Magnetic Fe3O4 nanoparticles (NPs) are promising as drug delivery vehicles for both diagnostic and therapeutic applications.1 The key to achieving these dual applications is that the drug-Fe3O4 NPs are stable in a biological circulation system, readily interact with cells or other biological units of interest, and are capable of releasing the drug once the selected targeting is realized.2 Currently, drug-Fe3O4 NP conjugates are made either by embedding the drug in the hydrophobic media in the double-layer coating of Fe3O4 NPs3 or by incorporating both drug and Fe3O4 NPs in the SiO2 matrix.4 Although the conjugates prepared from these methods show enhanced dispersion stability, they have a hydrodynamic diameter of 150 nm or larger. Such large NP delivery systems may have very limited extravasation ability and may be subject to easy uptake by the reticuloendothelial system (RES),5 unsuitable for target-specific delivery applications. Recently we reported that Fe3O4 NPs coated with dopamine (DPA) and COOH-terminated polyethylene glycol (PEG) are stable in cell culture media against macrophage cell uptake.6 The hydrodynamic sizes of the NPs are tuned by the length of the PEG molecules. These PEG-DPA-Fe3O4 NPs offer an ideal platform for drug coupling and delivery.
Here we report that 6-hydroxy-chromone-3-carbaldehyde (1a) can be readily coupled to these PEG-DPA-Fe3O4 NPs via a Schiff-base bond, as shown in Figure 1A, and released via a pH controlled manner. Chromones are a group of naturally occurring compounds containing core structure of benzopyranone and have been shown to be antifungal, antiviral, antihypertensive, and anticancer agents.7 However, their low solubility and short blood circulation time limit their usage for efficient therapeutic applications. We demonstrate that 1a coupled to 1c shows a dramatic increase in solubility from less than 2.5 for free chromone to 633 μg/mL for 1d. 1d is stable in neutral pH condition but releases free chromone very quickly in pH lower than 6, and inhibits HeLa cell proliferation more efficiently than the free chromone. This, plus the fluorescent chromone and superparamagnetic Fe3O4 NPs, renders 1d a powerful multifunctional delivery system for diagnostics and therapeutic applications.
Figure 1.
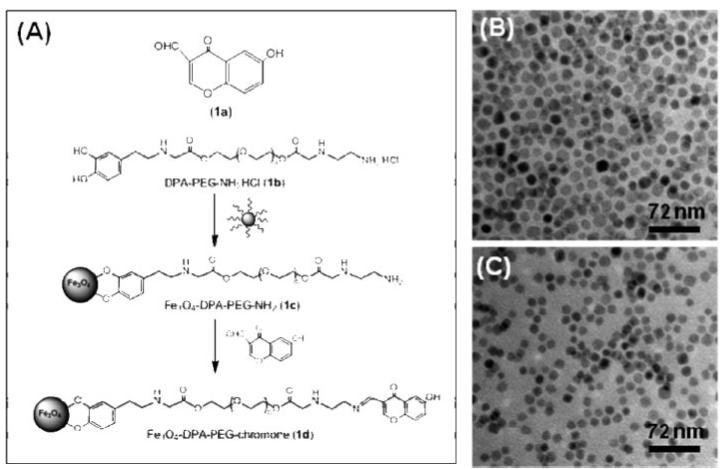
(a) Structure of chromone (1a) and the schematic illustration of the coupling between chromone and a Fe3O4 NP; TEM images of (b) the as-synthesized 12 nm Fe3O4 NPs from the hexane dispersion and (c) the chromone modified Fe3O4 NPs (1d) from water.
Fe3O4 NPs coated with a layer of oleate/oleylamine were synthesized through the decomposition of Fe(acac)3 with the core size around 12 nm (Figure 1B).8 The as-synthesized NPs were made biocompatible by replacing oleate/oleylamine with DPA-PEG (1b), giving 1c. 1a was loaded onto 1c via the formation of a Schiff-base bond.8 1d was readily dispersed in water with chromone solubility reaching 633 μg/ml,8 equal to ∼140 chromone molecules per Fe3O4 NP. The Fe3O4 core in 1d (Figure 1C) is similar to what is seen in Figure 1B, indicating no NP morphology change in the surfactant exchange process.
The Schiff-base bond is biodegradable via hydrolysis, and the process can be accelerated at low pH conditions.9 To examine the pH controlled release of chromone in 1d, we put the conjugate in the dialysis bag and incubated at different temperatures in the phosphate-buffered saline (PBS) plus 10% fetal bovine serum (FBS) with pH at 5 and 7.4. The detailed pH-dependent release in different buffers with pH ranging from 3 to 9 is given in the Supporting Information, Figure S4. The released chromone was quantified through its fluorescent signal.8 Figure 2 shows the percentage of chromone released from 1d at different pH values and temperatures. It can be seen that a few chromone are detached from 1d in the PBS of pH = 7.4 at both 25 and 37 °C culture conditions. However, in the PBS of pH = 5, the conjugate exhibits a drastic increase in free chromone and the higher incubation temperature (37 °C) results in higher chromone concentration in the solution. The slight increase in chromone release at 37 °C in pH = 5 indicates that the Schiff-base bond is subject to faster hydrolyzation at higher temperature. The hydrodynamic size of 1d is decreased in pH = 5, but those in pH = 7.4 are stable, as shown in Figure S5. This proves that chromone is released from 1d at low pH but is stable in the conjugate at pH = 7. From Figure S5, one can also see that 1c are stable in the incubation conditions and show no statistical hydrodynamic size change over the incubation time. The measured size increase from ∼60 to ∼110 nm in the presence of FBS is attributed to the adsorption of FBS onto the NP surface as reported previously.10
Figure 2.
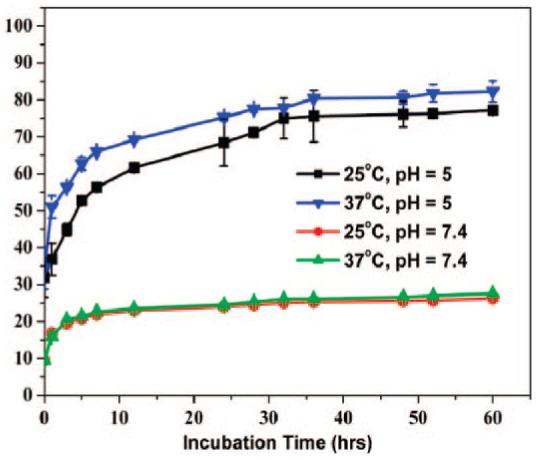
The percentage of the chromone released in PBS + 10% FBS from 1d with the incubation time under different pH values and temperatures in PBS.
The increased solubility of chromone present in 1d led to the enhanced uptake of 1d by HeLa cells. Figure S5 shows that at the same iron concentrations (7 μg/ml), more 1d than 1c are taken up by HeLa cells. Similar uptake enhancement is also observed for 1d over 1a. Figure 3 panels A and B are fluorescent images of the HeLa cells after their incubation with 1d and 1a in the same chromone concentration at 15 μg/ml. Because of the high chromone solubility in 1d, there exist more chromone molecules in solution interacting with HeLa cells, leading to the enhanced uptake and brighter image of the cells in Figure 3A. In contrast, the free chromone has very low solubility and with the same total amount of chromone added, the majority of the free chromone stays in the solid form and can be separated by centrifugation (8000 rpm). As a result, there is only small amount of free chromone in solution interacting with the cells, resulting in fewer uptakes and much darker fluorescent image (Figure 3B).
Figure 3.
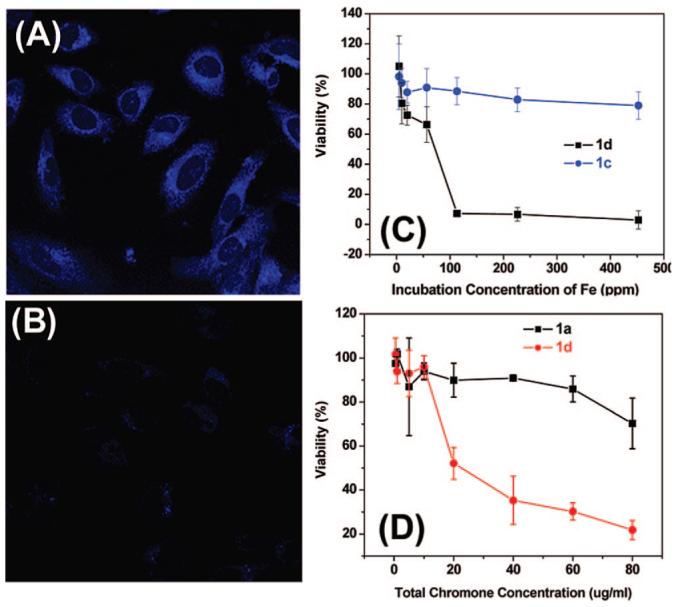
Fluorescent images of HeLa cells after incubated with (A) 1d and (B) 1a for 1 h; and viability of HeLa cells in the presence of (C) total iron concentration and (D) total chromone concentration.
The enhanced uptake of 1d leads to high toxicity to the HeLa cells. Figure 3 panels C and D are the HeLa cell viability data under the same iron (Figure 3C) concentration or the same amount of chromone added to the cell culture medium (Figure 3D). It can be seen that both 1c and 1a have very limited toxicity to HeLa cells as 1c has no chromone and 1a has the very low solubility in the buffer, preventing its uptake by the cells. In contrast, 1d are highly toxic with majority of the cells destroyed at ∼100 ppm iron concentration or at ∼40 μg chromone/ml. This high toxicity of 1d to the HeLa cells comes likely from their enhanced uptake by the HeLa cells and the controlled release of free chromone from 1d in the low pH cellular environment. The IC50, the half-maximal (50%) inhibitory concentration (IC), of 1d can be extracted from Figure 3D to be ∼21 μg/ml. Note that in the current experimental conditions, we could not get IC50 for 1a, 1b, and 1c because of their low solubility/low toxicity to HeLa cells. It should also be mentioned that the detailed toxicity mechanism for the chromone–Fe3O4 NPs is still unclear. However, from the fact that the free chromone can be released in a low pH environment and that the endosome/lysosome within a cell have the low pH's (<6), we can reasonably assume that the toxicity arises from the release of the free chromone from the chrome–Fe3O4 conjugate in the endosome/lysosome of the HeLa cells.
The current work demonstrates that chromone coupled to PEG-DPA-Fe3O4 NPs shows a dramatic increase in chromone solubility in cell culture medium from less than 2.5 to 633 μg/mL, and the free chromone can be released in a controlled manner at low pH conditions. The high chromone solubility in the chromone-Fe3O4 conjugate leads to the enhanced chromone uptake by HeLa cells, and as a result, the chromone shows a much more efficient inhibition to the HeLa cell proliferation. Although it is unclear how powerful/useful of the chromone molecule is as an anticancer agent, its high solubility achieved in the chromone-Fe3O4 NP conjugate should allow for more tests on this front. Furthermore, the reported concept should be readily extended to the synthesis of other anticancer drug–Fe3O4 NP conjugates. With controlled drug release only in the intercellular low pH conditions, such drug–Fe3O4 conjugates may offer much needed efficacy in successful cancer therapy.
Supplemental Material
Acknowledgment
The work was supported by Grant NIH/NCI 1R21CA1285-1. B.W. thanks the support from National Natural Science Foundation of China (20475023).
Footnotes
Supporting Information Available: Nanoparticle/surfactant synthesis and characterization. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Sun C, Lee JSH, Zhang M. Adv. Drug Delivery Rev. 2008;60:1252–65. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jun YW, Lee JH, Cheon J. Angew. Chem., Int. Ed. 2008;47:5122–5135. doi: 10.1002/anie.200701674. [DOI] [PubMed] [Google Scholar]; (c) Xu CJ, Sun SH. Polym. Int. 2007;56:821–826. [Google Scholar]
- 2.(a) Arruebo M, Fernandez-Pacheco R, Ibarra MR. Nano Today. 2007;2:22–32. [Google Scholar]; (b) Torchilin VP. Pharm. Res. 2007;24:2333–2334. doi: 10.1007/s11095-007-9463-5. [DOI] [PubMed] [Google Scholar]
- 3.Jain TK, Morales MA, Sahoo SK, Leslie-Pelecky DL, Labhasetwar V. Mol. Pharm. 2005;2:194–205. doi: 10.1021/mp0500014. [DOI] [PubMed] [Google Scholar]
- 4.(a) Kohler N, Sun C, Fichtenholtz A, Gunn J, Fang C, Zhang M. Small. 2006;2:785–792. doi: 10.1002/smll.200600009. [DOI] [PubMed] [Google Scholar]; (b) Son SJ, Reichel J, He B, Schuchman M, Lee SB. J. Am. Chem. Soc. 2005;127:7316–7317. doi: 10.1021/ja0517365. [DOI] [PubMed] [Google Scholar]
- 5.(a) Hu Y, Xie J, Tong YW, Wang CH. J. Controlled Release. 2007;118:7–17. doi: 10.1016/j.jconrel.2006.11.028. [DOI] [PubMed] [Google Scholar]; (b) Fang C, Shi B, Pei YY, Hong MH, Wu J, Chen HZ. Eur. J. Pharm. Sci. 2006;27:27–36. doi: 10.1016/j.ejps.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Xie J, Xu CJ, Kohler N, Hou YL, Sun SH. Adv. Mater. 2007;19:3163–3156. [Google Scholar]
- 7.(a) Edwards AM, Howell JBL. Clin. Exp. Allergy. 2000;30:756–774. doi: 10.1046/j.1365-2222.2000.00879.x. [DOI] [PubMed] [Google Scholar]; (b) Mukherjee AK, Basu S, Sarkar N, Ghosh AC. Curr. Med. Chem. 2001;8:1467–1486. doi: 10.2174/0929867013372094. [DOI] [PubMed] [Google Scholar]; (c) Barve V, Ahmed F, Adsule S, Banerjee S, Kulkarni S, Katiyar P, Anson CE, Powell AK, Padhye S, Sarkar FH. J. Med. Chem. 2006;49:3800–3808. doi: 10.1021/jm051068y. [DOI] [PubMed] [Google Scholar]; (d) Pisco L, Kordian M, Peseke K, Feist H, Michalik D, Estrada E, Carvalho J, Hamilton G, Rando D, Quincoces J. Eur. J. Med. Chem. 2006;41:401–407. doi: 10.1016/j.ejmech.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 8.See Supporting Information.
- 9.(a) Kratz F, Beyer U, Schutte MT. Crit. Rev. Ther. Drug Carrier Syst. 1999;16:245–288. doi: 10.1615/critrevtherdrugcarriersyst.v16.i3.10. [DOI] [PubMed] [Google Scholar]; (b) Saito H, Hoffman AS, Ogawa HI. J. Bioact. Compat. Polym. 2007;22:589–601. [Google Scholar]
- 10.Xu CJ, Xie J, Kohler N, Walsh EG, Chin YE, Sun SH. Chem. Asian J. 2008;3:548–552. doi: 10.1002/asia.200700301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


