Abstract
We determined if serum cystatin-C, a novel measure of kidney function that also co-localizes with brain β-amyloid, is associated with cognitive function among 3030 Black and White elders (mean age, 74 years). Elders with high cystatin-C (N=445, 15%) had worse baseline cognitive scores on 3MS (p=0.01) or DSST (p=0.02) compared to those with intermediate (N=1008, 33%) or low level (N=1577, 52%) and more pronounced decline over 7 years (p=0.002 and p=0.04). Multivariate adjustment led to similar results. Incident cognitive impairment (decline of ≥1.0 SD) was greatest among those with high cystatin-C (3MS: 38% vs. 25%, adjusted OR=1.92; 95%CI 1.37-2.69; DSST: 38% vs 26%, OR= 1.54; 95% CI 1.10-2.15).
BACKGROUND
Chronic kidney disease (CKD) and cognitive impairment commonly occur in older adults, yet the relation between CKD and cognitive function remains poorly understood. Cystatin-C, an inhibitor of cysteine proteases, is an innovative measure of kidney function. Importantly, cystatin-C colocalizes with β-amyloid in brains of patients with Alzheimer disease (AD)1. In addition, several studies have reported an association between a common polymorphism of the cystatin-C gene and risk of AD2, 3. We determined if cystatin-C was associated with baseline and 7-year decline in cognitive function among community-welling elders.
METHODS
Participants were part of the Health ABC study, a prospective study that enrolled 3075 community-dwelling older adults aged 70-79 years living in Memphis, TN or Pittsburgh, PA between 1997-984. To identify potential participants, a random sample of white and all black Medicare-eligible elders within designated zip code areas were contacted. To be eligible, participants had to report no difficulties performing activities of daily living, walking a quarter of a mile, or climbing 10 steps without resting. All participants signed an informed written consent, approved by the institutional review boards at the clinical sites. This study was approved by the institutional review boards of the University of Pittsburgh and University of Tennessee, Memphis and that of the coordinating center, the University of California San Francisco.
After excluding 31 elders missing cystatin-C and 14 missing baseline cognitive scores, we studied 3030 participants.
The Modified Mini-Mental State Examination (3MS) was administered during baseline (Year 1) and at the Year 3, 5 and 8 visits. It is a brief, cognitive test with components for orientation, concentration, language, praxis, and immediate and delayed memory5. The Digit Symbol Substitution Test (DSST) measures attention, psychomotor speed, and executive function 6 and was measured at Years 1, 5, and 8. We defined incident cognitive impairment as a score decline of ≥1.0 SD any time over follow-up.
Baseline cystatin-C level was measured from sera at the Health ABC core laboratory (University of Vermont, Burlington, VT) using a BNII nephelometer (Dade Behring Inc., Deerfield, IL) that used a particle-enhanced immunonepholometric assay (N Latex Cystatin C)7. The assay range is 0.195 to 7.330 mg/L. Among 61 healthy individuals with three cystatin C measurements over a 6-month period, the intra-individual coefficient of variation was 7.7%, reflecting long-term stability of the measurement8.
Potential covariates included sex, age, race, education, smoking and body mass index (BMI). Presence of diabetes mellitus and hypertension, and history of stroke or transient ischemic attack (TIA) were determined. Depressive symptoms were assessed with the Center for Epidemiologic Studies-Depression Scale (CES-D), with a score ≥ 16 consistent with possible depression9. Apolipoprotein E (APOE) genotype and serum level of high sensitivity C-reactive protein (CRP) were analyzed using standard techniques. We determined the estimated glomerular filtration rate (eGFR) for each participant using the MDRD equation10.
We categorized serum cystatin-C using established cut-points of ≤1.0; 1.0-1.25 and >1.25 mg/L11, 12. The associations among cystatin-C level and baseline and change in cognitive function over seven years by the 3MS (four measurements) and DSST (three measurements) were analyzed using mixed effects repeated measures models. Covariates in adjusted models included age, sex, education, race, baseline cognitive score and other covariates significantly associated (p<0.10) with cystatin-C and cognitive score. Next, we compared the incidence of cognitive impairment across cystatin-C group using logistic regression models. We repeated these models stratifying by those with eGFR <60 and ≥ 60 to determine if the association between cystatin-C and risk of cognitive impairment differed among those with and without the accepted criteria for CKD13. All analyses were conducted using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
Of the 3,030 participants, 445 (15%) had high, 1008 (33%) had intermediate and 1577 (52%) had low cystatin-C with a median level of 1.0 mg/L. Participants with higher cystatin-C level were more likely to be older, white, male, have a higher BMI and CRP and more likely to have hypertension, diabetes, or stroke (Table 1).
Table 1.
Baseline Characteristics of the Health ABC Participants by Cystatin-C group.
| Characteristic (Mean (sd) or %) | Cystatin-C (mg/L)
|
|||
|---|---|---|---|---|
| 0-1.00 (n=1577) | 1.01-1.25 (n=1008) | >1.25 (n=445) | P-value | |
| Age (years) | 73.2 (2.8) | 74.0 (2.9) | 74.3 (2.9) | <0.001 |
| White (%) | 54 | 64 | 63 | <0.001 |
| Male (%) | 41 | 56 | 56 | <0.001 |
| Education < high school (%) | 27 | 22 | 25 | 0.017 |
| Current smoker (%) | 9 | 11 | 12 | 0.177 |
| Depression score ≥16 (%) | 4 | 6 | 5 | 0.229 |
| Body mass index (kg/m2) | 26.9 (4.7) | 27.8 (4.7) | 28.2 (5.1) | <0.001 |
| C-reactive protein (ug/ml) | 2.6 (3.9) | 3.0 (4.5) | 4.4 (7.2) | <0.001 |
| Hypertension (%) | 59 | 64 | 76 | <0.001 |
| Diabetes (%) | 21 | 25 | 32 | <0.001 |
| Stroke (%) | 6 | 9 | 13 | <0.001 |
| APOE ε4 carrier (%) | 30 | 28 | 27 | 0.517 |
Elders with higher cystatin-C lhad worse baseline 3MS score (91.4 ± 8.3 vs 92.4 ± 8.4 and 92.4 ± 7.6 for high, intermediate and low cystatin-C, p=0.01) and DSST score (33.6 ± 14.4 vs 35.6 ± 15.0 and 35.4 ± 14.6, p=0.02). Adjustment for age, race, sex, education, baseline cognitive score, BMI, CRP level, hypertension, diabetes, and stroke led to similar results for 3MS but reduced the statistical significance for DSST (p=0.16). Over the 7 years of follow-up, those with high cystatin-C level had greater decline on 3MS (2.7 points vs 1.0 and 0.9 points for the intermediate and low groups, p=0.002) and on DSST (5.5 points vs 4.7 and 3.8 points, p=0.04). Multivariate adjustment led to similar results (3MS, p=0.001 and DSST, p=0.04) (Figure 1). The rate of loss to follow-up for repeated cognitive testing was 10%; those without follow-up were older, less educated, more likely to be male, had more comorbidities and had slightly higher cystatin-C (p<0.001). The incidence of cognitive impairment on the 3MS was greatest among those with high cystatin-C (38%, OR=1.90; 95%CI 1.42-2.56), but similar among those with intermediate (27%; OR=1.13; 95%CI 0.91-1.42) and low cystatin-C (25%; referent) (Table 2). Similarly, the incidence of cognitive impairment on DSST was greatest for high cystatin-C (38%, OR=1.71; 95% CI 1.25-2.32) and somewhat higher for intermediate level(31%, OR= 1.25; 95% CI 1.00-1.55) compared to low level (26%). Multivariate adjustment led to similar results.
Figure 1.
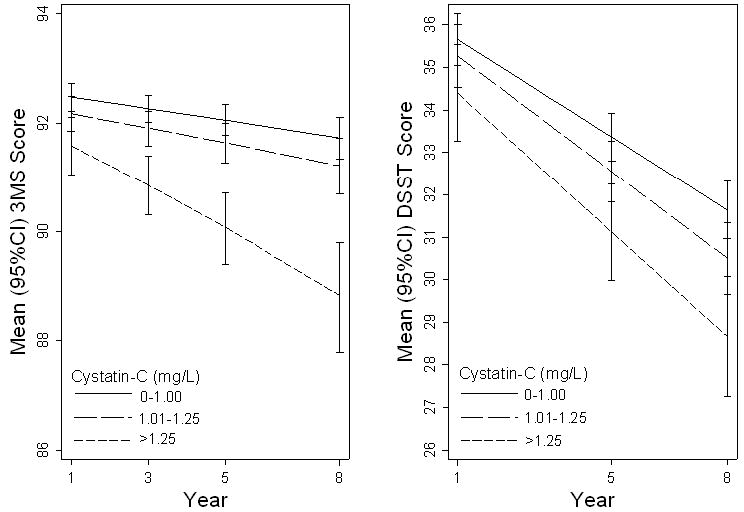
Mixed model predicted 3MS and DSST score as a function of cystatin-C level, adjusted for age, race, gender, education, baseline cognitive score, body mass index, C-reactive protein, hypertension, diabetes, and stroke. Error bars indicate 95% confidence intervals.
Table 2.
The likelihood of developing cognitive impairment over 7 years of follow-up by cystatin-C level.
| Cystatin-C (mg/L) | % Cognitive Impairment | Odds Ratio (95% Confidence Interval)
|
|
|---|---|---|---|
| Unadjusted | Adjusted* | ||
| 3MS | |||
| 0-1.00 | 25 | 1.00 (ref) | 1.00 (ref) |
| 1.01-1.25 | 27 | 1.13 (0.91-1.42) | 1.25 (0.98-1.61) |
| >1.25 | 38 | 1.90 (1.42-2.56) | 1.92 (1.37-2.69) |
| Digit Symbol | |||
| 0-1.00 | 26 | 1.00 (ref) | 1.00 (ref) |
| 1.01-1.25 | 31 | 1.25 (1.00-1.55) | 1.18 (0.93-1.50) |
| >1.25 | 38 | 1.71 (1.25-2.32) | 1.54 (1.10-2.15) |
Adjusted for age, race, sex, education, body mass index, C-reactive protein, hypertension, diabetes, and stroke.
In order to determine if the association between cystatin-C and cognitive decline was modified by sex, race or APOE e4 status, we added interaction terms to the random effects models but none were statistically significant (all p>0.1). We determined if the association between cystatin-C and incident impairment was evident only among elders with worse kidney function by stratifying the cohort based on eGFR< 60 or ≥60. Participants with high cystatin-C and with eGFR <60 had a roughly two-fold increase in likelihood of developing cognitive impairment compared to those with low level (3MS, 39% vs 22% OR= 2.27; 95% CI 1.22-4.23 and DSST, 36% vs 24% OR=1.78; 95% CI 0.94- 3.39). Interestingly, for those with high cystatin-C and with eGFR ≥ 60, the likelihood of developing cognitive impairment was also increased about 2-fold (3MS, 36% vs 25% OR=1.72; 95% CI 1.04-2.84 and DSST, 42% vs 27% OR=2.01; 95% CI 1.23- 3.29).
DISCUSSION
We determined that among community-resident elders, those with elevated cystatin-C had lower scores on cognitive tests and were more likely to experience decline in cognitive function over 7 years, independent of demographics and comorbidites. In addition, participants with elevated cystatin-C were approximately twice as likely to develop cognitive impairment.
The association between cystatin-C and cognition underscores the under-appreciated connection between CKD and cognitive function. Among participants in the Cardiovascular Health Cognition Study, those with CKD had an elevated risk of developing dementia14. Similarly, we found that elders with CKD from a clinic sample had poorer performance on executive function and verbal memory compared with published norms15. In another study of postmenopausal women, eGFR was associated with performance on tests of global cognitive function, executive function, language, and memory16. We previously reported in Health ABC, that CKD was associated with an increased risk of cognitive impairment 17.
Relative to serum creatinine or creatinine-based equations, cystatin-C concentrations may represent a more sensitive marker of CKD, particularly in the elderly. Cystatin-C was recently highly correlated with serial measurements of GFR by iothalamate clearance18. This likely reflects the fact that a low serum creatinine level can simultaneously reflect both good kidney function and also decreased muscle mass (often seen the elderly, especially with malnutrition or chronic illness).
Possible mechanisms underlying an association between CKD and cognitive impairment include increases in cardiovascular disease and inflammation. Cystatin-C has been found to correlate highly with subclinical brain infarcts in elders19. While we adjusted for cardiovascular disease risk factors (diabetes, hypertension, BMI and stroke) and the association between cystatin-C level and cognition remained, we did not have brain MRI measurements that might have depicted subclinical ischemic brain disease. In addition, despite adjustment for CRP level, the association of cystatin-C level and cognitive decline remained strong and independent.
It is also possible that there is an association between cystatin-C and cognition that is independent of kidney function. Cystatin-C is produced by nearly all human cells and cystatin-C colocalizes with β-amyloid in brains of patients with AD1 primarily in the hippocampus and entorhinal cortex20. In addition, several studies have reported an association between a common polymorphism of the cystatin-C gene and risk of AD suggesting that cystatin-C may have a direct effect on AD risk2. Supporting this possibility, when we stratified by conventional eGFR cutoffs used to classify CKD, elders with high cystatin-C had higher risks of cognitive impairment whether or not they had CKD. Alternatively, these results could reflect less misclassification of CKD by cystatin-C relative to eGFR, particularly among those with modest reductions in kidney function.
There are several important limitations to this study. We could not test all cognitive domains and are limited in the interpretation of our findings due to the lack of etiology of cognitive impairment. Participants in our cohort did not undergo a clinical evaluation or brain MRI to determine whether the impairment was due to vascular disease, AD or some other etiology. Some participants did not have follow-up cognitive testing and higher attrition rates among those with high cystatin C may have led to an underestimation of the association between cystatin-C and cognitive decline.
Our findings suggest that high serum cystatin-C is associated with impaired cognition in elders. Further studies should determine if this may be a useful biomarker for cognitive function and to determine the mechanisms underlying this association.
Acknowledgments
Funding/Support: AG-6-2101, AG-6-2103, AG-6-2106, and AG021918. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging. Dr. Yaffe was supported in part by DK069406 and an anonymous foundation.
Role of the Sponsor: In their role as coauthors, representatives of the NIH participated in the design and conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review, and approval of the manuscript.
References
- 1.Sastre M, Calero M, Pawlik M, et al. Binding of cystatin C to Alzheimer’s amyloid beta inhibits in vitro amyloid fibril formation. Neurobiol Aging. 2004;25:1033–1043. doi: 10.1016/j.neurobiolaging.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Finckh U, von der Kammer H, Velden J, et al. Genetic association of a cystatin C gene polymorphism with late-onset Alzheimer disease. Arch Neurol. 2000;57:1579–1583. doi: 10.1001/archneur.57.11.1579. [DOI] [PubMed] [Google Scholar]
- 3.Chuo LJ, Sheu WH, Pai MC, Kuo YM. Genotype and plasma concentration of cystatin C in patients with late-onset Alzheimer disease. Dement Geriatr Cogn Disord. 2007;23:251–257. doi: 10.1159/000100021. [DOI] [PubMed] [Google Scholar]
- 4.Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56:M644–649. doi: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- 5.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 6.Wechsler D. Wechsler Adult Intelligence Scale-Third Edition (WAIS-III) San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 7.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59:1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 8.Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 9.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977:385–401. [Google Scholar]
- 10.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 11.McManus D, Shlipak M, Ix JH, et al. Association of cystatin C with poor exercise capacity and heart rate recovery: data from the heart and soul study. Am J Kidney Dis. 2007;49:365–372. doi: 10.1053/j.ajkd.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarnak MJ, Katz R, Stehman-Breen CO, et al. Cystatin C concentration as a risk factor for heart failure in older adults. Ann Intern Med. 2005;142:497–505. doi: 10.7326/0003-4819-142-7-200504050-00008. [DOI] [PubMed] [Google Scholar]
- 13.Levey AS, Greene T, Sarnak MJ, et al. Effect of dietary protein restriction on the progression of kidney disease: long-term follow-up of the Modification of Diet in Renal Disease (MDRD) Study. Am J Kidney Dis. 2006;48:879–888. doi: 10.1053/j.ajkd.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 14.Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate renal impairment and risk of dementia among older adults: the Cardiovascular Health Cognition Study. J Am Soc Nephrol. 2004;15:1904–1911. doi: 10.1097/01.asn.0000131529.60019.fa. [DOI] [PubMed] [Google Scholar]
- 15.Kurella M, Chertow GM, Luan J, Yaffe K. Cognitive impairment in chronic kidney disease. J Am Geriatr Soc. 2004;52:1863–1869. doi: 10.1111/j.1532-5415.2004.52508.x. [DOI] [PubMed] [Google Scholar]
- 16.Kurella M, Yaffe K, Shlipak MG, et al. Chronic kidney disease and cognitive impairment in menopausal women. Am J Kidney Dis. 2005;45:66–76. doi: 10.1053/j.ajkd.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 17.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2005;16:2127–2133. doi: 10.1681/ASN.2005010005. [DOI] [PubMed] [Google Scholar]
- 18.Perkins BA, Nelson RG, Ostrander BE, et al. Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol. 2005;16:1404–1412. doi: 10.1681/ASN.2004100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seliger SL, Longstreth WT, Jr, Katz R, et al. Cystatin C and subclinical brain infarction. J Am Soc Nephrol. 2005;16:3721–3727. doi: 10.1681/ASN.2005010006. [DOI] [PubMed] [Google Scholar]
- 20.Deng A, Irizarry MC, Nitsch RM, et al. Elevation of cystatin C in susceptible neurons in Alzheimer’s disease. Am J Pathol. 2001;159:1061–1068. doi: 10.1016/S0002-9440(10)61781-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


