Abstract
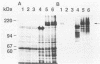
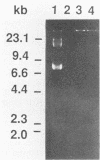
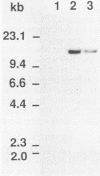
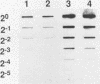
The pac gene of the serotype c strain Streptococcus mutans MT8148 encodes a cell surface protein antigen (PAc) of approximate 190 kilodaltons. The serotype c strain S. mutans GS-5 does not produce the 190-kilodalton PAc but produces a lower-molecular-weight protein that reacts with anti-PAc serum. The SphI-BamHI fragment of the pac gene was ligated with the S. mutans-Escherichia coli shuttle vector pSA3. The chimeric shuttle vector was transformed into strain GS-5, and two transformants (TK15 and TK18) were isolated. These transformants produced a large amount of cell-free and cell-bound PAc of 190 kilodaltons. No plasmid was isolated from these transformants, and the EcoRI fragments of their chromosomal DNA hybridized with the erythromycin resistance gene in the shuttle vector DNA, indicating insertion of the chimeric shuttle vector DNA into the chromosomal DNA. The cell hydrophobicity of strains TK15 and TK18 as well as PAc-defective mutants constructed by inserting an erythromycin resistance gene into the pac gene of strain MT8148 was analyzed. Strains MT8148, TK15, and TK18 were hydrophobic. On the other hand, strain GS-5 and PAc-defective MT8148 transformants were hydrophilic. Resting cells of the hydrophobic strains attached in larger numbers to saliva-coated hydroxyapatite than did the hydrophilic strains. Human whole saliva induced the aggregation of cells of the hydrophobic strains but not that of cells of the hydrophilic strains. These results suggest that cell surface PAc of S. mutans serotype c participates in attachment of the streptococcal cell to experimental pellicles.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermans F., Klein J. P., Cormont F., Bazin H., Ogier J. A., Frank R. M., Vreven J. Antibody specificity and antigen characterization of rat monoclonal antibodies against Streptococcus mutans cell wall-associated protein antigens. Infect Immun. 1985 Aug;49(2):344–350. doi: 10.1128/iai.49.2.344-350.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermans F., Klein J. P., Ogier J., Bazin H., Cormont F., Frank R. M. Purification and characterization of a saliva-interacting cell-wall protein from Streptococcus mutans serotype f by using monoclonal-antibody immunoaffinity chromatography. Biochem J. 1985 May 15;228(1):211–217. doi: 10.1042/bj2280211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayakawa G. Y., Bleiweis A. S., Crowley P. J., Cunningham M. W. Heart cross-reactive antigens of mutans streptococci share epitopes with group A streptococci and myosin. J Immunol. 1988 Jan 1;140(1):253–257. [PubMed] [Google Scholar]
- Bergmeier L. A., Lehner T. Lack of antibodies to human heart tissue in sera of rhesus monkeys immunized with Streptococcus mutans antigens and comparative study with rabbit antisera. Infect Immun. 1983 Jun;40(3):1075–1082. doi: 10.1128/iai.40.3.1075-1082.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao M. L., Ferretti J. J. Streptococcus-Escherichia coli shuttle vector pSA3 and its use in the cloning of streptococcal genes. Appl Environ Microbiol. 1985 Jan;49(1):115–119. doi: 10.1128/aem.49.1.115-119.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eifert R., Rosan B., Golub E. Optimization of an hydroxyapatite adhesion assay for Streptococcus sanguis. Infect Immun. 1984 May;44(2):287–291. doi: 10.1128/iai.44.2.287-291.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forester H., Hunter N., Knox K. W. Characteristics of a high molecular weight extracellular protein of Streptococcus mutans. J Gen Microbiol. 1983 Sep;129(9):2779–2788. doi: 10.1099/00221287-129-9-2779. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Adherent interactions which may affect microbial ecology in the mouth. J Dent Res. 1984 Mar;63(3):378–385. doi: 10.1177/00220345840630030401. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Cohen L., Hay D. I. Strains of Streptococcus mutans and Streptococcus sobrinus attach to different pellicle receptors. Infect Immun. 1986 May;52(2):555–561. doi: 10.1128/iai.52.2.555-561.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada S., Furuta T., Okahashi N., Nisizawa T., Yamamoto T., Chiba J. Characterization of a monoclonal antibody specific for lipoteichoic acid from various gram-positive bacteria. Microbiol Immunol. 1984;28(9):1009–1021. doi: 10.1111/j.1348-0421.1984.tb00757.x. [DOI] [PubMed] [Google Scholar]
- Hamada S., Koga T., Okahashi N. Characterization of a mutant of serotype g Streptococcus mutans strain 6715 lacking dextran-induced agglutination. Zentralbl Bakteriol Mikrobiol Hyg A. 1983 May;254(3):343–351. [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt R. G., Abiko Y., Saito S., Smorawinska M., Hansen J. B., Curtiss R., 3rd Streptococcus mutans genes that code for extracellular proteins in Escherichia coli K-12. Infect Immun. 1982 Oct;38(1):147–156. doi: 10.1128/iai.38.1.147-156.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M., Machardy S. M., Sheppard A. J., Woods N. C. Evidence for an immunological relationship between Streptococcus mutans and human cardiac tissue. Infect Immun. 1980 Feb;27(2):576–588. doi: 10.1128/iai.27.2.576-588.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga T., Asakawa H., Okahashi N., Hamada S. Sucrose-dependent cell adherence and cariogenicity of serotype c Streptococcus mutans. J Gen Microbiol. 1986 Oct;132(10):2873–2883. doi: 10.1099/00221287-132-10-2873. [DOI] [PubMed] [Google Scholar]
- Koga T., Toda Y., Moro I., Hamada S. Electron-microscopic observation of adherence of serotype c Streptococcus mutans to the enamel surface due to glucan synthesis. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Nov;269(4):492–500. doi: 10.1016/s0176-6724(88)80071-3. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leblanc D. J., Lee L. N. Rapid screening procedure for detection of plasmids in streptococci. J Bacteriol. 1979 Dec;140(3):1112–1115. doi: 10.1128/jb.140.3.1112-1115.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. F., Progulske-Fox A., Bleiweis A. S. Molecular cloning and expression of a Streptococcus mutans major surface protein antigen, P1 (I/II), in Escherichia coli. Infect Immun. 1988 Aug;56(8):2114–2119. doi: 10.1128/iai.56.8.2114-2119.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner T., Caldwell J., Smith R. Local passive immunization by monoclonal antibodies against streptococcal antigen I/II in the prevention of dental caries. Infect Immun. 1985 Dec;50(3):796–799. doi: 10.1128/iai.50.3.796-799.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner T., Russell M. W., Caldwell J., Smith R. Immunization with purified protein antigens from Streptococcus mutans against dental caries in rhesus monkeys. Infect Immun. 1981 Nov;34(2):407–415. doi: 10.1128/iai.34.2.407-415.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindler L. E., Macrina F. L. Characterization of genetic transformation in Streptococcus mutans by using a novel high-efficiency plasmid marker rescue system. J Bacteriol. 1986 May;166(2):658–665. doi: 10.1128/jb.166.2.658-665.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J. K., Smith R., Lehner T. Use of monoclonal antibodies in local passive immunization to prevent colonization of human teeth by Streptococcus mutans. Infect Immun. 1987 May;55(5):1274–1278. doi: 10.1128/iai.55.5.1274-1278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride B. C., Song M., Krasse B., Olsson J. Biochemical and immunological differences between hydrophobic and hydrophilic strains of Streptococcus mutans. Infect Immun. 1984 Apr;44(1):68–75. doi: 10.1128/iai.44.1.68-75.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro I., Russell M. W. Ultrastructural localization of protein antigens I/II and III in Streptococcus mutans. Infect Immun. 1983 Jul;41(1):410–413. doi: 10.1128/iai.41.1.410-413.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris E. J., McBride B. C. Aggregation of Streptococcus sanguis by a neuraminidase-sensitive component of serum and crevicular fluid. Infect Immun. 1983 Dec;42(3):1073–1080. doi: 10.1128/iai.42.3.1073-1080.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H., Kato H., Okahashi N., Takahashi I., Hamada S., Koga T. Characterization of a cell-surface protein antigen of hydrophilic Streptococcus mutans strain GS-5. J Gen Microbiol. 1989 Apr;135(4):981–988. doi: 10.1099/00221287-135-4-981. [DOI] [PubMed] [Google Scholar]
- Okahashi N., Koga T., Hamada S. Purification and immunochemical properties of a protein antigen from serotype g Streptococcus mutans. Microbiol Immunol. 1986;30(1):35–47. doi: 10.1111/j.1348-0421.1986.tb00919.x. [DOI] [PubMed] [Google Scholar]
- Okahashi N., Sasakawa C., Yoshikawa M., Hamada S., Koga T. Cloning of a surface protein antigen gene from serotype c Streptococcus mutans. Mol Microbiol. 1989 Feb;3(2):221–228. doi: 10.1111/j.1365-2958.1989.tb01811.x. [DOI] [PubMed] [Google Scholar]
- Okahashi N., Sasakawa C., Yoshikawa M., Hamada S., Koga T. Molecular characterization of a surface protein antigen gene from serotype c Streptococcus mutans, implicated in dental caries. Mol Microbiol. 1989 May;3(5):673–678. doi: 10.1111/j.1365-2958.1989.tb00215.x. [DOI] [PubMed] [Google Scholar]
- Peros W. J., Gibbons R. J. Evidence suggesting multiple binding sites in experimental pellicles for Streptococcus mutans JBP. J Dent Res. 1986 Nov;65(11):1332–1334. doi: 10.1177/00220345860650111001. [DOI] [PubMed] [Google Scholar]
- Rosan B., Malamud D., Appelbaum B., Golub E. Characteristic differences between saliva-dependent aggregation and adhesion of streptococci. Infect Immun. 1982 Jan;35(1):86–90. doi: 10.1128/iai.35.1.86-90.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W. Analysis of heart-reactive antibodies induced in rabbits by immunization with Streptococcus mutans. J Oral Pathol. 1987 May;16(5):234–240. doi: 10.1111/j.1600-0714.1987.tb01486.x. [DOI] [PubMed] [Google Scholar]
- Russell M. W., Bergmeier L. A., Zanders E. D., Lehner T. Protein antigens of Streptococcus mutans: purification and properties of a double antigen and its protease-resistant component. Infect Immun. 1980 May;28(2):486–493. doi: 10.1128/iai.28.2.486-493.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. W., Mansson-Rahemtulla B. Interaction between surface protein antigens of Streptococcus mutans and human salivary components. Oral Microbiol Immunol. 1989 Jun;4(2):106–111. doi: 10.1111/j.1399-302x.1989.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Russell R. R., Beighton D., Cohen B. Immunisation of monkeys (Macaca fascicularis) with antigens purified from Streptococcus mutans. Br Dent J. 1982 Feb 2;152(3):81–84. doi: 10.1038/sj.bdj.4804751. [DOI] [PubMed] [Google Scholar]
- Sasakawa C., Yoshikawa M. Transposon (Tn5)-mediated suppressive integration of ColE1 derivatives into the chromosome of Escherichia coli K12 (dnaA). Biochem Biophys Res Commun. 1980 Oct 16;96(3):1364–1370. doi: 10.1016/0006-291x(80)90101-1. [DOI] [PubMed] [Google Scholar]
- Sommer P., Bruyère T., Ogier J. A., Garnier J. M., Jeltsch J. M., Klein J. P. Cloning of the saliva-interacting protein gene from Streptococcus mutans. J Bacteriol. 1987 Nov;169(11):5167–5173. doi: 10.1128/jb.169.11.5167-5173.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanberg M., Westergren G., Olsson J. Oral implantation in humans of Streptococcus mutans strains with different degrees of hydrophobicity. Infect Immun. 1984 Mar;43(3):817–821. doi: 10.1128/iai.43.3.817-821.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I., Okahashi N., Sasakawa C., Yoshikawa M., Hamada S., Koga T. Homology between surface protein antigen genes of Streptococcus sobrinus and Streptococcus mutans. FEBS Lett. 1989 Jun 5;249(2):383–388. doi: 10.1016/0014-5793(89)80664-7. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Westergren G., Olsson J. Hydrophobicity and adherence of oral streptococci after repeated subculture in vitro. Infect Immun. 1983 Apr;40(1):432–435. doi: 10.1128/iai.40.1.432-435.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]