Abstract
Treatment of autoimmune diseases remains a challenge for immunological research. An ideal therapy should inhibit the immune reaction against the diseased organ and leave the rest of the immune response intact. Our previous studies showed that donor-derived dendritic cells (DCs) treated in vitro with mitomycin C (MMC) suppress rat heart allograft rejection if injected into recipients before transplantation. Here we analyze their efficacy in controlling autoimmunity. MMC-DCs loaded with myelin-basic-protein (MBP) inhibited specific T cells derived from multiple sclerosis patients in vitro. If coincubated with MMC-DCs, T cells were arrested in the G0/G1 cell cycle phase. Microarray gene scan showed that MMC influences the expression of 116 genes in DCs, one main cluster comprising apoptotic and the second cluster immunosuppressive genes. Apparently, the combination of apoptosis with expression of tolerogenic molecules renders MMC-DCs suppressive. MBP-loaded MMC-DCs also inhibited mouse T cells in vitro and, in contrast to MBP-loaded naïve DCs, did not induce experimental autoimmune encephalitis. Most importantly, mice vaccinated with inhibitory DCs became resistant to the disease. Whereas this is not the first report on generation of suppressive DCs, it delineates a method using a clinically approved drug at nontoxic concentrations, which yields irreversibly changed DCs, effective across species in vitro and in vivo.
Keywords: immunological tolerance, multiple sclerosis, immunosuppressive therapy
The role of autoantigens and autoreactive lymphocytes in the initiation and maintenance of autoimmune diseases has been controversially discussed (1). Even if self-antigens were not the primordial cause of some autoimmune conditions, they offer a way of directing an immunosuppressive effect to the diseased organ, and therefore constitute a promising alternative to the commonly used, broadly reactive immunosuppressants. The “holy grail” for autoimmunity is not the disease-causing antigen, but the disease-curing antigen (2). In a clinical trial, an immunomodulatory peptide derived from hsp60—one of the known target self-antigens—was injected into patients with newly diagnosed type 1 diabetes. Although the study was small, it suggested that the peptide treatment preserved endogenous insulin production (3). Tolerance induction was also attempted by oral administration of self-antigens. This has been tried with myelin in multiple sclerosis (MS), collagen in rheumatoid arthritis, and insulin in type I diabetes. Despite success with the prevention of diseases in animal models, clinical trials attempting to treat an ongoing disease in humans have thus far been unsuccessful (4). What has been learned from animal models and a few clinical studies is that autoantigens must be presented in a nonimmunogenic form, usually by altering their structure. A successful example for the latter strategy is treatment of MS patients with copaxone, a synthetic peptide that mimics the composition of the central nervous component, myelin basic protein (MBP) (4).
Dendritic cells (DCs) are commonly thought of as strongly stimulatory cells, but DCs can also suppress the immune response (5). Evidently, evolution has developed highly efficient mechanisms for protecting tissues and organs from self-destruction, one of them being mediated by DCs (5). What nature applies for protection from self-attack might be used by man for treatment of autoimmune diseases or graft rejection. Unfortunately, natural suppressive DCs are hard to define phenotypically, and therefore difficult to use for therapeutic applications. For example, it has been shown that immature DCs induce tolerance, whereas mature DCs activate immune responses (5). However, a growing body of recent evidence indicates that DC maturation per se is not a distinguishing feature of immunogenicity, as opposed to tolerogenicity (6). It is presently believed that the development of an immunogenic or tolerogenic response depends on the net effect of antigen dose, DC lineage and maturational status, DC stimulation by pathogen-derived products, and the cytokine milieu (5, 6). Given the hardly predictable suppressive function of natural DCs, a reasonable alternative for therapeutic purposes would be the in vitro generation of inhibitory DCs. Several methods have been described for designing suppressive DCs (7). A major risk of deliberately generated inhibitory DCs is their potential reversibility to a stimulatory status. Furthermore, their effectiveness may be limited to certain species. In previous studies we showed that treatment of DCs with mitomycin C (MMC) stably transforms stimulatory into inhibitory cells (8). A single injection of such donor DCs into the recipient before heart transplantation suppressed heart allograft rejection in rats (8).
The strategy for controlling autoimmune reactions envisaged herein is to load MMC-treated human or murine DCs with self-antigens and to use these “inhibitory bullets” for targeted suppression of specific self-reactive T cells in vitro and in vivo.
Results
Myelin-specific T cells have been proposed to play a role in the pathogenesis of MS (1, 4). Therefore, MBP represents a candidate antigen for specific immunotherapy in MS. In the following experiments we studied the capacity of MMC-DCs loaded with MBP to control the activity of specific T cells derived from MS patients in cell cultures, and we tested their action in a mouse experimental autoimmune encephalomyelitis (EAE) model.
MBP-Loaded MMC-DCs Inhibit Specific T Cells of MS Patients in Vitro.
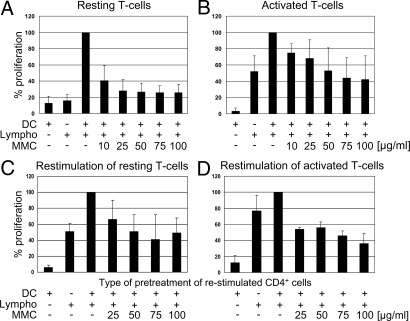
DCs derived from MS patients were loaded with MBP and co incubated with autologous T cells. In a parallel experiment, the DCs were loaded with MBP and treated with MMC. Whereas untreated DCs induced strong T cell stimulation, MMC-DCs did not (Fig. 1A).
Fig. 1.
Effect of MMC-treated DCs loaded with MBP on resting or activated T cells from MS patients. (A and B) Primary stimulation. DCs loaded with MBP and matured were treated with MMC in various concentrations and coincubated with resting (A) (n=8) or activated peripheral lymphocytes (clone ES-BP8T) (B) (n=7) at a ratio of ≤1:10. Mature DCs were CD14−, HLA-DR2+, CD802+, CD862+, CD83+ and constituted >80% of dendritic cells. Negative controls were MBP-loaded DCs or lymphocytes only, whereas positive controls were MBP-loaded MMC-untreated DCs coincubated with lymphocytes. Additional negative controls of experiment B (not shown) consisted of DCs only or DCs loaded with an irrelevant peptide, both coincubated with lymphocytes (none of them inducing T cell stimulation). (C and D) Restimulation. CD4+ cells were isolated from previous culture settings and restimulated with freshly prepared MBP-loaded DCs of the same donor. Legend to abscissa shows how CD4 cells were pretreated. Ordinate shows T cell proliferation. The first column represents DCs only (negative control). Cell proliferation was assessed by [3H]thymidine incorporation. Data represent mean ± SD and are expressed as percentage of positive control values (MBP-loaded DCs plus lymphocytes = 100%) (for all MMC-treated cells vs. untreated controls P < 0.05).
Therapy of patients with active MS should address already activated autoreactive T cells. To find out whether these lymphocytes can be controlled by inhibitory DCs, preactivated MBP-specific T cells derived from an MS patient were incubated with HLA-DR-matched, MBP-loaded MMC-DCs. The response of these T cells was significantly reduced (Fig. 1B). By contrast, MBP-loaded/MMC-untreated DCs strongly stimulated the T cells, whereas naïve DCs or DCs loaded with an irrelevant peptide did not result in stimulation (data not shown).
In the next experiment, we investigated whether suppressed T cells can be restimulated with naïve MBP-loaded DCs of the same donor. The results showed that, once suppressed by MMC-DCs, T cells cannot or can only weakly be reactivated (Fig. 1 C and D).
It is important to note that the suppressive capacity of DCs depends on MMC concentrations during pretreatment. Optimal T cell suppression is obtained at high doses.
Supernatants of Mitomycin-Treated DCs Do Not Inhibit the T Cells.
Upon treatment of DCs with MMC, a certain amount of substance might have diffused from the intracellular compartment into the culture medium and blocked T cell proliferation. To exclude that, supernatants of MMC-treated DCs were collected and used as medium in T cell proliferation assays. The results showed that T cell proliferation was not significantly affected [supporting information (SI) Fig. S1], indicating that leakage of MMC from treated cells is not the reason for suppression.
T Cells Are Blocked in the G0/G1 Phase.
When analyzing the mechanism of suppression, two aspects must be considered: the reaction of T cells to inhibitory DCs and the molecular changes of DCs induced by MMC-treatment.
As shown in the previous experiment, restimulation of suppressed T cells was not or only partially possible, indicating that the cells either became areactive or died. Cell cycle analysis revealed a significant accumulation in the G0/G1 phase of T cells coincubated with MMC-DCs (Fig. S2). This finding argues for induction of T cell areactivity.
Mitomycin C Does Not Inhibit the Expression of MHC-II and CD80/86 on DCs.
MMC might have changed the expression of MHC II or CD80/86. FACS analysis showed that MHC II and CD80/86 were not down-regulated upon incubation with MMC (mean channel of MMC-treated/untreated DCs: MHC II = 757/745; CD80 = 759/728; CD86 = 751/744); therefore, reduced antigen presentation by lower MHC II density, or less costimulation by lower CD80/CD86 expression, cannot serve as an explanation for the inhibited T cell proliferation.
Mitomycin C Modulates the Expression of Apoptotic and Immunoregulatory Genes of DCs.
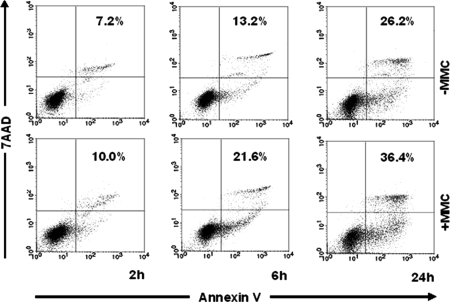
A comprehensive gene scan of 47,000 transcripts and additional variants was carried out by affymetrix microarray analysis. Genes whose expression was changed significantly upon treatment of DCs with MMC in three independent experiments were further analyzed. Based on this criterion, 116 genes were identified (Table S1). Among the affected genes, two main clusters were found: one involved in apoptosis and the other mediating immunosuppression. Functionally relevant genes are highlighted in Table S1 (column 3) and their full names as well as the proteins they are coding for are shown in column 4. Quantitative RT-PCR (data not shown) of the most important candidates confirmed the affymetrix microarray data. Among apoptosis-related genes, six proapoptotic genes were up-regulated (LRDD, TNFRSF 10b, PERP, FDXR, TRAF4, DDIT3) and five antiapoptotic genes down-regulated (NRG2, CFLAR, I-FLICE, Usurpin, FLAME-1), pointing to induction of cell death by apoptosis. It has been speculated that apoptotic cells are tolerogenic (12, 13). Therefore, it was important to verify by FACS whether the changed expression of these genes had repercussions on cell viability. As shown in Fig. 2, MMC-treated DCs enter earlier into apoptosis than untreated DCs. Interestingly, in parallel to apoptotic genes, well known immunosuppressive genes (ADM, TSC22D3, LILRB4) (14–16) were up-regulated along with a series of potentially inhibitory genes (MAFB, CSF2RA, MAP4K4, GAB2) (17–21). Taken together, these findings indicate induction of apoptosis and increased expression of immunosuppressive genes in DCs treated with MMC.
Fig. 2.
Flowcytometric analysis of apoptosis following treatment of DCs with MMC. DCs were treated with 50 μg/ml MMC and labeled with annexin-V-FITC and 7-AAD after 2, 6, and 24 h of incubation. Controls consisted of untreated DCs (− MMC). The lower and upper right quadrants show apoptosis. Percentages of apoptotic cells are displayed.
MBP-Loaded MMC-DCs Inhibit Specific Mouse T Cells in Vitro.
We addressed the question of whether the suppressive effect mediated by MMC-DCs in vitro also works in vivo. For clarifying this point, a mouse EAE model was chosen, a setting in which MBP-specific T cells cause an inflammatory disease, similar to the inflammation of MS in humans. A prerequisite for their effectiveness in vivo was that, similar to human DCs, MMC-treated mouse DCs were T cell suppressive in vitro. Cell culture studies showed that mouse DCs loaded with MBP and treated with MMC significantly suppress specific syngeneic T cells of Tg4 mice (MBP-MMC-DCs + T cells = 12,653 ± 923 versus MBP-DCs + T cells = 24,727 ± 3197; naïve DCs + T cells = 7,007 ± 1,591) (mean of cpm ± SEM) (P = 0.022).
Previous observations of Liu et al. (13) showed that, when injected into mice, antigen-loaded tolerogenic cells first drive antigen-specific T cells into cell-cycle, and subsequently the T cells are inactivated. This finding prompted us to trace the fate of the deleterious autoreactive T cells in animals treated with inhibitory DCs. MBP-specific T cells were labeled ex vivo with CFSE and injected into syngeneic mice. Thereafter, the animals were injected intravenously with MBP-loaded DCs treated with MMC, and T cells were isolated and analyzed by FACS. The MBP-specific T cells showed a significant degree of proliferation (MBP-MMC-DC = 25% versus MBP-DC = 22%). Evidently, despite initial stimulation, the T cells must have been subsequently inactivated because, as shown in the following in vivo experiment, they were not able to cause EAE. This finding is in line with the observation described by Liu et al. (13).
Vaccination with MBP-Loaded MMC-DCs Protects Mice from EAE.
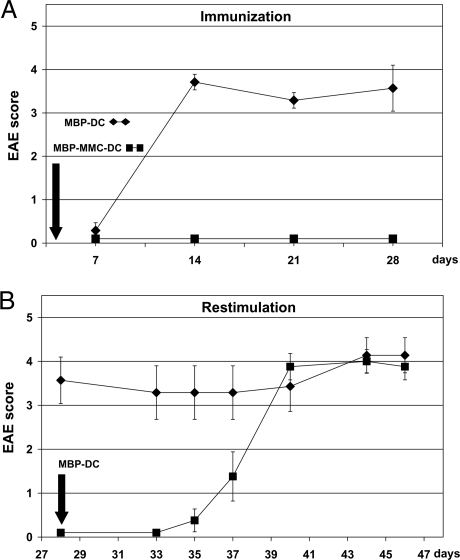
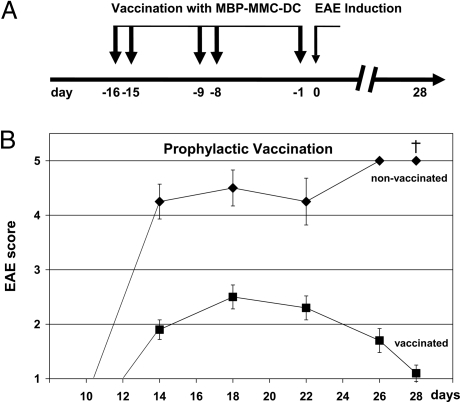
MBP-loaded untreated DCs were injected into animals and, as expected, severe EAE occurred within 2 to 3 weeks (Fig. 3A). If MBP-loaded DCs were pretreated with MMC and then injected, however, the animals remained completely free of symptoms, showing that MBP-specific T cells were not activated. An interesting question was whether the symptom-free animals became resistant to EAE. To this end, treated animals were rechallenged with MBP-DCs. To our disappointment, they developed a significant degree of EAE (Fig. 3B). One must keep in mind that the transgenic Tg4 mice used in our study carry >90% MBP-specific T cells, in contrast to only <0.0001% in normal rodents (9, 22). We suspected that it might be difficult to inactivate such a large number of T cells with one injection only. Therefore, in a subsequent experiment the animals were treated five times with MBP-MMC-DCs and then rechallenged (Fig. 4A). As shown in Fig. 4B, this time the result was positive: whereas controls, which had not been vaccinated with MBP-MMC-DCs developed severe EAE with lethal outcome, prevaccinated mice recovered after a mild episode of disease. Evidently, prophylactic vaccination with autoantigen-loaded DCs is possible.
Fig. 3.
Effect of MMC-treated, MBP-presenting dendritic cells in vivo. (A) Tg4 mice were injected intravenously with either MBP-DCs (♦) or MMC-treated MBP-DCs (■). (B) Mice immunized in experiment A with MMC-treated MBP-DCs (■) were challenged on day 28 with MBP-loaded DCs. Mice immunized in experiment A with MBP-DCs (♦) served as controls. Evaluation of EAE was performed according to the Coligan score. Data are shown as mean values ± SEM (n = 8 per group).
Fig. 4.
Prophylactic vaccination against EAE with MBP-loaded MMC-treated dendritic cells. (A) Tg4 mice were repetitively immunized with MBP-MMC-DCs. On day 0 these (■), as well as nonvaccinated mice (♦), were challenged with MBP-pulsed DCs. (B) The EAE severity (Coligan score) is shown, starting from day 10 after MBP-DC challenge. Data are displayed as mean ± SEM (n = 8 nonvaccinated, n = 10 vaccinated group).
Discussion
Attempts to generate regulatory DCs for control of autoimmune reactions have recently been described. Enk and colleagues generated suppressive DCs by incubating the cells in vitro with IL-10 and inhibited ovalbumin-specific CD4 T cell responses in naïve and sensitized mice (23). Huang et al. (24) observed that a subpopulation of immature bone marrow-derived DCs, if pulsed with MBP and injected into syngeneic rats, are protected from clinical EAE. Others showed that, to the contrary, mature but not immature DCs injected into mice with EAE reduced the severity of clinical signs and inflammation in the CNS (25). These conflicting findings stress the functional plasticity, from immunostimulation to suppression, of DCs under various conditions. Clinical signs of disease could also be reduced if rats or mice with incipient EAE were injected with IFN-γ-treated DCs (26). In neither of the latter two studies were antigen-specific DCs used. By contrast, Menges et al. (27) used TNF-α matured DCs pulsed with autoantigenic peptide and observed protection from EAE if the mice were injected before inductive immunization. Another experimental study suggested that inhibition of NF-kB by pharmacological agents enhanced the capacity of immature DCs to induce antigen-specific suppression to self-antigens in mice (28). When murine DCs were transduced with the gene for suppressor of cytokine signaling-3, they exhibited a DC2 phenotype that promoted Th2 cell differentiation and weakly influenced autoimmune reactions in vivo (29). The severity of EAE in mice could also be reduced with autoantigen-loaded DCs expressing TRAIL or PDL1 transgenes (30).
Different functional behaviors of DCs belonging to the same maturational stage (5, 6), the difficulty to standardize the generation of suppressive DCs by biological agents, and reversible modifications induced by cytokines or other biological agents, are all hurdles for the use of suppressive DCs in clinical trials, entailing the risk of stimulating instead of inhibiting the immune response. Ideally, inhibitory DCs for clinical application should be easily and reproducibly generated, stable in their suppressive action, and capable of irreversibly inactivating autoreactive T cells.
Based on our previous experience in rats (8), in which allograft rejection was successfully controlled by MMC-DCs, stably inhibitory DCs for control of autoimmunity were generated in the present study by treating the cells with MMC and loading with autoantigen. These cells protected animals from lethal EAE, showing that, in principle, effective prophylactic vaccination against T cell-mediated autoaggression is possible. A further question was whether MBP-MMC-DCs can inhibit induction of EAE if coadministered with the stimulatory MBP-DCs. Our findings showed that suppressive DCs have the capacity to inhibit the stimulatory action of MBP-DCs and limit the extent of subsequent EAE (data not shown). MMC is an alkylating agent used in cancer therapy that strongly binds to distinct DNA sites, cross-links the double helical strands, inhibits DNA synthesis, and consequently suppresses cell proliferation. In addition, MMC inhibits RNA and protein synthesis. Interestingly, alkylating agents not only inhibit but also activate pathways usually triggered by stimulatory agents (31). Therefore, it is not surprising that in our model the expression of certain DC-genes was up- and not down-regulated. Because of the irreversible interaction of MMC with intracellular compounds, cells do not release MMC upon incubation with this substance. This was confirmed by our finding that supernatants of MMC-treated DCs do not significantly suppress T cell reactions. Most importantly, in contrast to manipulations of DCs with biological agents (e.g., cytokines), MMC-treatment induces irreversibly suppressive DCs by induction of apoptosis, a feature that offers a potential for developing a stable therapeutic tool. Another advantage of this model is the use of nontoxic doses of a clinically approved drug. The therapeutic dose of MMC is 10 to 20 mg/m2; the concentration of MMC used in this study for incubation of cells was 0.05 to 0.100 mg/ml. Our analyses showed that after extensive washing the cell suspension contained, if at all, nonactive traces of MMC. No clinical side effects are expected at these minimal amounts of free MMC in the injected solution.
Our findings demonstrate that MMC-DCs are effective in controlling both mouse and human autoreactive T cells. Previous studies in our laboratory showed that MMC-DCs are strongly inhibitory in rats (8). In contrast with other models, the therapeutic tool described herein works across species. Moreover, in the present study the in vivo effect was tested under aggravating conditions. Normal rodents carry <10−6 MBP-reactive T cells in their repertoire (22). We used Tg4 transgenic mice with >90% MBP-reactive T cells and consequently with an extreme proneness to EAE (9). If this huge number of “dangerous” T cells can be kept in check, we can expect a reliable effect when lower numbers of autoreactive T cells are involved.
In former times, MMC was used in DC-induced T-cell activation experiments to suppress proliferation of contaminating cells in the DC preparation. Later on, MMC was replaced with ionizing irradiation of cells. Here, the question must be addressed why in those experiments MMC-treated DCs stimulated and in our experiments they suppressed T-cell proliferation. We also had certain degree of stimulation in our cultures. The extent of T-cell proliferation was dose dependent, i.e. the less MMC was used for treatment of DCs, the higher the stimulation. Apparently, in former experiments, this stimulation was sufficient to study T-cell responses. In the current series of experiments, we did the next step and analyzed restimulation of T cells cocultured with MMC-DCs. In contrast to T cells activated with untreated DCs, those cocultured with MMC-DCs showed significantly suppressed proliferation. This finding was further supported by our observation that CFSE-labelled T cells injected into mice treated with MMC-DCs proliferated first, but subsequently the animals became resistant to EAE. Apparently, MMC-DC treatment induces initial activation but thereafter MBP-specific T cells are inactivated. A similar finding was reported by Liu et al. regarding the induction of tolerance in mice by antigen-loaded tolerogenic cells (13). Other factors that possibly influence the extent of T-cell suppression in vitro and which might have varied among studies are the percentage of apoptotic cells in the MMC-DC preparation, the DC:T-cell ratio, and the maturational stage of DCs.
A previous study showed that exposure to necrotic tumor cells, in contrast to exposure to apoptotic cells, induces immunostimulation (12). This observation, as well as other observations (13), led to the hypothesis that necrotic cell death is immunogenic, whereas apoptotic cell death is poorly immunogenic or even tolerogenic. From a physiological view this makes sense, because apoptosis is the normal process of cell death in our tissues. Would apoptosis induce immune responses, it would lead to inflammation and autoimmunity. Liu et al. (13) used this phenomenon to actively induce tolerance. Dying apoptotic splenocytes were loaded with ovalbumin and injected into syngeneic mice. After an initial phase of T cell stimulation, the recipients became tolerant to ovalbumin (13). It is interesting to note the reports showing that DC lifespan has important consequences for DC-T cell interaction, and thus determines the immunological outcome. Hugues et al. concluded that stable interactions favor T cell priming, whereas brief contacts between DCs and T cells may contribute to the induction of T cell tolerance (32). In the present series of experiments, MMC accelerated the natural process of apoptosis, shortening the lifespan of injected DCs and thus their contact with T cells. This provides a possible explanation for the observed tolerogenic effect. Obeid et al. (33) have recently analyzed the immunogenic potential of tumor cells rendered apoptotic by various chemotherapeutic drugs and observed that anthracyclins generate stimulatory cells, whereas other drugs, such as mitomycin C, do not. If anthracyclins were used, the chaperon protein calreticulin was up-regulated and responsible for the stimulatory action. This finding is important for tumor therapy, which aims at killing malignant cells and concomitantly stimulating the immune response against the tumor. Our findings are interesting in this context by demonstrating that treatment with MMC renders the cells apoptotic but—as shown by affymetrix microarray—does not up-regulate calreticulin; instead, it up-regulates immunosuppressive molecules. Whereas the observation of Obeid et al. (33) may be used for improving chemotherapy in cancer, our observation has a therapeutic potential for controlling autoimmune disease or graft rejection.
In the present study, induction of apoptosis was suggested by up-regulation of proapoptotic genes, including LRDD (coding for PIDD), TNFRSF10b (coding for TRAIL-R2), PERP, FDXR, TRAF4, and DDIT3. Additionally, we noted down-regulation of genes that protect from apoptosis, such as NRG2 and CFLAR (coding for cFLIP and its variants I-FLICE, usurpin, FLAME-1). Most importantly, apoptosis of MMC-treated DCs was demonstrated by FACS.
Gene expression analysis showed that, concomitantly with induction of apoptosis, a series of strongly immunosuppressive genes were up-regulated. ADM (adrenomedullin), whose expression was increased 10 times, is a peptide that prevents sepsis-induced mortality, abrogates colitis, and provides highly effective therapy of arthritis by decreasing the presence of autoreactive Th1 cells, inducing regulatory T cells and inhibiting autoimmune and inflammatory responses (14). ADM also up-regulates TGF-β. Gene expression analysis showed unchanged TGF-β expression. However, this reflects the status 18 h following MMC-treatment, the point in time when gene scan was performed. It is conceivable that increased TGF-β expression occurs at a later point. TGF-β-mediated suppression might also play a role in another context. Fadok et al. (34) showed that phagocytosis of apoptotic cells by human macrophages increases their TGF-β expression. Transferred to our model, that means that administration of apoptotic DCs in vivo might increase TGF-β production and contribute to the described suppression of autoreactive T cells. On the other hand, Chen et al. (35) showed that apoptotic cells per se release TGF-β, thereby contributing to the generation of an immunosuppressive milieu. In their studies, TGF-β release was not associated with up-regulation of TGF-β transcription, suggesting release of existing intracellular stocks rather than de novo synthesis. Translated to our system, this indicates that MMC-DCs release their intracellular TGF-β without up-regulating its expression. Another gene whose expression was up-regulated by MMC was TSC22D3 (coding for GILZ). Interestingly, the same gene is up-regulated upon exposure of DCs to glucocorticoids, IL-10, or TGF-β, all well known immunological inhibitors (15). GILZ confers a suppressive phenotype to DCs and prevents them from activating T cells (15). A molecule induced by GILZ is LILRB4 (coding for ILT3), a protein which renders monocytes and DCs tolerogenic and has clinical relevance (16). Human heart transplant recipients with stable grafts have circulating T suppressor cells that up-regulate ILT3 in donor antigen-presenting cells (16). These findings demonstrate an important immunoregulatory function of ILT3. We found a significant increase of ILT3 expression in MMC-DCs. Other functionally relevant genes whose expression was modulated by MMC were MAFB (which directs differentiation away from DCs toward monocytes) (17), CSF2RA (which transduces GM-CSF signals) (18), MAP4K4 (which mediates TNF-α signaling and cell migration) (19, 20), and GAB2 (which transmits signals delivered by cytokine-, growth factor-, and antigen-receptors) (21). All of these genes might play a role in immunosuppression induced by MMC-treated DCs.
Taken together, the observations of Obeid et al. (33) and our team suggest that induction of apoptosis with concomitant up-regulation of activatory molecules renders cells immunogenic, whereas apoptosis and up-regulation of inhibitory molecules renders cells immunosuppressive.
With respect to the possibility of developing therapeutic applications, some critical points of the present model should be discussed. One point is the use of MBP, a potential autoantigen in MS, with the aim of controlling a polyspecific immune response. It is thought that some autoimmune diseases start with an immune reaction to a single epitope expressed on an organ-specific antigen and extend to neighboring epitopes on the same or nearby molecules (4). This process, termed “epitope spreading,” eventually draws in a polyspecific immune response with unspecific inflammatory processes. Controlling a polyspecific response is a challenge for all antigen-specific therapies. In the 1970s, a random copolymer of amino acids, termed “glatiramer acetate,” was developed to mimic the composition of MBP. In clinical trials, glatiramer slowed progression of disability and significantly reduced the relapse rate of MS (36). Studies showed that the copolymer tolerized against a variety of different myelin antigens. More recently, altered peptide ligands of MBP and other autoantigens constructed by substituting amino acids at the contact sites of these epitopes with the T cell receptor, showed similar effects in animal models (4). These and other observations (37) indicate that the use of a single epitope can inhibit a disease caused by reactivity to multiple self-epitopes by directing unspecific regulatory mechanisms toward a certain organ. Apparently, in contrast to epitope spreading, epitope containment is also possible. Based on these observations, it is conceivable that MMC-DCs loaded with MBP, although addressing the immune response to one antigen, can also control reactions to neighboring molecules. An elegant variant of our model would be to load the inhibitory DCs with glatiramer acetate or other altered peptides derived from autoantigens. The suppressive action of peptides would be expected to be amplified.
A critical issue is the use of DCs as a clinical immunosuppressive tool. DCs are strongly stimulatory cells, and even if rendered suppressive might convert into stimulatory cells and exacerbate an autoimmune disease. Importantly, in contrast to cytokines or other biomolecules, MMC, the substance used in this study, causes irreversible changes by induction of apoptosis. This makes it unlikely that MMC-DCs regain their stimulatory function. An alternative approach for minimizing the risk of stimulation would be the use of the patient's own blood DCs. It was speculated that DCs of patients with autoimmune diseases already carry the pathogenic autoantigens (7). These natural DCs could be isolated, rendered suppressive by treatment with MMC, and reinjected into the patient.
Our in vivo data are derived from studies in the murine EAE model. It has been questioned to which extent this model reflects the pathogenesis of MS in humans (1). Of course, no mouse data, including those derived from EAE studies, can be automatically extrapolated to humans. It is worth mentioning, however, that despite all criticism, three therapeutic compounds approved for use in MS—glatiramer acetate, mitoxantrone, and natlizumab—emerged directly from findings in the EAE model (38). Our observations in mice gain additional relevance by the finding that T cells of MS patients are also suppressed by MBP-loaded MMC-DCs.
Despite comprehensive gene scan detection of molecular candidates, the mechanism of suppression induced by MMC-DCs has not been fully clarified. Nevertheless, our study describes a simple method for generating suppressive DCs with a clinically approved drug at nontoxic concentrations. In contrast to many other approaches, the resulting inhibitory DCs are underway to apoptosis, an irreversible process that prevents a return to stimulatory activity. Importantly, MMC-DCs have the capacity of inactivating autoreactive T cells in vitro and in vivo across species. When speaking about T cell-mediated autoimmune encephalitis, there are three decisive phases that can be approached by immunotherapy: prevention of disease, blocking of induction, and inhibition of an ongoing disease. In this article we present a method for prophylactic vaccination against EAE and for control of disease induction. These results ought to be completed by experiments trying to inhibit an ongoing disease. We are aware that the present study does not solve all problems related to EAE, but it presents an exciting an easy to perform way to prophylactic vaccination, paving the way for targeted immunosuppressive therapy in autoimmune diseases.
Materials and Methods
Mice.
B10.PL mice were obtained from Jackson Laboratories. T cell receptor (TCR)-transgenic Tg4 mice (I-Au background) express a TCR derived from an encephalitogenic CD4+ T cell clone specific for a MBP peptide (amino acid 1–9) (9).
Generation of DCs.
Murine DCs were generated from bone marrow cells of B10.PL mice according to the protocol of Lutz et al. (10). The cells were cultured for 9 days in GM-CSF containing medium (RPMI supplemented with 10% FCS, 2mM l-glutamine, 100 units/ml penicillin/streptomycin, 0.1 mM 2-mercaptoethanol and 10% of GM-CSF containing supernatant). For activation, 0.5 μM CpG-ODN 1668 was added, and 90 min later nonadherent BMDCs were obtained. MMC (50 μg/ml) was added for the last 30 min of culture, and the cells (106/ml) were extensively washed. N-terminal acetylated MBP1–10 peptide Ac-ASQKRPSQRS (Ac1–10) was synthesized in house using standard Fmoc chemistry and HPLC purified. The MBP peptide was added at a concentration of 5 μM in combination with CpG-ODN. Maturation was defined by CD402+, CD80+, CD86+, and MHCII+ (the last 3 markers fluctuating in their expression). The percentage of mature DCs was >80%.
Human DCs were generated according to a standard protocol as previously described (11). Peripheral blood monocytes were cultured in the presence of 1000 units/ml rh IL-4 (Promocell, Heidelberg, Germany) and 666 units/ml rh GM-CSF (Sigma–Aldrich Chemie GmbH, Taufkirchen, Germany) in RPMI medium 1640 supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine, and penicillin (100 units/ml)/streptomycin (100 μg/ml). On day 6 of culture, nonadherent cells (immature DCs) were collected and matured for 36 h with a combination of 500 ng/ml CD40 Ligand (CD40L) (Alexis Biochemicals) and 5 μg/ml lipopolysaccharide (LPS) (Sigma–Aldrich). For myelin basic protein (MBP)-specific T cell studies, 30 μg/ml MBP (Sigma–Aldrich) was added to immature DCs from MS patients or HLA-DRB1*0301+ healthy blood donors until complete maturation of DCs. Maturation was defined by the following phenotype: CD14−, HLA-DR2+, CD802+, CD862+, and CD83+. The percentage of mature DCs was >80%. For certain studies, MMC (10–100 μg/ml) was added to the culture medium of mature DCs; after 30 min of incubation at 37 °C, the cells were extensively washed.
T Cell Studies in Vitro.
Murine lymphocytes were cultured with bone marrow-derived DCs. Human peripheral blood lymphocytes of MS patients were coincubated with autologous MBP-loaded DCs. In a parallel experiment, DRB1*0301-DCs from healthy donors were loaded with MBP and coincubated with MBP-specific CD4+ T cells (clone ES-BP8T) as previously described (11). In a control experiment, the DCs were loaded with an irrelevant peptide with comparable interaction with DRB1*0301. The HLA restriction was calculated by the SYFPEITHI software. Cocultures were performed at a DC:T cell ratio of ≤ 1:10. T cell proliferation was measured by [3H]thymidine incorporation.
EAE Model.
EAE was induced by i.v. injection of 5 × 106 activated DCs (in 0.2 ml) pulsed with 5 μM autoantigenic MBP peptide (+/− MMC). On day 1 and 2 after immunization, each mouse was injected intraperitonially with 200-ng pertussis toxin (Calbiochem) in 500-μl Dulbecco's PBS. Symptoms were evaluated daily according to the Coligan score.
Affymetrix Microarray.
Total RNA was extracted from DCs 18 hr after MMC treatment by the isothiocyanate method (RNeasy RNA isolation kit; Qiagen, Hilden, Germany) according to the manufacturer's instructions. DNase treatment was carried out by using DNase I (Applied Biosystems, Darmstadt, Germany). Total RNA (5 μg) was converted into ds-cDNA using T7-(dT)24 primers and the Superscript Choice system (Invitrogen, Karlsruhe, Germany). Biotin-labeled cRNA was generated from the cDNA sample by in vitro transcription using the BioArray HighYield RNA Transcript Labeling kit (Enzo Diagnostics, New York, NY). The purified and fragmented biotin-labeled cRNA was then hybridized to U133 Plus 2.0 gene chips (Affymetrix, High Wycombe, UK). The hybridized gene chips were stained with streptavidin-phycoerythrin (MoBiTec, Göttingen, Germany) and scanned using the GeneArray scanner (Affymetrix). RNA quality was confirmed by spectrophotometric examination and by assessing 5′/3′ ratios of control genes provided on the test 3 array. Microarray data of samples derived from 3 unrelated DC donors were analyzed. Untreated or MMC-treated DCs of the same healthy donor were used. Data analysis was performed according to instructions provided by Affymetrix using the Affymetrix Data Mining tool (DMT 4.0), the Affymetrix publishing tool (MDB 3.0), and the statistical data analysis software (Affymetrix Microarray Suite 5.0). Comparisons between human mitomycin C-treated and untreated DCs were done for genes with a positive detection call in at least one experimental group and with a fold change of at least 1.2 (corresponding to a signal log ratio between the two experimental groups of less than −0.3 or more than 0.3). Functional classifications from Gene Ontology (GO) Consortium (www.geneontology.org) were assigned to each identified gene.
FACS Analysis.
Human DCs were stained with fluorescein isothiocyanate (FITC)-labeled annexin V and 7-amino-actinomycin (7-AAD) to confirm apoptotic cell death.
DC staining was performed with fluorescence (FITC, PE)-labeled monoclonal antibodies (to MHC II, CD80, CD86,) to concentrations indicated by the manufacturers (BD Biosciences).
Approval for Animal and Human Studies.
Animal experiments were approved by the Animal Welfare Board of the Governmental Office Karlsruhe. Studies of human sera and cells were approved by the University of Heidelberg Ethics Committee.
Statistics.
Results are shown as mean ± SD or SEM as indicated. Single values of T cell proliferation represent the mean [3H]thymidine incorporation (cpm) of triplicate cultures and are given in percentage of the positive control (= 100% proliferation) or counts per minute. P values were calculated by the unpaired Student's t test using SigmaStat software (SPSS). Statistical significance was set at P < 0.05.
Supplementary Material
Acknowledgments.
We thank Helmut Simon, Stephanie Grimm, and Sanela Paljevic for their expert technical assistance. Tg4 mice were kindly provided by D.C. Wraith (Bristol, U.K.) and the T cell clone ES-BP8T was provided by Dr. Edgar Meinl (Department of Neuroimmunology, Max Planck Institute of Neurobiology, Martinsried, Germany). We thank Uwe Appelt for his generous assistance in preparation of the figures.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807185105/DCSupplemental.
References
- 1.Hemmer B, Archelos JJ, Hartung HP. New concepts in the immunopathogenesis of multiple sclerosis. Nat Rev Neurosci. 2002;3:291–301. doi: 10.1038/nrn784. [DOI] [PubMed] [Google Scholar]
- 2.Kamphuis S, Albani S, Prakken BJ. Heat-shock protein 60 as a tool for novel therapeutic strategies that target the induction of regulatory T cells in human arthritis. Expert Opin Biol Ther. 2006;6:579–589. doi: 10.1517/14712598.6.6.579. [DOI] [PubMed] [Google Scholar]
- 3.Raz I, et al. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet. 2001;358:1749–1753. doi: 10.1016/S0140-6736(01)06801-5. [DOI] [PubMed] [Google Scholar]
- 4.Steinman L. Immune therapy for autoimmune diseases. Science. 2004;305:212–216. doi: 10.1126/science.1099896. [DOI] [PubMed] [Google Scholar]
- 5.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Ann Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 6.Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108:1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 7.Xiao BG, Huang YM, Link H. Tolerogenic dendritic cells: the ins and outs of outcome. J Immunother. 2006;29:465–471. doi: 10.1097/01.cji.0000210387.55951.8b. [DOI] [PubMed] [Google Scholar]
- 8.Jiga L, Ehser S, Kleist C, Opelz G, Terness P. Inhibition of heart allograft rejection with mitomycin C-treated donor dendritic cells. Transplantation. 2007;83:347–350. doi: 10.1097/01.tp.0000248854.30016.11. [DOI] [PubMed] [Google Scholar]
- 9.Wraith DC, McDevitt HO, Steinman L, Acha-Orbea H. T cell recognition as the target for immune intervention in autoimmune disease. Cell. 1989;57:709–715. doi: 10.1016/0092-8674(89)90786-1. [DOI] [PubMed] [Google Scholar]
- 10.Lutz MB, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 11.Terness P, Chuang JJ, Bauer T, Jiga L, Opelz G. Regulation of human auto- and alloreactive T cells by indoleamine 2,3-dioxygenase (IDO)-producing dendritic cells: too much ado about IDO? Blood. 2005;105:2480–2486. doi: 10.1182/blood-2004-06-2103. [DOI] [PubMed] [Google Scholar]
- 12.Sauter B, et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu K, et al. Immune tolerance after delivery of dying cells to dendritic cells in situ. J Exp Med. 2002;196:1091–1097. doi: 10.1084/jem.20021215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varela N, Chorny A, Gonzalez-Rey E, Delgado M. Tuning inflammation with anti-inflammatory neuropeptides. Expert Opin Biol Ther. 2007;7:461–478. doi: 10.1517/14712598.7.4.461. [DOI] [PubMed] [Google Scholar]
- 15.Cohen N, et al. GILZ expression in human dendritic cells redirects their maturation and prevents antigen-specific T lymphocyte response. Blood. 2006;107:2037–2044. doi: 10.1182/blood-2005-07-2760. [DOI] [PubMed] [Google Scholar]
- 16.Chang CC, et al. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3:237–243. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 17.Bakri Y, et al. Balance of MafB and PU 1 specifies alternative macrophage or dendritic cell fate. Blood. 2005;105:2707–2716. doi: 10.1182/blood-2004-04-1448. [DOI] [PubMed] [Google Scholar]
- 18.Crosier K, Wong G, Mathey-Prevot B, Nathan D, Sieff C. A functional isoform of the human granulocyte/macrophage colony-stimulating factor receptor has an unusual cytoplasmic domain. Proc Natl Acad Sci USA. 1991;88:7744–7748. doi: 10.1073/pnas.88.17.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao Z, et al. A novel human STE20-related protein kinase, HGK, that specifically activates the c-Jun N-terminal kinase signaling pathway. J Biol Chem. 1999;274:2118–2125. doi: 10.1074/jbc.274.4.2118. [DOI] [PubMed] [Google Scholar]
- 20.Collins C, et al. A small interfering RNA screen for modulators of tumor cell motility identifies MAP4K4 as a promigratory kinase. Proc Natl Acad Sci USA. 2006;103:3775–3780. doi: 10.1073/pnas.0600040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishida K, et al. Gab-family adapter proteins act downstream of cytokine and growth factor receptors and T- and B-cell antigen receptors. Blood. 1999;93:1809–1816. [PubMed] [Google Scholar]
- 22.Sun D, Whitaker J, Wilson D. Regulatory T cells in experimental allergic encephalomyelitis. I. Frequency and specificity analysis in normal and immune rats of a T cell subset that inhibits disease. Int Immunol. 1999;11:307–315. doi: 10.1093/intimm/11.3.307. [DOI] [PubMed] [Google Scholar]
- 23.Müller G, et al. Interleukin-10-treated dendritic cells modulate immune responses of naive and sensitized T cells in vivo. J Invest Dermatol. 2002;119:836–841. doi: 10.1046/j.1523-1747.2002.00496.x. [DOI] [PubMed] [Google Scholar]
- 24.Huang YM, Yang JS, Xu LY, Link H, Xiao BG. Autoantigen-pulsed dendritic cells induce tolerance to experimental allergic encephalomyelitis (EAE) in Lewis rats. Clin Exp Immunol. 2000;122:437–444. doi: 10.1046/j.1365-2249.2000.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang G, Kishi M, Xu H, Rostami A. Mature bone marrow-derived dendritic cells polarize Th2 response and suppress experimental autoimmune encephalomyelitis. Multiple Sclerosis. 2002;8:463–468. doi: 10.1191/1352458502ms857oa. [DOI] [PubMed] [Google Scholar]
- 26.Xiao BG, et al. Therapeutic potential of IFN-gamma-modified dendritic cells in acute and chronic experimental allergic encephalomyelitis. Int Immunol. 2004;16:13–22. doi: 10.1093/intimm/dxh003. [DOI] [PubMed] [Google Scholar]
- 27.Menges M, et al. Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J Exp Med. 2002;195:15–22. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iruretagoyena MI, et al. Inhibition of nuclear factor-kappa B enhances the capacity of immature dendritic cells to induce antigen-specific tolerance in experimental autoimmune encephalomyelitis. J Pharmacol Exp Ther. 2006;318:59–67. doi: 10.1124/jpet.106.103259. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Chu N, Rostami A, Zhang GX. Dendritic cells transduced with SOCS-3 exhibit a tolerogenic/DC2 phenotype that directs type 2 Th cell differentiation in vitro and in vivo. J Immunol. 2006;177:1679–1688. doi: 10.4049/jimmunol.177.3.1679. [DOI] [PubMed] [Google Scholar]
- 30.Hirata S, et al. Prevention of experimental autoimmune encephalomyelitis by transfer of embryonic stem cell-derived dendritic cells expressing myelin oligodendrocyte glycoprotein peptide along with TRAIL or programmed death-1 ligand. J Immunol. 2005;174:1888–1897. doi: 10.4049/jimmunol.174.4.1888. [DOI] [PubMed] [Google Scholar]
- 31.Liu Z-G, et al. Three distinct signaling responses by murine fibroblasts to genotoxic stress. Nature. 1996;384:273–276. doi: 10.1038/384273a0. [DOI] [PubMed] [Google Scholar]
- 32.Hugues S, et al. Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. Nat Immunol. 2004;5:1235–1242. doi: 10.1038/ni1134. [DOI] [PubMed] [Google Scholar]
- 33.Obeid M, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 34.Fadok VA, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W, Frank ME, Jin W, Wahl SM. TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001;14:715–725. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 36.Johnson K, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45:1268–1276. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- 37.Lo J, Clare-Salzler MJ. Dendritic cell subsets and type I diabetes: focus upon DC-based therapy. Autoimmun Rev. 2006;5:419–423. doi: 10.1016/j.autrev.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Steinman L, Zamvil S. How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Ann Neurol. 2006;60:12–21. doi: 10.1002/ana.20913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.