Abstract
In rats, androgens in adulthood regulate the morphology of motoneurons in the spinal nucleus of the bulbocavernosus (SNB), including the size of their somata and the length of their dendrites. There are conflicting reports about whether androgens exert similar influences on SNB motoneurons in mice. We castrated or sham-operated C57BL6J mice at 90 days of age and, thirty days later, injected cholera toxin conjugated horseradish peroxidase into the bulbocavernosus muscle (to label SNB motoneurons) on one side, and into intrinsic foot muscles contralaterally (to label motoneurons of the retrodorsolateral nucleus (RDLN)). Castrated mice had significantly smaller SNB somas compared to sham operated mice while there were no differences in soma size of RDLN motoneurons. Dendritic length in C57BL6J mice, estimated in 3-dimensions, also decreased significantly after adult castration. In rats, androgens act directly through androgen receptors (AR) in SNB motoneurons to control soma size and nearly all their SNB motoneurons contain AR. Since SNB somata in C57BL6J mice shrank after adult castration, we used immunocytochemistry to characterize AR expression in SNB cells as well as motoneurons in the RDLN and dorsolateral nucleus (DLN). A pattern of labeling matched that seen previously in rats: the highest percentage of AR-immunoreactive motoneurons are in the SNB (98%), the lowest in the RDLN (25%), and an intermediate number in the DLN (78%). This pattern of AR labeling is consistent with the possibility that androgens also act directly on SNB motoneurons in mice to regulate soma size in mice.
Keywords: Androgen receptor, dendritic plasticity, immunocytochemistry, retrograde tracing, sexually dimorphic, neuromuscular
Introduction
The spinal nucleus of the bulbocavernosus (SNB) in mice, as in rats, is a sexually dimorphic group of motoneurons located in the lower lumbar spinal cord (Monks et al., 2003; Forger et al., 1997). SNB motoneurons innervate the bulbocavernosus (BC) and levator ani (LA) perineal muscles, which are involved in male copulatory behavior (Sachs, 1982). The size of both SNB motoneurons and their BC/LA muscle targets are maintained by testicular androgens secreted in adulthood. Castration in adulthood reduces SNB soma size and BC/LA muscle size in rats and also in some strains of mice, while androgen treatment reverses this effect (Breedlove & Arnold, 1981; Forger and Breedlove, 1986; Wee & Clemens, 1987). In rats, androgens act directly through androgen receptors in SNB motoneurons to regulate soma size (Watson et al., 2001). The length of SNB dendrites also decreases after castration of adult rats (Kurz et al., 1986). In this case, androgens act indirectly, through the BC/LA target muscles, to regulate the length of SNB dendrites (Rand and Breedlove, 1995).
The rat model has been most frequently used to investigate the hormone sensitivity of the SNB system. However, the availability of genetic murine models necessitates a re-characterization of the hormone sensitivity of the SNB system in mice. In particular, few studies have examined the hormone sensitivity of the SNB system in adult mice, and there are inconsistencies among the existing studies. Wee and Clemens (1987) examined the effects of long term castration (25 weeks) in 3 strains of mice (C57BL6J, DBA/2J, B6D2F1). They found that SNB somas shrank in DBA/2J and B6D2F1 strains but not in C57BL6 mice. Another study examining the effects of a shorter term castration (6 weeks) in B6D2F1 mice also show a decrease in SNB soma size (Park et al., 2002). The role of adult circulating androgens in maintaining SNB dendrites has been examined in two species of mice. Forger and Breedlove (1986) showed that in white footed mice (Peromyscus leucopus) SNB dendritic length decreased 7 weeks following castration. However, the B6D2F1 laboratory mouse failed to show a decrease in dendritic length after either 6 or 12 weeks of castration (Park et al., 2002).
We sought to investigate the androgen sensitivity of the adult SNB system in C57BL6J mice, a commonly used strain in genetic models. We examined the effects of short-term adult castration (30 days) on soma size and dendritic length of SNB motoneurons in C57BL6J mice (Experiment 1) and found that both the somata and dendrites of SNB motoneurons were sensitive to adult castration.
We also characterized expression of androgen receptors (ARs) in SNB motoneurons of C57BL6J mice (Experiment 2) using immunocytochemistry, since in rats androgens regulate soma size by activating ARs in the motoneurons themselves. We compared AR expression in SNB motoneurons to two other groups of motoneurons in the same spinal segments: the retrodorsolateral nucleus (RDLN), and the dorsolateral nucleus (DLN). The RDLN motoneurons, which innervate muscles of the foot (Leslie et al., 1991), are largely hormone insensitive (Jordan et al., 2002) and are therefore often used as a control when determining the hormone sensitivity of SNB motoneurons. DLN motoneurons are moderately hormone-sensitive (Jordan et al., 1982) and innervate the ischiocavernosus muscles (McKenna & Nadelhaft, 1986), and are also involved in copulatory reflexes in rodents (Sachs, 1982). In rats, approximately 80–95% of SNB motoneurons contain AR-immunopositive nuclei, while DLN motoneurons contain a slightly smaller proportion, and about 30% of RDLN motoneurons have AR-immunopositive nuclei (Jordan et al., 1997; Jordan, 1997). We found AR-immunoreactivity in all three pools of motoneurons in mice, with proportions of AR-positive cells across the pools approximating that seen in rats.
Method
Experiment 1
Animals
70–90 day old male C57BL6J mice obtained from Jackson Laboratories (Bar Harbor, Maine) were used in the experiment, and all procedures were approved by the Michigan State University IACUC. Mice were housed in a vivarium with a 12/12 light/dark cycle with food and water available ad libitum. Animals were randomly assigned to one of two treatment groups (castration or sham operation) and were housed for at least one week before surgery. Mice were anesthetized with isoflurane and bilateral incisions were made in the scrotum to expose the testes, which were removed in some animals (castrated group) and left intact in others (sham group). In the castrated group the testes were excised from the scrotum, tied off with silk suture and removed. For both castrated and shams, incisions in the scrotum and skin were closed using wound clips.
Histology
Thirty days following castration or sham surgery mice were re-anesthetized with isoflurane and the left BC muscle was exposed and injected with CT-HRP (1 μL, 0.2 % CT-HRP per injection; List Biological, Campbell, CA) to retrogradely label SNB motoneurons. An intrinsic muscle of the right foot was also injected (1 μL, 0.2 % CT-HRP) directly through the skin on the ventral surface of the foot, to retrogradely label RDLN neurons. Forty-eight hours following CT-HRP injections, animals were deeply anesthetized with sodium pentobarbital and perfused with 0.9% saline followed by cold 0.1 M phosphate buffer (pH = 7.4) containing 0.8% paraformaldehyde and 1.25% glutaraldehyde. Spinal cords were dissected out and placed in the same fixative for 5 hours at 4°C, after which they were transferred to a 10% phosphate-buffered sucrose solution and held overnight at 4°C. The following day, spinal cords were transversely sectioned on a freezing, sliding microtome at 40 μm. Sections were reacted with tetramethylbenzidine for histological visualization of HRP according to Park et al. (2002). After the reaction, alternate sections were mounted onto three separate gelatin-coated slides. The next day 2/3 of the sections were dehydrated, defatted, and coverslipped, and the other third were counterstained with neutral red before dehydrating, defatting, and coverslipping.
Morphological Analysis
Soma Area
Cross sectional areas of CT-HRP-labeled SNB and RDLN cell bodies were measured using StereoInvestigator (MicroBrightField, Inc.) and a Zeiss Axioplan-2 light microscope equipped with a video camera. Twenty-30 labeled motoneurons were traced throughout the rostrocaudal extent of each motor pool for each animal, using a 63x objective. Measurements of SNB and RDLN somal areas for each animal were then averaged. These means were averaged across animals within a group to obtain a mean ± standard error of the mean (SEM) for each group with n = number of animals per group (8 castrated, 11 sham).
Dendritic Length
Dendritic length was estimated in 3-dimensions using the “space balls” stereological probe in the same material used for estimating soma size. Every third section (120 μm apart) was used to assess SNB dendritic length. Approximately 250 sites (grid spacing; 75 × 75 μm) were sampled in each section through an estimated 24 μm of tissue depth, using a 100x oil-immersion objective. The average tissue thickness was determined by focusing through two randomly chosen sections from each animal, which were then averaged to generate a mean thickness of 24 ± 2 μm (Mean ± SEM). In order to avoid artifact at the surface, 3 μm guard zones were set, leaving the effective probing depth at 18 μm. We estimated dendritic length by applying the isotropic “space balls” probe (Mouton et al., 2002), where the number of dendritic intersections with the surface area of a virtual sphere in a known volume of tissue is proportional to the total length within that volume, following the formula of Smith and Guttman (1953). Using the fractionator approach (West et al., 1991), total length in the reference space was then calculated. This procedure ensures that SNB labeled dendrites are equally likely to be sampled, regardless of orientation, in three dimensions. The formula for this procedure is: total estimated Length = 2*(v/s)*ΣI*1/ssf*1/asf*1/tsf. Where (v/s) is the ratio of the sampling box volume (18*18*18 = 5832) to surface area of the spherical probe (4πr2 = 1018), ΣI is the sum of intersections counted per animal, ssf = (sections sample/total sections), asf = (area frame/area x-y step), tsf = (sampling box height/section thickness). In order to minimize the potential bias of treating dendritic processes as two dimensional, however thin (~1um in diameter), we employed a relatively large sampling sphere, and criterion where the center of the process was used to mark an intersection. This technique does not account for shrinkage during tissue processing, nor can we be certain that shrinkage occurred equally in all three planes, so our estimates probably do not reflect the actual length of dendrites in vivo. The total length of dendrite per SNB motoneuron was determined by dividing the total estimated length of dendrites by the number of CT-HRP labeled cell bodies in those same sections. The sampling coefficient of error (CE) for all cases was less than 0.05.
Due to the lateral location of the RDLN motor pool and the midline distribution of SNB motoneurons, in a few cases SNB dendrites extended into the region occupied by dendrites of RDLN motoneurons. In these instances a marker was placed on the dendrite of interest and the magnification was decreased from 100x to 10x at which point the origin of the dendrite (SNB or RDLN) could be determined. In theory CT-HRP injections into muscle may also label sensory 1a afferents, which may appear in the dorsal horn, but we found no evidence of such labeling.
Experiment 2
AR immunocytochemistry for adult spinal motoneurons in mice
We used the rabbit PG-21 antiserum (0.158 μg/ml) directed against the first 21 amino acids of the androgen receptor (kindly provided by Gail Prins) and followed methods used previously (Jordan, 1997), to characterize the expression of androgen receptors in lumbar motoneurons of adult male mice. In brief, adult gonadally intact male C57BL6J mice obtained from Jackson Laboratories were overdosed with an i.p. injection of sodium pentobarbital and perfused intracardially with saline followed by 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4). Spinal cords were harvested, postfixed for one hour and transferred to 20% phosphate buffered sucrose for overnight at 4º C. Spinal cords were sectioned using a freezing sliding microtome, generating cross sections at 30μm thick. Every section was collected and immunoreacted as free floating sections in nylon-lined staining grids. Every third section collected was stained without the primary and served as the “no primary” control. ARs were visualized using a peroxidase Elite ABC kit (Vector Laboratories), and a biotinylated goat anti-rabbit secondary (1:400) and nickel-enhanced 0.025% diaminobenzidine in 0.05M tris (pH 7.2) as the chromogen and 0.006% vol/vol hydrogen peroxide. Tissue sections were mounted onto gel-subbed slides, dehydrated, cleared and coverslipped with Permount. Following the initial microscope analysis to estimate the number of AR-immunopositive motoneurons in the SNB, DLN and RDLN, coverslips were soaked off and tissue was counterstained with Neutral Red to reveal any motoneurons with nuclei that were not immunocytochemically labeled.
Microscope analysis
We followed previously published methods for estimating the percentage of SNB, DLN and RDLN motoneurons that are AR-positive (Jordan et al., 1997). The location of these three motor pools in the mouse lumbar spinal cord is comparable to that in the rat, as results from experiment 1 using CT-HRP to retrogradely label motoneurons in the SNB and RDLN confirmed. The DLN is the only other distinct pool of motoneurons at this rostrocaudal level of the lumbar spinal cord. Within each pool, motoneurons were judged to have dark or light AR immunoreactivity in their nuclei in sections that did not yet have the Nissl counterstain. Individual nuclei were assigned as having a light or dark stain based on subjective criteria, with intermediately stained nuclei assigned to the light category. Sections were then counterstained with Neutral Red to reveal all motoneurons, and the total number of motoneuronal nuclei was counted for each of the three pools. In each case, sections were analyzed throughout the rostrocaudal extent of the SNB, DLN and RDLN. These two sets of counts were used to estimate the percentage of AR-positive motoneurons in the SNB, DLN, and RDLN.
Statistics
Parametric statistical analyses were used with N = number of animals and only two-tailed p values reported. T-tests were used to assess the effects of castration on motoneuronal soma size, dendritic length, and seminal vesicle and BC/LA muscle weights. A one-way repeated measures analysis of variance was used to assess regional differences in AR expression among the three different motoneuronal groups (SNB, RDLN and DLN).
Results
Experiment 1
BC/LA Muscle and Seminal Vesicle Weights
BC/LA muscle weights were significantly reduced in castrated mice (51.3 ± 5.2 mg) compared to sham operated controls (115.8 ± 6.4 mg; p < .001). Seminal vesicle weights were also significantly reduced in castrated mice (19.5 ± 3.8 mg) compared to sham operated mice (184.6 ± 10.4 mg; p < .001).
Soma Area and Dendritic Length
CT-HRP injected into the bulbocavernosus muscles of C57BL6J male mice reveal a distribution of SNB motoneurons comparable to rats, with CT-HRP labeled motoneurons in mice located primarily in the dorsomedial aspect of the ventral horn just ventral to the central canal (Fig 1A). However, compared to rats, in which SNB motoneurons tend to tightly cluster, and remain such throughout the rostracaudal extent of the nucleus, SNB motoneurons in C57BL6J mice have a more dispersed distribution, tending to be spread along the medial border of the gray matter of the ventral horn. This more dispersed distribution becomes particularly evident in more rostral sections of the mouse SNB.
Figure 1.
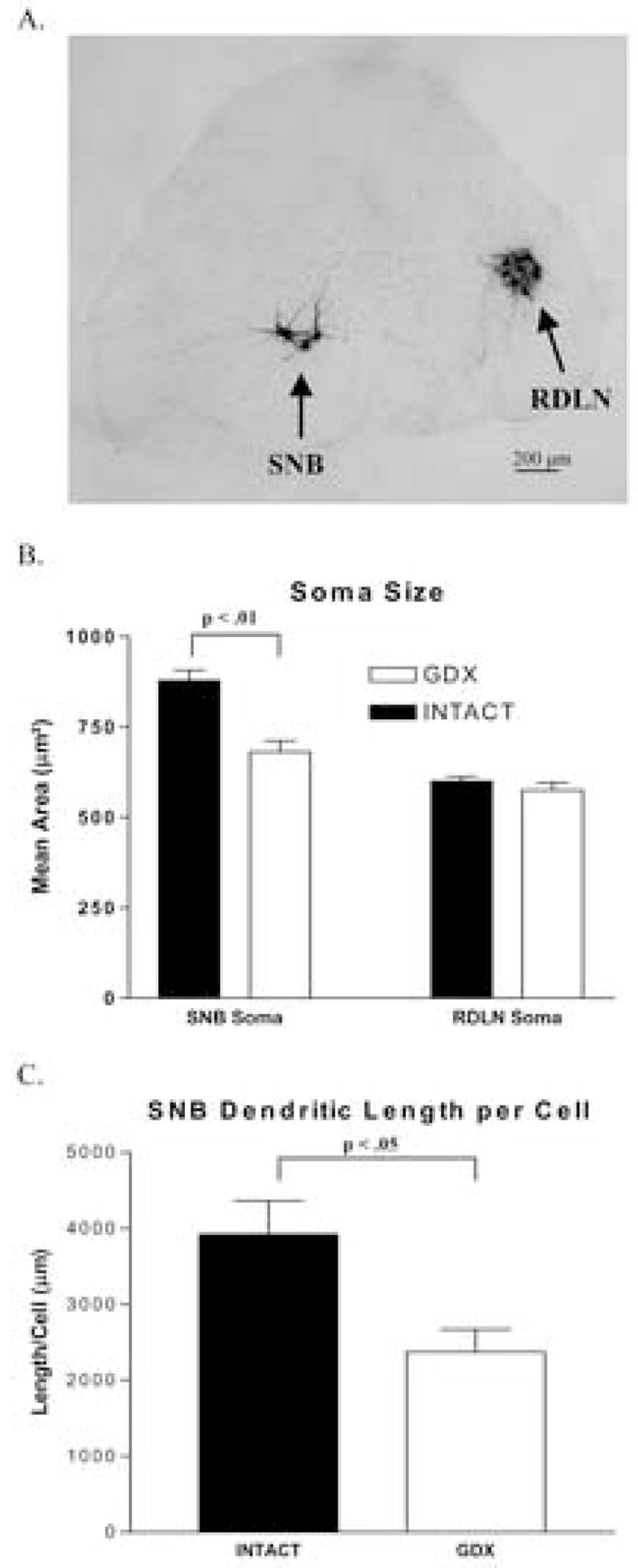
(A) Representative photomicrograph of motoneurons retrogradely labeled by CT-HRP in the SNB and RDLN motor pools of the lower lumbar spinal cord from an adult C57BL6J male mouse. (B) SNB and RDLN soma area in adult male C57BL6J mice 30 days after gonadectomy or sham surgery. SNB motoneuron soma area was reduced in gonadectomized compared to sham operated mice (p< 0.01) but RDLN soma area was not. (C) Total estimated length of dendrites per SNB motoneuron following gonadectomy or sham surgery. The length of SNB dendrites was also reduced in male C57BL6J mice gonadectomized as adults compared to gonadally intact (sham-operated) controls (p< 0.05). Mean ± SEM are depicted.
Adult castration affected the cross sectional area of SNB but not RDLN motoneurons. Male mice castrated for 30 days had smaller SNB somata compared to sham-operated controls (p < .01; Figure 1B) whereas the size of RDLN somas did not differ between castrated and sham-operated animals(p > .05; Figure 1B). The average overall length of dendrite per SNB motoneuron was also significantly decreased in castrated compared to sham-operated mice (p < .05; Figure 1C).
Experiment 2
Motoneuronal nuclei stained robustly for AR with little or no staining evident in the cytoplasmic compartment of motoneurons (Figure 2A). Furthermore, no nuclear staining was observed in control tissue that was stained without the AR antiserum (data not shown). Quantitative analysis of AR-stained motoneurons in the SNB, RDLN and DLN of gonadally intact male mice revealed a pattern of labeling in mouse motoneuronal pools that matches that seen in rats: the highest percentage of (light + dark labeled) AR-immunoreactive (AR-IR) motoneurons is in the SNB (98%), the lowest in the RDLN (25%), and an intermediate number in the DLN (78%; Figure 2B). For total (light+dark) AR-IR motoneurons, we found a significant main effect of motoneuron pool {F(2,17)= 18.4 p<.001}. Post hoc analysis revealed that the SNB and DLN contain more light + dark AR-IR motoneurons than the RDLN (p<0.001 and p<0.01, respectively), however the SNB and DLN did not significantly differ in percentage of total AR-IR motoneurons. For dark AR-IR motoneurons, we again found a significant main effect of motoneuron pool {F(2,17)= 20.7 p<.001}. Post hoc analysis revealed a larger percentage of darkly-labeled motoneurons in the SNB and DLN were AR-IR than in the RDLN (p<0.001 and p<0.05, respectively). Furthermore, the SNB contains a greater percentage of dark AR-IR motoneurons than the DLN (p<.05).
Figure 2.
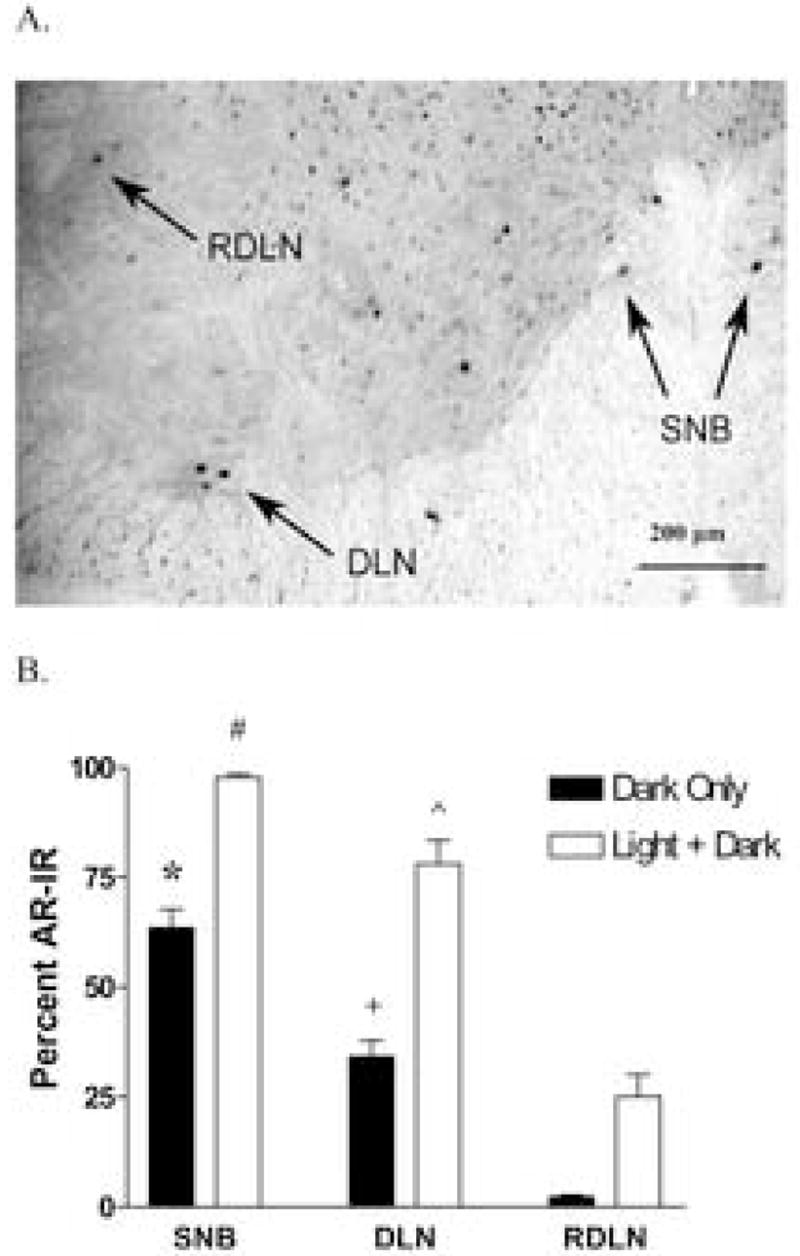
(A) Representative photomicrograph showing AR-IR in motoneuronal nuclei of the SNB, DLN, and RDLN motoneurons in a normal adult C57BL6J male mouse. (B) Percentage of AR-immunopositive motoneurons (Dark only or Light + Dark) in the SNB, DLN, and RDLN pools. The greatest percentage of AR-IR is found in the SNB, the least in the RDLN, and an intermediate amount in the DLN. (* p<.05 and p<.001 compared to dark DLN and RDLN respectively, + p<.05 compared to dark SNB and RDLN, # p<.001 compared to light+dark RDLN, ^ p<.01 compared to light+dark RDLN).
Discussion
SNB soma size and dendritic length, and BC/LA muscle mass were all reduced in castrates, suggesting that all these morphological endpoints of the SNB system are maintained by adult gonadal androgens in C57BL6J male mice. The relative decrease in BC/LA muscle and seminal vesicle mass after adult castration are comparable to previous findings in both mice and rats (Wainman & Shipounoff, 1941; Park et al., 2002; Forger & Breedlove 1987; Jordan et al., 2002).
Our finding that SNB soma size decreased 30 days after castration is in contrast to a previous study in which SNB soma size did not decrease following a longer castration period (25 weeks) in C57BL6J mice (Wee and Clemens, 1987). It is possible that the difference between the two studies in time after castration is responsible for the discrepancy. No one has worked out the relationship between levels of circulating androgens with age and SNB soma size in mice so it is possible that a decline in secreted androgens in gonadally intact mice with age might erase the effect of castration on SNB soma size that is seen in younger animals after shorter periods of castration. Another possible explanation for the different results of these two studies is the different label used to visualize motoneurons. In our study, we measured the size of SNB motoneurons that were labeled with CT-HRP, whereas Wee and Clemens measured the size of SNB motoneurons in Nissl stained sections. Perhaps SNB motoneurons are more difficult to identify in Nissl stained sections due to their more dispersed distribution in mice compared to rats. Moreover, many SNB motoneurons may have shrunk so severely after several months of castration that their typically dark Nissl-staining may have decreased, making it difficult to identify SNB motoneurons and detect an effect of castration on their size. This would explain why, in the same study, fewer SNB motoneurons were counted in castrated males than in gonadally intact C57BL6J male mice.
One major concern about the observed difference in dendritic length is that gonadal androgens might have facilitated the transport of CT-HRP in mice, causing an apparent but not actual difference in SNB dendritic length between gonadally intact and castrated adult male mice. There are several reasons why we do not think this is the case. In rats, androgens have been shown to affect overall dendritic length without affecting the radial extent of the SNB dendritic field or rostrocaudal extent (Kurz et al., 1991; Fargo and Sengelaub, 2004). In our analysis of dendritic length, we included a rough measure of radial extent in which the area occupied by SNB dendrites in each section was traced, and found no difference in the means (± SEM) between castrated (389340 ± 11711 μm²) and intact animals (406961 ± 15848 μm²). Similarly, the mean (± SEM) rostrocaudal extent of the SNB system did not differ between groups (castrated: 1560 ± 59 μm; intact: 1440 ± 74 μm). Thus, it is difficult to explain how androgens might have enhanced CT-HRP transport to increase the apparent length of dendritic branches without affecting either radial or rostrocaudal extent. We therefore conclude that differences in the transport of CT-HRP did not contribute to the group difference in total dendritic length. Since we did not include an androgen replacement group, it is also possible that the decrease in soma size and dendritic length after castration could be due to the loss of some testicular factor other than testosterone. However, this seems unlikely based on responses of SNB motoneurons in rats (Kurz et al., 1986).
We also found that a large majority of mouse SNB motoneurons express ARs, comparable to that found in rats, a species in which SNB soma size decreases following adult castration. Given that adult SNB motoneurons in C57BL6J mice contain ARs and that the size of their cell bodies also depends on adult levels of androgen, androgen may also act directly via ARs in SNB motoneurons to regulate _soma size in C57BL6J mice, as it does in rats (Watson et al, 2001). We also found that the percentage of AR-IR motoneurons in C57BL6J mice was highest in the SNB, lowest in the RDLN and intermediate in the DLN, matching the rank order of AR expression levels previously described for rats (Jordan et al, 1997).
This is also the first demonstration in mice that a period of castration as short as 30 days can decrease SNB somal area. Others have found effects at 6–7 weeks after castration (Park et al., 2002; Forger & Breedlove 1987). The significant decrease in soma size after 30 days of castration in mice provides another example of how the SNB system of C57BL6J mice is comparable to the SNB system in rats, in which SNB soma size also decreases 30 days after castration (Breedlove and Arnold, 1981).
We found that the length of SNB dendrites decreased by 38% following 30 days of castration. This finding is in line with greater than 50% reductions in the dendritic arborization of SNB motoneurons in rats following castration (Kurz et al., 1986). These results are also comparable to those found for the feral white footed mouse in which SNB dendritic length decreased by 27% after 7 weeks of castration (Forger & Breedlove, 1986). However, they are in contrast to findings in the only other laboratory mouse strain (B6D2F1) in which the androgen-sensitivity of SNB dendrites has been examined. In B6D2F1 mice, the length of SNB dendrites did not appear to be dependent on adult levels of androgen. While there is no information to date on the mechanisms underlying the androgenic regulation of dendrites in mice, we can draw again from evidence obtained from rats which suggests that androgens in adulthood act directly on the BC/LA muscles to indirectly regulate the length of SNB dendrites (Rand & Breedlove, 1995) and this androgen action may be mediated via some muscle-derived factor such as brain derived neurotrophic factor (Yang et al., 2004). We report that BC/LA muscle mass in adult C57BL6J mice is highly androgen-dependent, as in rats (Venable, 1966). Recent unpublished observations in our lab also indicate that the LA muscle in C57BL6J mice has a similar pattern of AR expression as previously described for rats (Monks et al, 2004). Thus, androgen may act through a similar pathway to regulate SNB dendrites in C57BL6J mice.
We suggest two reasons why adult SNB dendrites in C57BL6J mice and B6D2F1 mice may vary in their androgen-dependence. Such differences may reflect (1) genuine differences in androgen-sensitivity introduced by the different strains or reflect (2) differences in the techniques used to estimate dendritic length. In the present study, we employed a non-biased probe to estimate dendritic length in three dimensions, which has previously been shown to effectively estimate length (Mouton et al., 2000), while Park et al (2002) used several other methods to estimate dendritic length. However, even their direct measures of dendrites in three dimensions failed to reveal any androgen-related changes in the length of dendrites in B6D2F1 mice, making it likely that strain differences account for the different patterns of results.
If indeed the difference in SNB dendritic length represents a strain difference between C57BL6J and B6D2F1 mice, it is difficult to speculate why one strain responds to androgens differently than the other. However, there is evidence to suggest that the B6D2F1 strain may be less sensitive to changes in endogenous hormone levels than other lab mouse strains. The B6D2F1 strain is something of an anomaly in that a moderate percentage of males retain copulatory function 25 weeks after castration, whereas C57BL6J and DBA/2J males show a rapid decline in copulatory function after castration (Clemens et al., 1988). It is possible that the retention of copulatory behavior in castrated B6D2F1 mice is a functional reflection of the ability of the SNB system to retain its dendritic arborization independent of androgen. Such differences in androgen dependence may also be related to differences in AR expression. There may be fewer ARs in BC/LA muscles of B6D2F1 mice, rendering their dendrites less dependent on androgens.
Finding that the length of SNB dendrites can be altered by circulating hormones in adulthood is the first demonstration of this kind for laboratory mice. Therefore, the C57BL6J background may be a useful mouse model for manipulating the genome to dissect out the various mechanisms that underlie the androgen-regulated plasticity of the SNB system.
Acknowledgments
This work was supported by NIH grant R01 NS045195, as well as a postdoctoral fellowship (DAM) from the Canadian Institute of Health Research (CIHR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res. 1981;225(2):297–307. doi: 10.1016/0006-8993(81)90837-4. [DOI] [PubMed] [Google Scholar]
- Clemens LG, Wee BE, Weaver DR, Roy EJ, Goldman BD, Rakerd B. Retention of masculine sexual behavior following castration in male B6D2F1 mice. Physiol Behav. 1988;42(1):69–76. doi: 10.1016/0031-9384(88)90262-4. [DOI] [PubMed] [Google Scholar]
- Fargo KN, Sengelaub DR. Testosterone manipulation protects motoneurons from dendritic atrophy after contralateral motoneuron depletion. J Comp Neurol. 2004;469(1):96–106. doi: 10.1002/cne.10991. [DOI] [PubMed] [Google Scholar]
- Forger NG, Breedlove SM. Seasonal variation in mammalian striated muscle mass and motoneuron morphology. J Neurobiol. 1986;18(2):155–65. doi: 10.1002/neu.480180204. [DOI] [PubMed] [Google Scholar]
- Forger NG, Howell ML, Bengston L, MacKenzie L, DeChiara TM, Yancopoulos GD. Sexual dimorphism in the spinal cord is absent in mice lacking the ciliary neurotrophic factor receptor. J Neurosci. 1997;17(24):9605–12. doi: 10.1523/JNEUROSCI.17-24-09605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan C. Androgen receptor (AR) immunoreactivity in rat pudendal motoneurons: implications for accessory proteins. Horm Behav. 1997;32(1):1–10. doi: 10.1006/hbeh.1997.1397. [DOI] [PubMed] [Google Scholar]
- Jordan CL, Breedlove SM, Arnold AP. Sexual dimorphism and the influence of neonatal androgen in the dorsolateral motor nucleus of the rat lumbar spinal cord. Brain Res. 1982;249(2):309–14. doi: 10.1016/0006-8993(82)90065-8. [DOI] [PubMed] [Google Scholar]
- Jordan CL, Christensen SE, Handa RJ, Anderson JL, Pouliot WA, Breedlove SM. Evidence that androgen acts through NMDA receptors to affect motoneurons in the rat spinal nucleus of the bulbocavernosus. J Neurosci. 2002;22(21):9567–72. doi: 10.1523/JNEUROSCI.22-21-09567.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CL, Padgett B, Hershey J, Prins G, Arnold A. Ontogeny of androgen receptor immunoreactivity in lumbar motoneurons and in the sexually dimorphic levator ani muscle of male rats. J Comp Neurol. 1997;379(1):88–98. [PubMed] [Google Scholar]
- Kurz EM, Sengelaub DR, Arnold AP. Androgens regulate the dendritic length of mammalian motoneurons in adulthood. Science. 1986;232(4748):395–8. doi: 10.1126/science.3961488. [DOI] [PubMed] [Google Scholar]
- Kurz EM, Brewer RG, Sengelaub DR. Hormonally mediated plasticity of motoneuron morphology in the adult rat spinal cord: a cholera toxin-HRP study. J Nerobiol. 1991;22(9):976–88. doi: 10.1002/neu.480220909. [DOI] [PubMed] [Google Scholar]
- Leslie M, Forger NG, Breedlove SM. Sexual dimorphism and androgen effects on spinal motoneurons innervating the rat flexor digitorum brevis. Brain Res. 1991;561(2):269–73. doi: 10.1016/0006-8993(91)91603-x. [DOI] [PubMed] [Google Scholar]
- McKenna KE, Nadelhaft I. The organization of the pudendal nerve in the male and female rat. J Comp Neurol. 1986;248(4):532–49. doi: 10.1002/cne.902480406. [DOI] [PubMed] [Google Scholar]
- Monks DA, O’Bryant EL, Jordan CL. Androgen receptor immunoreactivity in skeletal muscle: enrichment at the neuromuscular junction. J Comp Neurol. 2004;473(1):59–72. doi: 10.1002/cne.20088. [DOI] [PubMed] [Google Scholar]
- Monks DA, Xu J, O’Malley BW, Jordan CL. Steroid receptor coactivator-1 is not required for androgen-mediated sexual differentiation of spinal motoneurons. Neuroendocrinology. 2003;78(1):45–51. doi: 10.1159/000071705. [DOI] [PubMed] [Google Scholar]
- Mouton PR, Gokhale AM, Ward NL, West MJ. Stereological length estimation using spherical probes. J Microscoscopy. 2002;206(Pt 1):54–64. doi: 10.1046/j.1365-2818.2002.01006.x. [DOI] [PubMed] [Google Scholar]
- Park JJ, Zup SL, Verhovshek T, Sengelaub DR, Forger NG. Castration reduces motoneuron soma size but not dendritic length in the spinal nucleus of the bulbocavernosus of wild-type and BCL-2 overexpressing mice. J Neurobiol. 2002;53(3):403–12. doi: 10.1002/neu.10103. [DOI] [PubMed] [Google Scholar]
- Rand MN, Breedlove SM. Androgen alters the dendritic arbors of SNB motoneurons by acting upon their target muscles. J Neurosci. 1995;15(6):4408–16. doi: 10.1523/JNEUROSCI.15-06-04408.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs BD. Role of striated penile muscles in penile reflexes, copulation, and induction of pregnancy in the rat. J Reprod Fertil. 1982;66(2):433–43. doi: 10.1530/jrf.0.0660433. [DOI] [PubMed] [Google Scholar]
- Smith CS, Guttman L. Measurement of internal boundaries in three dimensional structures by random sectioning. Trans AIME. 1953;197:81–92. [Google Scholar]
- Venable JH. Morphology of the cells of normal, testosterone-deprived and testosterone-stimulated levator ani muscles. Am J Anat. 1966;119(2):271–301. doi: 10.1002/aja.1001190206. [DOI] [PubMed] [Google Scholar]
- Wainman P, Shipounoff GC. The effects of castration and testosterone propionate on the striated perineal musculature of the rat. Endocrinology. 1941;29:975–8. [Google Scholar]
- Watson NV, Freeman LM, Breedlove SM. Neuronal size in the spinal nucleus of the bulbocavernosus: direct modulation by androgen in rats with mosaic androgen insensitivity. J Neurosci. 2001;21(3):1062–6. doi: 10.1523/JNEUROSCI.21-03-01062.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee BE, Clemens LG. Characteristics of the spinal nucleus of the bulbocavernosus are influenced by genotype in the house mouse. Brain Res. 1987;424(2):305–10. doi: 10.1016/0006-8993(87)91475-2. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJG. Unbiased sterological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–97. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Yang LY, Verhovshek T, Sengelaub DR. Brain-derived neurotrophic factor and androgen interact in the maintenance of dendritic morphology in a sexually dimorphic rat spinal nucleus. Endocrinology. 2004;145(1):161–8. doi: 10.1210/en.2003-0853. [DOI] [PubMed] [Google Scholar]


