Abstract
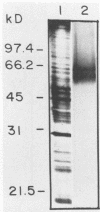
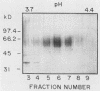
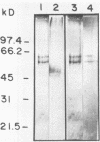
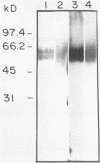
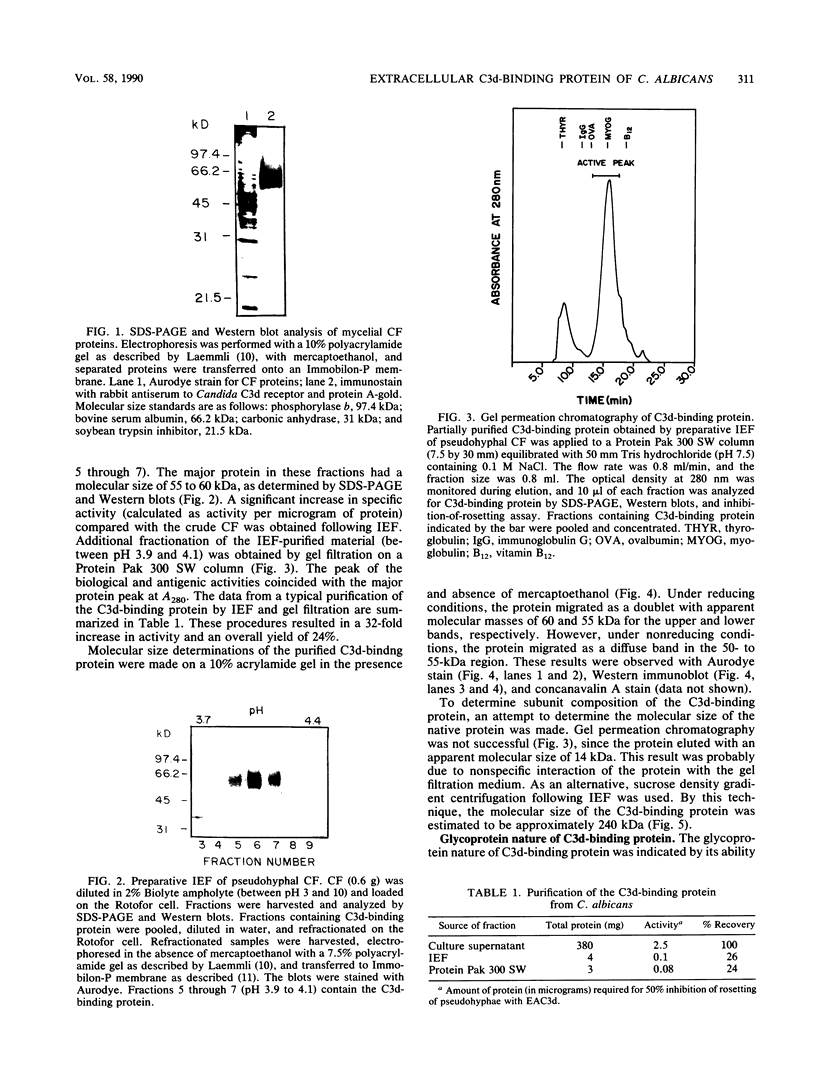
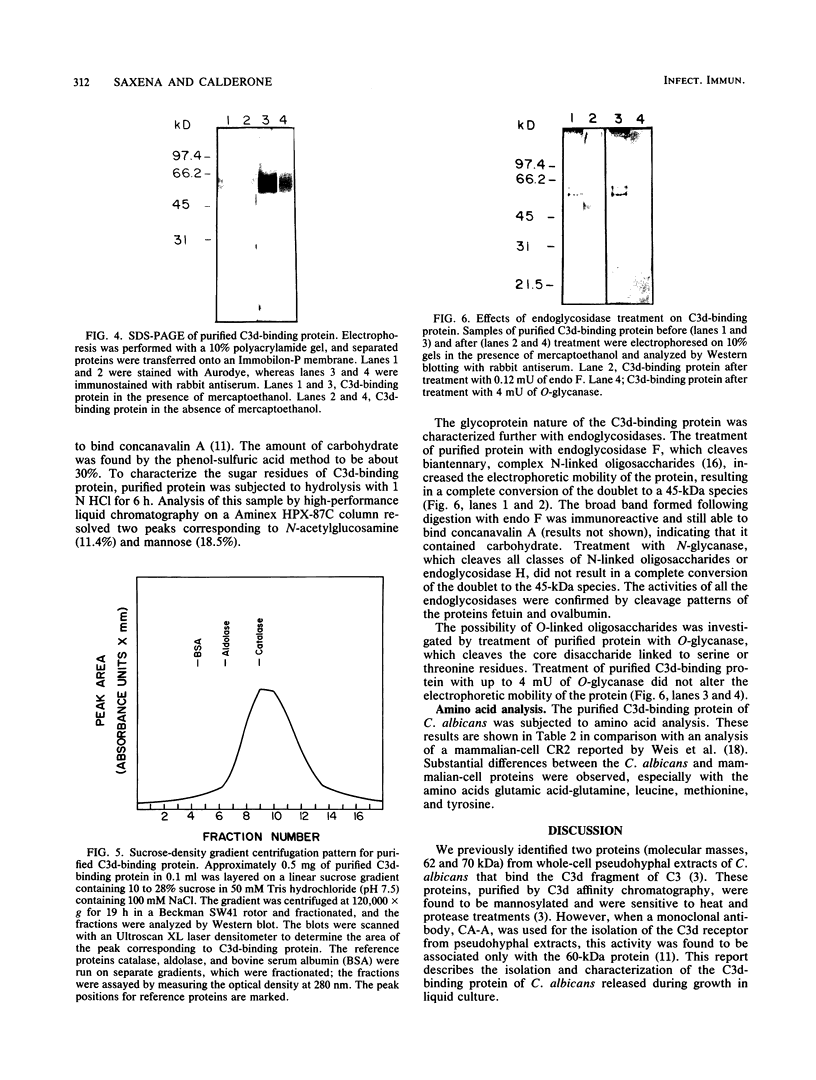
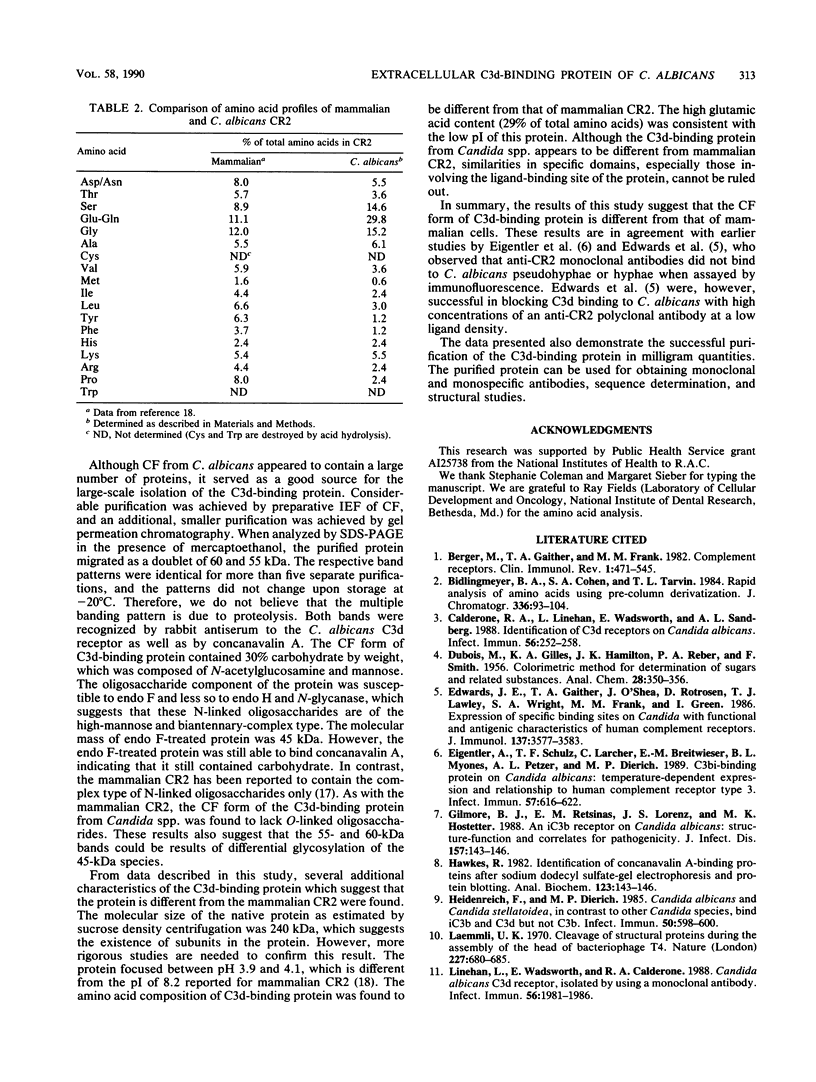
A C3d-binding glycoprotein was purified from the culture filtrate of Candida albicans by preparative isoelectric focusing. The protein possessed a pI of 3.9 to 4.1 and could inhibit rosetting of EAC3d (sheep erythrocytes conjugated to C3d) by pseudohyphae of C. albicans. When analyzed by polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate and mercaptoethanol, the protein migrated as a doublet with apparent molecular masses of 55 and 60 kilodaltons (kDa) and as a 50-kDa band in nonreducing gels. These results were observed with Aurodye stain for proteins. Western immunoblot, and concanavalin A stain, which indicates that both bands contain carbohydrate as well as antigenic determinants. The treatment of purified glycoprotein with endoglycosidase F but not endoglycosidases H, N, and O resulted in a complete conversion of the doublet into a faster-migrating broad band with an apparent molecular mass of 45 kDa. When the amino acid analysis of the C3d-binding protein was compared with that of the CR2 from B lymphocytes, significant differences were observed. These data indicate that C. albicans secretes a C3d-binding protein during growth in vitro which appears to be different from the mammalian C3d receptor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger M., Gaither I. A., Frank M. M. Complement receptors. Clin Immunol Rev. 1981;1(4):471–545. [PubMed] [Google Scholar]
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Calderone R. A., Linehan L., Wadsworth E., Sandberg A. L. Identification of C3d receptors on Candida albicans. Infect Immun. 1988 Jan;56(1):252–258. doi: 10.1128/iai.56.1.252-258.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. E., Jr, Gaither T. A., O'Shea J. J., Rotrosen D., Lawley T. J., Wright S. A., Frank M. M., Green I. Expression of specific binding sites on Candida with functional and antigenic characteristics of human complement receptors. J Immunol. 1986 Dec 1;137(11):3577–3583. [PubMed] [Google Scholar]
- Eigentler A., Schulz T. F., Larcher C., Breitwieser E. M., Myones B. L., Petzer A. L., Dierich M. P. C3bi-binding protein on Candida albicans: temperature-dependent expression and relationship to human complement receptor type 3. Infect Immun. 1989 Feb;57(2):616–622. doi: 10.1128/iai.57.2.616-622.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes R. Identification of concanavalin A-binding proteins after sodium dodecyl sulfate--gel electrophoresis and protein blotting. Anal Biochem. 1982 Jun;123(1):143–146. doi: 10.1016/0003-2697(82)90634-0. [DOI] [PubMed] [Google Scholar]
- Heidenreich F., Dierich M. P. Candida albicans and Candida stellatoidea, in contrast to other Candida species, bind iC3b and C3d but not C3b. Infect Immun. 1985 Nov;50(2):598–600. doi: 10.1128/iai.50.2.598-600.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linehan L., Wadsworth E., Calderone R. Candida albicans C3d receptor, isolated by using a monoclonal antibody. Infect Immun. 1988 Aug;56(8):1981–1986. doi: 10.1128/iai.56.8.1981-1986.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Manning M., Mitchell T. G. Strain variation and morphogenesis of yeast- and mycelial-phase Candida albicans in low-sulfate, synthetic medium. J Bacteriol. 1980 May;142(2):714–719. doi: 10.1128/jb.142.2.714-719.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson M. A., Belshe R. B., Orvell C., Norrby E. Respiratory syncytial virus epidemics: variable dominance of subgroups A and B strains among children, 1981-1986. J Infect Dis. 1988 Jan;157(1):143–148. doi: 10.1093/infdis/157.1.143. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. The release of intact oligosaccharides from specific glycoproteins by endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1974 Feb 10;249(3):818–824. [PubMed] [Google Scholar]
- Weis J. J., Fearon D. T. The identification of N-linked oligosaccharides on the human CR2/Epstein-Barr virus receptor and their function in receptor metabolism, plasma membrane expression, and ligand binding. J Biol Chem. 1985 Nov 5;260(25):13824–13830. [PubMed] [Google Scholar]
- Weis J. J., Richards S. A., Smith J. A., Fearon D. T. Purification of the B lymphocyte receptor for the C3d fragment of complement and the Epstein-Barr virus by monoclonal antibody affinity chromatography, and assessment of its functional capacities. J Immunol Methods. 1986 Aug 21;92(1):79–87. doi: 10.1016/0022-1759(86)90506-5. [DOI] [PubMed] [Google Scholar]