Abstract
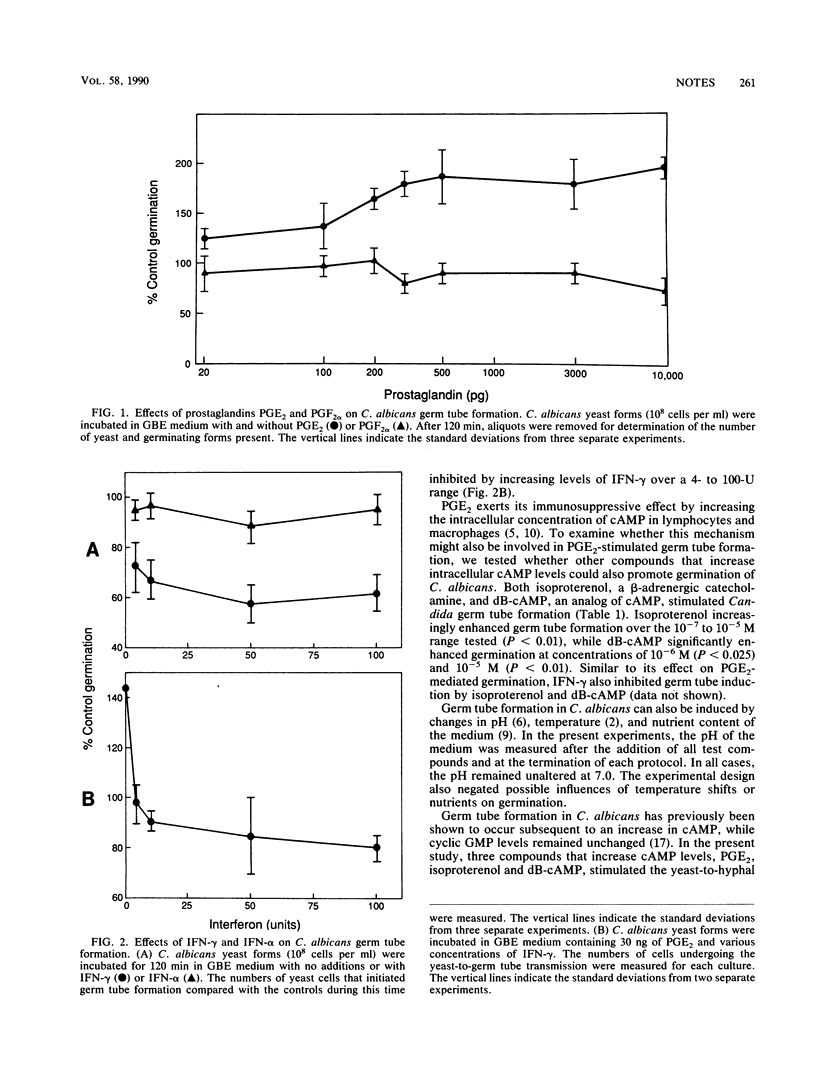
Prostaglandin E2 (PGE2), an immunosuppressive monokine that increases intracellular cyclic AMP (cAMP) levels, stimulated Candida albicans germ tube formation. Dibutyryl cAMP (dB-cAMP) and isoproterenol, other compounds that increase cAMP levels, also stimulated germination. Gamma interferon (IFN-gamma), a product of cellular immune system activation, inhibited Candida germ tube formation, even in the presence of PGE2, dB-cAMP, and isoproterenol. Thus, PGE2 and IFN-gamma as well as having opposing roles in the suppression or activation of cell-mediated immunity, are also antagonists for the yeast-to-hyphal transition of C. albicans.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. T. Prostaglandins and follicular functions. J Reprod Fertil. 1981 May;62(1):283–291. doi: 10.1530/jrf.0.0620283. [DOI] [PubMed] [Google Scholar]
- Auger P., Joly J. Factors influencing germ tube production in Candida albicans. Mycopathologia. 1977 Oct 28;61(3):183–186. doi: 10.1007/BF00468014. [DOI] [PubMed] [Google Scholar]
- Boraschi D., Censini S., Tagliabue A. Interferon-gamma reduces macrophage-suppressive activity by inhibiting prostaglandin E2 release and inducing interleukin 1 production. J Immunol. 1984 Aug;133(2):764–768. [PubMed] [Google Scholar]
- Bourne H. R., Lichtenstein L. M., Melmon K. L., Henney C. S., Weinstein Y., Shearer G. M. Modulation of inflammation and immunity by cyclic AMP. Science. 1974 Apr 5;184(4132):19–28. doi: 10.1126/science.184.4132.19. [DOI] [PubMed] [Google Scholar]
- Buffo J., Herman M. A., Soll D. R. A characterization of pH-regulated dimorphism in Candida albicans. Mycopathologia. 1984 Mar 15;85(1-2):21–30. doi: 10.1007/BF00436698. [DOI] [PubMed] [Google Scholar]
- Chouaib S., Welte K., Mertelsmann R., Dupont B. Prostaglandin E2 acts at two distinct pathways of T lymphocyte activation: inhibition of interleukin 2 production and down-regulation of transferrin receptor expression. J Immunol. 1985 Aug;135(2):1172–1179. [PubMed] [Google Scholar]
- Elger W., Fähnrich M., Beier S., Qing S. S., Chwalisz K. Endometrial and myometrial effects of progesterone antagonists in pregnant guinea pigs. Am J Obstet Gynecol. 1987 Oct;157(4 Pt 2):1065–1074. doi: 10.1016/s0002-9378(87)80134-5. [DOI] [PubMed] [Google Scholar]
- Evans E. G., Odds F. C., Richardson M. D., Holland K. T. The effect of growth medium of filament production in Candida albicans. Sabouraudia. 1974 Mar;12(1):112–119. doi: 10.1080/00362177485380151. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Bromberg S., Messner R. P. Studies on the cyclic AMP response to prostaglandin in human lymphocytes. Cell Immunol. 1981 May 15;60(2):298–307. doi: 10.1016/0008-8749(81)90271-9. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Ceuppens J. Regulation of the immune response by prostaglandins. J Clin Immunol. 1983 Oct;3(4):295–315. doi: 10.1007/BF00915791. [DOI] [PubMed] [Google Scholar]
- Heidenreich F., Dierich M. P. Candida albicans and Candida stellatoidea, in contrast to other Candida species, bind iC3b and C3d but not C3b. Infect Immun. 1985 Nov;50(2):598–600. doi: 10.1128/iai.50.2.598-600.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. M., Russell J. K., Torres B. A. Second messenger role of arachidonic acid and its metabolites in interferon-gamma production. J Immunol. 1986 Nov 15;137(10):3053–3056. [PubMed] [Google Scholar]
- Loose D. S., Feldman D. Characterization of a unique corticosterone-binding protein in Candida albicans. J Biol Chem. 1982 May 10;257(9):4925–4930. [PubMed] [Google Scholar]
- Moon Y. S., Duleba A. J., Kim K. S., Yuen B. H. Effects of prostaglandins E2 and F2 alpha on progesterone metabolism by rat granulosa cells. Biochem Biophys Res Commun. 1986 Mar 28;135(3):764–769. doi: 10.1016/0006-291x(86)90994-0. [DOI] [PubMed] [Google Scholar]
- Niimi M., Niimi K., Tokunaga J., Nakayama H. Changes in cyclic nucleotide levels and dimorphic transition in Candida albicans. J Bacteriol. 1980 Jun;142(3):1010–1014. doi: 10.1128/jb.142.3.1010-1014.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page S., Odds F. C. Binding of plasma proteins to Candida species in vitro. J Gen Microbiol. 1988 Oct;134(10):2693–2702. doi: 10.1099/00221287-134-10-2693. [DOI] [PubMed] [Google Scholar]
- Sandin R. L., Rogers A. L., Patterson R. J., Beneke E. S. Evidence for mannose-mediated adherence of Candida albicans to human buccal cells in vitro. Infect Immun. 1982 Jan;35(1):79–85. doi: 10.1128/iai.35.1.79-85.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel J. D., Muller G., Buckley H. R. Critical role of germ tube formation in the pathogenesis of candidal vaginitis. Infect Immun. 1984 Jun;44(3):576–580. doi: 10.1128/iai.44.3.576-580.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin S. S., Hirsch J., Ledger W. J. A macrophage defect in women with recurrent Candida vaginitis and its reversal in vitro by prostaglandin inhibitors. Am J Obstet Gynecol. 1986 Oct;155(4):790–795. doi: 10.1016/s0002-9378(86)80022-9. [DOI] [PubMed] [Google Scholar]
- Witkin S. S., Jeremias J., Ledger W. J. A localized vaginal allergic response in women with recurrent vaginitis. J Allergy Clin Immunol. 1988 Feb;81(2):412–416. doi: 10.1016/0091-6749(88)90909-8. [DOI] [PubMed] [Google Scholar]


