control of graded persistent activity in lateral amygdala neurons. Eur J Neurosci. 2006;24:3183–3194. doi: 10.1111/j.1460-9568.2006.05200.x. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Kuperstein M, Fagan A, Nagode J. Cue-sampling and goal-approach correlates of hippocampal unit activity in rats performing an odor-discrimination task. J Neurosci. 1987;7:716–732. doi: 10.1523/JNEUROSCI.07-03-00716.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ene FA, Kalmbach A, Kandler K. Metabotropic glutamate receptors in the lateral superior olive activate TRP-like channels: age- and experience-dependent regulation. J Neurophysiol. 2007;97:3365–3375. doi: 10.1152/jn.00686.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler MA, Sidiropoulou K, Ozkan ED, Phillips CW, Cooper DC. Corticolimbic expression of TRPC4 and TRPC5 channels in the rodent brain. PLoS ONE. 2007;2:e573. doi: 10.1371/journal.pone.0000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank LM, Brown EN. Persistent activity and memory in the entorhinal cortex. Trends Neurosci. 2003;26:400–401. doi: 10.1016/S0166-2236(03)00176-0. [DOI] [PubMed] [Google Scholar]
- Fransén E, Alonso AA, Hasselmo ME. Simulations of the role of the muscarinic-activated calcium-sensitive nonspecific cation current INCM in entorhinal neuronal activity during delayed matching tasks. J Neurosci. 2002;22:1081–1097. doi: 10.1523/JNEUROSCI.22-03-01081.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransén E, Tahvildari B, Egorov AV, Hasselmo ME, Alonso AA. Mechanism of graded persistent cellular activity of entorhinal cortex layer v neurons. Neuron. 2006;49:735–746. doi: 10.1016/j.neuron.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Gee CE, Benquet P, Gerber U. Group I metabotropic glutamate receptors activate a calcium-sensitive transient receptor potential-like conductance in rat hippocampus. J Physiol. 2003;546:655–664. doi: 10.1113/jphysiol.2002.032961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hagenston AM, Fitzpatrick JS, Yeckel MF. MGluR-Mediated Calcium Waves that Invade the Soma Regulate Firing in Layer V Medial Prefrontal Cortical Pyramidal Neurons. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm075. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. Neuromodulation: acetylcholine and memory consolidation. Trends Cogn Sci. 1999;3:351–359. doi: 10.1016/s1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Eichenbaum H. Hippocampal mechanisms for the context-dependent retrieval of episodes. Neural Netw. 2005;18:1172–1190. doi: 10.1016/j.neunet.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Stern CE. Mechanisms underlying working memory for novel information. Trends Cogn Sci. 2006;10:487–493. doi: 10.1016/j.tics.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Yoshihara T, Ichitani Y. Involvement of hippocampal metabotropic glutamate receptors in radial maze performance. Neuroreport. 2007;18:719–723. doi: 10.1097/WNR.0b013e3280d9e880. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Bursting of prefrontal cortex neurons in awake rats is regulated by metabotropic glutamate 5 (mGlu5) receptors: rate-dependent influence and interaction with NMDA receptors. Cereb Cortex. 2006;16:93–105. doi: 10.1093/cercor/bhi087. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature. 2003;426:285–291. doi: 10.1038/nature02162. [DOI] [PubMed] [Google Scholar]
- Klink R, Alonso A. Muscarinic modulation of the oscillatory and repetitive firing properties of entorhinal cortex layer II neurons. J Neurophysiol. 1997;77:1813–1828. doi: 10.1152/jn.1997.77.4.1813. [DOI] [PubMed] [Google Scholar]
- Kloosterman F, Witter MP, Van Haeften T. Topographical and laminar organization of subicular projections to the parahippocampal region of the rat. J Comp Neurol. 2003;455:156–171. doi: 10.1002/cne.10472. [DOI] [PubMed] [Google Scholar]
- Koller G, Satzger W, Adam M, Wagner M, Kathmann N, Soyka M, Engel R. Effects of scopolamine on matching to sample paradigm and related tests in human subjects. Neuropsychobiology. 2003;48:87–94. doi: 10.1159/000072883. [DOI] [PubMed] [Google Scholar]
- Major G, Tank D. Persistent neural activity: prevalence and mechanisms. Curr Opin Neurobiol. 2004;14:675–684. doi: 10.1016/j.conb.2004.10.017. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Koene RA, Eichenbaum H, Hasselmo ME. Cholinergic deafferentation of the entorhinal cortex in rats impairs encoding of novel but not familiar stimuli in a delayed nonmatch-to-sample task. J Neurosci. 2005;25:10273–10281. doi: 10.1523/JNEUROSCI.2386-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami A, Masuoka T, Yasuda M, Yamamoto Y, Kamei C. Participation of cholinergic system in memory deficits induced by blockade of hippocampal mGlu(1) receptors. Eur J Pharmacol. 2007;575:82–86. doi: 10.1016/j.ejphar.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Moran MM, Xu H, Clapham DE. TRP ion channels in the nervous system. Curr Opin Neurobiol. 2004;14:362–329. doi: 10.1016/j.conb.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Naber PA, Lopes da Silva FH, Witter MP. Reciprocal connections between the entorhinal cortex and hippocampal fields CA1 and the subiculum are in register with the projections from CA1 to the subiculum. Hippocampus. 2001;11:99–104. doi: 10.1002/hipo.1028. [DOI] [PubMed] [Google Scholar]
- Naie K, Manahan-Vaughan D. Regulation by metabotropic glutamate receptor 5 of LTP in the dentate gyrus of freely moving rats: relevance for learning and memory formation. Cereb Cortex. 2004;14:189–198. doi: 10.1093/cercor/bhg118. [DOI] [PubMed] [Google Scholar]
- Oestreich J, Dembrow NC, George AA, Zakon HH. A “sample-and-hold” pulse-counting integrator as a mechanism for graded memory underlying sensorimotor adaptation. Neuron. 2006;49:577–588. doi: 10.1016/j.neuron.2006.01.027. [DOI] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H. Complementary roles of the orbital prefrontal cortex and the perirhinal-entorhinal cortices in an odor-guided delayed-nonmatching-to-sample task. Behav Neurosci. 1992;106:762–775. doi: 10.1037//0735-7044.106.5.762. [DOI] [PubMed] [Google Scholar]
- Penetar DM, McDonough JH., Jr Effects of cholinergic drugs on delayed match-to-sample performance of rhesus monkeys. Pharmacol Biochem Behav. 1983;19:963–967. doi: 10.1016/0091-3057(83)90399-4. [DOI] [PubMed] [Google Scholar]
- Perrier JF, Hounsgaard J. Ca(2+)-activated nonselective cationic current (I(CAN)) in turtle motoneurons. J Neurophysiol. 1999;82:730–735. doi: 10.1152/jn.1999.82.2.730. [DOI] [PubMed] [Google Scholar]
- Reboreda A, Alonso A, Seguela P. Characterization of Ican role on the firing pattern of layer V neurons from rat entorhinal cortex under cholinergic modulation. FENS Abstr. 2006;3:A153.13. [Google Scholar]
- Reboreda A, Raouf R, Alonso A, Seguela P. Development of cholinergic modulation and graded persistent activity in layer v of medial entorhinal cortex. J Neurophysiol. 2007;97:3937–3947. doi: 10.1152/jn.01233.2006. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Semple J, Kumar R, Truman MI, Shorter J, Ferraro A, Fox B, McKay G, Matthews K. Effects of scopolamine on delayed-matching-to-sample and paired associates tests of visual memory and learning in human subjects: comparison with diazepam and implications for dementia. Psychopharmacology (Berl) 1997;134:95–106. doi: 10.1007/s002130050430. [DOI] [PubMed] [Google Scholar]
- Scharfman HE. Hyperexcitability of entorhinal cortex and hippocampus after application of aminooxyacetic acid (AOAA) to layer III of the rat medial entorhinal cortex in vitro. J Neurophysiol. 1996;76:2986–3001. doi: 10.1152/jn.1996.76.5.2986. [DOI] [PubMed] [Google Scholar]
- Schon K, Atri A, Hasselmo ME, Tricarico MD, LoPresti ML, Stern CE. Scopolamine reduces persistent activity related to long-term encoding in the parahippocampal gyrus during delayed matching in humans. J Neurosci. 2005;25:9112–9123. doi: 10.1523/JNEUROSCI.1982-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon K, Hasselmo ME, Lopresti ML, Tricarico MD, Stern CE. Persistence of parahippocampal representation in the absence of stimulus input enhances long-term encoding: a functional magnetic resonance imaging study of subsequent memory after a delayed match-to-sample task. J Neurosci. 2004;24:11088–11097. doi: 10.1523/JNEUROSCI.3807-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Miller EK, Desimone R. Object and place memory in the macaque entorhinal cortex. J Neurophysiol. 1997;78:1062–1081. doi: 10.1152/jn.1997.78.2.1062. [DOI] [PubMed] [Google Scholar]
- Svirskis G, Hounsgaard J. Transmitter regulation of plateau properties in turtle motoneurons. J Neurophysiol. 1998;79:45–50. doi: 10.1152/jn.1998.79.1.45. [DOI] [PubMed] [Google Scholar]
- Tahvildari B, Fransén E, Alonso AA, Hasselmo ME. Switching between “On” and “Off” states of persistent activity in lateral entorhinal layer III neurons. Hippocampus. 2007;17:257–263. doi: 10.1002/hipo.20270. [DOI] [PubMed] [Google Scholar]
- Tozzi A, Bengtson CP, Longone P, Carignani C, Fusco FR, Bernardi G, Mercuri NB. Involvement of transient receptor potential-like channels in responses to mGluR-I activation in midbrain dopamine neurons. Eur J Neurosci. 2003;18:2133–2145. doi: 10.1046/j.1460-9568.2003.02936.x. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O, Hinz U, Unsicker K, Egorov AV. Distribution of TRPC1 and TRPC5 in medial temporal lobe structures of mice. Cell Tissue Res. 2005;322:201–206. doi: 10.1007/s00441-005-0004-4. [DOI] [PubMed] [Google Scholar]
- Wyart C, Cocco S, Bourdieu L, Léger JF, Herr C, Chatenay D. Dynamics of excitatory synaptic components in sustained firing at low rates. J Neurophysiol. 2005;93:3370–3380. doi: 10.1152/jn.00530.2004. [DOI] [PubMed] [Google Scholar]
- Young BJ, Otto T, Fox GD, Eichenbaum H. Memory representation within the parahippocampal region. J Neurosci. 1997;17:5183–5195. doi: 10.1523/JNEUROSCI.17-13-05183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]