Abstract
Human Papillomavirus (HPV) is responsible for 99.7% of cervical cancer cases and an estimated 5% of all cancers worldwide. The largest burden from HPV-associated cervical cancers is in developing nations where effective cervical cancer screening programs are non-existent. Even in developed nations, diagnosis and treatment of cervical precancers continue to be large economic burdens. Prophylactic vaccination against HPV is an ideal method for the prevention of cervical cancer and other HPV associated diseases. Safe and effective virus-like-particle-derived prophylactic vaccines are available to most nations. The high cost of the current vaccines makes it out of reach for most developing nations. Since millions of women are already infected with HPV and have serious disease, therapeutic HPV vaccines are being developed to treat these women. The article presents the natural history, oncogenesis, and host immune interactions of HPV and associated diseases. The article also discusses the safety and efficacy of commercially available prophylactic vaccines against HPV, as well as novel prophylactic and therapeutic vaccine delivery strategies in early clinical development.
INTRODUCTION
Human papillomaviruses (HPVs) cause 5% of all cancers worldwide, which is similar to the burden caused by both hepatitis B and Helicobacter pylori [1]. These three viral infections result in the development of 15% of all cancers, which are responsible for over three-quarters of the cancers caused by known infectious diseases. The greatest burden of HPV-induced cancers occurs in developing countries. Although cervical cancer makes up the majority of these HPV-induced cancers, HPV is also responsible for the majority of penile, vaginal, vulvar, anal, and some oropharyngeal cancers [2–4].
Rates of cervical cancer have plummeted in countries where cervical cancer screening has been implemented. Unfortunately, most developing countries continue to lack resources for cervical cancer screening. This lack of screening results in the diagnosis of approximately 450,000 cervical cancer cases each year [5]. In the United States (US), approximately 11,000 cases of cervical cancer occur each year; the majority of which are found in women who have not had adequate cervical cancer screening (i.e., Papanicolaou [Pap] smears). In addition to the costs associated with cervical cancer, current screening strategies lead to huge medical costs associated with referrals and treatment of precancerous lesions. Huge medical costs are also incurred for treatment of external genital warts. These costs combined have been estimated at $4 billion (US dollars) annually [6]. Clearly, the impact for preventive vaccines could be powerful.
Since it is estimated that over 1.5 million women currently have HPV-associated disease in the US (i.e., condyloma, cervical intraepithelial neoplasia [CIN], and cancer), therapeutic vaccines have a great potential to result in life-saving and cost-saving therapy. In the US, standard treatment of HPV-associated disease is already quite costly and invasive. Certain populations, such as immunocompromised individuals, have high rates of recurrent disease after standard treatment, creating costly and frustrating scenarios. Therefore, alternate strategies such as vaccines for treatment of HPV infected women have been sought. In developing countries, even standard treatment is often unavailable. If therapeutic vaccines were cost-effective, then access to treatment could be dramatically improved.
HPV types associated with cancer
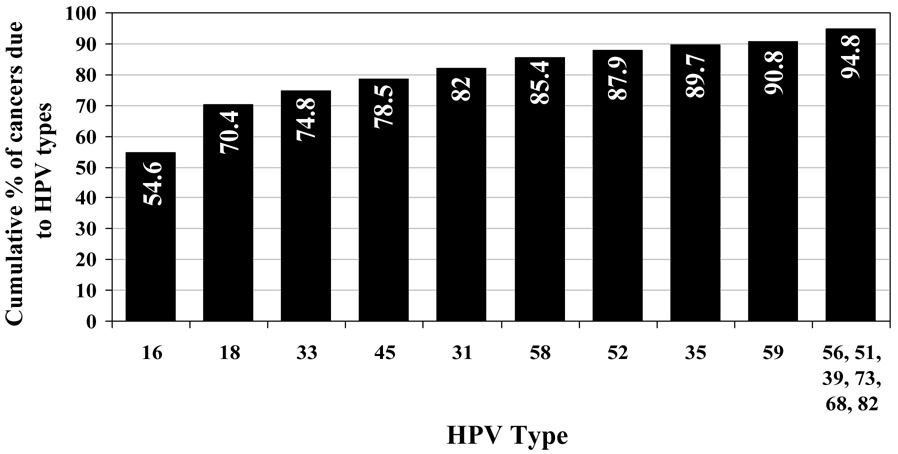
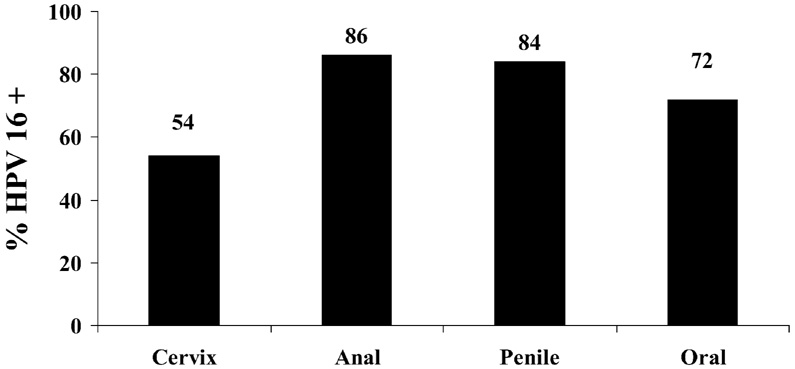
Figure 1 shows the predominant types of high-risk (HR) HPV in cervical cancer [7]. HPV 16 is also quite common in anal, penile, and oropharyngeal cancers (Figure 2) [3, 4, 8, 9]. It is estimated that vaccines which target HPV 16 and 18 would prevent 70% of cervical cancers; if HPV 45 and 31 were added, approximately 80% would be prevented. The potential role of other HPV types in causing cancer in vaccinated individuals is unknown. Some speculate that these “other” HPV types will simply replace HPV 16 and 18. Currently, there is no epidemiologic evidence to suggest this is true. HPV 16 and 18 have been consistently found at similar prevalence rates in studies of cervical cancer worldwide [10]. The detection of HPV 16 and 18 in women carries a 5-fold greater risk of developing the precancer CIN 2/3 than detection of other HR types [11]. Surprisingly, the overall efficacy of the current vaccine in the prevention of disease from all HPV types is not as dramatic as expected (see below: Current Vaccines). This suggests that other HPV types will continue to cause high-grade lesions [12]. Their role in causing cancers after vaccination remains to be defined.
Figure 1.
Cumulative percent of cervical cancer due to high-risk HPV types [7].
Figure 2.
Natural history of HPV
To understand the role of vaccines in HPV control, it is important to review the basics which include expression of viral proteins and the importance of immunologic responses to HPV control.
In order to initiate a productive acute infection, HPV infects basal epithelial cells via a wound or where they are anatomically accessible, such as the columnar epithelium of the cervix. Infection occurs when capsid proteins L1 and L2 attach to host cell receptors [13–15]. After attachment, the viral genome makes its way to the nucleus through ways not yet completely defined. The virus then uses the host cell DNA replication machinery to begin its own replication process. Whether there is a latency phase prior to active replication remains controversial. These viral genomes appear to persist in the nucleus of the infected stem cell and are distributed during mitosis to the daughter cells [16]. Early on in the replication process, E6 and E7 are expressed in the parabasalar cell layer. The two late proteins L1 and L2, which are responsible for the virion assembly and DNA packaging, are expressed quite late in the mature squamous cells. The final production of an infectious virion requires terminal differentiation of the infected epithelial cell [17, 18]. Release of infectious virus occurs at the natural end of the epithelial cell life through normal cell desquamation. Since this process is non-lytic, there is little induction of inflammatory processes. Viral replication during cell differentiation may result in typical cytopathologic changes, which are considered benign (i.e., CIN 1) [19, 20]. There is much controversy surrounding whether all women with HPV develop these cytopathic changes.
Clearly, viral persistence as defined by repeated DNA detection is the key to the development of cervical cancer. However, the term “persistence” remains a relatively elusive definition. Some individuals define persistence as the presence of the same virus present over a 6-month period; others require longer definitions. None of these definitions have credible biologic meaning since CIN 2 and 3 have been shown to occur in some women within months of HPV acquisition [21, 22], whereas some women carry HPV for years prior to clearance without ever developing CIN 2/3 [23–25]. Epidemiology studies show that the mean age of invasive cancer is several decades after the peak of detected infection [2]. Other cofactors seriously considered are smoking, oral contraceptive use, parity, and C. trachomatis infections [2]. However, compared with the risk associated with HPV persistence (which is in the order of 400 to 800-fold) [26], these other risk factors contribute a much lower risk in the order of 2 to 3-fold and are not independent of HPV persistence. These epidemiologic observations all have plausible biologic pathways that are discussed elsewhere [27–31].
Oncogenesis
The mechanisms involved in oncogenesis are thought to be primarily associated with the early expressed proteins E6 and E7 [32]. Although the relationships are complex, E6 works mostly through its ability to enhance p53 degradation. E7 causes disruption of the E2F/pRb complex, activating E2F, an important cellular transcription factor. Consequently, both E6 and E7 interfere with cell cycle control and cause cells to replicate even during times of DNA damage. E6 also has an important role in activating telomerase, an enzyme responsible for telomere lengthening, resulting in the prolonged life of epithelial cells. E7 is also involved in the blockade of apoptosis. E6 and E7 are expressed quite early in the life cycle of HPV, primarily in the basal and parabasal cell layer. In low-grade lesions, such as CIN 1, the expression level is rather low and confined to the lower levels, but as lesions progress, E6 and E7 become expressed throughout the epithelium. Because their expression is maintained during progression to high-grade lesions, these proteins have been main targets for therapeutic vaccines. The morphologic changes of CIN 2/3 (aneuploidy, altered chromatin texture, and increased nuclear volume) suggest that CIN 2/3 changes are due, in part, to the expression of these viral genes in epithelial stem cells which have lost the capacity to differentiate [33]. The epithelial stem cells experience mutagenic consequences (i.e., damage of chromosomal integrity and recombination of diverse DNA and viral integration) [34]. CIN 3 lesions and carcinomas are also found to have a higher number of HPV genomic integrations which, in turn, promote E6 and E7 expression [35, 36].
Immune responses to HPV
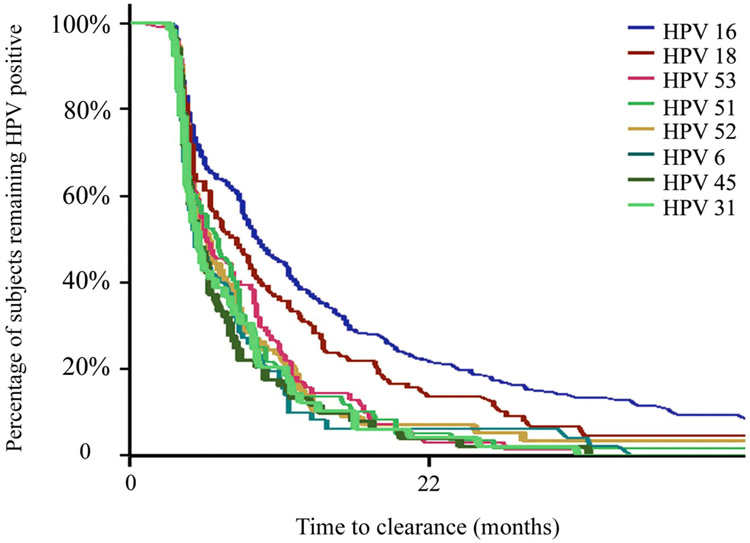
Curves depicting the natural history of HPV (Figure 3) [24] demonstrate that clearance likely involves both innate and adaptive immunity. Clearance for many women is seen within days to weeks and is likely due to innate mechanisms. In contrast, many women clear after months to years of infection, which is likely due to adaptive cell-mediated immune responses. The innate immune system reflects a complex system to recognize pathogens, including innate effector mechanisms (e.g., inflammation and chemotaxis), and acts as a bridge to adaptive immunity. One of the most important aspects of the innate immune system is the role of Toll-like receptors (TLRs) which are found on cells of the innate system, particularly dendritic cells and macrophages. These TLRs respond to pathogen-associated molecular patterns on a broad range of pathogens [37–40]. Ten human TLRs have been identified to date. The innate effector mechanisms resulting from TLR activation are mainly mediated through the production of cytokines that have direct anti-viral effects (e.g., type 1 interferons [IFNs] [IFN-α and -β]), promote inflammation (e.g., IL-1α, IL-1β, IL-6, and tumor necrosis factor), and chemotaxis (e.g., IL-8). TLRs also shape the adaptive response. The role of TLRs in control of HPV is unknown, but in vitro studies suggest that TLR 9 and 4 are important in initial recognition [41, 42]. Other innate factors thought to be important include lysis of infected cells by natural killer cells, inhibition of major histocompatibility complex class I proteins, and inhibition of signaling by type I IFNs [43].
Figure 3.
Natural history of HPV type-specific clearance (personal communication, Moscicki, University of California San Francisco).
Cell-mediated immune responses to HPV
Empirical evidence for the role of cell-mediated immunity in controlling cervical HPV infection comes from observations of increased persistence of HPV infection and associated disease in immunosuppressed individuals [44–47]. Although cell-mediated immune responses to HPV have been measured in a variety of ways [48–52], it appears that both CD4+ and CD8+ T-cell responses to E6 and E7 are important in modulating HPV infection and HPV-associated disease [52–55]. Recently, the role of T-regulatory cells in dampening immune responses has been recognized as potentially important in lesion progression [56, 57].
Integration of E6 and E7 promotes expression of these oncoproteins. It remains unclear whether viral integration occurs in women with viral persistence who continue to have normal cytology. Most studies show that viral integration is not seen in women with HPV persistence who remain healthy [58]. These findings may suggest that immune control through anti-E6 and -E7 effects may stop integration.
Recent attention has been drawn to the importance of cell-mediated responses to other viral proteins, including E2 (enhances viral replication and controls E6 and E7 expression) [59–61], E4 (binds cytokeratins; assists in blocking apoptosis) [62, 63], E5 (induces cellular proliferation) [64, 65], and L1/L2 (major capsid proteins) in viral clearance [66].
Mechanisms to evade the immune response
HPV is non-lytic and thereby does not elicit pro-inflammatory signals that would activate dendritic cells. In addition, viral proteins are not secreted; they are expressed in the nucleus of infected cells. The production of the capsid proteins, which are highly immunogenic, is limited to the terminally differentiated outer layers that are well away from the basal cell and subepithelial epithelium. Consequently, HPV L1 and L2 have little contact with immune responses at the epithelium.
HPV has numerous mechanisms to escape immune recognition. HPV has been shown to downregulate the expression of TLR, MCP-1, and IL-8, as well as block IFN-α function. In addition, major histocompatibility complex (MHC) class II expression is dampened by both HPV 16 and 18. E5 has been shown to enhance acidification of endosomes, disrupting trafficking of exocytic and endocytic pathways of MHC expression. E7 represses the MHC class I heavy chain promoter. Mechanisms used by HPV to escape the host immune response have been reviewed in depth by Kanodia et al [67].
Mucosal immune responses and HPV
There is certainly evidence that HPV can induce local immune responses. This is not surprising since HPV is not a systemic infection; rather, it remains an infection limited to epithelial cells. Secretory IgA and IgG specific to HPV can be found in cervical mucous [68]. Cytokines from cervical samples suggest that both innate and adaptive immunity is elicited by HPV in cervical immune cells [69]. HPV also appears to dampen and evade the immune response in mucosal cells. Whether these responses play any role in protection from re-infection is not known.
CURRENT VACCINES
Virus-like particle-based vaccines
Gardasil® (Merck & Co, Inc.; Whitehouse Station, New Jersey) is one preventive vaccine approved by the Food and Drug Administration (FDA). Another vaccine, Cervarix® (GlaxoSmithKline Biologicals; Rixensart, Belgium), was filed with the FDA in March 2007.
Both of these vaccines are composed of non-infectious, recombinant HPV virus-like particles (VLP). These icosahedral structures are composed of the major capsid protein, L1. When L1 is expressed in cells, it self-assembles to reflect the naturally occurring viral capsid. Since the VLP capsid assembles without viral DNA, the vaccine is non-infectious. This VLP has been shown to elicit strong systemic immune responses, both neutralizing antibodies and cellmediated immunity [8, 70–72]. Human studies have shown that high titers of specific antibodies are present in cervical secretions of women receiving HPV 16 L1 VLP [73]. However, it is thought that these HPV-specific antibodies are present in cervical secretions secondary to transudation or exudation at the site of microtrauma rather than local synthesis.
Efficacy of the quadrivalent vaccine
Gardasil® is a quadrivalent HPV VLP vaccine, which includes VLPs of HPV types 6, 11, 16, and 18. The VLPs are produced in yeast and the vaccine contains an adjuvant of aluminum hydroxyphosphate sulfate [12].
Several publications regarding Gardasil’s® efficacy in preventing HPV acquisition, persistence, and precancerous lesions have now been published [12, 66, 74–77]. None of the studies (either Gardasil ® or Cervarix ® ) have shown that the vaccine prevents cervical cancer. Clearly, it would not be ethical to have cervical cancer as an outcome since prevention of cancers through screening and treatments is standard of care. Rather, the studies were designed to see if the vaccine prevents HPV acquisition, persistence, and HPV-associated vulvar, vaginal, and cervical premalignant disease. These studies carefully followed women with cytology and HPV testing. The primary interest for most clinicians is reducing CIN 2/3 rates, which are considered true precancerous lesions. Results from the two largest trials, FUTURE I and FUTURE II, are summarized in Table 1 [12, 77]. The studies were designed to have answers regarding protection against HPV 6, 11, 16, 18-associated CIN 2/3 within 2 to 3 years after completion of the vaccination series. In order to see differences between groups in a limited period, women needed to be sexually active. However, in order to find unexposed women, women were required to have a limited number of sexual partners. HPV is acquired by most young women within 4 to 5 years after the onset of sexual activity, and the number of partners highly influences exposure rates. Consequently, entry criteria for FUTURE I and II limited the generalizability of the findings. In FUTURE I, women were excluded if they had more than 4 lifetime sexual partners, had a history of abnormal cytology or genital warts, or were pregnant. FUTURE II allowed women with a history of abnormal Pap smears or genital warts, but excluded women with more than 4 lifetime sexual partners and those who were pregnant. Both studies enrolled women aged 15 to 26 years. In comparison, US data show that on average, sexually active women aged 19 to 21 years have had 4 or more sexual partners [78].
Table 1.
Vaccine efficacy of Gardasil® against external genital, vaginal, and vulvar lesions associated with HPV 6, 11, 16, or 18, or regardless of HPV type.
| Vaccine (per protocol*) % (95% CI) | USP** (Unrestricted susceptible population) % (95% CI) | ITT*** (Vaccine types only) % (95% CI) | ITT*** (any HPV) % (95% CI) | |
|---|---|---|---|---|
| FUTURE I | ||||
| Condyloma | 100 (92 – 100) | 96 (86 – 99) | 76 (61 – 86) | 51 (32 – 65) |
| VIN† 1 or VAIN‡ 1 | 100 (49 – 100) | 82 (16 – 98) | 63 (<0 – 88) | 18 (<0 – 46) |
| VIN† 2/3 or VAIN‡ 2/3 | 100 (49 – 100) | 91 (37 – 100) | 62 (<0 – 89) | 26 (<0 – 63) |
| CIN 1 | 100 (92 – 100) | 97 (89 – 100) | 62 (46 – 74) | 25 (12 – 36) |
| CIN 2 | 100 (81 – 100) | 100 (86 – 100) | 30 (<0 – 56) | 13 (<0 – 34) |
| CIN 3 | 100 (76 – 100) | 100 (83 – 100) | 12 (<0 – 44) | −9 (<0 – 22) |
| AIS†† | 100 (15 – 100) | 100 (15 – 100) | 83 (<0 – 100) | 83 (<0 – 100) |
| FUTURE II | ||||
| CIN 2 | 100 (86 – 100) | 99 (93 – 100) | 57 (38 – 71) | 17 (1 – 31) |
| CIN 3 | 97 (79 – 100) | 97 (90 – 100) | 45 (23 – 61) | 22 (3 – 38) |
| AIS†† | 100 (<0 – 100) | 100 (55 – 100) | 28 (<0 – 82) | 21 (<0 – 38) |
FUTURE I included 2,723 vaccinated women and 2,732 control women; FUTURE II included 6,087 vaccinated women and 6,080 control women
The per protocol susceptible population was defined as subjects who were negative on PCR analysis and serologic testing for the relevant HPV type at enrollment, remained negative on PCR analysis for the same HPV type through 1 month after administration of the third dose of vaccine or placebo, received 3 doses of vaccine or placebo within 1 year, and did not have protocol violations.
Unrestricted susceptible population was defined as subjects who were negative by PCR analysis and serologic testing at enrollments, were allowed major protocol violations and received at least 1 dose. Case counting occurred after the first dose.
ITT = intention-to-treat. The intention-to-treat population was defined as subjects who underwent randomization, including those with prevalent anogenital disease or infections caused by any high- or low-risk HPV type before the administration of vaccine or placebo.
VIN = vulvar intraepithelial neoplasia
VAIN = vaginal intraepithelial neoplasia
AIS = adenocarcinoma in situ
The analyses were then separated into a per protocol and intention-to-treat analysis. Per protocol analysis included women who appeared HPV naïve to the 4 HPV types (i.e., were seronegative and DNA negative to the relevant vaccine HPV type at Day 1), remained polymerase chain reaction (PCR) negative to the relevant vaccine HPV type during the vaccination phase, had no protocol violations, and received all 3 vaccine doses (0-, 2-, and 6- month schedule). Cases were not counted until 1 month after the third dose. This was done to reflect a truly naïve population — the HPV virgin. This would also reflect a population who had not initiated sexual intercourse. The intention-to-treat analysis attempted to generalize the data to a larger group, which included women with a history of exposure by either PCR or serology and those treated with only 1 to 2 doses of the vaccine, and began counting cases after the first vaccination. The efficacy of Gardasil® to prevent CIN 2/3 associated with HPV 6, 11, 16, and 18 in women who appear naïve to HPV is close to 100%. Overall efficacy to prevent vulvar lesions including warts and VIN was also close to 100%. However, the overall efficacy of the vaccine to prevent CIN 2/3 was lowered dramatically when women already exposed to this virus were allowed to be vaccinated (Table 1), underscoring the importance of immunizing groups of women not exposed to the virus. Given the high rate of HPV acquisition, even with one partner, the vaccine has the best chance to be successful in non-sexually active youth.
One finding worth mentioning is the extremely low efficacy found in the analysis examining the prevention of CIN 2/3 for all HPV types. Certainly, it was expected that types other than HPV 16 and 18 would continue to cause abnormal cytology. It was expected that the vaccine would prevent 40% to 50% of CIN 2/3 cases, which is the proportion caused by HPV types 16 and 18 [3]. Data was not given for those in the per protocol analysis for CIN 2/3 among all HPV types. However, efficacy against CIN 2/3 lesions in the intention-to-treat analysis against any HPV type showed no statistical efficacy against CIN 3 (21%; 95% CI, <0% to 38%), and a reduction of 22% (95% CI, 3% to 38%) for CIN 2 (Table 1) [77].
Closer examination of the group that was already exposed to HPV revealed that once exposed to HPV, there is no protection for that specific type. From the FUTURE II data, the vaccine effectiveness against HPV 16- or 18-related CIN 2/3 and adenocarcinoma in situ for the intention-to-treat group was reported for those with a history of HPV exposure. They showed that the percent reduction was 10.6% (95% CI, <0% to 46%) if the women were serostatus negative and DNA positive by Day 1, and 1.2% (95% CI, <0% to 35%) if the women were serostatus positive and DNA positive by Day 1. For women who were serostatus positive but DNA negative, the percent reduction was 100% (0 vs. 4 cases in vaccine vs. placebo, respectively), but this was not statistically significant (95% CI, <0% to 100%).
Other immune responses
Although cell-mediated immune responses are measurably higher after vaccination with VLP-based vaccine and cell-mediated immune responses correlate with neutralizing antibody levels [70, 71], vaccination with Gardasil® does not appear to have any therapeutic benefits for CIN lesions. This is not surprising, as vaccines based on VLPs were never intended to be therapeutic vaccines because L1 and L2 are not abundantly expressed in CIN 2/3 lesions.
Efficacy of the bivalent vaccine
Cervarix® is a bivalent VLP vaccine (HPV types 16 and 18) manufactured in an insectcell system, and contains a proprietary adjuvant, aluminum hydroxide with 3-deacylated monophosphoryl lipid A (AS04). Several articles from the Phase 2b trials have been published for Cervarix® [2, 79].
Recently, data was published from the Phase 3 trials for the efficacy of the GlaxoSmithKline bivalent vaccine for HPV-associated cervical precancers. An interim analysis was scheduled for the Phase 3 trial when 23 cases of CIN 2/3 were diagnosed. Similar to the Merck trials, strict entry criteria limits the generalizability of other data. Women aged 15 to 25 years who reported no more than 6 lifetime sexual partners were examined. Women who had a history of colposcopy, who were pregnant or breastfeeding, or who had autoimmune disease or immunodeficiency were excluded. Those included in this interim analysis were subjects who were DNA negative and seronegative for the vaccine types and who received at least 1 vaccine dose. Subjects who had protocol deviations were allowed. This analysis was similar to the unrestricted susceptible population of the quadrivalent vaccine study as case counting began after dose 1 (Table 1). There were 9,258 subjects in the vaccine group and 9,267 individuals in the control group (who received hepatitis A vaccine). Data were calculated for a period of 15 months. The seropositivity was 99.5% for both HPV 16 and 18 and titers (as measured by ELISA) were 200 to 300 times higher than natural infection at Month 7 (1 month after the last dose). The bivalent vaccine demonstrated 90.4% (97.9% CI, 53.4% to 99.3%) efficacy against CIN 2+ lesions associated with HPV 16 and 18 in this analysis (Table 2). Efficacy against type 16 and 18 CIN lesions was 93.3% (97.9% CI, 47% to 99%) and 83.3% (97.9% CI, −78.8% to 99.9%), respectively [80]. Similar to data published by the FUTURE II study group, Cervarix® has also been shown to have no therapeutic effect for those already infected with the HPV vaccine types [81].
Table 2.
Vaccine efficacy of Cervarix® against CIN 1 and CIN 2+ due to vaccine types 16, or 18 and 6 month persistent infection with vaccine types 16, or 18, or non-vaccines types 31, 45, and 52.
| Efficacy analysis endpoint (Paavonen et al. 2007) | Vaccine efficacy%* (97.9% CI) |
|---|---|
| CIN 1 | 89 (59 – 99) |
| CIN 2+ | 90 (53 – 99) |
| CIN 2+ Due to type 16 | 93 (47 – 100) |
| CIN 2+ Due to type 18 | 83 (−79 – 100) |
| 6-month persistent infection type 16 | 84 (74 – 91) |
| 6-month persistent infection type 18 | 74 (49 – 88) |
| 6-month persistent infection type 31 | 36 (1 – 60) |
| 6-month persistent infection type 45 | 60 (3 – 85) |
| 6-month persistent infection type 52 | 32 (4 – 52) |
| 12-month persistent infection type 16 | 80 (48 – 94) |
| 12-month persistent infection type 18 | 66 (−33 – 94) |
| 12-month persistent infection type 31 | 11 (−115 – 64) |
| 12-month persistent infection type 45 | 62 (−93 – 95) |
| 12-month persistent infection type 52 | 47 (−12 – 76) |
Primary analysis of efficacy was initiated at post dose 1
Cross-protection
Since many of the HPV viral types have more DNA homology than others, cross-protection is certainly feasible, specifically with HPV types 31, 33, 35, 52, and 58, which are in the same species as HPV 16 (A9), and HPV types 39, 45, and 59, which are in the same species as HPV 18 (A7). In vitro evidence of the potential of cross-protection has been demonstrated [82]. In one publication, Cervarix® showed some protection against the acquisition of HPV 45 and 31 [2]. Recently published data focused on efficacy to prevent persistent infection [80]. Although there appeared to be some protection against 6-month persistent infection for HPV 45, 31, 33, and 52 (Table 2), only HPV 52 remained statistically significant for protection against 12-month persistent infection. The vaccinated group had an efficacy of 46.5% (97.9% CI, −12.3% to 75.8%; p=.0533) to prevent HPV 52 persistence compared with the control group. The efficacy against all oncogenic HPV persistence other than the vaccine types was 27.1% (97.9% CI, 0.5% to 46.8%; p=0.0174) [80].
Recent data from Gardasil® also showed cross-protection [83]. The analysis showed that the vaccine had a 27% (95% CI, 8% to 42%) efficacy in preventing CIN 1–3 associated with non-vaccine types (HPV types 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59). This was predominantly associated with the A9 HPV types (HPV 31, 33, 35, 52, and 58) with an efficacy of 34% (95% CI, 8% to 52%). The efficacy for the A7 types (HPV 39, 45, and 59) was not statistically significant. The analysis was not powered to examine single type infections. The Cervarix® analysis was not powered to examine histology [80]. The findings described for both trials may be due to true immunologic cross-protection.
Safety
Overall, both Gardasil® and Cervarix® appear to be well-tolerated. In the Gardasil® trials, injection site side effects were the most common side effects reported and were more common in the vaccine group than placebo group. Erythema, pain, and swelling, were the most common side effects (87% and 77% for vaccine and placebo, respectively), and were reported as more severe in the vaccine group than in the placebo group. Systemic adverse experiences were reported by a similar proportion of the vaccine and placebo groups. There were no deaths in the trial that were considered secondary to vaccine; serious vaccine-related adverse events were similar (1.8% and 1.7% for vaccine and placebo, respectively). Specific serious adverse events are outlined in the supplemental text of the FUTURE I and II publications [12, 77]. Pregnant women were excluded from the study and were counseled not to become pregnant during the trial. However, approximately 2,800 women became pregnant during the combined trials (around 1,400 in each group [vaccine and placebo]). Pregnancy outcomes were evaluated with respect for time from the injection to the onset of pregnancy. Sixty-six percent of the vaccine recipients and 63% of the placebo recipients who became pregnant had a live birth. One hundred twelve women receiving Gardasil® and 114 women receiving placebo became pregnant within 30 days of the injection. Spontaneous abortions occurred in 17% and 22% of vaccine and placebo groups, respectively, and in 23% in both groups of women who became pregnant within 30 days after the injection. Among infants born to women who became pregnant within 30 days of vaccination, 7.1% had congenital anomalies in contrast to none of the infants born to women receiving placebo. The anomalies were unrelated in type and were thought not likely related to the vaccine. Congenital anomalies affect 2% to 4% of all US live-born infants. Consequently, the lower rate in the placebo group was thought to be more unusual than the rate in the vaccinated group. It was noted, however, that 12.9% of infants from the vaccinated group (<30 days) had other medical conditions compared with 4.9% in the placebo group. Medical conditions were not described.
In the Phase 2b studies of Cervarix®, the vaccine also appears to be generally safe and well-tolerated. Similar to Gardasil®, injection site adverse events, including pain, redness or swelling, were reported more often among the vaccine group than the placebo group. The number of women with systemic or serious adverse events, including new onset autoimmune disorders, was similar in both groups. Serious adverse events occurred in 4% of the vaccine group and 5% of the placebo group. There were no deaths in the trial considered to be secondary to vaccine. Pregnancy and congenital anomaly data for this vaccine are not yet available to the public.
Duration of protection
Both vaccines have been shown to elicit strong systemic immune responses measured by antibody titers [2, 77]. Immunobridging studies have demonstrated greater immunogenicity in females as young as 9 years of age, compared with females in the clinical efficacy studies (15 to 25 years of age) [84–86]. It is noted that antibody titers after natural infection do not necessarily confer protection, as women with cervical cancer were much more likely to have antibodies to HPV than control women [53]. However, studies show that the antibody titers after immunization are almost 60-fold higher than those seen in natural history studies supporting its role in protection from vaccination.
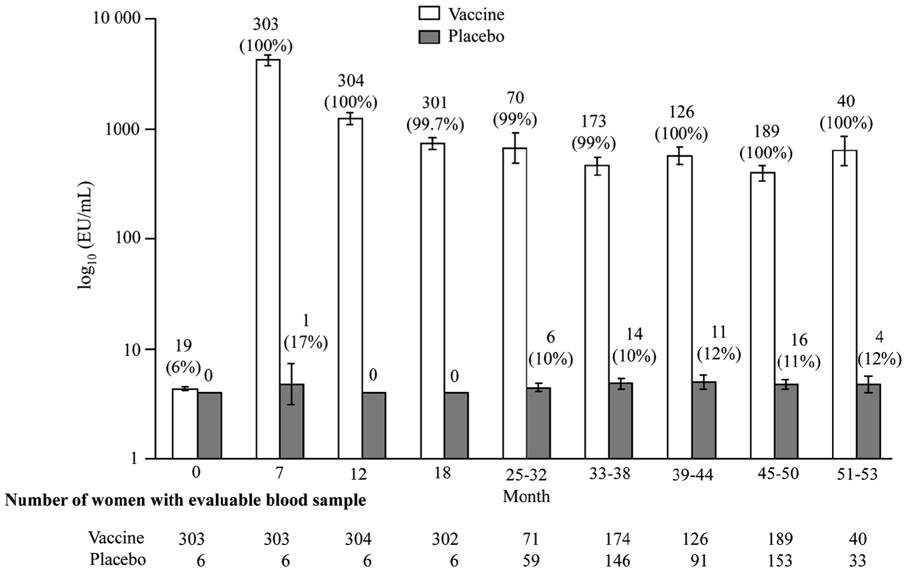
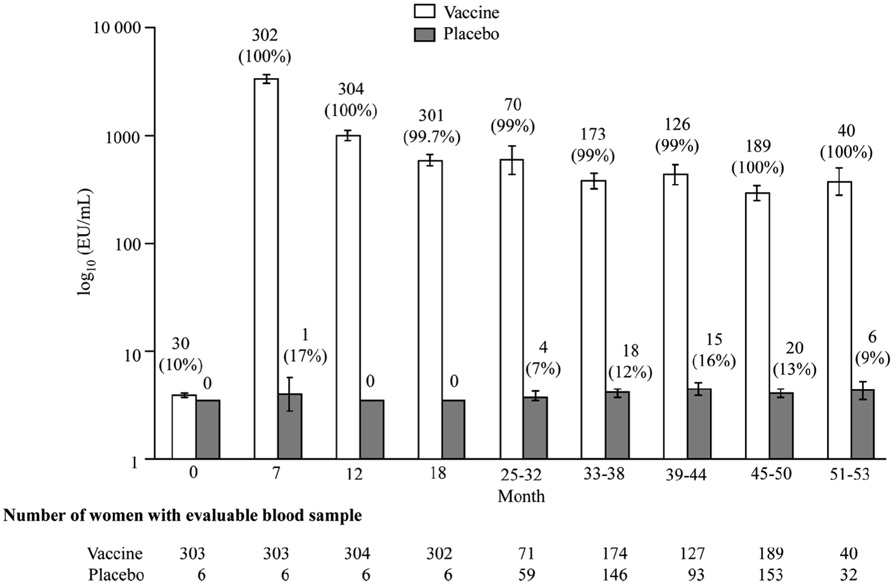
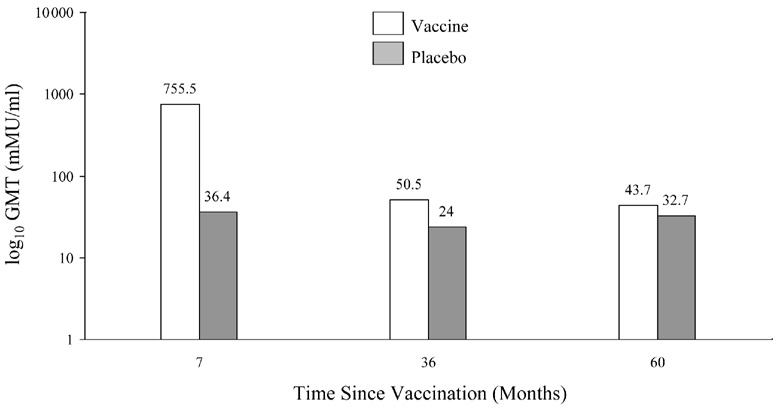
The measurement of antibody titers in each of the trials was performed in separate laboratories, according to different protocols, so no direct comparison can be made regarding specific titers. GlaxoSmithKline is currently performing a side-by-side trial of Cervarix® and Gardasil® to examine immunogenicity using the same method, but data is not available at this time. With both vaccines, seroconversion occurs in over 98% of recipients. Peak antibody titers are achieved 1 month after the third dose with a decline seen until around Month 18, where titers appear to stabilize. Harper et al. showed that high titers associated with Cervarix® were well above those induced by natural infections and remained quite high up to 5.5 years after immunization (Figure 4) [2, 87].
Figure 4.
Figure 4a. Geometric mean titers and seropositivity rates for HPV 16 according to group in according-to-protocol analyses for immunogenicity. (Reprinted with permission from Elsevier [Figure 2, page 1250 in: Harper, D. M., E. L. Franco, C. M. Wheeler, A. B. Moscicki, B. Romanowski, C. M. Roteli-Martins, D. Jenkins, A. Schuind, S. A. Costa Clemens, and G. Dubin. 2006. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 367:1247-55])
Figure 4b. Geometric mean titers and seropositivity rates fir HPV 16 according to group in according-to-protocol analyses for immunogenicity (Reprinted with permission from Elsevier [Figure 2, page 1250 in: Harper, D. M., E. L. Franco, C. M. Wheeler, A. B. Moscicki, B. Romanowski, C. M. Roteli-Martins, D. Jenkins, A. Schuind, S. A. Costa Clemens, and G. Dubin. 2006. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 367:1247-55])
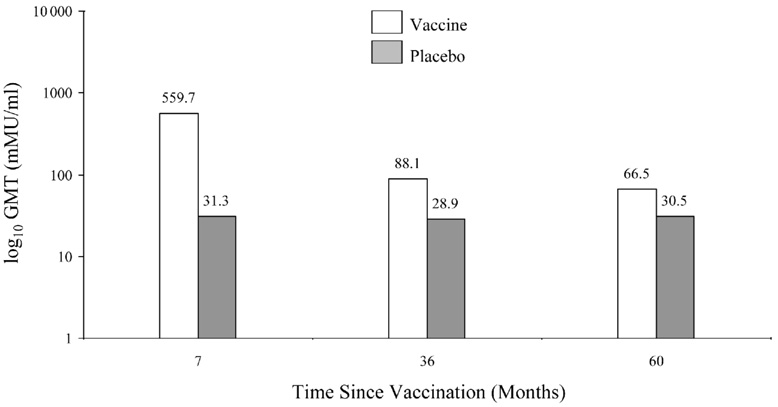
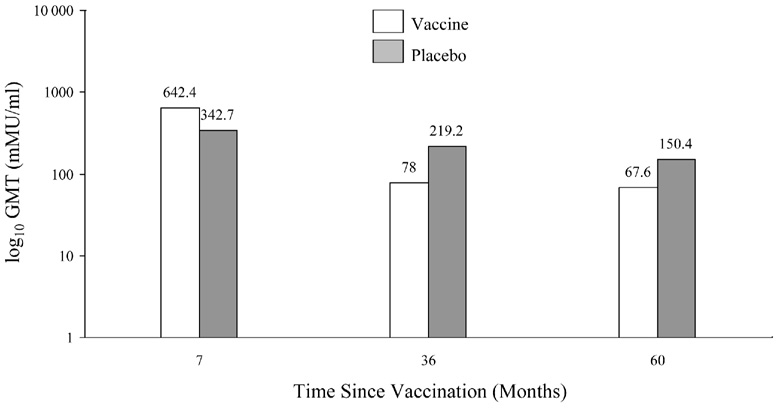
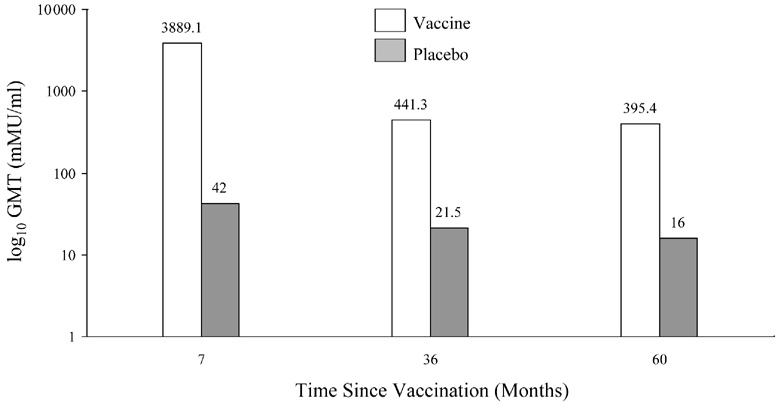
Data released in the FUTURE II study show that after 2 years, 96% of subjects were seropositive for HPV 6, 97% for HPV 11, and 99% for HPV 16. In comparison, only 68% of subjects were seropositive for HPV 18, as measured by the competitive Luminex immunoassays. GMTs induced by Gardasil® in a Phase 2 study through 5 years are also shown in Figure 5 [3]. These differences in antibody induction will need to be monitored. Since many women who become infected with HPV do not develop antibodies, there is some question on the reliability of following antibody titers in vaccinated women. Olsson et al. examined the response to the quadrivalent vaccine in a subgroup augmented with a booster vaccination [80]. The study showed that the titers for HPV 11 and 18 declined to reach similar titers as the placebo group (positive serostatus at entry). Titers to HPV 16 remained highly elevated and HPV 6 remained above the baseline seropositive group. They noted that despite the waning immunity, there were no breakthrough cases of confirmed HPV 6, 11, 16, or 18. The group then challenged the women with a booster at Month 60. Among the vaccinated subjects who became anti-HPV 6, 11, and 18 seronegative, 75%, 86%, and 97%, respectively, became seropositive to the relevant vaccine 1 month post challenge. Women that were seropositive had higher titer levels 1 month post challenge compared with the 7-month titers after the first 3 initial doses [80]. This study suggests a strong immune memory.
Figure 5.
Geometric mean titers of Gardasil according to HPV type through five years (Adapted by permission from MacMillan Publishers LTD. [Table 4 page 1464 in: Villa LL, Costa RL, Petta CA, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. Dec 4 2006;95(11):1459-1466 copyright 2006])
Figure 5a. GMT for HPV 6
Figure 5 b. GMTs for HPV 11
Figure 5 c. GMTs for HPV 16
Figure 5 d. GMTs for HPV 18
SECOND GENERATION HPV VACCINES
VLP vaccines
As the current vaccines only cover 70% of oncogenic HPV types, the need for additional coverage is being actively sought. Certainly, adding VLP types to the current vaccines is attractive. Some experts question increasing the number of types, as it may decrease typespecific antibody induction. As mentioned above, data from the quadrivalent vaccine show that some women lose detectable antibodies for HPV 18 two years after vaccination [77]. The authors noted that this loss did not correlate with loss of protection and it appears that re-exposure to the antigen through vaccination can result in immune induction [80]. It is likely that the antibody assay has a low sensitivity to detect HPV 18-specific antibodies.
Preclinical strategies
Bacteria and plant strategies
The current cost of the first generation vaccines is prohibitive in many developing countries. Cost may be reduced by producing the VLPs of similar capsomeres in either bacteria or plants. Although no human trials have begun, schemes have included producing VLPs in E. coli and transgenic plants. Maclean et al. [88] has developed a novel transgenic plant that expresses a human codon-optimizing gene that is linked to a chloroplast-targeted signal enhancing production of the protein.
Novel methods of delivery
Potential approaches to reducing costs may also include delivery of a single dose and removing the need for cold storage. Currently, the VLP-based vaccines require storage at 2°C to 8 °C for conformational stability. Developing vaccines that are lyophilized to a powder allows for easier storage and delivery [89].
Mucosal delivery vaccines may be advantageous for several reasons. Needle-free vaccines may reduce the cost of vaccine delivery and decrease the risk of exposure to unclean needles. Mucosal delivery would hopefully induce mucosal and systemic immune responses. There is data that show that the induction of mucosal immune responses, such as at the nasal mucous membranes, can “cross-talk” to other mucosal sites (i.e., the vagina) [90]. In a Phase 1 study, HPV 16 L1 VLP was directly administered into the upper respiratory tract using a nebulizer. The study group showed that neutralizing antibody responses could be elicited and were equal to those produced by intramuscular injection [91]. No data on clinical outcomes are available, but further development is underway [81].
Oral delivery is another sought out strategy; however, current studies show that large amounts of VLPs would be required to induce immune responses, making this strategy too costly. Sasagawa et al. reported oral delivery of an HPV 16 L1 vaccine produced by yeast (Schizosaccharomyces pombe) with a mucosal adjuvant heat-labile toxin L1 to mice [92]. Unfortunately, the trial showed relatively poor immunogenicity.
Another route that has been investigated is intravaginal administration. Intravaginal preparations of HPV 16 VLPs used in mice were capable of inducing systemic IgG and mucosal IgA [93]. However, preventive vaccines are targeted towards females who are not yet sexually active. Consequently, such strategies would likely not be acceptable.
Viral vectors
Viral vectors have been used in many delivery systems. Both adenovirus 5 and adenoassociated virus have been used as potential prophylactic vaccine candidates in preclinical trials [94, 95]. The use of viral vectors is attractive since the gene of interest is delivered more efficiently. In addition, viral vectors are usually highly immunogenic. Disadvantages to viral vectors include safety issues, specifically with DNA viruses where promoter insertion mutagenesis is a risk [73].
Bacterial vectors
Live bacteria vaccines have been pursued, as they are relatively inexpensive to manufacture. There are several bacterial-based vaccines in development. One is the L1 recombinant Bacille Calmette-Guérin vaccine, which showed full protection in the cotton-tailed rabbit papillomavirus[96]. Nardelli-Haefliger and colleagues have developed a recombinant Salmonella organism that assembles HPV 16 VLPs and is capable of inducing high titers of neutralizing antibodies in mice after a single nasal or oral immunization with the live bacterial vaccine [97]. Clinical trials (for a similar vaccine based on recombinant Salmonella) using oral delivery are planned in the near future [81, 98].
L2-based vaccines
L2 is the minor capsid protein. Vaccines including this protein have been examined since L2 appears to induce cross-neutralization more commonly than L1 which is relatively type-specific. Consequently, a monovalent vaccine could potentially protect a broad range of HPV types. Unfortunately, the neutralizing antibody titers are much lower than the ones generated by the L1 VLPs [99]. Better adjuvants may improve L2’s prospects as a vaccine target. Kawana et al. [100] performed a small pilot study examining the vaccination of women with a synthetic peptide of HPV 16 L2. The nasal inoculations generated anti-L2 antibodies that bound to both HPV 16 and 52 L1/L2-capsids in 4 of 5 recipients. Sera from the 4 recipients showed neutralizing activity against HPV 16 and 52. Optimization of L2-based vaccines is underway, with clinical trials planned in the near future [81].
Therapeutic vaccines
Several therapeutic vaccines have been in early trials. Most of the therapeutic vaccines are based on either E6 or E7 expression since E6 and E7 are consistently expressed in pre-malignancies and malignancies and have well known oncogenic properties reviewed earlier. The importance of cell-mediated immune responses to these proteins is well documented [53, 54, 101].
DNA vaccines
Native E7 has been disappointing in many studies showing its inability to confer resistance to tumor challenge. In addition, using non-mutated DNA has safety concerns. Brulet et al. recently published a study examining a novel E7 vaccine based on plasmids expressing E7 protein specifically in the endosomal compartment [102]. The plasmid encodes for an E7 protein fused to a domain of the MHC class II-associated invariant chain. This strategy to improve class II presentation of an antigen has been previously employed for other antigens [103]. In this trial, the E7 fusion protein was able to protect more than 60% of the vaccinated mice against E7-expressing tumors. This protection correlated with CD8+ T-cell responses but required the presence of CD4+ T-cells. This type of vaccine construct may allow more efficient targeting of the MHC class II pathway. Hung et al. recently published another study based on this strategy [104]. The group constructed a DNA vaccine encoding the invariant chain in which the class II-associated invariant chain peptide region is replaced with a CD4+ T-helper epitope (PADRE: invariant Pan HLA-DR reactive epitope). In preclinical studies, administration of this vaccine significantly showed both preventive and therapeutic responses.
Peptide-based DNA strategies
Few peptide-based vaccines have been used for therapeutic intervention since these vaccines are usually human leukocyte antigen (HLA)-specific. One study developed an HLA-A2-specific vaccine for women with HPV 16-associated disease. Women were treated with escalating doses of a vaccine consisting of a 9-amino acid peptide from residues 12–20 encoded by the E7 gene emulsified with incomplete Freund's adjuvant [105]. Unfortunately, the clinical results were not encouraging.
Another study used HLA-A2 restricted epitopes derived from HPV 16 E7 protein encapsulated in biodegradable polymer microparticles in patients with CIN and found a response rate of 33%; no controls were available for comparison [106]. A different version of the vaccine was developed which was based on plasmid DNA vaccine encoding protein peptides derived from the E6 and E7 proteins of HPV 16 and HPV 18. Although well-tolerated, overall, the vaccine did not show statistically different rates of regression of CIN lesions. Interestingly, in women under 25 years of age, the vaccine promoted resolution of CIN 2/3 more commonly than placebo (70% vs. 23% in vaccine group vs. placebo group, respectively). This could reflect the use of such vaccines early in the natural history of HPV infection. Once other mutations occur or there is evidence of a single clonal expansion, reversal may be more difficult [9]. Failure to induce remission could also reflect T-cell tolerance to E6/E7. No further trials are currently underway.
Protein-based strategies
Lu et al. developed an E7 fusion protein consisting of Mycobacterium tuberculosis heat shock protein 70 (TBhsp70) with HPV 16 mE6 delta/mE7 (mutated E6 and E7) [81]. It is well known that heat shock protein boosts immune responses [107, 108]. In preclinical trials, this vaccine induced tumor apoptosis and inhibition of tumor growth in mice. HPV 16 mE6 delta/E7 alone did not change tumor development. The vaccine was developed so that full E7 was expressed, leading to presentation of epitopes in the context of various HLAs, making it applicable to any patient regardless of HLA restriction. mE6 was truncated so that it would not have any transformation potential [81]. Another fusion protein, PD-E7, was comprised of a mutated HPV 16 E7 linked to the first 108 amino acids of Haemophilus influenzae protein D, formulated in the GlaxoSmithKline Biologicals’ adjuvant, AS02B. A small pilot study showed that the majority of women developed immune responses. However, efficacy data has not been reported [109].
A trial is currently ongoing at Johns Hopkins University, involving women with HPV 16- associated high-grade squamous intraepithelial lesions of the cervix and patients with advanced HPV-associated head and neck cancers. E7-heat shock fusion protein-based vaccine, pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine is being used in the study. pNGVL4a-Sig/E7(detox)/HSP70 DNA vaccine administered via gene gun generated the highest number of E7-specific CD8+ T-cells in comparison with other methods of delivery and vaccine combinations. No published data is available on clinical outcomes.
Another vaccine was developed by Stressgen Biotechnologies (Collegeville, PA) and was based on a fusion protein, Mycobacterium bovis, heat shock protein 65, covalently linked to HPV 16 and E7 [110]. Although the early clinical trials were promising for CIN treatment and recurrent laryngeal papillomatosis, Nventa Biopharmaceuticals Corporation (Victoria, British Columbia) has since reformulated the vaccine and is planning new clinical trials.
Viral-based strategies
Several reports have used viral vector strategies to deliver intracellular antigens for HPV therapeutic vaccines. Baez-Astua et al. used human adenovirus type 5 to express the fusion protein HPV 16 E7-hepatitis B surface antigen (HbsAg) and showed that this vaccine was able to induce high levels of E7-specific humoral and cellular immune responses in mice [94]. Brandsma et al. reported on the use of a recombinant vesicular stomatitis virus (VSV) vector expressing cotton-tailed rabbit E1, E2, E6, and E7 [111]. Although all 4 vaccines significantly reduced papilloma volumes, VSV-E7 was the most effective, ultimately eliminating all of the disease in the immunized rabbits. One of the concerns of using viral vectors such as VSV is the safety of its use in immunocompromised individuals. Another group developed a recombinant vaccinia virus encoding modified HPV 16 and 18 E6 and E7 [61]. A small number of women with high-grade vulvar intraepithelial neoplasia were immunized with this vaccine. Although the responders had higher levels of CD4+, CD8+, and CD1a+-immune infiltration cells than non-responders, none of the women had a complete clinical response, and less than 50% had some response.
Another strategy uses Modified Virus Ankara (MVA) E6-E7-IL-2 vaccine (Transgene; Strasbourg, France). MVA is a non-propagative attenuated vaccinia virus strain. An advantage of MVA is its cytoplasmic location, which prevents the risk of integration into the host genome. The vaccinia virus contains modified nucleotidic sequences that encode for HPV 16 E6 and E7 antigens and immunoregulatory cytokine interleukin-2 (IL-2). IL-2 is expected to enhance both specific and non-specific cellular immune responses. An unblinded Phase 2 trial in 21 CIN 2/3 patients who were given 3 subcutaneous injections showed promising results, with almost 50% (10) of women having complete regression. Tests for HPV 16 E6 and E7 mRNA expression were also negative. Seven of the women remained untreated at 12 months and were found to be free of CIN 2/3 [112].
E2 vaccines
E2 has important control over E6 and E7 transcription and is highly expressed in lesions. Consequently, some vaccines have been directed towards generating anti-E2 responses. A Phase 2 trial of a vaccinia virus recombinant MVA E2 vaccine was performed in 54 women [113]. Treatment consisted of weekly injections directly into the uterine cervix in a radial clockwise fashion at points corresponding to 3, 6, 9, and 12 o’clock over a 6-week period. They showed that 59% of patients with CIN 2/3 had complete regression. No similar follow-up was available for the control group, and MVA alone was not used as a control. High antibody titers to E2 were shown in all vaccinated patients. Few adverse events, such as headache and flu-like symptoms, were reported. The invasive procedure requires injections to be administered to the uterine cervix, which may limit the widespread use of such a vaccine.
Chimeric-based strategies
Another strategy is to use VLPs as vehicles to deliver unique epitopes such as chimeric E7 VLPs. Chimeric VLPs are papillomavirus VLPs composed of L1 or L1/L2 fused with a foreign epitope or polypeptides. Because VLPs induce strong T-helper cell responses, they are considered good adjuvants. Papillomavirus VLPs can be taken up by immature dendritic cells, which results in activation. Maturation of dendritic cells results in the up-regulation of co-stimulatory molecules. It has been shown that fusion proteins of L1 can also self-assemble into VLPs. Up to 60 amino acids can be fused to the C-terminus of a truncated papillomavirus L1 without disrupting the assembly of VLPs. Larger proteins can be placed into certain areas of L1 or L2 that are not important for assembly. Another strategy is to fuse epitopes into the loop regions of L1 that are exposed to the outer surface. The idea is that these epitopes are efficiently delivered to immune cells to induce epitope-specific immune responses [114]. These strategies have been used to create several chimeric vaccines.
Results from two clinical trials using chimeric vaccines have been published. An HPV L1-E7 chimeric VLP vaccine targeting HPV 16-associated high-grade squamous intraepithelial lesions (sponsored by Medigene; Martinsried, Germany) found no statistical effect on regression [72]. Another clinical trial included the Cantab/Xenova TA-CIN trial, which examined an HPV 16 L2-E6-E7 fusion protein, which does not form VLPs [60]. No clinical effect on CIN regression was found. E2 has also been used in a chimeric vaccine with VLP in mouse models and showed strong immunogenic responses to both L1 and E2 [73]. Several strategies to vaccinate women with advanced stage cervical cancer are also in preclinical phases but are beyond the scope of this review.
Summary
Prophylactic vaccines look quite promising for cervical cancer prevention. However, as per the Advisory Committee of Immunization Practices’ recommendation, targeting non-sexually active women will be critical for cervical cancer prevention. Evidence suggests that the benefit in sexually active women currently remains marginal, at best. Nonetheless, targeting non-sexually active women in the public health domain, specifically in countries that have no cervical cancer screening, has the potential to greatly decrease the burden associated with HPV. High cost of the current vaccines has driven researchers to continue to seek new methods of production and delivery. Most methods are in preclinical or early pilot phases. In addition to preventive strategies, therapeutic strategies remain of interest for the millions of women who currently are infected and/or have diseases requiring treatment. Several therapeutic strategies have focused on HPV E6 and E7. The few clinical trials to date have not shown the same success as the prevention trials.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006 Aug 21;24 Suppl 3:S11–S25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 2.Harper DM, Franco EL, Wheeler CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006 Apr 15;367(9518):1247–1255. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- 3.Clifford G, Franceschi S, Diaz M, et al. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine. 2006;24 Suppl 3:S26–S34. doi: 10.1016/j.vaccine.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 4.D'Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007 May 10;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 5.Globocan. The GLOBOCAN 2002 database. 2002 [cited; Available from: http://www-dep.iarc.fr/globocan/database.htm.
- 6.Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics. 2005;23(11):1107–1122. doi: 10.2165/00019053-200523110-00004. [DOI] [PubMed] [Google Scholar]
- 7.Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: A meta-analysis update. Int J Cancer. 2007 Aug 1;121(3):621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 8.Coeshott CM, Smithson SL, Verderber E, et al. Pluronic F127-based systemic vaccine delivery systems. Vaccine. 2004 Jun 23;22(19):2396–2405. doi: 10.1016/j.vaccine.2003.11.064. [DOI] [PubMed] [Google Scholar]
- 9.Klencke B, Matijevic M, Urban RG, et al. Encapsulated plasmid DNA treatment for human papillomavirus 16-associated anal dysplasia: a Phase I study of ZYC101. Clin Cancer Res. 2002 May;8(5):1028–1037. [PubMed] [Google Scholar]
- 10.Muñoz N, Bosch FX, De Sanjose S, et al. Epidemiological Classification of Human Papillomavirus Types Associated with Cervical Cancer. NEJM. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 11.Castle PE, Solomon D, Schiffman M, et al. Human papillomavirus type 16 infections and 2-year absolute risk of cervical precancer in women with equivocal or mild cytologic abnormalities. J Natl Cancer Inst. 2005;97(14):1066–1071. doi: 10.1093/jnci/dji186. [DOI] [PubMed] [Google Scholar]
- 12.Garland SM, Hernandez-Avila M, Wheeler CM, et al. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med. 2007 May 10;356(19):1928–1943. doi: 10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 13.Giroglou T, Florin L, Schafer F, et al. Human papillomavirus infection requires cell surface heparan sulfate. J Virol. 2001;75(3):1565–1570. doi: 10.1128/JVI.75.3.1565-1570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shafti-Keramat S, Handisurya A, Kriehuber E, et al. Different heparan sulfate proteoglycans serve as cellular receptors for human papillomaviruses. J Virol. 2003;77(24):13125–13135. doi: 10.1128/JVI.77.24.13125-13135.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang R, Day PM, Yutzy WHt, et al. Cell surface-binding motifs of L2 that facilitate papillomavirus infection. J Virol. 2003;77(6):3531–3541. doi: 10.1128/JVI.77.6.3531-3541.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doeberitz MVK. New markers for cervical dysplasia to visualize the genomic chaos created by aberrant oncogenic papillomavirus infections. Eur J Cancer. 2002;38:2229–2242. doi: 10.1016/s0959-8049(02)00462-8. [DOI] [PubMed] [Google Scholar]
- 17.Doorbar J, Foo C, Coleman N, et al. Characterization of events during the late stages of HPV16 infection in vivo using high-affinity synthetic Fabs to E4. Virology. 1997;238(1):40–52. doi: 10.1006/viro.1997.8768. [DOI] [PubMed] [Google Scholar]
- 18.Frattini MG, Lim HB, Doorbar J, et al. Induction of human papillomavirus type 18 late gene expression and genomic amplification in organotypic cultures from transfected DNA templates. J Virol. 1997;71(9):7068–7072. doi: 10.1128/jvi.71.9.7068-7072.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sherman ME, Kurman RJ. The role of exfoliative cytology and histopathology in screening and triage. Obstet and Gynecol Clin of North America. 1996;23:641–655. [PubMed] [Google Scholar]
- 20.Jenson AB, Kurman RJ, Lancaster WD. Tissue effects of and host response to human papillomavirus infection. Obstet Gyn Clinics N Am. 1987;14:397–406. [PubMed] [Google Scholar]
- 21.Winer RL, Kiviat NB, Hughes JP, et al. Development and duration of human papillomavirus lesions, after initial infection. J Infect Dis. 2005;191(5):731–738. doi: 10.1086/427557. [DOI] [PubMed] [Google Scholar]
- 22.Woodman CB, Collins S, Winter H, et al. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet. 2001;357(9271):1831–1836. doi: 10.1016/S0140-6736(00)04956-4. [DOI] [PubMed] [Google Scholar]
- 23.Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA. 2000;286(24):3106–3114. doi: 10.1001/jama.286.24.3106. [DOI] [PubMed] [Google Scholar]
- 24.Moscicki AB. 2007 Unpublished data. [Google Scholar]
- 25.Farhat S, Lee CKK, et al. HPV 16 Detection in Peripheral Blood: EliSpot Instead of CTL. 21st HPV International Conference and Clinical Workshop in Papillomavirus; 2004; Mexico City. 2004. [Google Scholar]
- 26.Kjaer SK, van den Brule AJ, Paull G, et al. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ. 2002;325(7364):572. doi: 10.1136/bmj.325.7364.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arbeit JM, Howley PM, Hanahan D. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. Proc Natl Acad Sci U S A. 1996;93(7):2930–2935. doi: 10.1073/pnas.93.7.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruutu M, Wahlroos N, Syrjanen K, et al. Effects of 17beta-estradiol and progesterone on transcription of human papillomavirus 16 E6/E7 oncogenes in CaSki and SiHa cell lines. Int J Gynecol Cancer. 2006;16(3):1261–1268. doi: 10.1111/j.1525-1438.2006.00563.x. [DOI] [PubMed] [Google Scholar]
- 29.Yuan F, Auborn K, James C. Altered growth and viral gene expression in human papillomavirus type 16-containing cancer cell lines treated with progesterone. Cancer Invest. 1999;17(1):19–29. [PubMed] [Google Scholar]
- 30.Scott ME, Ma Y, Farhat S, et al. Covariates of cervical cytokine mRNA expression by real-time PCR in adolescents and young women: effects of Chlamydia trachomatis infection, hormonal contraception, and smoking. J Clin Immunol. 2006;26(3):222–232. doi: 10.1007/s10875-006-9010-x. [DOI] [PubMed] [Google Scholar]
- 31.Schlott T, Eiffert H, Bohne W, et al. Chlamydia trachomatis modulates expression of tumor suppressor gene caveolin-1 and oncogene C-myc in the transformation zone of non-neoplastic cervical tissue. Gynecol Oncol. 2005 Sep;98(3):409–419. doi: 10.1016/j.ygyno.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 32.Scheffner M, Werness BA, Huibregste JM, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. doi: 10.1016/0092-8674(90)90409-8. [DOI] [PubMed] [Google Scholar]
- 33.Munger K, Werness BA, Dyson N, et al. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. Embo J. 1989;8:4099–4105. doi: 10.1002/j.1460-2075.1989.tb08594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duensing S, Duensing A, Flores ER, et al. Centrosome abnormalities and genomic instability by episomal expression of human papillomavirus type 16 in raft cultures of human keratinocytes. J Virol. 2001;75(16):7712–7716. doi: 10.1128/JVI.75.16.7712-7716.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Durst M, Kleinheinz A, Hotz M, et al. The physical state of human papillomavirus type 16 DNA in benign and malignant genital tumours. J Gen Virol. 1985;66(7):1515–1522. doi: 10.1099/0022-1317-66-7-1515. [DOI] [PubMed] [Google Scholar]
- 36.Schwarz E, Freese UK, Gissmann L, et al. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985;314(6006):111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- 37.Janeway CAJ, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 38.Compton T, Kurt-Jones EA, Boehme KW, et al. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J Virol. 2003;77(8):4588–4596. doi: 10.1128/JVI.77.8.4588-4596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 40.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 41.Hasan UA, Bates E, Takeshita F, et al. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J Immunol. 2007 Mar 1;178(5):3186–3197. doi: 10.4049/jimmunol.178.5.3186. [DOI] [PubMed] [Google Scholar]
- 42.Yan M, Peng J, Jabbar IA, et al. Activation of dendritic cells by human papillomavirus-like particles through TLR4 and NF-kappaB-mediated signalling, moderated by TGF-beta. Immunol Cell Biol. 2005 Feb;83(1):83–91. doi: 10.1111/j.1440-1711.2004.01291.x. [DOI] [PubMed] [Google Scholar]
- 43.Woodworth CD. HPV Innate Immunity. Front Biosci. 2002;7(7):d2058–d2071. doi: 10.2741/A898. [DOI] [PubMed] [Google Scholar]
- 44.Sillman FH, Stanek A, Sedlis A, et al. The relationship between human papillomavirus and lower genital intraepithelial neoplasia in immunosuppressed women. J Obstet Gynecol. 1984;150:300. doi: 10.1016/s0002-9378(84)90369-7. [DOI] [PubMed] [Google Scholar]
- 45.Penn I. Cancers of the anogenital regions in renal transplant recipients. Cancer. 1986;1986(58) doi: 10.1002/1097-0142(19860801)58:3<611::aid-cncr2820580303>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 46.Palefsky JM, Gonzales J, Greenblatt RM, et al. Anal intraepithelial neoplasia and anal papillomavirus infection among homosexuals males with group IV HIV disease. JAMA. 1990;263:2911–2916. [PubMed] [Google Scholar]
- 47.Sun XW, Kuhn L, Ellerbrock TV, et al. Human papillomavirus infection in women infected with the human immunodeficiency virus. NEJM. 1997;337:1343–1349. doi: 10.1056/NEJM199711063371903. [DOI] [PubMed] [Google Scholar]
- 48.Haftek M, Jablonska S, Orth G. Specific cell-mediated immunity in patients with epidermodysplasia verruciformis and plane warts. Dermatologica. 1985;170:213. doi: 10.1159/000249535. [DOI] [PubMed] [Google Scholar]
- 49.Iwatsuki K, Tagami H, Takagawa M, et al. Plane warts under spontaneous regression. Arch Derm. 1996;122:655. doi: 10.1001/archderm.122.6.655. [DOI] [PubMed] [Google Scholar]
- 50.Rogozinski TT, Jablonska S, Jarzabek-Chorzelska M. Role of cell-mediated immunity in spontaneous regression of plane warts. Int J Dermatol. 1988;27:322–326. doi: 10.1111/j.1365-4362.1988.tb02362.x. [DOI] [PubMed] [Google Scholar]
- 51.Nakagawa M, Stites DP, Farhat S, et al. T cell proliferative response to human papillomavirus type 16 peptides: Relationship to cervical intraepithelial neoplasia. J Clin Diag Lab Immunol. 1996;3:205–210. doi: 10.1128/cdli.3.2.205-210.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kadish AS, Ho GY, Burk RD, et al. Lymphoproliferative responses to human papillomavirus (HPV) type 16 proteins E6 and E7: outcome of HPV infection and associated neoplasia. J Natl Cancer Inst. 1997;89(17):1285–1293. doi: 10.1093/jnci/89.17.1285. [DOI] [PubMed] [Google Scholar]
- 53.Stanley M. Immune responses to human papillomavirus. Vaccine. 2006 Mar 30;24 Suppl 1:S16–S22. doi: 10.1016/j.vaccine.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 54.Nakagawa M, Stites D, Patel S, et al. Persistence of Human Papillomavirus 16 infection is associated with lack of cytotoxic T lymphocyte response to the E6 antigen. J Infect Dis. 2000;182:595–598. doi: 10.1086/315706. [DOI] [PubMed] [Google Scholar]
- 55.Welters MJ, van der Logt P, van den Eeden SJ, et al. Detection of human papillomavirus type 18 E6 and E7-specific CD4+ T-helper 1 immunity in relation to health versus disease. Int J Cancer. 2006 Feb 15;118(4):950–956. doi: 10.1002/ijc.21459. [DOI] [PubMed] [Google Scholar]
- 56.van der Burg SH, Piersma SJ, de Jong A, et al. Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proc Natl Acad Sci U S A. 2007 Jul 17;104(29):12087–12092. doi: 10.1073/pnas.0704672104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Molling JW, de Gruijl TD, Glim J, et al. CD4(+)CD25hi regulatory T-cell frequency correlates with persistence of human papillomavirus type 16 and T helper cell responses in patients with cervical intraepithelial neoplasia. Int J Cancer. 2007 Oct 15;121(8):1749–1755. doi: 10.1002/ijc.22894. [DOI] [PubMed] [Google Scholar]
- 58.Hudelist G, Manavi M, Pischinger KI, et al. Physical state and expression of HPV DNA in benign and dysplastic cervical tissue: different levels of viral integration are correlated with lesion grade. Gynecol Oncol. 2004;92(3):873–880. doi: 10.1016/j.ygyno.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 59.Bontkes HJ, de Gruijl TD, Bijl A, et al. Human papillomavirus type 16 E2-specific T-helper lymphocyte responses in patients with cervical intraepithelial neoplasia. J Gen Virol. 1999;80(Pt 9):2453–2459. doi: 10.1099/0022-1317-80-9-2453. [DOI] [PubMed] [Google Scholar]
- 60.de Jong A, O'Neill T, Khan AY, et al. Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine. 2002 Oct 4;20(29–30):3456–3464. doi: 10.1016/s0264-410x(02)00350-x. [DOI] [PubMed] [Google Scholar]
- 61.Davidson EJ, Boswell CM, Sehr P, et al. Immunological and clinical responses in women with vulval intraepithelial neoplasia vaccinated with a vaccinia virus encoding human papillomavirus 16/18 oncoproteins. Cancer Res. 2003 Sep 15;63(18):6032–6041. [PubMed] [Google Scholar]
- 62.Wadler S, Levy D, Frederickson HL, et al. A phase II trial of interleukin-12 in patients with advanced cervical cancer: clinical and immunologic correlates. Eastern Cooperative Oncology Group study E1E96. Gynecol Oncol. 2004;92(3):957–964. doi: 10.1016/j.ygyno.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 63.Steele JC, Roberts S, Rookes SM, et al. Detection of CD4(+)- and CD8(+)-T-cell responses to human papillomavirus type 1 antigens expressed at various stages of the virus life cycle by using an enzyme-linked immunospot assay of gamma interferon release. J Virol. 2002;76(12):6027–6036. doi: 10.1128/JVI.76.12.6027-6036.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leykauf K, Salek M, Schluter H, et al. Identification of membrane proteins differentially expressed in human papillomavirus type 16 E5-transfected human keratinocytes by nanoelectrospray ionization mass spectrometry. J Gen Virol. 2004;85(Pt 6):1427–1431. doi: 10.1099/vir.0.79844-0. [DOI] [PubMed] [Google Scholar]
- 65.Tsai TC, Chen SL. The biochemical and biological functions of human papillomavirus type 16 E5 protein. Arch Virol. 2003;148(8):1445–1453. doi: 10.1007/s00705-003-0111-z. [DOI] [PubMed] [Google Scholar]
- 66.Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347(21):1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 67.Kanodia S, Fahey LM, Kast WM. Mechanisms used by human papillomaviruses to escape the host immune response. Curr Cancer Drug Targets. 2007 Feb;7(1):79–89. doi: 10.2174/156800907780006869. [DOI] [PubMed] [Google Scholar]
- 68.Bierl C, Karem K, Poon AC, et al. Correlates of cervical mucosal antibodies to human papillomavirus 16: results from a case control study. Gynecol Oncol. 2005;99(3 Suppl 1):S262–S268. doi: 10.1016/j.ygyno.2005.07.100. [DOI] [PubMed] [Google Scholar]
- 69.Scott M, Nakagawa M, Moscicki AB. Cell-mediated immune response to human papillomavirus infection. Clin Diagn Lab Immunol. 2001;8(2):209–220. doi: 10.1128/CDLI.8.2.209-220.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pinto LA, Castle PE, Roden RB, et al. HPV-16 L1 VLP vaccine elicits a broad-spectrum of cytokine responses in whole blood. Vaccine. 2005 May 20;23(27):3555–3564. doi: 10.1016/j.vaccine.2005.01.146. [DOI] [PubMed] [Google Scholar]
- 71.Pinto LA, Edwards J, Castle PE, et al. Cellular immune responses to human papillomavirus (HPV)-16 L1 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. J Infect Dis. 2003 Jul 15;188(2):327–338. doi: 10.1086/376505. [DOI] [PubMed] [Google Scholar]
- 72.Schreckenberger C, Kaufmann AM. Vaccination strategies for the treatment and prevention of cervical cancer. Curr Opin Oncol. 2004 Sep;16(5):485–491. doi: 10.1097/00001622-200409000-00013. [DOI] [PubMed] [Google Scholar]
- 73.Qian J, Dong Y, Pang YY, et al. Combined prophylactic and therapeutic cancer vaccine: enhancing CTL responses to HPV16 E2 using a chimeric VLP in HLA-A2 mice. Int J Cancer. 2006 Jun 15;118(12):3022–3029. doi: 10.1002/ijc.21781. [DOI] [PubMed] [Google Scholar]
- 74.Mao C, Koutsky LA, Ault KA, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 2006;107(1):18–27. doi: 10.1097/01.AOG.0000192397.41191.fb. [DOI] [PubMed] [Google Scholar]
- 75.Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005 May;6(5):271–278. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 76.Emeny RT, Wheeler CM, Jansen KU, et al. Priming of human papillomavirus type 11-specific humoral and cellular immune responses in college-aged women with a virus-like particle vaccine. J Virol. 2002 Aug;76(15):7832–7842. doi: 10.1128/JVI.76.15.7832-7842.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.The FUTURE II Study Group. Quadrivalent Vaccine against Human Papillomavirus to Prevent High-Grade Cervical Lesions. NEJM. 2007;356(19):1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 78.Mosher WD, Chandra A, Jones J. Sexual behavior and selected health measures: men and women 15–44 years of age, United States, 2002. Adv Data. 2005;362:1–55. [PubMed] [Google Scholar]
- 79.Harper DM, Franco EL, Wheeler C, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004 Nov 13–19;364(9447):1757–1765. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 80.Olsson SE, Villa LL, Costa RL, et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine. 2007 Apr 20; doi: 10.1016/j.vaccine.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 81.Hildesheim A, Herrero R, Wacholder S, et al. Effect of human papillomavirus 16/18 L1 viruslike particle vaccine among young women with preexisting infection: a randomized trial. Jama. 2007 Aug 15;298(7):743–753. doi: 10.1001/jama.298.7.743. [DOI] [PubMed] [Google Scholar]
- 82.Stanley M. Prophylactic HPV Vaccines. J Clin Pathol. 2007 Jan 26; doi: 10.1136/jcp.2006.040568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brown D. HPV Type 6/11/16/18 Vaccine: First Analysis of Cross-Protection Against Persistent Infection, Cervical Intraepithelial Neoplasia (CIN), and Adenocarcinoma In Situ (AIS) Caused By Oncogenic HPV Types In Addition To 16/18. 47th Annual ICAAC - American Society for Microbiology; Chicago, IL. 2007. [2007 September 17–20]. [Google Scholar]
- 84.Block SL, Nolan T, Sattler C, et al. Comparison of the immunogenicity and reactogenicity of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in male and female adolescents and young adult women. Pediatrics. 2006 Nov;118(5):2135–2145. doi: 10.1542/peds.2006-0461. [DOI] [PubMed] [Google Scholar]
- 85.Pedersen C, Petaja T, Strauss G, et al. Immunization of early adolescent females with human papillomavirus type 16 and 18 L1 virus-like particle vaccine containing AS04 adjuvant. J Adolesc Health. 2007 Jun;40(6):564–571. doi: 10.1016/j.jadohealth.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 86.Reisinger KS, Block SL, Lazcano-Ponce E, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J. 2007 Mar;26(3):201–209. doi: 10.1097/01.inf.0000253970.29190.5a. [DOI] [PubMed] [Google Scholar]
- 87.Gall SA, Teixeira J, Wheeler C, et al. Substantial impact on precancerous lesions and HPV infections through 55 years in women vaccinated with the HPV-16/18 L1 VLP ASO4 candidate vaccine American Association for Cancer Research. Los Angeles: 2007. Apr 14–18, Substantial impact on precancerous lesions and HPV infections through 5.5 years in women vaccinated with the HPV-16/18 L1 VLP ASO4 candidate vaccine. [Google Scholar]
- 88.Maclean J, Koekemoer M, Olivier AJ, et al. Optimization of human papillomavirus type 16 (HPV-16) L1 expression in plants: comparison of the suitability of different HPV-16 L1 gene variants and different cell-compartment localization. J Gen Virol. 2007 May;88(Pt 5):1460–1469. doi: 10.1099/vir.0.82718-0. [DOI] [PubMed] [Google Scholar]
- 89.Roden RB, Lowy DR, Schiller JT. Papillomavirus is resistant to desiccation. J Infect Dis. 1997 Oct;176(4):1076–1079. doi: 10.1086/516515. [DOI] [PubMed] [Google Scholar]
- 90.Manuri PR, Nehete B, Nehete PN, et al. Intranasal immunization with synthetic peptides corresponding to the E6 and E7 oncoproteins of human papillomavirus type 16 induces systemic and mucosal cellular immune responses and tumor protection. Vaccine. 2007 Apr 30;25(17):3302–3310. doi: 10.1016/j.vaccine.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nardelli-Haefliger D, Lurati F, Wirthner D, et al. Immune responses induced by lower airway mucosal immunisation with a human papillomavirus type 16 virus-like particle vaccine. Vaccine. 2005 May 25;23(28):3634–3641. doi: 10.1016/j.vaccine.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 92.Sasagawa T, Tani M, Basha W, et al. A human papillomavirus type 16 vaccine by oral delivery of L1 protein. Virus Res. 2005 Jun;110(1–2):81–90. doi: 10.1016/j.virusres.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 93.Park JS, Oh YK, Kang MJ, et al. Enhanced mucosal and systemic immune responses following intravaginal immunization with human papillomavirus 16 L1 virus-like particle vaccine in thermosensitive mucoadhesive delivery systems. J Med Virol. 2003;70(4):633–641. doi: 10.1002/jmv.10442. [DOI] [PubMed] [Google Scholar]
- 94.Baez-Astua A, Herraez-Hernandez E, Garbi N, et al. Low-dose adenovirus vaccine encoding chimeric hepatitis B virus surface antigen-human papillomavirus type 16 E7 proteins induces enhanced E7-specific antibody and cytotoxic T-cell responses. J Virol. 2005 Oct;79(20):12807–12817. doi: 10.1128/JVI.79.20.12807-12817.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu DW, Chang JL, Tsao YP, et al. Co-vaccination with adeno-associated virus vectors encoding human papillomavirus 16 L1 proteins and adenovirus encoding murine GMCSF can elicit strong and prolonged neutralizing antibody. Int J Cancer. 2005 Jan 1;113(1):93–100. doi: 10.1002/ijc.20530. [DOI] [PubMed] [Google Scholar]
- 96.Govan VA, Christensen ND, Berkower C, et al. Immunisation with recombinant BCG expressing the cottontail rabbit papillomavirus (CRPV) L1 gene provides protection from CRPV challenge. Vaccine. 2006 Mar 15;24(12):2087–2093. doi: 10.1016/j.vaccine.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 97.Baud D, Ponci F, Bobst M, et al. Improved efficiency of a Salmonella-based vaccine against human papillomavirus type 16 virus-like particles achieved by using a codon-optimized version of L1. J Virol. 2004 Dec;78(23):12901–12909. doi: 10.1128/JVI.78.23.12901-12909.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McNeil C. Search for HPV treatment vaccine heats up, researchers optimistic. J Natl Cancer Inst. 2006 Jul 19;98(14):954–955. doi: 10.1093/jnci/djj314. [DOI] [PubMed] [Google Scholar]
- 99.Pastrana DV, Gambhira R, Buck CB, et al. Cross-neutralization of cutaneous and mucosal Papillomavirus types with anti-sera to the amino terminus of L2. Virology. 2005 Jul 5;337(2):365–372. doi: 10.1016/j.virol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 100.Kawana K, Yasugi T, Kanda T, et al. Safety and immunogenicity of a peptide containing the cross-neutralization epitope of HPV16 L2 administered nasally in healthy volunteers. Vaccine. 2003 Oct 1;21(27–30):4256–4260. doi: 10.1016/s0264-410x(03)00454-7. [DOI] [PubMed] [Google Scholar]
- 101.Peng S, Trimble C, Wu L, et al. HLA-DQB1*02-restricted HPV-16 E7 peptide-specific CD4+ T-cell immune responses correlate with regression of HPV-16-associated high-grade squamous intraepithelial lesions. Clin Cancer Res. 2007 Apr 15;13(8):2479–2487. doi: 10.1158/1078-0432.CCR-06-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brulet JM, Maudoux F, Thomas S, et al. DNA vaccine encoding endosome-targeted human papillomavirus type 16 E7 protein generates CD4+ T cell-dependent protection. Eur J Immunol. 2007 Feb;37(2):376–384. doi: 10.1002/eji.200636233. [DOI] [PubMed] [Google Scholar]
- 103.Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- 104.Hung CF, Tsai YC, He L, et al. DNA Vaccines Encoding Ii-PADRE Generates Potent PADRE-specific CD4(+) T-Cell Immune Responses and Enhances Vaccine Potency. Mol Ther. 2007 Jun;15(6):1211–1219. doi: 10.1038/sj.mt.6300121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Muderspach L, Wilczynski S, Roman L, et al. A phase I trial of a human papillomavirus (HPV) peptide vaccine for women with high-grade cervical and vulvar intraepithelial neoplasia who are HPV 16 positive. Clin Cancer Res. 2000 Sep;6(9):3406–3416. [PubMed] [Google Scholar]
- 106.Sheets EE, Urban RG, Crum CP, et al. Immunotherapy of human cervical high-grade cervical intraepithelial neoplasia with microparticle-delivered human papillomavirus 16 E7 plasmid DNA. Am J Obstet Gynecol. 2003 Apr;188(4):916–926. doi: 10.1067/mob.2003.256. [DOI] [PubMed] [Google Scholar]
- 107.Pockley AG, Muthana M, Calderwood SK. The dual immunoregulatory roles of stress proteins. Trends Biochem Sci. 2008 Feb;33(2):71–79. doi: 10.1016/j.tibs.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 108.Morris LF, Ribas A. Therapeutic cancer vaccines. Surg Oncol Clin N Am. 2007 Oct;16(4):819–831. doi: 10.1016/j.soc.2007.07.007. ix. [DOI] [PubMed] [Google Scholar]
- 109.Hallez S, Simon P, Maudoux F, et al. Phase I/II trial of immunogenicity of a human papillomavirus (HPV) type 16 E7 protein-based vaccine in women with oncogenic HPV-positive cervical intraepithelial neoplasia. Cancer Immunol Immunother. 2004 Jul;53(7):642–650. doi: 10.1007/s00262-004-0501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Goldstone SE, Palefsky JM, Winnett MT, et al. Activity of HspE7, a novel immunotherapy, in patients with anogenital warts. Dis Colon Rectum. 2002;45(4):502–507. doi: 10.1007/s10350-004-6229-6. [DOI] [PubMed] [Google Scholar]
- 111.Brandsma JL, Shylankevich M, Su Y, et al. Vesicular stomatitis virus-based therapeutic vaccination targeted to the e1, e2, e6, and e7 proteins of cottontail rabbit papillomavirus. J Virol. 2007 Jun;81(11):5749–5758. doi: 10.1128/JVI.02835-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brun JL. Eurogin 2006: Human Papilliomavirus Infection and Global Prevention of Cevical Cancer; 2006. Paris: 2006. Apr 23–26, In a phase II study with HPV 16 CIN 2/3 patients, Transgene's TG4001 induces clinical regression and HPV 16 transcription inhibition. In a phase II study with HPV 16 CIN 2/3 patients, Transgene's TG4001 induces clinical regression and HPV 16 transcription inhibition. [Google Scholar]
- 113.Garcia-Hernandez E, Gonzalez-Sanchez JL, Andrade-Manzano A, et al. Regression of papilloma high-grade lesions (CIN 2 and CIN 3) is stimulated by therapeutic vaccination with MVA E2 recombinant vaccine. Cancer Gene Ther. 2006 Jun;13(6):592–597. doi: 10.1038/sj.cgt.7700937. [DOI] [PubMed] [Google Scholar]
- 114.Xu YF, Zhang YQ, Xu XM, et al. Papillomavirus virus-like particles as vehicles for the delivery of epitopes or genes. Arch Virol. 2006 Nov;151(11):2133–2148. doi: 10.1007/s00705-006-0798-8. [DOI] [PubMed] [Google Scholar]