Abstract
During the female reproductive cycle, the neuroendocrine action of estradiol switches from negative feedback to positive feedback to initiate the preovulatory GnRH and subsequent LH surges. Estrogen receptor-α (ERα) is required for both estradiol negative and positive feedback regulation of LH. ERα may signal through estrogen response elements (EREs) in DNA and/or via ERE-independent pathways. Previously, a knock-in mutant allele (ERα−/AA) that selectively restores ERE-independent signaling onto the ERα−/− background was shown to confer partial negative but not positive estradiol feedback on serum LH. The current study investigated the roles of the ERE-dependent and ERE-independent ERα pathways for estradiol feedback at the level of GnRH neuron firing activity. The above ERα genetic models were crossed with GnRH-green fluorescent protein mice to enable identification of GnRH neurons in brain slices. Targeted extracellular recordings were used to monitor GnRH neuron firing activity using an ovariectomized, estradiol-treated mouse model that exhibits diurnal switches between negative and positive feedback. In wild-type mice, GnRH neuron firing decreased in response to estradiol during negative feedback and increased during positive feedback. In contrast, both positive and negative responses to estradiol were absent in GnRH neurons from ERα−/− and ERα−/AA mice. ERE-dependent signaling is thus required to increase GnRH neuron firing to generate a GnRH/LH surge. Furthermore, ERE-dependent and -independent ERα signaling pathways both appear necessary to mediate estradiol negative feedback on serum LH levels, suggesting central and pituitary estradiol feedback may use different combinations of ERα signaling pathways.
GnRH NEURONS FORM the final common pathway in the neuroendocrine regulation of reproduction. GnRH stimulates the secretion of the pituitary gonadotropins LH and FSH. During most of the female reproductive cycle, ovarian estradiol exerts negative feedback to reduce gonadotropin release (1,2). In the late follicular phase, in response to sustained high levels of estradiol from preovulatory follicles, the action of estradiol switches from negative to positive feedback, resulting in a surge release of GnRH, that is likely due to increased GnRH neuron firing activity (3). The GnRH surge triggers a surge of LH secretion to initiate ovulation (4,5,6,7).
The α-isoform of the estrogen receptor-α (ERα) appears to be critical for estradiol feedback. ERα, but not ERβ, knockout mice have elevated LH, indicating a lack of estradiol negative feedback, and also lack the positive feedback LH surge response to estradiol (2,8). A neuron-specific ERα knockout mouse also lacks estradiol positive feedback (9), suggesting estradiol action is at least in part via a neural mechanism.
ERα exerts its cellular effects by interacting with multiple signaling pathways and transcription factors (10). In the classical genomic pathway, ERα translocates into the nucleus and binds and recruits cofactors to estrogen response element (ERE) regulatory sites in DNA to alter gene transcription (11,12). Alternatively, ERα may signal through ERE-independent genomic pathways via protein-protein interactions to alter transcription of genes at non-ERE DNA sites (13,14,15,16). Estradiol effects may also be triggered via membrane-initiated signaling cascades (17,18,19,20). Physiologically, these pathways are not mutually exclusive and may converge to modulate specific genes or cellular responses (17).
Previously, a mutant receptor (E07A/G208A; AA) with disrupted DNA binding but intact ERE-independent activity (13) was used to develop a nonclassical ER knock-in (NERKI) mouse model (16). The AA mutant ERα allele was bred onto an ERα null (ERαKO) background (ERα−/−) to generate a mouse model with isolated ERE-independent ERα signaling (ERα−/AA). This model was used to characterize the distinct in vivo roles for ERα pathways in mediating estradiol effects in bone (21,22), uterus (23), the male neuroendocrine axis, and reproductive behavior (24). Additionally, in the female, ERE-independent ERα signaling was found to be capable of conveying partial estradiol negative feedback on serum LH, whereas positive feedback required ERE-dependent ERα signaling (25).
The purpose of the current study was to examine whether ERE-dependent and/or ERE-independent ERα pathways are needed for estradiol feedback at the level of GnRH neuron firing activity. GnRH-green fluorescent protein (GFP) transgenic mice (26) were crossed with the NERKI and ERαKO mouse models to allow single-unit recordings of GnRH neurons in living brain slices. We used an estradiol treatment regimen in which wild-type (WT) mice exhibit daily switches between estradiol negative feedback, with low LH levels and low GnRH neuron activity, and estradiol positive feedback, with high LH levels and high GnRH neuron activity (3). Because the ERα−/AA and ERα−/− genotypes do not exhibit normal estrous cycles (8,9,16,25), it was necessary to use a surge-induction protocol. Ovariectomy and treatment with a constant physiological level of estradiol causes WT mice to exhibit daily switches between low GnRH neuron activity with low LH levels and high GnRH neuron activity with high LH levels (3). The present study indicates that ERE-dependent signaling is required for both negative and positive feedback regulation of GnRH neuron firing but that both ERE-dependent and ERE-independent signaling are needed for full suppression of serum LH.
Materials and Methods
Animals
All procedures were approved by the Animal Care and Use Committees of Northwestern University and the University of Virginia. Mice were maintained on a 14-h light, 10-h dark photoperiod (Virginia: lights off at 1630 h EST) with food (Northwestern: Harlan Teklad 7912, Indianapolis, IN; Virginia: Harlan 2916) and water available ad libitum.
Introduction of the GnRH-GFP transgene into NERKI and ERαKO mouse models
NERKI mice were on a 129SvJ background (16) and ERαKO mice on a C57BL/6 background (27). To produce congenic strains, the NERKI allele was previously outcrossed 14 generations to the ERαKO. GnRH-GFP mice (26) were transferred from the University of Virginia to Northwestern University, where they were outcrossed to NERKI and ERαKO colonies. It was assumed that the GnRH transgene was present on one chromosome. Thus, male progeny from this union were considered obligate heterozygotes for the GnRH-GFP transgene. Those heterozygous for NERKI (ERα+/AA) or ERαKO (ERα+/−) alleles were backcrossed to GnRH-GFP founder, and thus considered homozygous, female mice. Genetic test crosses were performed on male heterozygous NERKI (ERα+/AA) and ERαKO (ERα+/−) progeny by outcrossing them to WT C57BL/6J female mice (000664; Jackson Laboratory, Bar Harbor, ME). Male mice that transmitted the GnRH transgene to 100% of at least 20 pups were selected as founders for new NERKI and ERαKO colonies that stably express the GnRH-GFP transgene. To prepare additional female breeding stock, ERαKO GnRH-GFP founder males were outcrossed to the original GnRH-GFP founder female mice. Resultant female progeny heterozygous for the ERαKO allele (ERα+/−) were bred to either NERKI (ERα+/AA) or ERKO (ERα+/−) GnRH-GFP founder males identified by genetic test cross. This produced NERKI/ERαKO (ERα−/AA), homozygous ERαKO (ERα−/−), and WT littermate control (ERα+/+) mice expressing the GnRH-GFP transgene. Mice were transferred to the University of Virginia between 6 and 8 wk of age.
Animal surgery and treatment to induce estradiol negative and positive feedback
After a 1-month quarantine period, mice were ovariectomized (OVX) and either simultaneously implanted with an estradiol capsule (OVX+E: WT n = 15; ERα−/AA n = 16; ERα−/− n = 17 mice) or not treated further (OVX: WT n = 6; ERα−/AA n = 5; ERα−/− n = 5 mice). OVX+E treatment in this manner generates daily LH surges that peak around the time of lights off, with LH levels showing a significant increase beginning at 1500 h (3). For electrophysiology experiments, mice were euthanized at either 0900–1030 h (negative feedback groups) or 1430–1500 h (positive feedback groups). Uterine weight is known to increase in response to estradiol and was measured at the time of brain slice preparation to confirm endocrine status. The average weight of uteri from WT mice was significantly increased in response to estradiol treatment (OVX 31.2 ± 1.8 mg, OVX+E WT 117.1 ± 0.1 mg; P < 0.0001). In contrast, uteri from ERα−/− and ERα−/AA mice were hypoplastic and did not respond to estradiol with increased weight (P > 0.6), confirming previous results (23). For LH level measurements, trunk blood was collected either at the time of brain slice preparation or after CO2 anesthesia. Serum was separated and stored at −20 C until assay. Serum LH concentration was determined using a two-site mouse LH sandwich RIA at the Center for Research in Reproduction Ligand and Assay Core at the University of Virginia (28,29). All samples were run in the same assay with an intraassay coefficient of variation of 3.9%, and level of detection was 0.04 ng/ml; all samples read within the reportable range.
Brain slice preparation
Reagents were purchased from Sigma Chemical Co. (St. Louis, MO) unless noted. Two to four days after surgery, sagittal brain slices were prepared using slight modifications (3,30) of previous descriptions (31). For recording, slices were placed in a recording chamber and continuously superfused with oxygenated normal saline maintained at 30–32 C with an in-line heating unit (Warner Instruments, Hamden, CT). Estradiol treatment was solely in vivo, and estradiol was not present in any recording solutions.
Recordings
Targeted single-unit extracellular recordings (loose-patch) were used in this study because this configuration allows recording with minimal impact on the behavior of the recorded cell (31). GFP-GnRH neurons in the preoptic area and ventral hypothalamus were identified by brief illumination at 470 nm. Recording pipettes (1–3 mΩ) were filled with normal HEPES-buffered solution (31). Seal resistances (5–50 mΩ) were monitored at least every 30 min during recording. Recordings were made in voltage-clamp mode with the pipette holding potential at 0 mV, and signals were filtered at 10 kHz. After a recording stabilization period of several minutes after electrode placement, cells were recorded for 30–60 min. If no firing was observed after 60 min, 15 mm KCl was added to the bath solution to depolarize cells and induce firing. If no firing occurred with KCl treatment, the data were discarded because it was not possible to confirm recording integrity or cell health. Experiments were performed using an EPC 8 amplifier (HEKA Electronics, Lambrecht/Pfalz, Germany) with the Pulse Control XOP (Instrutech, Port Washington, NY) running in Igor Pro software (Wavemetrics, Lake Oswego, OR) on a G4 Macintosh computer. Signals were digitized at 16-bit resolution through an ITC-18 acquisition interface (Instrutech). Action currents (events), the membrane currents associated with action potential firing, were detected using Event Tracker software in Igor Pro. For simplicity, we use the terms firing rate or firing activity to refer to currents recorded extracellularly.
Recordings were performed between 1030 and 1400 h (negative feedback groups) or 1600 and 1930 h (positive feedback groups). No more than three cells per animal and only one cell per slice were recorded. Within-animal variance was of the same magnitude as between-animal variance, and thus cells are considered as independent observations. Similarly, values from d 2–4 after surgery were grouped together because variance among days was similar to variance within values for each day.
Data analysis
Each time the recording trace crossed the threshold for event detection, 10 msec worth of data centered on the threshold crossing were recorded and stored to a digital file. Events were detected offline using custom programs in Igor Pro (31) and binned at 1-min intervals. Binned data were transferred to Excel (Microsoft, Redmond, WA) and Prism4 (GraphPad, San Diego, CA) for further analysis. Data were analyzed for mean firing rate, percentage of time spent in quiescence, and longest duration of quiescent periods. Mean firing rate (in Hertz) was calculated by dividing the total number of events over the duration of recording in seconds. Quiescent time was defined as 1-min bins containing no more than one event. The percentage of bins that were quiescent (a measure of overall cell activity) and the longest duration of consecutive quiescent bins (a measure of firing pattern) were calculated for each cell. Data were log transformed and group means compared using two-way ANOVA followed by Bonferroni multiple-comparisons tests for effects of estradiol treatment and genotype. Data are presented as means ± sem. Statistical significance was set at P < 0.05.
Results
The daily surge estradiol replacement paradigm confirms LH data
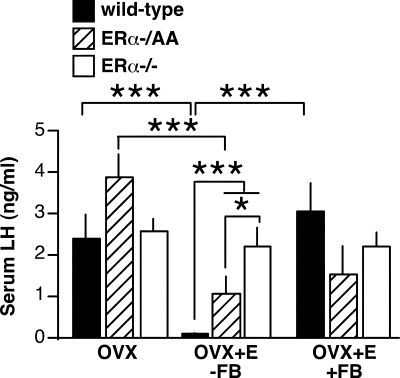
Previous experiments in which OVX WT, ERα−/AA, and ERα−/− mice were treated with an estradiol capsule for 1 wk, followed by a subsequent estradiol benzoate injection showed that ERE-independent ERα signaling partially restores negative but not positive feedback on LH levels (25). In the current study, a 2- to 4-d constant estradiol treatment regimen (3) was used to examine diurnal shifts between negative and positive feedback on LH levels in WT, ERα−/AA, and ERα−/− mice [two-way ANOVA, F(4,38) = 12.4; P < 0.0001). During negative feedback (measured between 0900 and 1030 h), LH levels in OVX+E WT mice showed a decrease in comparison with OVX animals (P < 0.001) (Fig. 1). Estradiol treatment significantly lowered LH in ERα−/AA mice (P < 0.01), although LH levels in OVX+E ERα−/AA animals were still significantly higher than in WT mice (P < 0.001). This result agrees with a previous report (25) indicating the ERE-independent ERα signaling pathway can convey partial estradiol negative feedback but that complete suppression of LH requires ERE-dependent ERα signaling as well. As previously observed (25,32), estradiol had no effect on LH levels in ERα−/− mice, indicating ERα is required to mediate estradiol negative feedback.
Figure 1.
ERE-independent ERα signaling partially restores negative feedback but not positive feedback on serum LH levels. Bars represent serum LH concentrations (mean ± sem). Serum samples were collected during the times of negative (−FB) or positive (+FB) feedback in WT (black), ERα−/AA (hatched), and ERα−/− (white). OVX n = 4–6 mice per group; OVX+E −FB n = 6–7 mice per group; OVX+E +FB n = 4 mice per group. *, P < 0.05; ***, P < 0.001.
During positive feedback (measured between 1600 and 1630 h), LH levels in WT OVX+E mice increased 32-fold (P < 0.001) compared with levels during negative feedback (Fig. 1). The LH increase was absent in ERα−/AA and ERα−/− mice, confirming a requirement for ERE-dependent signaling to convey estradiol positive feedback on serum LH (25). As the daily surge estradiol treatment paradigm recapitulated both the negative and positive feedback serum LH results previously reported for this genetic model, it was used for all experiments described here.
GnRH neuron firing is not altered by ligand-independent ERα effects
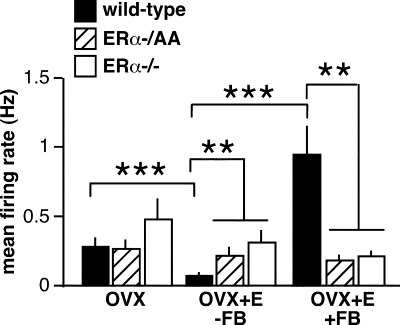
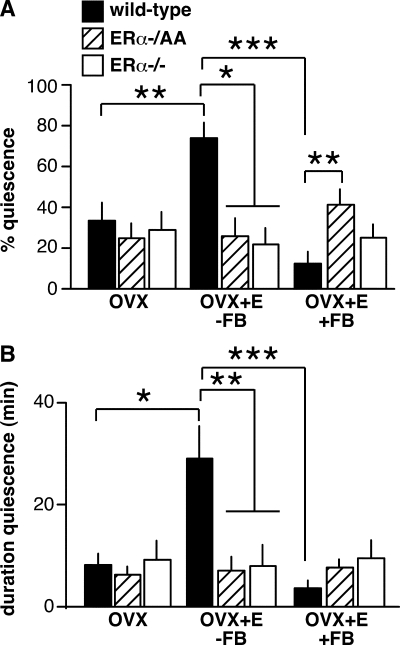
To investigate whether these changes in LH levels are associated with alterations in GnRH neuron firing activity, targeted single-unit recordings were used to monitor the firing of GnRH neurons from WT, ERα−/AA, and ERα−/− mice [two-way ANOVA: mean firing rate F(4,80) = 8.75, P < 0.0001; percentage of time in quiescence F(4,80) = 5.69, P = 0.0004; duration of quiescent time F(4,80) = 4.36, P = 0.0031). GnRH neurons from WT OVX mice not treated with estradiol show no diurnal changes in GnRH neuron firing activity (3). To examine whether genotype alone in the absence of gonadal steroids altered the firing of GnRH neurons, recordings of firing activity of OVX cells were collected during the time of negative feedback (WT n = 9 cells from six mice; ERα−/AA n = 9 cells from five mice; ERα−/− n = 11 cells from five mice) (Fig. 2). There was no effect of genotype on GnRH neurons from OVX mice on mean firing rate (Fig. 3, P > 0.9), percentage of time in quiescence (Fig. 4A, P > 0.6), or duration of quiescent time (Fig. 4B, P > 0.9). This suggests neither the long-term absence of ERα nor ligand-independent ERα signaling alters GnRH neuron firing activity in this model (33,34). Consistent with this interpretation, elevated serum LH was reported in a ligand-independent ER knock-in mouse, indicating ligand-independent functions of this receptor are not sufficient to convey negative feedback (35).
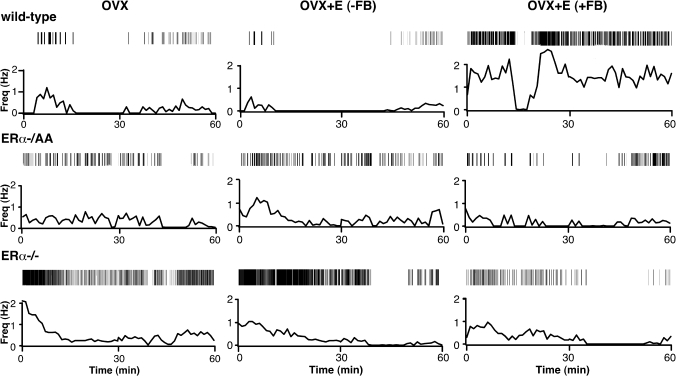
Figure 2.
Representative recordings of cells from OVX (left column), OVX+E negative feedback (−FB) (middle column) and positive feedback (+FB) (right column) WT (top row), ERα−/AA (middle row), and ERα−/− (bottom row) mice. Firing rate is displayed at 1-min intervals. Vertical lines at the top of each panel illustrate timing of individual action currents detected. Recordings were chosen as representative on the basis of mean firing rate.
Figure 3.
Summary of effects of genotype and estradiol treatment on mean firing rate of GnRH neurons (mean ± sem for each group). n = 9–12 cells per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Figure 4.
Summary of effects of genotype and estradiol treatment on percentage of time spent in quiescence (A) and longest duration of quiescent time (B) of GnRH neurons (mean ± sem for each group). n = 9–12 cells per group. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Estradiol negative feedback effects on GnRH neuron firing activity require ERE-dependent ERα signaling
LH levels in OVX+E and OVX mice during negative feedback confirmed that the isolated ERE-independent signaling pathway was able to mediate a significant, although incomplete, decrease in pituitary output (25) (Fig. 1). Estradiol-induced decreases in LH levels in WT mice were previously shown to correlate with decreased GnRH neuron activity (3), an observation repeated in the present study. In cells from WT mice (n = 10 cells from six mice), estradiol decreased mean firing rate (Fig. 3, P < 0.001) and increased measures of quiescence (Fig. 4, P < 0.05). In contrast, cells from ERα−/AA and ERα−/− mice showed no response to estradiol treatment in any of the firing parameters examined during the time of negative feedback (ERα−/AA n = 9 cells from four mice; ERα−/− n = 10 cells from five mice) (Figs. 3 and 4). Consistent with this, the firing of both ERα−/AA and ERα−/− OVX+E cells were greater than WT cells (P < 0.05, all parameters) and not different from each other. Thus, although the ERE-independent pathway is sufficient to mediate some degree of estradiol negative feedback on LH levels, ERE-dependent signaling is required for negative effects on GnRH neuron firing.
Estradiol positive feedback effects on GnRH neuron firing activity require ERE-dependent ERα signaling
The lack of an estradiol-induced LH surge in both ERα−/AA and ERα−/− mice (2,8,9,25) (Fig. 1) may be due to a failure of estradiol to increase GnRH neuron activity, GnRH release, and/or pituitary response. To examine the first possibility, firing activity of cells from OVX+E animals was recorded during the time of positive feedback (WT n = 10 cells from five mice; ERα−/AA n = 9 cells from six mice; ERα−/− n = 12 cells from six mice) (Fig. 2). As reported (3), the mean firing rate of WT OVX+E cells increased (Fig. 3, P < 0.001), and the percentage and duration of quiescence decreased (Fig. 4, P < 0.001) during positive compared with negative feedback. In contrast, there was no change in any measured parameter of GnRH neuron activity from OVX+E ERα−/AA or ERα−/− mice (Figs. 3 and 4). Moreover, the mean firing rates of OVX+E ERα−/AA and ERα−/− OVX+E GnRH neurons were lower than WT neurons during positive feedback (Fig. 3, P < 0.01). Compared with WT, ERα−/AA cells showed increased percentage of time in quiescence (Fig. 4A, P < 0.01) but no difference in the duration of quiescence (Fig. 4B). ERα−/− cells showed no differences in quiescence compared with either WT or ERα−/AA cells (Fig. 4). Thus, consistent with the lack of LH surges, the overall level of firing activity is significantly lower in these genotypes. These data indicate genomic ERE-dependent ERα signaling is required for estradiol to increase GnRH neuron firing during positive feedback.
Discussion
ERα signaling is crucial for estradiol negative and positive feedback regulation of LH. ERα can signal via ERE-dependent or -independent genomic, as well as ERE-independent nongenomic mechanisms (37). Previously, and as confirmed in Fig. 1, the ERE-independent ERα signaling pathway was shown to convey partial estradiol negative feedback on serum LH levels, whereas the ERE-dependent ERα signaling pathway was required for estradiol positive feedback regulation of LH (25). Here, the relative roles of these ERα signaling pathways in mediating estradiol effects on GnRH neuron firing activity were directly examined. In mice with isolated ERE-independent ERα signaling, no estradiol-induced changes in GnRH neuron activity were observed. These data suggest ERE-dependent ERα signaling is required for both positive and negative estradiol feedback effects on GnRH neuron activity in this animal model. Together with the LH data, these results suggest that negative feedback on serum LH levels is differentially mediated by both ERE-dependent and ERE-independent signaling. ERE-dependent ERα signaling appears to suppress LH with a concomitant reduction in GnRH neuron activity. In contrast, ERE-independent ERα signaling partially suppresses serum LH via a mechanism not reflected by decreased GnRH neuron firing.
The ERE-independent pathway was able to convey significant but incomplete suppression of serum LH but not GnRH neuron firing activity. There are several explanations for this dissociation. First, ERE-independent signaling may act at the pituitary to decrease responsiveness to GnRH. In this regard, LH release is rapidly lost after injection of estradiol benzoate in OVX ewes; this loss occurs before the loss of GnRH pulses, consistent with differential action of estradiol on the release of these two hormones (38). In male mice, steroid milieux producing reduced serum LH release but increased GnRH neuron activity have been reported, again suggesting differential regulation (39). In a previous report, female mice with isolated ERE-independent ERα signaling did not exhibit a decreased pituitary LH response to exogenous GnRH (25). That study, however, used a single supraphysiological dose of GnRH, which may not have revealed decreased pituitary sensitivity to endogenous or pulsatile GnRH stimulation. Second, although neural activity has been associated with hormone release (40,41), ERE-independent signaling may alter the amount of GnRH released per action potential. Estradiol negative feedback can reduce GnRH pulse amplitude (38,42,43), which may combine with negative actions on pituitary gonadotropes to reduce gonadotropin release (38,44,45,46,47). Third, the ERα−/AA genotype could alter the coordination of GnRH neurons that is likely needed to produce pulses of hormone that reach the pituitary. Finally, neuroanatomical changes such as alterations in glial ensheathment of axon terminals at the median eminence (48) may play a role in decreasing GnRH release in the absence of a decrease in GnRH neuron activity. Thus, altered GnRH release may not have been reflected by GnRH neuron firing and the present methodology. It is important to point out, however, that GnRH neuron activity did accurately parallel LH release in both the WT and ERα−/− conditions.
The lack of normal cycles in the genetic models altering estradiol signaling mandated use of a GnRH/LH surge induction protocol to gather these data. We chose a model in which removal of the ovaries and replacement with a constant physiological estradiol level provided via an implant produces daily switches between negative and positive feedback (3). Daily surges have been demonstrated in several rodent species (3,49,50) and are postulated to reveal that the central signal for ovulation can occur on a daily basis if estradiol levels are sufficient. Consistent with this, the rise in estradiol production by the developing follicles to levels sufficient to induce a GnRH/LH surge appears to be an important factor contributing to the difference in length of the follicular phase in mammals. This daily surge model has now been used to examine several parameters associated with surge induction including GnRH activity, neurotransmission, and neuromodulation (3,51,52). Although no differences have been observed in any of these parameters within the day range used (daily surge d 2–4), it is important to point out that we cannot exclude the possibility that these daily LH surges differ in mechanism from one another. It is also possible that the mechanisms of negative and/or positive feedback in this model differ from those during the natural cycle.
The location of ERα needed for feedback responses of GnRH neurons is under debate. Although ERα has been shown to be expressed in immortalized GnRH neuronal cell lines (53,54), it has not yet been detected in native adult GnRH neurons (55). Estradiol effects may be conveyed indirectly to GnRH neurons through transsynaptic contacts with ERα-expressing neurons, astrocytes, or other glial or endothelial cells (2,56). The anteroventral periventricular area, in particular, appears to be a source of ERα-expressing neural afferents that is critical for GnRH regulation (9,57,58,59). Direct action of estradiol on GnRH neurons may also be possible via ERβ (60,61), which is expressed in all genotypes used in the current study. The lack of an effect of estradiol on GnRH neuron firing in the ERα−/− indicates a requirement for ERα signaling but does not exclude a potential modulatory role for ERβ (62), for example via rapid effects of estradiol on GnRH neurons (63,64,65,66).
A role for synaptic inputs in transmitting estradiol feedback is supported by recent work indicating estradiol feedback on GnRH neuron firing activity may be mediated through changes in γ-aminobutyric acid (GABA) transmission. Although controversial (67,68), there is considerable evidence that GABAA receptor activation can excite GnRH neurons (69,70,71). Furthermore, estradiol decreases GABA transmission to GnRH neurons during negative feedback but increases it during positive feedback (51). In addition, neuromodulators such as kisspeptin (72), vasoactive intestinal polypeptide (52,73,74), gonadotropin-inhibitory hormone (75), vasopressin (76,77), catecholamines (78), and neuropeptide Y (36) have been implicated in negative feedback and/or surge generation. Thus, a likely primary role for ERE-dependent signaling is to drive changes in neurotransmission via fast synaptic transmission and/or neuromodulatory factors that appear critical to negative and positive feedback regulation of GnRH neurons.
In summary, the present studies demonstrate estradiol positive and negative feedback on GnRH neuron firing activity require signaling via ERα, and specifically via the ERE-dependent genomic pathway. Furthermore, estradiol negative feedback is distinctly conveyed through ERE-dependent and -independent ERα signaling pathways and dissociable at the levels of GnRH neuron firing activity and serum LH. Future studies will be necessary to elucidate the sites of estrogen action that lead to LH suppression and to identify the molecular targets of ERE-dependent and ERE-independent pathways in the hypothalamus and pituitary.
Acknowledgments
ERαKO mice were a generous gift from Dr. Pierre Chambon. We thank Debra Fisher, Lisa Hurley, and Lisa Fisher for excellent technical assistance and Dr. Jon E. Levine for comments on this manuscript.
Footnotes
This work was supported by National Institute of Child Health and Human Development/National Institutes of Health Grants R01 HD41469 (S.M.M.) and P01 HD21921 (J.L.J.) and Cooperative Agreement U54 HD28934 (Center for Research in Reproduction Ligand and Assay Core at the University of Virginia). C.A.C. was supported by National Institute of Neurological Disorders and Stroke National Research Service Award F31 NS53253, and C.G-K. was supported by National Institutes of Health Training Grant T32 GM008061.
Disclosure Statement: The authors have nothing to disclose.
First Published Online July 17, 2008
See editorial p. 5325.
Abbreviations: ERα, Estrogen receptor-α; ERE, estrogen response element; ERαKO, ERα null; GABA, γ-aminobutyric acid; GFP, green fluorescent protein; NERKI, nonclassical ER knock-in; OVX, ovariectomized; WT, wild-type.
References
- Levine JE 1997 New concepts of the neuroendocrine regulation of gonadotropin surges in rats. Biol Reprod 56:293–302 [DOI] [PubMed] [Google Scholar]
- Herbison AE 1998 Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons. Endocr Rev 19:302–330 [DOI] [PubMed] [Google Scholar]
- Christian CA, Mobley JL, Moenter SM 2005 Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA 102:15682–15687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar DK, Chiappa SA, Fink G, Sherwood NM 1976 Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature 264:461–463 [DOI] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Locatelli A, Karsch FJ 1991 Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology 129:1175–1182 [DOI] [PubMed] [Google Scholar]
- Pau KY, Berria M, Hess DL, Spies HG 1993 Preovulatory gonadotropin-releasing hormone surge in ovarian-intact rhesus macaques. Endocrinology 133:1650–1656 [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Thomas GB, Yao B, Cummins JT 1987 GnRH secretion throughout the ovine estrous cycle. Neuroendocrinology 46:82–88 [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS 1999 Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20:358–417 [DOI] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone H-J, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE 2006 Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley BW, Tsai MJ 1992 Molecular pathways of steroid receptor action. Biol Reprod 46:163–167 [DOI] [PubMed] [Google Scholar]
- Tsai MJ, O'Malley BW 1994 Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem 63:451–486 [DOI] [PubMed] [Google Scholar]
- McKenna NJ, Lanz RB, O'Malley BW 1999 Nuclear receptor coregulators: cellular and molecular biology. Endocr Rev 20:321–344 [DOI] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Weiss J, Chien PY, Gehm BD, Jameson JL 2001 Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J Biol Chem 276:13615–13621 [DOI] [PubMed] [Google Scholar]
- Ray A, Prefontaine KE, Ray P 1994 Down-modulation of interleukin-6 gene expression by 17β-estradiol in the absence of high affinity DNA binding by the estrogen receptor. J Biol Chem 269:12940–12946 [PubMed] [Google Scholar]
- Stein B, Yang MX 1995 Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-κB and C/EBPβ. Mol Cell Biol 15:4971–4979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL 2002 An estrogen receptor (ER)α deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol 16:2188–2201 [DOI] [PubMed] [Google Scholar]
- Ronnekleiv OK, Kelly MJ 2005 Diversity of ovarian steroid signaling in the hypothalamus. Front Neuroendocrinol 26:65–84 [DOI] [PubMed] [Google Scholar]
- Song RX, McPherson RA, Adam L, Bao Y, Shupnik M, Kumar R, Santen RJ 2002 Linkage of rapid estrogen action to MAPK activation by ERα-Shc association and Shc pathway activation. Mol Endocrinol 16:116–127 [DOI] [PubMed] [Google Scholar]
- Navarro CE, Saeed SA, Murdock C, Martinez-Fuentes AJ, Arora KK, Krsmanovic LZ, Catt KJ 2003 Regulation of cyclic adenosine 3′,5′-monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotrophin-releasing hormone neurons. Mol Endocrinol 17:1792–1804 [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER 2006 Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol 20:1996–2009 [DOI] [PubMed] [Google Scholar]
- Syed FA, Fraser DG, Spelsberg TC, Rosen CJ, Krust A, Chambon P, Jameson JL, Khosla S 2007 Effects of loss of classical estrogen response element signaling on bone in male mice. Endocrinology 148:1902–1910 [DOI] [PubMed] [Google Scholar]
- Syed FA, Modder UI, Fraser DG, Spelsberg TC, Rosen CJ, Krust A, Chambon P, Jameson JL, Khosla S 2005 Skeletal effects of estrogen are mediated by opposing actions of classical and nonclassical estrogen receptor pathways. J Bone Miner Res 20:1992–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, Korach KS, Weiss J, Jameson JL 2006 Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor α binding to classical estrogen response elements. J Biol Chem 281:26683–26692 [DOI] [PubMed] [Google Scholar]
- McDevitt MA, Glidewell-Kenney C, Weiss J, Chambon P, Jameson JL, Levine JE 2007 Estrogen response element-independent estrogen receptor (ER)-α signaling does not rescue sexual behavior but restores normal testosterone secretion in male ERα knockout mice. Endocrinology 148:5288–5294 [DOI] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL 2007 Nonclassical estrogen receptor alpha signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci USA 104:8173–8177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter KJ, Song WJ, Sampson TL, Wuarin JP, Saunders JT, Dudek FE, Moenter SM 2000 Genetic targeting of green fluorescent protein to gonadotropin-releasing hormone neurons: characterization of whole-cell electrophysiological properties and morphology. Endocrinology 141:412–419 [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M 2000 Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 2000:4277–4291 [DOI] [PubMed] [Google Scholar]
- Krieg RJ, Tokieda K, Chan JC, Veldhuis JD 2000 Impact of uremia on female reproductive cyclicity, ovulation, and luteinizing hormone in the rat. Kidney Int 58:569–574 [DOI] [PubMed] [Google Scholar]
- Haavisto AM, Pettersson K, Bergendahl M, Perheentupa A, Roser JF, Huhtaniemi I 1993 A supersensitive immunofluorometric assay for rat luteinizing hormone. Endocrinology 132:1687–1691 [DOI] [PubMed] [Google Scholar]
- Chu Z, Moenter SM 2005 Endogenous activation of metabotropic glutamate receptors modulates GABAergic transmission to gonadotropin-releasing hormone neurons and alters their firing rate: a possible local feedback circuit. J Neurosci 25:5740–5749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunemaker CS, DeFazio RA, Moenter SM 2002 Estradiol-sensitive afferents modulate long-term episodic firing patterns of GnRH neurons. Endocrinology 143:2284–2292 [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Haisenleder DJ, Lubahn DB, Rissman EF 1999 Steroid feedback on gonadotropin release and pituitary gonadotropin subunit mRNA in mice lacking a functional estrogen receptor alpha. Endocrine 11:137–143 [DOI] [PubMed] [Google Scholar]
- Newton CJ, Buric R, Trapp T, Brockmeier S, Pagotto U, Stalla GK 1994 The unliganded estrogen receptor (ER) transduces growth factor signals. J Steroid Biochem Mol Biol 48:481–486 [DOI] [PubMed] [Google Scholar]
- White R, Sjoberg M, Kalkhoven E, Parker MG 1997 Ligand-independent activation of the oestrogen receptor by mutation of a conserved tyrosine. EMBO J 16:1427–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkevicius KW, Burdette JE, Woloszyn K, Hewitt SC, Hamilton K, Sugg SL, Temple KA, Wondisford FE, Korach KS, Woodruff TK, Greene GL 2008 An estrogen receptor α knock-in mutation provides evidence of ligand-independent signaling and allows modulation of ligand-induced pathways in vivo. Endocrinology 149:2970–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno M, Gearing M, Terasawa E 2000 The role of neuropeptide Y in the progesterone-induced luteinizing hormone-releasing hormone surge in vivo in ovariectomized female rhesus monkeys. Endocrinology 141:1772–1779 [DOI] [PubMed] [Google Scholar]
- Hall JM, Couse JF, Korach KS 2001 The multifaceted mechanisms of estradiol and estrogen receptor signaling. J Biol Chem 276:36869–36872 [DOI] [PubMed] [Google Scholar]
- Caraty A, Locatelli A, Martin GB 1989 Biphasic response in the secretion of gonadotrophin-releasing hormone in ovariectomized ewes injected with oestradiol. J Endocrinol 123:375–382 [DOI] [PubMed] [Google Scholar]
- Pielecka J, Moenter SM 2006 Effect of steroid milieu on gonadotropin-releasing hormone-1 neuron firing pattern and luteinizing hormone levels in male mice. Biol Reprod 74:931–937 [DOI] [PubMed] [Google Scholar]
- Nunemaker CS, DeFazio RA, Geusz ME, Herzog ED, Pitts GR, Moenter SM 2001 Long-term recordings of networks of immortalized GnRH neurons reveal episodic patterns of electrical activity. J Neurophysiol 86:86–93 [DOI] [PubMed] [Google Scholar]
- Dutton A, Dyball RE 1979 Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol 290:433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar DK, Fink G 1980 Luteinizing hormone releasing factor in pituitary stalk plasma from long-term ovariectomized rats: effects of steroids. J Endocrinol 86:511–524 [DOI] [PubMed] [Google Scholar]
- Evans NP, Dahl GE, Glover BH, Karsch FJ 1994 Central regulation of pulsatile gonadotropin-releasing hormone (GnRH) secretion by estradiol during the period leading up to the preovulatory GnRH surge in the ewe. Endocrinology 134:1806–1811 [DOI] [PubMed] [Google Scholar]
- Nakai Y, Plant TM, Hess DL, Keogh EJ, Knobil E 1978 On the sites of the negative and positive feedback actions of estradiol in the control of gonadotropin secretion in the rhesus monkey. Endocrinology 102:1008–1014 [DOI] [PubMed] [Google Scholar]
- Libertun C, Orias R, McCann SM 1974 Biphasic effect of estrogen on the sensitivity of the pituitary to luteinizing hormone-releasing factor (LRF). Endocrinology 94:1094–1100 [DOI] [PubMed] [Google Scholar]
- Strobl FJ, Levine JE 1988 Estrogen inhibits luteinizing hormone (LH), but not follicle-stimulating hormone secretion in hypophysectomized pituitary-grafted rats receiving pulsatile LH-releasing hormone infusions. Endocrinology 123:622–630 [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT 1984 Direct pituitary effects of estrogen and progesterone on gonadotropin secretion in the ovariectomized ewe. Neuroendocrinology 39:267–274 [DOI] [PubMed] [Google Scholar]
- Prevot V 2002 Glial-neuronal-endothelial interactions are involved in the control of GnRH secretion. J Neuroendocrinol 14:247–255 [DOI] [PubMed] [Google Scholar]
- Legan SJ, Karsch FJ 1975 A daily signal for the LH surge in the rat. Endocrinology 96:57–62 [DOI] [PubMed] [Google Scholar]
- Norman RL, Blake CA, Sawyer CH 1973 Estrogen-dependent 24-hour periodicity in pituitary LH release in the female hamster. Endocrinology 93:965–970 [DOI] [PubMed] [Google Scholar]
- Christian CA, Moenter SM 2007 Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. J Neurosci 27:1913–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian CA, Moenter SM 2008 Vasoactive intestinal polypeptide can excite gonadotropin-releasing hormone neurons in a manner dependent on estradiol and gated by time of day. Endocrinology 149:3130–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy D, Angelini NL, Belsham DD 1999 Estrogen directly respresses gonadotropin-releasing hormone (GnRH) gene expression in estrogen receptor-α (ERα)- and ERβ-expressing GT1-7 GnRH neurons. Endocrinology 140:5045–5053 [DOI] [PubMed] [Google Scholar]
- Radovick S, Ticknor CM, Nakayama Y, Notides AC, Rahman A, Weintraub BD, Cutler Jr GB, Wondisford FE 1991 Evidence for direct estrogen regulation of the human gonadotropin-releasing hormone gene. J Clin Invest 88:1649–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, Pape JR 2001 New evidence for estrogen receptors in gonadotropin-releasing hormone neurons. Front Neuroendocrinol 22:292–308 [DOI] [PubMed] [Google Scholar]
- Langub Jr MC, Watson Jr RE 1992 Estrogen receptor-immunoreactive glia, endothelia, and ependyma in guinea pig preoptic area and median eminence: electron microscopy. Endocrinology 130:364–372 [DOI] [PubMed] [Google Scholar]
- Watson RE, Langub MC, Engle MG, Maley BE 1995 Estrogen-receptive neurons in the anteroventral periventricular nucleus are synaptic targets of the suprachiasmatic nucleus to the gonadotropin-releasing hormone system: combined tracing and light and electron microscopic immunocytochemical studies. Brain Res 689:254–264 [DOI] [PubMed] [Google Scholar]
- Ottem EN, Godwin JG, Krishnan S, Petersen SL 2004 Dual-phenotype GABA/glutamate neurons in adult preoptic area: sexual dimorphism and function. J Neurosci 24:8097–8105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Carr AM, Zee MC, Lorang D 1996 Ovarian steroid regulation of estrogen and progesterone receptor messenger ribonucleic acid in the anteroventral periventricular nucleus of the rat. J Neuroendocrinol 8:45–56 [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabas K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z 2001 Estrogen receptor-β immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 142:3261–3264 [DOI] [PubMed] [Google Scholar]
- Skynner MJ, Sim JA, Herbison AE 1999 Detection of estrogen receptor α and β messenger ribonucleic acids in adult gonadotropin-releasing hormone neurons. Endocrinology 140:5195–5201 [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Walker VR, Korach KS 2003 Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) null mice reveals hypergonadism and endocrine sex reversal in females lacking ERα but not ERβ. Mol Endocrinol 17:1039–1053 [DOI] [PubMed] [Google Scholar]
- Temple JL, Laing E, Sunder A, Wray S 2004 Direct action of estradiol on gonadotropin-releasing hormone-1 neuronal activity via a transcription-dependent mechanism. J Neurosci 24:6326–6333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagrange AH, Ronnekleiv OK, Kelly MJ 1995 Estradiol-17β and μ-opioid peptides rapidly hyperpolarize GnRH neurons: a cellular mechanism of negative feedback? Endocrinology 136:2341–2344 [DOI] [PubMed] [Google Scholar]
- Abraham IM, Han SK, Todman MG, Korach KS, Herbison AE 2003 Estrogen receptor β mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci 23:5771–5777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe H, Terasawa E 2005 Firing pattern and rapid modulation of activity by estrogen in primate luteinizing hormone releasing hormone-1 neurons. Endocrinology 146:4312–4320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Abraham IM, Herbison AE 2002 Effect of GABA on GnRH neurons switches from depolarization to hyperpolarization at puberty in the female mouse. Endocrinology 143:1459–1466 [DOI] [PubMed] [Google Scholar]
- Han SK, Todman MG, Herbison AE 2004 Endogenous GABA release inhibits the firing of adult gonadotropin-releasing hormone neurons. Endocrinology 145:495–499 [DOI] [PubMed] [Google Scholar]
- DeFazio RA, Heger S, Ojeda SR, Moenter SM 2002 Activation of A-type γ-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol 16:2872–2891 [DOI] [PubMed] [Google Scholar]
- Moenter SM, DeFazio RA 2005 Endogenous γ-aminobutyric acid can excite gonadotropin-releasing hormone neurons. Endocrinology 146:5374–5379 [DOI] [PubMed] [Google Scholar]
- Yin C, Tanaka N, Kato M, Sakuma Y 2006 GABA depolarizes GnRH neurons isolated from adult GnRH-EGFP transgenic rats. Neurosci Res 55S:S159 [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA 2006 Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 26:6687–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harney JP, Scarbrough K, Rosewell KL, Wise PM 1996 In vivo antisense antagonism of vasoactive intestinal peptide in the suprachiasmatic nuclei causes aging-like changes in the estradiol-induced luteinizing hormone and prolactin surges. Endocrinology 137:3696–3701 [DOI] [PubMed] [Google Scholar]
- van der Beek EM, Swarts HJ, Wiegant VM 1999 Central administration of antiserum to vasoactive intestinal peptide delays and reduces luteinizing hormone and prolactin surges in ovariectomized, estrogen-treated rats. Neuroendocrinology 69:227–237 [DOI] [PubMed] [Google Scholar]
- Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R 2006 Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci USA 103:2410–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm IF, van der Beek EM, Wiegant VM, Buijs RM, Kalsbeek A 1999 Vasopressin induces a luteinizing hormone surge in ovariectomized, estradiol-treated rats with lesions of the suprachiasmatic nucleus. Neuroscience 93:659–666 [DOI] [PubMed] [Google Scholar]
- Miller BH, Olson SL, Levine JE, Turek FW, Horton TH, Takahashi JS 2006 Vasopressin regulation of the proestrous luteinizing hormone surge in wild-type and Clock mutant mice. Biol Reprod 75:778–784 [DOI] [PubMed] [Google Scholar]
- Temel S, Lin W, Lakhlani S, Jennes L 2002 Expression of estrogen receptor-α and cFos in norepinephrine and epinephrine neurons of young and middle-aged rats during the steroid-induced luteinizing hormone surge. Endocrinology 143:3974–3983 [DOI] [PubMed] [Google Scholar]