Abstract
Objective
Our first purpose was to determine whether there was a proximal to distal gradient in motor deficits in nine segments of the affected upper extremity (shoulder, elbow, forearm, wrist, and 5 fingers) post stroke. Our second purpose was to determine which upper extremity segments made the greatest contributions to hand function.
Methods
33 subjects were tested on average 18.6 (± 5.6) days after stroke. The ability to move each segment was measured by active range of motion (AROM). Hand function was measured by a battery of standardized clinical tests which were synthesized into a single, sensitive score for hand function using principal components analysis.
Results
AROM at all nine segments of the upper extremity was reduced and there was no evidence of a proximal to distal gradient in AROM values. Strength of each segment was reduced and there was also no evidence of a gradient in strength values. AROM at each segment was strongly correlated with hand function scores (range 0.76 – 0.94). General multiple regression analysis showed that AROM explained 82% of the variance in hand function, with most of the variance shared across proximal, middle, and distal segments. Hierarchical regression analysis showed that shoulder AROM alone could explain 88% of the variance in hand function.
Conclusion
Early after stroke a proximal to distal gradient of motor deficits was not present, and loss of hand function was due to a loss of ability to move many segments of the upper extremity and not just the distal ones.
Significance
These results suggest that a change in the clinical perception of motor deficits post stroke is needed. Our finding that shoulder AROM predicted almost all the variance in hand function opens up the possibility that this quick, simple measure may be predictive of future hand function. This would be of high economic and clinical utility compared to other ongoing efforts attempting to predict outcomes post stroke (e.g. fMRI, MEG).
Keywords: gradient, hand function, hemiparesis, stroke, upper extremity
Introduction
The most common neurological impairment caused by stroke is paresis, i.e. a reduced ability to voluntarily activate spinal motoneurons. The classical perception of clinicians about individuals who have recently suffered a stroke is that paresis of distal upper extremity segments is more severe than paresis of proximal upper extremity segments (Twitchell, 1951;Saladin, 1996). Based on this clinical perception, it is often presumed that the loss of distal segment movement is responsible for the loss of hand function after stroke (Muellbacher et al., 2002). The perception is consistent with disruption to the corticospinal system which is known to have greater input to distal cervical motoneuron pools compared to proximal cervical motoneuron pools (Palmer and Ashby, 1992;Porter and Lemon, 1993;McKiernan et al., 1998;Turton and Lemon, 1999). Indeed, the severity of motor deficits post stroke is strongly related to the degree of corticospinal system damage (Pineiro et al., 2000). Quantitative data have shown the existence of greater strength deficits in the more distal versus the more proximal upper extremity segments in a small sample of people with chronic hemiparesis (Colebatch and Gandevia, 1989). An early qualitative study however is in conflict with the above evidence, suggesting that a proximal to distal gradient of motor deficits is not present post stroke (Bard and Hirschberg, 1965).
We recently investigated movement control in the entire upper extremity and how loss of segmental movement control was related to hand function in a sample of people with chronic hemiparesis post stroke (Lang and Beebe, 2007). We found that all upper extremity segments were similarly affected, and movement at all segments was related to hand function. This study was important because it was the first to systematically quantify how movement control is affected across the entire upper extremity in the same sample. A limitation to this study was that it was a cross-sectional sample from a chronic stroke population (average of two years post stroke). Thus it was not possible to determine if a proximal to distal gradient in motor deficits was present and then changed due to recovery, or if a proximal to distal gradient in motor deficits was not present early after stroke.
The purposes of this study were to investigate, in the first few weeks after stroke, how stroke affects the ability to move at nine segments of the upper extremity (shoulder, elbow, forearm, wrist, and five fingers), and then to determine how loss of movement control at various segments contributes to loss of hand function. Hand function is defined as the ability to use the hand and fingers for activities of daily living or occupation, and includes the ability to move the upper limb in all dimensions to position and orient the hand in the environment (Kapandji, 1981;Cooper et al., 1993). We chose to use active range of motion (AROM) as our measure of the ability to move each segment because AROM was the variable that was most strongly related to hand function in our previous sample (Lang and Beebe, 2007), and because it can easily be measured clinically. Conceptually, AROM in people post stroke can be considered a quick measure of the ability to voluntarily activate the spinal motoneuron pools that move a given segment (Hislop and Montgomery, 2002).
We hypothesized that proximal and distal segments would be similarly affected at this early time point following stroke. This hypothesis is based on the premise that, despite the greater corticospinal system input to distal cervical motoneuron pools than to proximal, the proportion of input to distal motoneuron pools damaged following stroke may be similar to the proportion of input damaged in the proximal motoneuron pools, resulting in similar loss of movement control across all segments. For example, if proximal and distal motoneuron pools lost half their input, then one might expect to see proximal and distal movement capabilities reduced by half as well. We also hypothesized that more proximal segments would make equally strong contributions to hand function as more distal segments. This second hypothesis is based on the premise that functional use of the hand is dependent on not only manipulating objects with the fingers but also dependent on appropriately positioning and orienting the hand in the environment.
Methods
Subjects
Thirty-three subjects with hemiparesis due to stroke participated in this study. Subjects were recruited from the Cognitive Rehabilitation Research Group Stroke Registry based on the presence of hemiparesis. Subjects were included if they 1) had a diagnosis of ischemic or hemorrhagic stroke by a stroke neurologist within one month of onset, 2) had CT or MRI imaging data consistent with clinical presentation, 3) had persistent hemiparesis with a score of 1 to 4 on the Motor Arm item of the National Institutes of Health Stroke Scale (NIHSS), 4) had evidence of preserved cognition as indicated by a score of 0 or 1 on the Consciousness and Communication item of the NIHSS, and 5) had the ability to follow 2-step commands. Patients were excluded from the study if they 1) had orthopaedic or other medical conditions that limited the affected upper extremity prior to the stroke, 2) had a prior history of hemiparesis or stroke, 3) had hemispatial neglect as evidenced by a score of 2 on the Extinction and Inattention item of the NIHSS, 4) had severe aphasia as evidenced by a score of 2 or 3 on the Language item of the NIHSS, 5) had complete hemianopsia as evidenced by a score of 2 or 3 on the Visual item of the NIHSS, or 6) the subject was unable to give informed consent. Characteristics of the group are provided in Table 1. This study was approved by the Washington University Human Research Protection Office, and all participants provided informed consent prior to participation.
Table 1.
Subject Characteristics (n = 33)
| mean ± SD | range/percent | normal | |
|---|---|---|---|
| Age (years) | 56.9 ± 10.2 | (31–77) | - |
| Gender | 19 males 14 females |
(58%) (42%) |
- |
| Time since stroke (days) | 18.6 ± 5.57 | (8–30) | - |
| Hand affected | 14 left 19 right |
(42%) (58%) |
- |
| Type of stroke | 32 ischemic 1 hemorrhagic |
(97%) (3%) |
- - |
| Subjects with identifiable acute lesiona | 20/33 | (61%) | - |
| Lesion location in subjects withidentifiable acute lesion | 3 Superficial cortical 10 Deep cortical Superficial & Deep 2 Brainstem |
(15%) (50%) (25%) (10%) |
- - - - |
| Lesion Size (largest aperture diameter)b | 7 ≤ 1.5cm 10 1.6-3.0 cm 3 > 3.0 cm |
(35%) (50%) (15%) |
- - - |
| Dominant hand affected | 17 | (52%) | - |
| Composite strength | 0.35 ± 0.36 | (0 – 1) | 0.93 – 1.07 |
| Shoulder pain | 0.76 ± 1.64 | (0 – 6) | 0 |
| Index finger joint position sense | 27 normal 6 impaired |
(90%) (10%) |
- - |
| Modified Ashworth Scale | 0.58 ± 0.67 | (0 – 3) | 0 |
| Action Research Arm Test | 26.4 ± 23.9 | (0 – 57) | 57 |
| Jebsen Test of Hand function (secs) | 418 ± 316 | (38 – 720) | 24 – 36 |
| Grip Strength (% unaffected side) | 29.6 ± 31.2 | (0 – 89) | 0.85 – 1.15 |
| Pinch Strength (% unaffected side) | 43.1 ± 43.4 | (0 – 152) | 0.94 – 1.06 |
| 9-Hole Peg test (pegs/sec) | 0.20 ± 0.27 | (0.0 – 0.79) | 0.77 – 0.99 |
| Stroke Impact Scale: Hand function | 19.9 ± 28.0 | (0 – 85) | 100 |
Values are means ± SD (range), or number (%)
20 Subjects had a definite acute lesion as seen on clinical CT or MR scan. 13 subjects were without a definitive acute lesion. These 13 subjects had clinical CT scans upon admission to the acute neurology service only, making it unlikely that an acute ischemic lesion could have been detected.
For multiple lesions, diameter of largest lesion reported.
Testing paradigm
Subjects were tested for their ability to use their hand for functional activities, and for their ability to move nine upper extremity segments (active range of motion; AROM). Testing was completed, in most cases, during a single testing session lasting approximately two hours. In a few cases, testing was completed in two separate one hour sessions within a 24-hour period.
Measurement of hand function
All subjects underwent a battery of six standardized clinical tests of hand function. A battery of tests was used to measure hand function in multiple ways (e.g. criterion-rated, timed performance, self-report). This was because a single test may not suitably capture and quantify hand function across this patient population (Wade et al., 1983;Duncan et al., 2000). The tests were selected based on published data regarding reliability, validity, normative values, and appropriateness for use with people with stroke. All clinical tests were performed on both sides such that the unaffected upper extremity served as the matched control for the affected side. The following tests were used.
Jebsen Test of Hand function
(Jebsen et al., 1969;Hummel et al., 2005) is a functional assessment scored by the times to complete seven common tasks. A lower time indicates faster task completion, and thus better hand function. The first task, writing a sentence, was not used (Hummel et al., 2005) because it is dependent on hand dominance and education level. Normal performance, as measured by the summed time to complete 6 tasks, for healthy adults in their 60s is between 24 and 36 seconds (Agnew and Maas, 1982;Hackel et al., 1992). Subjects were allowed a maximum of 120 seconds to complete the test (Duncan et al., 1998).
Action Research Arm Test
(Lyle, 1981;Hsieh et al., 1998;Van der Lee et al., 2001a;Van der Lee et al., 2001b;Lang et al., 2006) is a criterion-rated assessment of functional upper extremity gross movement, grasping, gripping and pinching. A maximum score of 57 indicates normal function.
9-Hole Peg Test
(Mathiowetz et al., 1985b) is a finger coordination measure of timed performance to insert and remove nine pegs. Scores were expressed as the number of pegs placed per second. Normal performance for healthy adults in their 60s is between 0.77 and 0.99 pegs per second (Mathiowetz et al., 1985b).
Stroke Impact Scale
(Duncan et al., 1999;Duncan et al., 2001) is a self-report questionnaire to measure the impact of stroke in multiple domains. The maximum score of 100 indicates normal function. Data on all domains of the Stroke Impact Scale were collected, but only the hand function subscale was part of our hand function battery.
Grip Strength
(Schmidt and Toews, 1970) is a dynamometer measurement of the maximum amount of force produced during a five-finger grip, and has been proposed as a surrogate measure of hand functional outcome (Boissy et al., 1999). Scores were expressed as a ratio value of affected/unaffected side where higher ratios mean affected side strength is more similar to unaffected side strength. In healthy individuals, the ratio between the strength on the two sides of the body is typically between 0.85 and 1.15 (Mathiowetz et al., 1985a).
Pinch Strength
(Mathiowetz et al., 1985a) is a dynamometer measurement of the maximum amount of force produced during a three-fingered key pinch. Scores were expressed as a ratio value of affected/unaffected side where higher ratios mean affected side strength is more similar to unaffected side strength. In healthy individuals, the ratio between the strength on the two sides of the body is typically between 0.94 and 1.06 (Mathiowetz et al., 1985a).
The results of the test battery were synthesized to yield a single measure of hand function for each subject using principal components analysis (Ward et al., 2003a;Ward et al., 2003b;Lang and Beebe, 2007). Principal components analysis reduces a number of correlated variables into a few, uncorrelated variables, or principal components using a covariation or correlation matrix. As before (Lang and Beebe, 2007), the first principal component explained a large portion (87.5%) of the variance in test scores and the weighted linear coefficients of this first component were used to generate a single score of hand function for each subject.
Additional testing
Additional clinical tests were conducted to provide a more thorough description of the sample (Table 1). To quantify upper extremity strength, a hand-held dynamometer was used to assess both flexion and extension of the shoulder, elbow, wrist, and index finger using standardized, reliable test positions (Andrews et al., 1996). Intra-tester reliability of handheld dynamometry has been shown to be between 0.93 and 0.98 for these upper extremity muscle groups (Andrews et al., 1996). Both affected and unaffected sides were tested and the results were expressed as a percentage of unaffected side where normal non-dominant to dominant is between 0.93 and 0.98 (calculated from (Andrews et al., 1996). Affected side shoulder pain was assessed using a standard 11 point pain scale, where 0 = no pain. Joint position sense was evaluated on both sides at the index finger and wrist using standard clinical technique where normal = correct on ≥ 3/5 trials. Lastly, spasticity was evaluated using the Modified Ashworth Scale at four joints on the affected side: the metacarpophalangeal joints of hand, the wrist, the elbow, and the shoulder. Modified Ashworth scores were expressed as an average across all joints, where normal equals 0.
Measurement of active range of motion
Kinematic techniques were used to quantify segmental movement of nine upper extremity segments on the affected side, contralateral to the lesion. Three-dimensional movements of the upper extremity were captured using an electromagnetic tracking system (The Motion Monitor, Innovative Sports Training Inc, Chicago IL). Nine sensors were attached to the trunk: mid-sternum (1 sensor), upper arm: proximal to the lateral epicondyle, bisecting the upper arm mass (1), forearm: mid-point between the radial and ulnar styloids on the dorsum of the forearm (1), hand: mid-point of the 3rd metacarpal on the dorsum of the hand (1), and fingers: on the nail of each digit (5). While seated, subjects were instructed to make movements of one segment while keeping the other upper extremity segments still. The starting position for each movement was with the upper extremity hanging down by the side and care was taken to ensure that the tested upper extremity did not contact or otherwise be obstructed by the side edge of the chair. Subjects were reasonably good at keeping their uninstructed segments still, especially the proximal segments during distal movement due to gravity and the testing position. Some subjects were better than others, and similar to previous work, those with greater AROM were better able to isolate movements (Lang and Beebe, 2007). The nine instructed movements were: shoulder flexion, elbow flexion, forearm pronation/supination, wrist flexion/extension, thumb flexion, index finger flexion, middle finger flexion, ring finger flexion, and little finger flexion. Subjects were allowed to move at a self-selected pace and instructed to move the segment as far as they could then return to the starting position. Two trials of each movement were recorded. Due to upgrades in the data collection system during the study, data was collected at either 60 Hz or 100 Hz and stored offline for subsequent analyses.
Kinematic data were low-pass filtered at 6 Hz using a second-order Butterworth filter. Motion Monitor software (Innovative Sports Training Inc, Chicago IL) was used to calculate and extract segmental position and angle data from the sensor data used standard rigid body methodology (Wu et al., 2005). For each trial, the main variable extracted was AROM. AROM was used as a measure of how far a segment can be moved against gravity, and was calculated as the angular excursion through which a segment moved when it was the instructed one. The Motion Monitor software allowed us to compute angular excursion irrespective of movement at non-instructed segments, i.e. elbow excursion was the range through which the elbow moved regardless of whether the shoulder and the wrist also moved. For finger AROM, finger angular excursions were calculated from finger angle data (Theverapperuma et al., 2006;Lang and Beebe, 2007), a Euler angle calculation that represents the rotation at all three joints of each finger. Because the start position was with the upper extremity hanging down by the side, two movements, forearm supination/pronation, and thumb flexion were tested in a gravity eliminated position. In these two movements, the moment arms were negligible such that gravity was not likely to have a substantial influence in a subject’s ability to perform the movements. Custom-written software in MATLAB (The Mathworks, Nadick, MA) was used to do subsequent analyses.
The nine different segments have different anatomical ranges of motion. In order to examine how one segment may be affected compared to another, AROM values were converted from degrees to percent of normal range during the same task. Normal range values were obtained from our previous sample of segmental AROM in the unaffected (ipsilesional) upper extremity of similarly aged people with chronic hemiparesis performing the same movements, under the same conditions (Lang and Beebe, 2007). In this sample (n = 28), AROM values were not different from values obtained during pilot testing in a group of healthy, young, neurologically-intact adults.
Data analysis
SPSS version 13 was used for all statistical analyses and the criterion for statistical significance was set at p < 0.05. Distributions of variables were examined for normality using the Kolmogorov-Smirnov test. The six subtest scores included from the Jebsen Test of Hand function were not normally distributed and were transformed for further statistical analyses. Each subtest score was transformed using the natural log function. All subsequent analyses using these six variables were done with the transformed data.
A repeated measures within subjects ANOVA, was used to determine if AROM at the nine upper extremity segments were similarly or differentially affected. A second repeated measures ANOVA was used to determine if strength at four joints (shoulder, elbow, wrist, index finger) were similarly or differentially affected. When significant effects were found, Bonferroni corrected post hoc t-tests were used to detect where the differences existed. Pearson product moment correlations were used to evaluate the relationships between hand function (score from principal components analysis) and AROM at all nine segments. Based on our sample size, correlation coefficients with an absolute value greater than 0.34 were statistically significant at the p < 0.05 level.
Two multiple regression analyses were used to determine which segments made the greatest contributions to the variance in hand function. For the first analysis, we divided the nine segments into three functional groupings, 1) proximal (shoulder and elbow), 2) middle (forearm and wrist, and 3) distal (thumb, index, middle, ring and little fingers) (Lang and Beebe, 2007). The grouping values were calculated by taking the average amount of normalized movement for each group, e.g. distal grouping = sum of finger AROM for all five fingers divided by 5. Functionally, the proximal grouping translates the arm toward an object, the middle grouping orients the hand to an object, and the distal grouping allows for manipulation of an object (Lang and Beebe, 2007). The dependent variable in the general regression model was the hand function score obtained from the principal components analysis. The independent variables were the three groupings, proximal, middle, and distal and were entered simultaneously into the model. Squared semi-partial correlation coefficients were used to determine the unique variance associated with each grouping. This first analysis was used to determine the relative importance of AROM in the proximal versus middle versus distal upper extremity segments for hand function. For the second analysis, hierarchical regression was used to determine the most parsimonious combination of segments that could explain the variance in hand function scores. The dependent variable in this model was the hand function score. The independent variables were the nine upper extremity segments. We used results obtained from the correlational analysis to determine which order the segments would be entered into the regression. The second analysis was used in an attempt to develop a simple model of how the inability to move segments translates to loss of hand function.
Results
Thirty-three subjects with acute hemiparesis were included in the study (Table 1). Average age was 56.9 (± 10.2) years old, and average time since stroke was 18.6 (± 5.6) days. Within this sample, there were varying degrees of hemiparesis from nearly complete plegia to just barely detectable paresis (Table 1). The subjects had minimal shoulder pain, and minimal spasticity throughout their upper extremity. Only six subjects had impaired joint position sense at the index finger and/or wrist. Lesion information is included in Table 1. A radiologist inspected clinical CT or MR images for each subject and determined that 1) lesion location within the motor system was highly variable; this is consistent with our recruitment based on the presence of hemiparesis versus recruitment based on lesion location, 2) lesions varied in size, and 3) 97% of the lesions were ischemic.
Active range of motion of the affected upper extremity
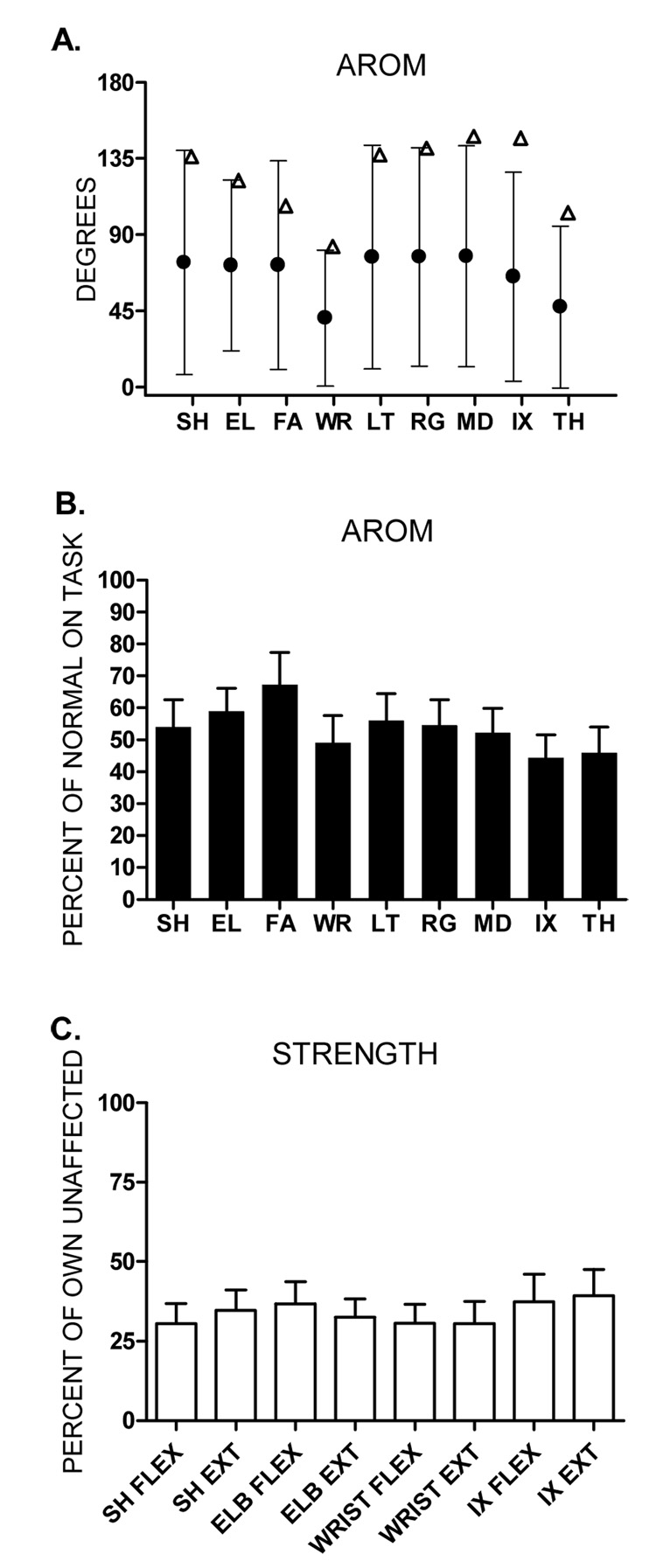
AROM at all nine upper extremity segments was decreased and highly variable across subjects (Figure 1A; filled circles). To determine if differences existed between segments, we normalized the data to the mean AROM of the unaffected side of comparable aged individuals with stroke performing the same task under the same conditions (see Methods; open triangles Figure 1A). Average normalized AROM for each segment of the upper extremity ranged from 44% to 67% (Figure 1B). Using a repeated measures ANOVA on the normalized values, we found a significant main effect of segment (p = 0.001), indicating there was a difference between the degree to which some segments were affected. Post hoc comparisons indicated that the lone difference was that the forearm AROM was greater than the index finger AROM (p = 0.004). Overall, the AROM data did not indicate that the more distal segments were affected to a greater degree than the more proximal segments.
Figure 1.
A: Group means ± SDs for affected side AROM (●) values for each of the 9 segments. (Δ) represents normal AROM for each of the 9 segments. Normal AROM values were obtained from the unaffected side of comparable aged individuals with stroke performing the same task (Figure 5A, Lang & Beebe, 2007) and are shown only to appreciate how the percent of normal AROM was calculated. B: Bars represent mean percent of normal AROM ± SEs. C. Group means ± SEs for the percentage of affected to unaffected side strength. SH: shoulder, EL: elbow, FA: forearm, WR: wrist, LT: little finger, RG: ring finger, MD: middle finger, IX: index finger, TH: thumb, FLEX: flexion, EXT: extension
To further investigate this issue, we looked for a proximal to distal gradient in the strength measurements. A repeated measures ANOVA was used to test if strength was more or less affected across four joints of the upper extremity. Average strength percentages across the four joints ranged from 30% to 39% (Figure 1C). We found no difference in the degree to which strength was affected across the four joints (main effect of joint, p = 0.31). These data are consistent with the AROM data, indicating that the stroke had a similar effect on all the upper extremity segments.
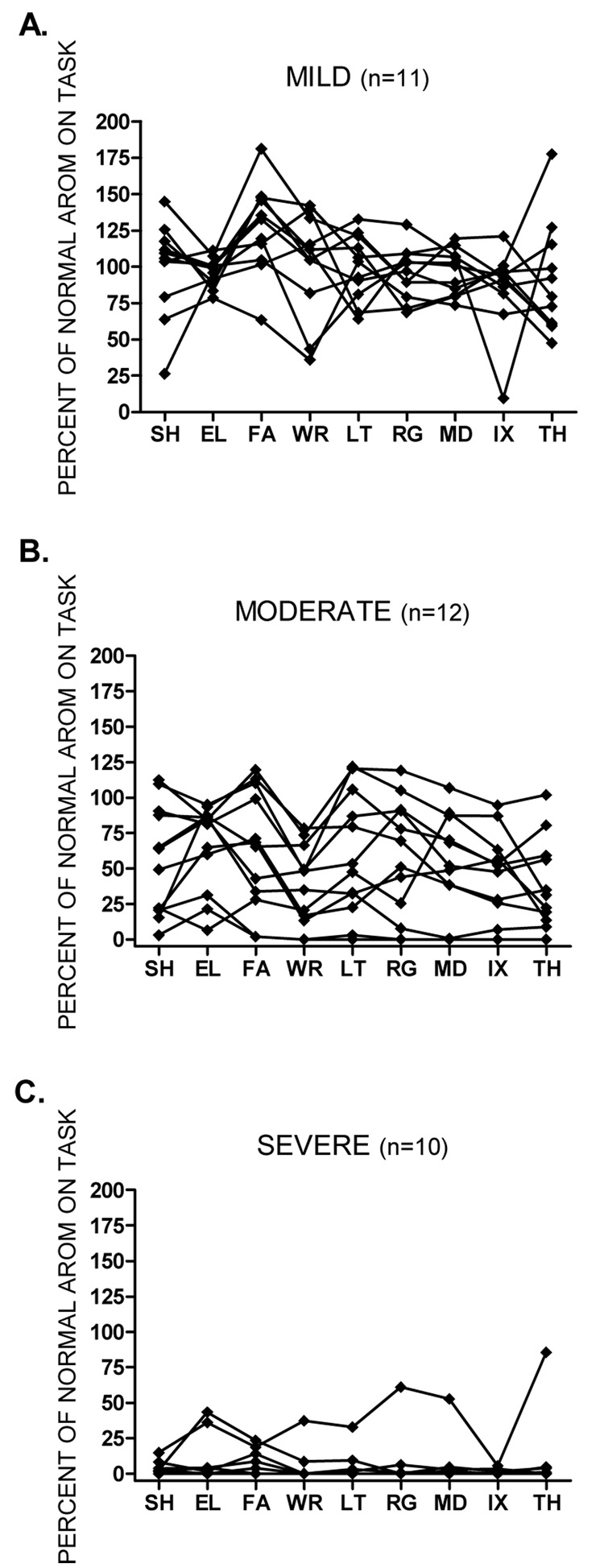
A secondary inspection of individual AROM data also suggested a lack of proximal to distal gradient (Figure 2). For graphing purposes, strength measurements were used to stratify the subjects into three groups, mild: affected side ≥ 50% strength of unaffected side (n = 11; Figure 2A), moderate: affected side < 50% strength of unaffected side (n = 12; Figure 2B), and severe: affected side = 0% strength of unaffected side (n = 10; Figure 2C). Within each subgroup, the normalized AROM of each segment was examined for each subject. Despite the variability of movement within each subgroup, no subject demonstrated a proximal to distal gradient.
Figure 2.
Figures A, B, and C show individual data for percent of normal AROM at each segment of the upper extremity. Values may be greater than 100% if a subject moved a particular segment much more than the average of the healthy sample. Subjects were stratified according to the percent strength in their unaffected arm. Figure A: mild ≥ 50% strength of unaffected side. Figure B: moderate < 50% strength of unaffected side. Figure C: severe = 0% strength of unaffected side. It is possible to have 0% strength, but still have some AROM because a person was given a score of 0 at a segment if they were unable to hold the test position against gravity. If distal segments were affected to a greater degree than proximal segments, we would have expected to see lines sloping downward from left to right. Abbreviations same as in figure 1.
Hand function relative to active range of motion
Within the first month after stroke, AROM was strongly correlated with hand function scores (Table 2, HF column). All correlations were significant at the p < 0.01 level. Overall, the correlation between hand function and shoulder AROM was the largest (0.94), and the correlation between hand function and thumb AROM was the smallest (0.76). Consistent with the fact that AROM at all nine segments was correlated with hand function, we found similarly strong correlations between the AROM values at each segment (Table 2, remaining columns) The largest correlation coefficient was between the ring and middle finger (0.93), while the smallest correlation coefficient was between the index finger and thumb (0.68). Nonetheless, all between segment correlations were moderate to strong (all p values < 0.01), indicating that the degree to which upper extremity movement was affected co-varied across segments.
Table 2.
Pearson Product Moment Correlations (n = 33)
| HF | Shoulder | Elbow | Forearm | Wrist | Little | Ring | Middle | Index | Thumb | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shoulder | .94 | 1.0 | |||||||||
| Elbow | .79 | .84 | 1.0 | ||||||||
| Forearm | .81 | .87 | .88 | 1.0 | |||||||
| Wrist | .86 | .86 | .84 | .90 | 1.0 | ||||||
| Little | .81 | .84 | .86 | .87 | .83 | 1.0 | |||||
| Ring | .80 | .83 | .87 | .85 | .82 | .91 | 1.0 | ||||
| Middle | .79 | .85 | .81 | .85 | .85 | .89 | .93 | 1.0 | |||
| Index | .81 | .90 | .81 | .86 | .81 | .81 | .79 | .89 | 1.0 | ||
| Thumb | .76 | .73 | .76 | .68 | .83 | .73 | .80 | .78 | .68 | 1.0 |
Left: Correlations between AROM at each segment of the upper extremity and the Hand function Score (HF).
Right: Correlation matrix between each segment of the upper extremity.
All correlation coefficients are significant at the p < 0.01 level.
Multiple regression analysis was used to determine how loss of AROM affected hand function in people with acute hemiparesis. Segments were grouped into proximal, middle, and distal groupings (see Methods). We used a general regression model to analyze the groupings because we wanted to determine the relative contributions of proximal, middle, and distal segments to loss of hand function. AROM values explained 82% of the variance in hand function scores (F3,29= 45.19, p < 0.001), with the majority of this variance being shared between the proximal, middle, and distal groupings. In other words, the proportion of explained variance in hand function was not unique to any one grouping. The shared variance supports the results of the correlation analysis and suggests that no one segment or grouping is more important for hand function. We then used a hierarchical regression model to determine if a more parsimonious set of variables could be useful for predicting hand function. Based on the results of the correlational analysis, the shoulder was entered first into the regression equation followed by the other segments in descending order. The shoulder segment alone explained 88% of the variance in hand function scores at this early time point after stroke (F1,31= 229.8, p < 0.001), and no other variables entered into the equation.
Discussion
In people with hemiparesis due to a recent stroke, the ability to move each segment was generally similar across nine upper extremity segments. This was a consistent finding when assessed with AROM measurements, with strength measurements, and with visual inspection of individual data. The ability to move each segment was correlated with hand function, and surprisingly the shoulder had the highest correlation coefficient (r= 0.94). Early after stroke, loss of hand function was due to losses in the ability to move all upper extremity segments and not just the distal ones. The most parsimonious regression model required only a single variable – shoulder AROM – to explain nearly all the variance in hand function (R2= 0.88). These data support our first hypothesis, suggesting that a proximal to distal gradient of motor deficits is not present early after stroke. Our second hypothesis was also supported, indicating that the ability to move the proximal segments is as equally important for hand function as the ability to move the distal segments, even at this early time point post stroke.
Our findings build upon the existing literature that examines one or a few segments post stroke (Bourbonnais et al., 1989;Dewald et al., 1995;Boissy et al., 1999;Lang and Schieber, 2003;Lang and Schieber, 2004;Mercier and Bourbonnais, 2004;Zackowski et al., 2004;Mirbagheri et al., 2005), by examining the ability to move at nine upper extremity segments in the same sample. Previous work looking across more than a few upper extremity segments has been done on samples of people with chronic hemiparesis post stroke (Colebatch and Gandevia, 1989;Lang and Beebe, 2007). Here, we extend that work in a sample of people tested an average of 3 weeks post stroke. Our findings also expand upon an earlier qualitative study (Bard and Hirschberg, 1965) by quantifying the loss of ability to move each segment and by investigating how loss of movement at various segments translates to loss of hand function.
The absence of a proximal to distal gradient in motor deficits
Quantitative measures of both AROM and strength, taken early after stroke, indicate that all segments of the upper extremity are similarly affected. AROM and strength can be considered indirect measures of the ability to volitionally activate the spinal motoneuron pools (Hislop and Montgomery, 2002). AROM measures may be better able to capture deficits at the lower end of spectrum, i.e. can the muscles be activated enough to move the segment through the range. Strength measures may be better able to capture deficits at the higher end of the spectrum, i.e. can the muscles be activated sufficiently to produce force against externally imposed loads. With both measures, there appeared to be no sparing of proximal versus distal upper extremity segments. Our finding is consistent with previous work showing no proximal to distal gradient in the ability to fractionate movement, as quantified by the individuation index (Zackowski et al., 2004;Lang and Beebe, 2007). Our finding is in conflict with the common clinical teaching that the distal segments are more impaired (Twitchell, 1951;Saladin, 1996) and in conflict with previous results showing a proximal to distal gradient in strength deficits (Colebatch and Gandevia, 1989). We now discuss possible explanations for disagreement between our results and others and a potential mechanism for these results.
A possible explanation for the mismatch in results is that a proximal to distal gradient of motor deficits exists in some but not all patients post stroke. For example, a gradient could be present in those with more moderate to severe paresis as found previously (Colebatch and Gandevia, 1989). There are several differences in their landmark work compared to our study that merit discussion. Their subjects were tested later after stroke (approximately 3 months) than ours were (ave. 18.6 days). They used a more precise methodology and tested muscle strength in different muscle groups. We tested shoulder flexion/extension, and index finger flexion/extension, while Colebatch and Gandevia tested shoulder abduction/adduction, and index through small finger flexion/extension. It is possible that the combination of more moderate-to-severe subjects tested in different combinations of muscle groups later after stroke can account for the proximal to distal gradient seen in their results but not in ours.
Hatakenaka and colleagues recently showed that some subcortical lesions may produce a proximal to distal gradient, while other subcortical lesions may produce a distal to proximal gradient (Hatakenaka et al., 2007). In this study, patients were categorized into “proximal” and “distal” groups based on whether individual subjects scored higher on the proximal vs. distal part of an upper extremity clinical test. In comparing the scores of the proximal and distal groups however (p. 350), it appears that both groups had proximal and distal paresis. Proximal paresis was actually worse in the distal group (2.2 points on a 0 – 5 point scale, where a lower score is worse) than in the proximal group (2.9 points). Distal paresis was worse in the distal group (1.4 points) than the proximal group (3.3 points). Like in their study, our sample included a wide range of deficits, from those with very mild paresis to nearly complete plegia. Our AROM measurements may have been more sensitive in capturing deficits than their clinical rating scale, while their imaging data was vastly better than the data to which we had access. Our subjects had lesions affecting cortical and/or subcortical structures, while their subjects had subcortical lesions exclusively. Relative to the two studies discussed above, it is possible that our group averages masked individual proximal to distal and/or distal to proximal gradients of motor deficits. We find this possibility unlikely however, since inspection of individual data (figure 2) did not reveal deficit gradients in either direction.
Another possible explanation for the mismatch between clinical perception and our results is that clinicians are likely to see that which they are looking for (Sotos, 2007). For example, when a patient is asked to move the shoulder, he/she may be able to perform a shoulder shrug, and when asked to move their hand or fingers, the fingers flex ever so slightly. If the clinician expects to see greater distal motor deficits, then the shoulder shrug may be considered to reflect greater movement capabilities than the trace finger flexion. Both movements in this example are only a small portion of the AROM and are equally impaired. Furthermore, our results suggest that both movements would contribute little, if any, to functional use of the hand.
The general severity of motor deficits post stroke is due to the extent of damage to the corticospinal system (Pineiro et al., 2000;Stinear et al., 2007). It has been thought that the proximal to distal gradient in movement deficits stems from disruption of this system, which sends more numerous projections to the distal cervical motoneuron pools (Palmer and Ashby, 1992;Porter and Lemon, 1993;McKiernan et al., 1998;Turton and Lemon, 1999). If compensatory control was exerted by other descending motor pathways, e.g. reticulospinal tract, that exert control over more proximal limb musculature (Davidson and Buford, 2004), then a gradient of movement deficits might be more apparent. It is possible that our finding of similar movement deficits across upper extremity segments reflects lesions that disrupt similar proportions of proximal and distal inputs. A similar proportion of inputs could be disrupted following stroke because motor cortical territory for proximal and distal muscles is strongly overlapping (Park et al., 2001) and as the axons descend through the subcortical structures, they are densely packed together (Morecraft et al., 2007). Additionally, the output from the motor cortical areas is divergent to multiple spinal motoneuron pools (Fetz and Cheney, 1980;Shinoda et al., 1981;Schieber, 2001), some of which may control more proximal muscles and some of which may control more distal muscles. Thus, in our sample with heterogeneous lesions, damage to many locations within the motor system may result in a similar disruption to proximal and distal inputs.
Relationships between the ability to move each segment and hand function
Correlational analyses indicated that the ability to move each segment was related to hand function early after stroke. Using a general regression analysis, we found that AROM measures from proximal, middle, and distal segments together explained 82% of the variance in hand function. The majority of the explained variance was shared across the three groupings and not unique to any one grouping. In other words, hand function is equally dependent on the ability to move the proximal segments as it is the middle and distal segments. This finding is consistent with our recent results from a cross-sectional sample of people with chronic hemiparesis post stroke, where R2 = 73% (Lang and Beebe, 2007). Taken together, these results suggest two things. First, replication of the strong correlations and regression models indicate that our findings are likely to be real, versus a random sample-dependent occurrence that can occasionally happen with these analyses (Cohen and Cohen, 1983). Second, the similarity in the magnitudes of the correlation and regression coefficients suggest that the relationship between the ability to move a segment and hand function stays relatively stable over time in people with stroke. These findings are exciting because the ability to move a segment (AROM) is easy to measure clinically. Although we used a motion capture system, the same AROM measurements could be obtained with an inexpensive goniometer at bedside or in the clinic. The obtained values can not only provide information about the ability to move segments, but can also provide information about the functional capacity of the limb.
Shoulder AROM alone acts as a simple predictor of hand function
To determine if a more parsimonious set of variables could explain hand function we used a hierarchical regression model with each of the nine segments as independent variables. Shoulder AROM was the only variable to enter the regression equation, explaining 88% of the variance in hand function early after stroke. We were surprised that such a large proportion of the variance was explained by a single variable, and surprised that it was shoulder AROM. Although the relationships were somewhat weaker, we were able to find a precedent for this in the literature, where the strength of the shoulder flexor muscles was correlated with scores on hand function tests in a sample of people with chronic hemiparesis (Mercier and Bourbonnais, 2004). The ability to move the shoulder appears to be a vital component of hand function (Kapandji, 1981). Shoulder movement is necessary for translating the hand in the environment so that the fingers may approach and contact an object.
Because the ability to move co-varied across segments (see right columns in table 2), there may be no need to make measurements of the ability to move all upper extremity segments. Measurements of one or two segments may be sufficient to quantify the motor deficits post stroke. Our results point to the importance of selecting the shoulder as a segment to measure because it also provides considerable information about hand function at this early time point after stroke. Measuring AROM of the shoulder and one, more distal segment, could capture upper extremity movement abilities more comprehensively, allow classification of the patients (Hatakenaka et al., 2007), and lead to more focused rehabilitation more quickly. For example, if shoulder AROM is minimal, efforts could be made to focus on improving shoulder motion because it is most highly correlated with hand function in the present study, and in chronic hemiparesis (Lang and Beebe, 2007). In people whose rehabilitation was focused on the proximal upper extremity, a large majority of patients regained functional use of their upper extremity (Volpe et al., 1999;Volpe et al., 2000). Lastly, our results open up the possibility that shoulder AROM measures taken early after stroke could be predictive of later hand function.
Limitations to our findings
Our subjects were recruited based on the presence of acute hemiparesis following a clinical diagnosis of a cerebrovascular accident. The inclusion/exclusion criteria resulted in a heterogeneous sample of subjects with a wide range of movement abilities and a range of lesion locations and sizes within the motor system. Recruiting based on the presence of hemiparesis and excluding based on the presence of severe aphasia, hemi-neglect, and hemianopsia likely restricted the range of possible lesion locations and sizes that we could have encountered. Other work from our lab has shown that, when recruited based on the presence of hemiparesis, upper extremity muscle activation patterns and their recovery are similar between those subjects with a lesion specifically identified in the corticospinal system and those subjects with lesions that are unidentified or include other structures in addition to the corticospinal system (Wagner et al., 2007a;Wagner et al., 2007b). Our sample size and lack of research level imaging data in the current study prevented any meaningful examination of lesion characteristics with respect to our results. Thus, we can make conclusions about the ability to move the segments and their relationship to hand function, but we can only speculate about how the results relate to corticospinal system damage at present.
We assessed nine segments of the upper extremity to determine if there was a proximal to distal gradient of motor deficit, and how the loss of movement control related to hand function. We did not test every movement direction at every segment. Some movements, such as shoulder abduction, elbow extension, wrist radial and ulnar deviation, and finger extension were not tested. Since time did not permit testing all possible movements, we had to select a reasonable representation of movements that spanned the proximal to distal musculature. For example, we chose to test shoulder flexion over shoulder abduction because it could be tested against gravity in a sitting position and because many upper extremity activities are performed in the front of the body where flexion is the required motion. We chose to assess finger flexion instead of finger extension because, with the arm hanging down by the side in the test position with the wrist held in neutral, finger flexion was against gravity while finger extension was not. Forearm pronation/supination and wrist flexion/extension were tested as combined movements because movements in both directions were equally unaffected (pronation/supination) or equally affected (flexion/extension) by gravity in the test position. It is possible that assessment of movements not tested or separate assessment of movement directions (supination vs. pronation) tested together would have produced different results. We consider this possibility unlikely however because the tested movements were strongly correlated with each other, making it likely that the ability to move in a different, untested direction at the same segment would also be strongly correlated to the tested movements. For those movements tested in combinations (e.g. pronation/supination and wrist flexion/extension), it is unlikely that separate testing would have produced different results because separate testing of strength in each movement direction did not reveal a directional bias in deficits (figure 1C) (Gowland et al., 1992;Fellows et al., 1994a;Fellows et al., 1994b).
Interestingly, some subjects in the mild and moderate groups attained percentages of normal AROM in some segments that exceeded 100%. This is likely due to the fact that we normalized by a previous group average, creating a comparison of each hemiparetic subject’s data to the average data of another group. Within the group used to compute “normal” values, there was considerable variability as to how far individuals could move specific segments. In addition, we had a wide range of ages in the current study (31 – 77 years, table 1). Increasing age is often linked with decreased AROM (Boone and Azen, 1979). Thus, because of the normalization to a group values and the age range of our sample, it is not unreasonable that some hemiparetic subjects could attain percentages of AROM that were above 100%.
Finally, we measured movement at each segment independently to determine how the ability to move segments was affected and how the segmental ability to move related to hand function. We recognize that in order to use the upper extremity for functional activities it is important to be able to coordinate movement simultaneously at many segments. Our tested movements were only a small set of relatively isolated and not particularly functional movements. Our data cannot shed light on how people with hemiparesis post stroke coordinate movement between segments, but that has been elegantly investigated by others (Beer et al., 1999;Cirstea and Levin, 2000;Beer et al., 2000;Levin et al., 2002;Beer et al., 2004;Keller et al., 2005). Based on our data, we hypothesize that the ability to coordinate movement between segments may be directly related to the ability to move an individual segment at this early time point post stroke. If a segment has a limited ability to move, it follows that the ability to coordinate its movement with other segments would also be limited. Given the large amount of variance in hand function that can be predicted by AROM measures at this early time point after stroke however, we speculate that other combinations of movements would not explain more. In our previous chronic sample (Lang and Beebe, 2007), AROM predicted a still substantial but slightly smaller amount of variance in hand function. Over the course of time and recovery, it may be that other combinations of movements may take on a measurable role in explaining the variance in hand function. This would be consistent with the demonstrated difficulty in coordinating upper extremity segments as shown by others (Levin, 1996;Beer et al., 1999;Cirstea and Levin, 2000;Beer et al., 2000;Reisman and Scholz, 2003;Beer et al., 2004;Reisman and Scholz, 2006).
Conclusions
Our data show that early after stroke, the ability to move each segment of the upper extremity was similarly affected. A proximal to distal gradient of motor deficits was not present within the first few weeks of stroke. These results suggest that a change in the clinical perception of motor deficits post stroke is needed. Our data also show that loss of hand function post stroke is due to a loss of the ability to move many segments of the upper extremity and not just the distal ones. Our finding that shoulder AROM predicted almost all the variance in hand function opens up the possibility that this quick, simple measure may be predictive of future hand function. This would be of high economic and clinical utility compared to other ongoing efforts attempting to predict outcomes post stroke (e.g. fMRI, MEG). Further longitudinal studies are underway to determine if AROM values are indeed predictive of later hand function and if their predictive value is strong enough to be used to estimate hand function prognosis in individual patients.
Acknowledgments
We thank Lucy Morris, MD, MPH, for her assistance with lesion data analysis, and Dustin Hardwick, DPT, for his assistance with data collection. Supported by National Institutes of Health HD047669, and Foundation for Physical Therapy Promotion of Doctoral Studies II Scholarship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Agnew PJ, Maas F. Hand function related to age and sex. Archives of Physical Medicine and Rehabilitation. 1982;63:269–271. [PubMed] [Google Scholar]
- Andrews AW, Thomas MW, Bohannon RW. Normative values for isometric muscle force measurements obtained with hand-held dynamometers. Phys Ther. 1996;76:248–259. doi: 10.1093/ptj/76.3.248. [DOI] [PubMed] [Google Scholar]
- Bard G, Hirschberg GG. Recovery of voluntary motionin upper extremity following hemiplegia. Arch Phys Med Rehabil. 1965;46:567–567. [PubMed] [Google Scholar]
- Beer RF, Dewald JP, Dawson ML, Rymer WZ. Target-dependent differences between free and constrained arm movements in chronic hemiparesis. Experimental Brain Research. 2004;156:458–470. doi: 10.1007/s00221-003-1807-8. [DOI] [PubMed] [Google Scholar]
- Beer RF, Dewald JP, Rymer WZ. Deficits in the coordination of multijoint arm movements in patients with hemiparesis: evidence for disturbed control of limb dynamics. Experimental Brain Research. 2000;131:305–319. doi: 10.1007/s002219900275. [DOI] [PubMed] [Google Scholar]
- Beer RF, Given JD, Dewald JP. Task-dependent weakness at the elbow in patients with hemiparesis. Archives of Physical Medicine and Rehabilitation. 1999;80:766–772. doi: 10.1016/s0003-9993(99)90225-3. [DOI] [PubMed] [Google Scholar]
- Boissy P, Bourbonnais D, Carlotti MM, Gravel D, Arsenault BA. Maximal grip force in chronic stroke subjects and its relationship to global upper extremity function. Clinical Rehabilitation. 1999;13:354–362. doi: 10.1191/026921599676433080. [DOI] [PubMed] [Google Scholar]
- Boone DC, Azen SP. Normal range of motion of joints in male subjects. The Journal of Bone and Joint Surgery American volume. 1979;61:756–759. [PubMed] [Google Scholar]
- Bourbonnais D, Vanden NS, Carey KM, Rymer WZ. Abnormal spatial patterns of elbow muscle activation in hemiparetic human subjects. Brain. 1989;112(Pt 1):85–102. doi: 10.1093/brain/112.1.85. [DOI] [PubMed] [Google Scholar]
- Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000;123(Pt 5):940–953. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Hillsdale: Lawrence Erlbaum Associates, Inc; 1983. [Google Scholar]
- Colebatch JG, Gandevia SC. The distribution of muscular weakness in upper motor neuron lesions affecting the arm. Brain. 1989;112(Pt 3):749–763. doi: 10.1093/brain/112.3.749. [DOI] [PubMed] [Google Scholar]
- Cooper JE, Shwedyk E, Quanbury AO, Miller J, Hildebrand D. Elbow joint restriction: effect on functional upper limb motion during performance of three feeding activities. Arch Phys Med Rehabil. 1993;74:805–809. doi: 10.1016/0003-9993(93)90005-u. [DOI] [PubMed] [Google Scholar]
- Davidson AG, Buford JA. Motor outputs from the primate reticular formation to shoulder muscles as revealed by stimulus-triggered averaging. Journal of Neurophysiology. 2004;92:83–95. doi: 10.1152/jn.00083.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewald JP, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118(Pt 2):495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- Duncan PW, Lai SM, Keighley J. Defining post-stroke recovery: implications for design and interpretation of drug trials. Neuropharmacology. 2000;39:835–841. doi: 10.1016/s0028-3908(00)00003-4. [DOI] [PubMed] [Google Scholar]
- Duncan PW, Richards L, Wallace D, Stoker-Yates J, Pohl P, Luchies C, Ogle A, Studenski S. A randomized, controlled pilot study of a home-based exercise program for individuals with mild and moderate stroke. Stroke. 1998;29:2055–2060. doi: 10.1161/01.str.29.10.2055. [DOI] [PubMed] [Google Scholar]
- Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30:2131–2140. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]
- Duncan PW, Wallace D, Studenski S, Lai SM, Johnson D. Conceptualization of a new stroke-specific outcome measure: the stroke impact scale. Topics of Stroke Rehabilitation. 2001;8:19–33. doi: 10.1310/BRHX-PKTA-0TUJ-UYWT. [DOI] [PubMed] [Google Scholar]
- Fellows SJ, Kaus C, Ross HF, Thilmann AF. Agonist and antagonist EMG activation during isometric torque development at the elbow in spastic hemiparesis. Electroencephalogr Clin Neurophysiol. 1994a;93:106–112. doi: 10.1016/0168-5597(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Fellows SJ, Kaus C, Thilmann AF. Voluntary movement at the elbow in spastic hemiparesis. Ann Neurol. 1994b;36:397–407. doi: 10.1002/ana.410360311. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Cheney PD. Postspike facilitation of forelimb muscle activity by primate corticomotoneuronal cells. J Neurophysiol. 1980;44:751–772. doi: 10.1152/jn.1980.44.4.751. [DOI] [PubMed] [Google Scholar]
- Gowland C, deBruin H, Basmajian JV, Plews N, Burcea I. Agonist and antagonist activity during voluntary upper-limb movement in patients with stroke. Phys Ther. 1992;72:624–633. doi: 10.1093/ptj/72.9.624. [DOI] [PubMed] [Google Scholar]
- Hackel ME, Wolfe GA, Bang SM, Canfield JS. Changes in hand function in the aging adult as determined by the Jebsen Test of Hand Function. Physical Therapy. 1992;72:373–377. doi: 10.1093/ptj/72.5.373. [DOI] [PubMed] [Google Scholar]
- Hatakenaka M, Miyai I, Sakoda S, Yanagihara T. Proximal paresis of the upper extremity in patients with stroke. Neurology. 2007;69:348–355. doi: 10.1212/01.wnl.0000266387.43527.bd. [DOI] [PubMed] [Google Scholar]
- Hislop HJ, Montgomery J. Daniel's and Worthingham's Muscle Testing. Saunders; 2002. [Google Scholar]
- Hsieh CL, Hsueh IP, Chiang FM, Lin PH. Inter-rater reliability and validity of the action research arm test in stroke patients. Age Ageing. 1998;27:107–113. doi: 10.1093/ageing/27.2.107. [DOI] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, Cohen LG. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Arch Phys Med Rehabil. 1969;50:311–319. [PubMed] [Google Scholar]
- Kapandji IA. The upper limb as logistical support for the hand. Saunders: Philadelphia; 1981. [Google Scholar]
- Keller T, Ellis MD, Dewald JP. Overcoming abnormal joint torque patterns in paretic upper extremities using triceps stimulation. Artificial Organs. 2005;29:229–232. doi: 10.1111/j.1525-1594.2005.29041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CE, Beebe JA. Relating movement control at 9 upper extremity segments to loss of hand function in people with chronic hemiparesis. Neurorehabilitation Neural Repair. 2007;21:279–291. doi: 10.1177/1545968306296964. [DOI] [PubMed] [Google Scholar]
- Lang CE, Schieber MH. Differential impairment of individuated finger movements in humans after damage to the motor cortex or the corticospinal tract. Journal of Neurophysiology. 2003;90:1160–1170. doi: 10.1152/jn.00130.2003. [DOI] [PubMed] [Google Scholar]
- Lang CE, Schieber MH. Human finger independence: limitations due to passive mechanical coupling versus active neuromuscular control. Journal of Neurophysiology. 2004;92:2802–2810. doi: 10.1152/jn.00480.2004. [DOI] [PubMed] [Google Scholar]
- Lang CE, Wagner JM, Dromerick AW, Edwards DF. Measurement of upper-extremity function early after stroke: properties of the action research arm test. Arch Phys Med Rehabil. 2006;87:1605–1610. doi: 10.1016/j.apmr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Levin MF. Interjoint coordination during pointing movements is disrupted in spastic hemiparesis. Brain. 1996;119(Pt 1):281–293. doi: 10.1093/brain/119.1.281. [DOI] [PubMed] [Google Scholar]
- Levin MF, Michaelsen SM, Cirstea CM, Roby-Brami A. Use of the trunk for reaching targets placed within and beyond the reach in adult hemiparesis. Experimental Brain Research. 2002;143:171–180. doi: 10.1007/s00221-001-0976-6. [DOI] [PubMed] [Google Scholar]
- Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. International Journal of Rehabilitation Research. 1981;4:483–492. doi: 10.1097/00004356-198112000-00001. [DOI] [PubMed] [Google Scholar]
- Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: normative data for adults. Archives of Physical Medicine and Rehabilitation. 1985a;66:69–74. [PubMed] [Google Scholar]
- Mathiowetz V, Weber K, Kashman N, Volland G. Adult norms for the nine hole peg test of finger dexterity. The Occupational Therapy Journal of Research. 1985b;5:24–38. [Google Scholar]
- McKiernan BJ, Marcario JK, Karrer JH, Cheney PD. Corticomotoneuronal postspike effects in shoulder, elbow, wrist, digit, and intrinsic hand muscles during a reach and prehension task. Journal of Neurophysiology. 1998;80:1961–1980. doi: 10.1152/jn.1998.80.4.1961. [DOI] [PubMed] [Google Scholar]
- Mercier C, Bourbonnais D. Relative shoulder flexor and handgrip strength is related to upper limb function after stroke. Clinical Rehabilitation. 2004;18:215–221. doi: 10.1191/0269215504cr724oa. [DOI] [PubMed] [Google Scholar]
- Mirbagheri MM, Rymer WZ, Tsao C, Settle K. Evolution of reflexive and muscular mechanical properties in stroke-induced spasticity; Conf Proc IEEE Eng Med Biol Soc; 2005. pp. 4393–4395. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, McNeal DW, Stilwell-Morecraft KS, Dvanajscak Z, Ge J, Schneider P. Localization of arm representation in the cerebral peduncle of the non-human primate. J Comp Neurol. 2007;504:149–167. doi: 10.1002/cne.21438. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Richards C, Ziemann U, Wittenberg G, Weltz D, Boroojerdi B, Cohen L, Hallett M. Improving hand function in chronic stroke. Archives of neurology. 2002;59:1278–1282. doi: 10.1001/archneur.59.8.1278. [DOI] [PubMed] [Google Scholar]
- Palmer E, Ashby P. Corticospinal projections to upper limb motoneurones in humans. The Journal of Physiology. 1992;448:397–412. doi: 10.1113/jphysiol.1992.sp019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MC, Belhaj-Saif A, Gordon M, Cheney PD. Consistent features in the forelimb representation of primary motor cortex in rhesus macaques. J Neurosci. 2001;21:2784–2792. doi: 10.1523/JNEUROSCI.21-08-02784.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineiro R, Pendlebury ST, Smith S, Flitney D, Blamire AM, Styles P, Matthews PM. Relating MRI changes to motor deficit after ischemic stroke by segmentation of functional motor pathways. Stroke. 2000;31:672–679. doi: 10.1161/01.str.31.3.672. [DOI] [PubMed] [Google Scholar]
- Porter R, Lemon R. Corticospinal function and voluntary movement. New York: Oxford University Press; 1993. [Google Scholar]
- Reisman DS, Scholz JP. Aspects of joint coordination are preserved during pointing in persons with post-stroke hemiparesis. Brain. 2003;126:2510–2527. doi: 10.1093/brain/awg246. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Scholz JP. Workspace location influences joint coordination during reaching in post-stroke hemiparesis. Exp Brain Res. 2006;170:265–276. doi: 10.1007/s00221-005-0209-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladin LK. Cerebrovascular Disease: Stroke. In: Fredricks CM, Saladin LK, editors. Pathophysiology of the Motor Systems: Principles and Clinical Presentation. Philadelphia: F.A Davis; 1996. pp. 486–512. [Google Scholar]
- Schieber MH. Constraints on somatotopic organization in the primary motor cortex. J Neurophysiol. 2001;86:2125–2143. doi: 10.1152/jn.2001.86.5.2125. [DOI] [PubMed] [Google Scholar]
- Schmidt RT, Toews JV. Grip strength as measured by the Jamar dynamometer. Archives of Physical Medicine and Rehabilitation. 1970;51:321–327. [PubMed] [Google Scholar]
- Shinoda Y, Yokota J, Futami T. Divergent projection of individual corticospinal axons to motoneurons of multiple muscles in the monkey. Neurosci Lett. 1981;23:7–12. doi: 10.1016/0304-3940(81)90182-8. [DOI] [PubMed] [Google Scholar]
- Sotos JG. Zebra Cards. MoveBusy Press; 2007. [Google Scholar]
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- Theverapperuma LS, Hendrix CM, Mason CR, Ebner TJ. Finger movements during reach-to-grasp in the monkey: amplitude scaling of a temporal synergy. Exp Brain Res. 2006;169:433–448. doi: 10.1007/s00221-005-0167-y. [DOI] [PubMed] [Google Scholar]
- Turton A, Lemon RN. The contribution of fast corticospinal input to the voluntary activation of proximal muscles in normal subjects and in stroke patients. Experimental Brain Research. 1999;129:559–572. doi: 10.1007/s002210050926. [DOI] [PubMed] [Google Scholar]
- Twitchell TE. The restoration of motor function following hemiplegia in man. Brain. 1951;74:443–480. doi: 10.1093/brain/74.4.443. [DOI] [PubMed] [Google Scholar]
- Van der Lee JH, Beckerman H, Lankhorst GJ, Bouter LM. The responsiveness of the Action Research Arm test and the Fugl-Meyer Assessment scale in chronic stroke patients. Journal of Rehabilitation Medicine. 2001a;33:110–113. doi: 10.1080/165019701750165916. [DOI] [PubMed] [Google Scholar]
- Van der Lee JH, De G V, Beckerman H, Wagenaar RC, Lankhorst GJ, Bouter LM. The intra- and interrater reliability of the action research arm test: a practical test of upper extremity function in patients with stroke. Archives of Physical Medicine and Rehabilitation. 2001b;82:14–19. doi: 10.1053/apmr.2001.18668. [DOI] [PubMed] [Google Scholar]
- Volpe BT, Krebs HI, Hogan N, Edelstein OL, Diels C, Aisen M. A novel approach to stroke rehabilitation: robot-aided sensorimotor stimulation. Neurology. 2000;54:1938–1944. doi: 10.1212/wnl.54.10.1938. [DOI] [PubMed] [Google Scholar]
- Volpe BT, Krebs HI, Hogan N, Edelsteinn L, Diels CM, Aisen ML. Robot training enhanced motor outcome in patients with stroke maintained over 3 years. Neurology. 1999;53:1874–1876. doi: 10.1212/wnl.53.8.1874. [DOI] [PubMed] [Google Scholar]
- Wade DT, Langton-Hewer R, Wood VA, Skilbeck CE, Ismail HM. The hemiplegic arm after stroke: measurement and recovery. J Neurol Neurosurg Psychiatry. 1983;46:521–524. doi: 10.1136/jnnp.46.6.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JM, Dromerick AW, Sahrmann SA, Lang CE. Upper extremity muscle activation during recovery of reaching in subjects with post-stroke hemiparesis. Clinical Neurophysiology. 2007a;118:164–176. doi: 10.1016/j.clinph.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner JM, Lang CE, Sahrmann SA, Edwards DF, Dromerick AW. Sensorimotor impairments and reaching performance in subjects with poststroke hemiparesis during the first few months of recovery. Phys Ther. 2007b;87:751–765. doi: 10.2522/ptj.20060135. [DOI] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003a;126:2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of outcome after stroke: a cross-sectional fMRI study. Brain. 2003b;126:1430–1448. doi: 10.1093/brain/awg145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, van der Helm FC, Veeger HE, Makhsous M, Van RP, Anglin C, Nagels J, Karduna AR, McQuade K, Wang X, Werner FW, Buchholz B. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion--Part II: shoulder, elbow, wrist and hand. Journal of Biomechanics. 2005;38:981–992. doi: 10.1016/j.jbiomech.2004.05.042. [DOI] [PubMed] [Google Scholar]
- Zackowski KM, Dromerick AW, Sahrmann SA, Thach WT, Bastian AJ. How do strength, sensation, spasticity and joint individuation relate to the reaching deficits of people with chronic hemiparesis? Brain. 2004;127:1035–1046. doi: 10.1093/brain/awh116. [DOI] [PubMed] [Google Scholar]