Abstract
In the setting of insulin resistance, agonists of peroxisome proliferator-activated receptor (PPAR)-γ restore insulin action in muscle and promote lipid redistribution. Mice with muscle-specific knockout of PPARγ (MuPPARγKO) develop excess adiposity, despite reduced food intake and normal glucose disposal in muscle. To understand the relation between muscle PPARγ and lipid accumulation, we studied the fuel energetics of MuPPARγKO mice. Compared with controls, MuPPARγKO mice exhibited significantly increased ambulatory activity, muscle mitochondrial uncoupling, and respiratory quotient. Fitting with this latter finding, MuPPARγKO animals compared with control siblings exhibited a 25% reduction in the uptake of the fatty acid tracer 2-bromo-palmitate (P < 0.05) and a 13% increase in serum nonesterified fatty acids (P = 0.05). These abnormalities were associated with no change in AMP kinase (AMPK) phosphorylation, AMPK activity, or phosphorylation of acetyl-CoA carboxylase in muscle and occurred despite increased expression of fatty acid transport protein 1. Palmitate oxidation was not significantly altered in MuPPARγKO mice despite the increased expression of several genes promoting lipid oxidation. These data demonstrate that PPARγ, even in the absence of exogenous activators, is required for normal rates of fatty acid uptake in oxidative skeletal muscle via mechanisms independent of AMPK and fatty acid transport protein 1. Thus, when PPARγ activity in muscle is absent or reduced, there will be decreased fatty acid disposal leading to diminished energy utilization and ultimately adiposity.
ACTIVATION OF THE transcription factor peroxisome proliferator-activated receptor (PPAR)-γ by thiazolidinediones (TZDs) exerts beneficial effects on insulin sensitivity, hyperglycemia (1), and diabetes prevention (2). PPARγ expression is highest in adipose tissue (3), in which direct action of PPARγ up-regulates genes involved in fatty acid uptake and storage (3), ultimately promoting an increase in fat mass. The improvement of insulin-stimulated glucose disposal that occurs with use of the TZDs, in contrast, is localized primarily to the skeletal muscle (4,5,6). It has therefore been postulated that the insulin-sensitizing effects of the TZDs on skeletal muscle are largely mediated indirectly via changes in the secreted products of adipose tissue (1,7), including altered adipokine expression and reduced fatty acid release (3).
PPARγ is expressed at low levels in the skeletal muscle of humans and rodents (4,8). In studies using transgenic mice with muscle-selective deletion of PPARγ (MuPPARγKO), it has been shown that muscle PPARγ contributes to the TZD-induced insulin sensitization in this tissue (9) but is not required for whole-body improvements in glucose levels and insulin sensitivity induced by TZD treatment (9,10). More interestingly, muscle PPARγ also contributes to normal lipid homeostasis, even in the absence of external ligand stimulation (9,10). Therein MuPPARγKO mice unexpectedly develop elevated serum lipids (9), enlarged fat pads (9,10), obesity on high-fat diet (10), and whole-body insulin resistance (9,10). Lipid overload appears to be a primary event in the insulin resistance pathology of these mice because adiposity is observed before the development of overt hyperglycemia or hyperinsulinemia and the obesity in these mice develops despite reduced dietary intake (10).
These latter findings suggest that PPARγ in muscle contributes significantly to energy homeostasis. This notion is further supported by the observation that the intramyocellular lipid content of skeletal muscle decreases upon whole-body treatment with TZDs (11,12), although the mechanism is unclear. PPARγ in other tissues is known to regulate energy homeostasis, including genes involved in fatty acid transport and mitochondrial uncoupling (13,14,15,16,17). The two other members of the PPAR family, PPARα and PPARδ, are likewise important in energy homeostasis. PPARα up-regulates genes involved in lipid oxidation in the liver and heart (18,19) and has been suggested to do the same in skeletal muscle (20,21), and PPARδ promotes catabolism of carbohydrates in liver and lipids in skeletal muscle (22,23,24).
To better define the role of PPARγ in muscle, we sought to determine the mechanism producing obesity and lipid overload in MuPPARγKO mice. To this end, we investigated the energetics, uncoupling, and fatty acid handling of skeletal muscle from these animals. We focused on younger mice in the absence of TZD treatment to get at primary mechanisms reflecting the endogenous activity of muscle PPARγ in the absence of external stimulation and before the development of overt obesity, glucose intolerance, or hyperinsulinemia. We found that under these conditions, muscle PPARγ does indeed play a role in control of muscle lipid update that can have a significant effect on whole-body energy expenditure when disrupted.
Materials and Methods
Animals
Mice with muscle selective loss of PPARγ (MuPPARγKO) were bred on a mixed background and genotyped as described (10). All mice carried two copies of the PPARγ-loxP allele. Breedings were set up such that about half of the mice inherited the muscle creatine kinase-cre (MCK-cre) allele, thus acquiring muscle-specific loss of PPARγ (10). Sibling mice carrying no MCK-cre allele, termed Flox, served as controls. Mice were of the F2 or subsequent generations. Animals were housed in pathogen-free facilities and exposed to a 12-h light, 12-h dark cycle. The mice were fed either standard rodent chow containing 8% fat by weight or high-fat chow containing 29.3% fat, 25.2% protein, and 28.8% carbohydrate by weight (Harlan Teklad, Madison, WI). In some experiments, animals on high-fat diets were treated with rosiglitazone (ROSI) delivered as a 0.006% food admixture amounting to 3.9 ± 0.1 mg/kg·d or by gavage at 5 mg/kg·d using 0.5 methylcellulose as vehicle. These dosing approaches led to systemic TZD action as evidenced by increased white and brown fat mass and/or reduced random blood glucose in ob/ob mice (data not shown). Plasma nonesterified fatty acid (NEFA) and triglyceride levels were measured as described (10). All protocols were approved by the relevant Animal Care and Use Committee of Joslin Diabetes Center or the University of Iowa, and adhered to National Institute of Health guidelines.
Energetic monitoring
Indirect calorimetry and locomotor activity were simultaneously measured in 10-wk-old male mice as described (25) using the comprehensive laboratory animal monitoring system equipped with Oxymax and OPTO-M3 sensors (Columbus Instruments, Columbus, OH). The mice were acclimated to the monitoring cages for 48 h before initiating measurements. Ambulatory activity was estimated by summing the number of sequential beam interruptions measured independently in orthogonal horizontal directions. Indirect calorimetry was performed through measurement of O2 consumption and CO2 production. The respiratory exchange ratio (RER) was taken as the ratio of CO2 production to O2 consumption. Heat production was estimated as (3.815 + 1.232 · RER) · oxygen consumption (VO2) (26).
Mitochondrial uncoupling
Mitochondria were isolated as described (27) from tissues collected from mice euthanized at age 37–48 wk (mean control 42.9 ± 1.5, knockout 42.7 ± 1.7). Mice weights were not different between the two genotypes, as expected on normal chow (10). Mitochondrial respiration was measured using a Clark miniature oxygen electrode at 37 C in fatty-acid-free respiratory media. Mitochondrial inner membrane potential was determined from electrode-based measurement of tetraphenyl phosphonium concentrations external to the mitochondrial matrix according to the Nernst equation, Δ Ψ = RT/zF · ln Ce/Ci, where z = valence of cation, R designates the gas constant, T the temperature, and F Faraday’s constant. Mitochondria were incubated under conditions wherein H+ conductance is solely proton leak dependent. Succinate (5 mm) was the fuel source. The membrane potential was varied through incremental amounts of malonate. The membrane potential and oxygen consumption were recorded simultaneously, thus allowing determination of the degree of proton leak for any given membrane potential (27).
AMP kinase (AMPK) protein content, activity, and Thr172 phosphorylation
Gastrocnemius muscle samples were rapidly dissected and processed for determination of AMPK signaling components. Mice were fasting for 10–12 h at the time of euthanasia. Lysates and immunoprecipitates were prepared as described (28) for measurement of AMPK isoform-specific levels and activities. Phosphospecific antibodies were used to determine levels of AMPK Thr172 phosphorylation (28) and acetyl-CoA carboxylase (ACC) phosphorylation (29). Studies were performed on male mice maintained on normal chow diet at 22.0 ± 3.6 wk of age or after 7 wk of high-fat diet with or without ROSI admixture at 6 months of age.
Fatty acid uptake and oxidation
Fatty acid uptake and oxidation were studied in male mice at 22.0 ± 3.6 wk of age. After a 10- to 12-h fast, mice were euthanized, and soleus and extensor digitorum longus (EDL) muscles were rapidly and carefully dissected free and placed in preoxygenated incubation buffer at 37 C with ongoing exposure of the liquid to 95% O2-5% CO2. The incubation buffer contained 5.6 mm glucose, 0.2 mm palmitic acid, and 0.45 mm (3 g/dl) fatty acid-free BSA prepared in Krebs-Ringer bicarbonate buffer [117 mm NaCl, 4.7 mm KCl, 2.5 mm CaCl2, 1.2 mm KH2PO4, 1.2 mm MgSO4, 24.6 mm NaHCO3 (pH 7.4)]. Palmitic acid dissolved in ethanol was added slowly to actively stirred albumin containing buffer. The solution rapidly clarified, and the final ethanol concentration was less than 0.16% (vol/vol). After 30 min the muscle explants were transferred to tubes containing fresh incubation buffer that also contained 0.2 μCi/ml [1-14C] palmitic acid (PerkinElmer Life Sciences, Inc., Boston, MA) and 0.2 μCi/ml [9,10-3H]R-2-bromopalmitic acid (American Radiolabeled Chemicals, Inc., St. Louis, MO). After 10 min of incubation with ongoing O2 exposure, the tubes were capped for 50 min. The muscle explants were then moved to ice-cold incubation buffer for 10 min and subsequently snap frozen, weighed, and dissolved in 1 m NaOH and neutralized with 1 m HCl. Isotope-specific radioactivity in the resultant supernatant was determined by dual-channel scintillation counting. CO2 production was determined by capture in center wells containing hyamine hydroxide of 14CO2 counts released by acidification of the incubation media, followed by scintillation counting.
Measurement of gene expression
RNA was isolated from mixed hind-limb skeletal muscle as described (10). Real-time PCR was performed using a MX-3000 (Stratagene, La Jolla, CA). Total RNA was reverse transcribed and amplified in a one-step reaction using full-velocity SYBR Green QRT-PCR master mix kit (Stratagene). Gene-specific primers are published as supplemental data on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org. β-Actin was used as a reference control. All samples were run in triplicates. Size-based identities of PCR products were confirmed by agarose gel electrophoresis.
Statistics
Values are reported as group means ± se. Comprehensive laboratory animal monitoring system data were analyzed by repeated-measure ANOVA (30), assessing for interaction between genotype and measured parameters, treating time of measurement as a within-subject factor and siblingship as a between-subject factor. Uncoupling data were analyzed for interaction between genotype and measured responses by multivariate ANOVA using Wilks’ λ test. Pairwise comparisons were performed by t test, pairing by siblingship when possible.
Results
Energetic responses to high-fat diet
When placed on a high-fat diet for 7 wk, MuPPARγKO mice gain an excess of 2.8 g weight over 7 wk, equivalent to an extra 8% of their initial body weight, compared with Flox control mice, with the excess weight accounted entirely by an increase in whole-body triglyceride (10). This occurred despite decreased dietary intake in the MuPPARγKO mice. Given that the sole genetic defect in MuPPARγKO mice is localized to muscle, we hypothesized that the excess weight gain was due to either inherently impaired muscle energy expenditure or a loss of locomotor activity induced by loss of muscle PPARγ. To test these possibilities, MuPPARγKO mice and sibling Flox controls (n = 4/group) were placed in metabolic monitoring cages. Measurement of locomotor activity and indirect calorimetry were initiated after 2 d of acclimation to the metabolic cage environment and 4 d after being switched to high-fat diet (29.3% by weight). This time after switch to high-fat diet corresponds with the point at which MuPPARγKO mice begin to accumulate excess weight compared with controls (10), thus minimizing effects that would be secondary to the eventual obesity had the mice been studied at a later time point.
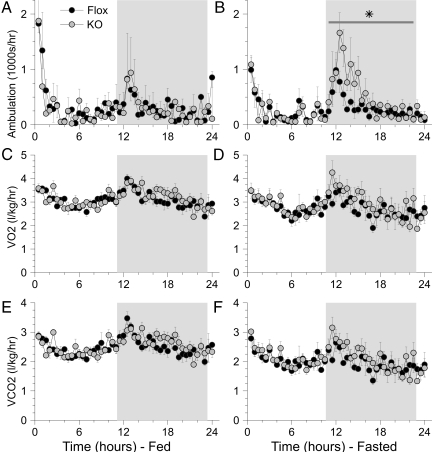
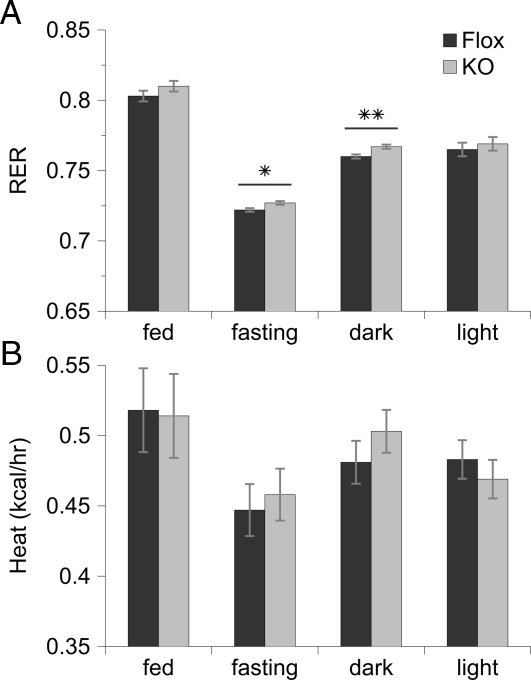
During the calorimeter study, there were no significant differences in mouse weight, change in weight, or food consumed between the two genotypes. Interestingly, whereas there were no differences in locomotor activity during the light and fed periods, the MuPPARγKO mice exhibited a significant increase (P < 0.05) in locomotor activity during the dark cycle in the fasted state compared with control siblings (Fig. 1, A and B). The MuPPARγKO mice exhibited a higher RER during the fasting (P < 0.05) and dark (P < 0.01) cycles compared with control siblings (Fig. 2A). However, there were no measurable differences in VO2 or carbon dioxide production between the two mice genotypes (Fig. 1, C–F) and no differences in heat production (Fig. 2B).
Figure 1.
Ambulation (A and B), oxygen consumption (VO2) (C and D), and carbon dioxide production (VCO2) (E and F) were measured in MuPPARγKO (n = 4, gray circles) vs. Flox control (n = 4, black circles) siblings. Data were collected for 24 h of ad libitum food access (A, C, and E) followed by 24 h of fasting (B, D, and F). Ambulation was estimated by summing sequential beam interruptions measured independently in orthogonal horizontal directions. The diurnal dark period is indicated by the gray background. *, P < 0.05 for increased ambulation in MuPPARγKO mice compared with Flox siblings during the fasting dark period.
Figure 2.
RER (A) and heat production (B) as calculated from indirect calorimetry measurements detailed in Fig. 1, for MuPPARγKO (gray bars) and Flox (black bars) siblings. *, P < 0.05 and **, P < 0.01 for a difference between genotypes.
Mitochondrial coupling
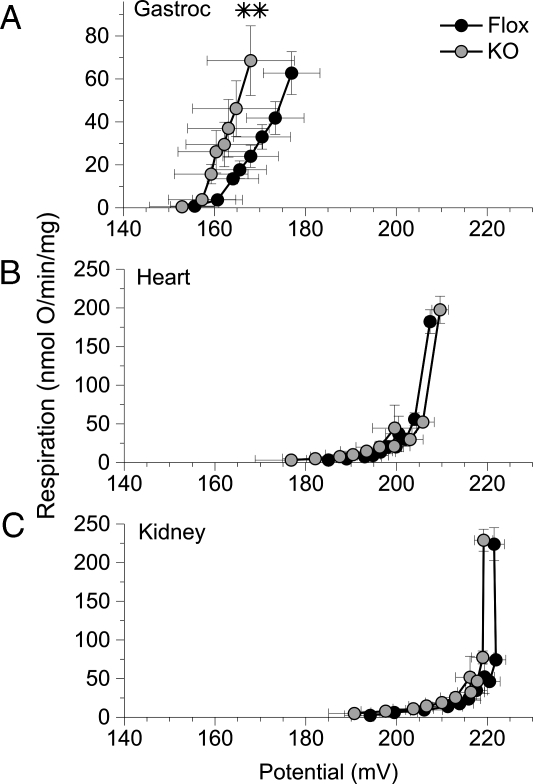
Uncoupling protein (UCP)-1 and -3 have upstream PPARγ response elements (14,31), and UCP2 undergoes an increase in expression upon the exposure of selected tissues to PPAR activators (17). We therefore postulated that muscle PPARγ might be involved a similar regulation in skeletal muscle, such that MuPPARγKO mice would have diminished levels of skeletal muscle uncoupling proteins and demonstrate decreased mitochondrial uncoupling. Such a state could be expected to reduce whole-body energy expenditure and thus contribute to a propensity to obesity. However, in contrast to our postulate, mitochondria isolated from the gastrocnemius muscle of MuPPARγKO mice had increased uncoupling compared with that of control mice (P < 0.01), as exhibited by a leftward shift in the oxygen flux across many mitochondria inner membrane potential levels (Fig. 3A). By contrast, there were no statistical differences in the degree of uncoupling between MuPPARγKO and control-sibling mice among mitochondria isolated from heart (Fig. 3B) or kidney (Fig. 3C).
Figure 3.
Respiratory uncoupling was determined from the kinetics of the proton leak in mitochondria isolated from MuPPARγKO (gray circles) vs. Flox control (black circles) mice. Oxygen consumption was measured as a function of mitochondrial inner membrane potential. Mitochondria were collected from gastrocnemius (n = 7–8) (A), myocardium (n = 6–7) (B), and kidney (n = 6–7) (C). **, P < 0.01 for global difference in the membrane potential vs. respiration curves between MuPPARγKO and Flox mice.
AMPK signaling
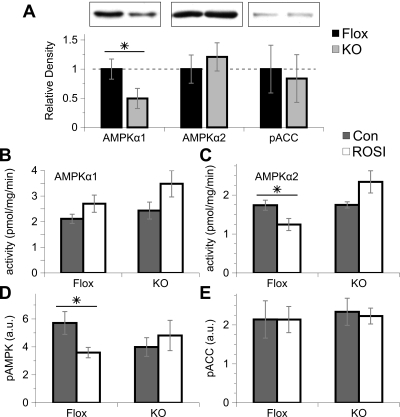
AMPK coordinates several energetic pathways, including the regulation of fatty acid β-oxidation via phosphorylation of ACC. It is postulated that TZDs may act in part via AMPK stimulation (32,33). We therefore measured AMPK levels and ACC phosphorylation in freshly isolated muscle samples from mice on a normal chow diet. Although AMPKα1 protein levels were reduced by about 50% in MuPPARγKO mice compared with control siblings, there was no change in expression of AMPKα2 or in phosphorylation of ACC (Fig. 4A). Furthermore, freshly isolated muscle samples from MuPPARγKO and control mice on high-fat diet showed no differences in AMPKα1 or AMPKα2 activity (Fig. 4, B and C, gray bars). Likewise, there were no differences in the phosphorylation of AMPK or ACC (Fig. 4, D and E, gray bars) between Flox and MuPPARγKO mice.
Figure 4.
Effect of muscle PPARγ and ROSI treatment on AMPK levels, activity, and phosphorylation as measured in freshly isolated muscle (n = 4–5/genotype). Muscle lysates from MuPPARγKO (gray bars) and Flox (black bars) mice on normal-chow diet were immunoblotted for AMPKα1, AMPKα2, or phospho-ACC (pACC), with inset representative blots (A). AMPK activity was assayed in immunoprecipitates prepared using isoform-specific antibodies against AMPKα1 (B) or AMPKα2 (C) in muscle samples from MuPPARγKO (KO) and Flox mice on high-fat diet with (white bars) or without (gray bars) ROSI. Con Control. Likewise, phosphorylation of AMPK-Thr172 (D) and ACC (E) were determined in muscle lysates using phosphospecific antibodies. *, P < 0.05.
We also tested the impact of ROSI treatment on muscle AMPK action in mice on high-fat diet. AMPKα1 activity was unaffected by ROSI, whereas AMPKα2 activity was interestingly decreased by ROSI in Flox but not MuPPARγKO mice (Fig. 4, B and C). Likewise, AMPK phosphorylation was diminished by ROSI in Flox but not MuPPARγKO muscle (Fig. 4D). However, phosphorylation of ACC was unaffected (Fig. 4E).
Impact of muscle PPARγ on fatty acid uptake and oxidation
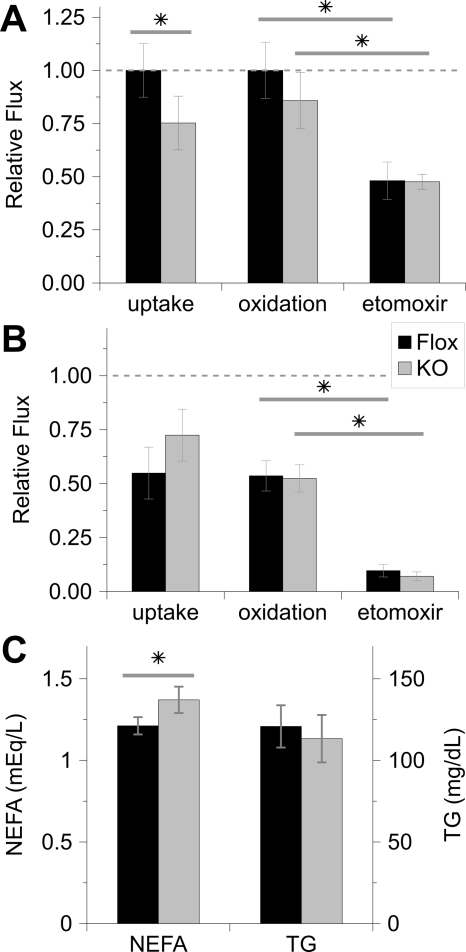
Given that obesity in MuPPARγKO mice was associated with increased RER, we postulated that loss of muscle PPARγ would impair the muscle-based disposal of fatty acids. To this end, we measured the oxidation of the saturated fatty acid palmitate as well as the uptake of the fatty acid uptake tracer R-2-bromopalmitate, an analog of palmitate that is not subject to β-oxidation. These studies were performed in explanted soleus and EDL muscles, representative of oxidative and nonoxidative muscle, respectively. To account for the genetic and phenotypic variance introduced by mixed-strain breeding, we paired MuPPARγKO and Flox-control siblings.
There was a 25% decrease in the uptake of the palmitate analog in soleus explants from MuPPARγKO compared with Flox mice (Fig. 5A, P < 0.05). Likewise, tissue uptake and incorporation of 14C-palmitate was decreased in MuPPARγKO soleus at 0.86 ± 0.08-fold of that in Flox soleus (P = 0.05, n = 7–8). Oxidation of palmitate was not consistently altered in the soleus of MuPPARγKO mice (Fig. 5A). Fatty acid uptake or oxidation was not impaired in the EDL of MuPPARγKO compared with control mice (Fig. 5B), although the total uptake and oxidation in EDL was lower than that of soleus, as expected. Etomoxir, a chemical that blocks the β-oxidation of fatty acids by inhibiting of carnitine palmitoyl transferase-I, reduced palmitate oxidation in Flox animals in both muscle types to a similar degree in both MuPPARγKO and control mice (Fig. 5, A and B). Consistent with an impairment of tissue uptake of fatty acids, serum NEFAs were elevated in MuPPARγKO vs. Flox mice on normal chow diet, whereas triglyceride levels were unaffected (Fig. 5C). NEFA levels were not different between the two genotypes when on a high-fat diet (data not shown).
Figure 5.
Fatty acid handling is perturbed in oxidative muscle on loss of PPARγ. Measurements were made in soleus (A) or EDL (B) muscle explants using 3H-2-bromo-palmitate and 14C-palmitate to trace uptake (n = 5 sibling pairs) and oxidation (n = 7–8 sibling pairs), respectively. Oxidation was also measured after inhibition of β-oxidation with etomoxir. Uptake and oxidation are shown relative to that experienced in uninhibited Flox soleus. Serum NEFAs and triglycerides (TG) in Flox (black bar) and MuPPARγKO (gray bar) mice on normal chow (C). *, P < 0.05.
We also assessed the impact of ROSI administration on the fuel energetics of isolated muscle. ROSI had no significant effect on palmitate analog uptake or palmitate oxidation in the soleus or EDL muscle of Flox mice (Table 1). Interestingly, there was a small but statistically significant effect of ROSI to increase palmitate-analog uptake in MuPPARγKO soleus, although there was no effect in EDL (Table 1). Otherwise, ROSI had no significant effect on palmitate oxidation in soleus or EDL of MuPPARγKO mice.
Table 1.
Effect of ROSI pretreatment on fatty acid and glucose energetics in skeletal muscle ex vivo
| 2-Br-palmitate uptake (relative units)
|
Palmitate oxidation (relative units)
|
||||
|---|---|---|---|---|---|
| Genotype | Treatment | Soleus | EDL | Soleus | EDL |
| n | 4–5 | 4–5 | 4–5 | 4–5 | |
| Flox | Vehicle | 1.00 | 0.68 ± 0.05 | 1.00 | 0.48 ± 0.14 |
| Flox | ROSI | 0.81 ± 0.14 | 0.68 ± 0.05 | 0.96 ± 0.20 | 0.63 ± 0.14 |
| n | 2 | 4–5 | 2–3 | 4–5 | |
| KO | Vehicle | 1.00 | 0.69 ± 0.07 | 1.00 | 0.99 ± 0.12 |
| KO | ROSI | 1.07 ± 0.01a | 0.54 ± 0.07 | 1.30 ± 1.01 | 1.09 ± 0.32 |
Uptake was studied in muscle isolated from mice on normal chow treated with ROSI or vehicle only by gavage for 5 d before study. Confidence limits represent standard errors of measurement. Relative units are in comparison with that measured in soleus of vehicle-treated animals.
P < 0.05 vs. vehicle, otherwise P value is nonsignificant for all ROSI vs. vehicle comparisons.
Expression of genes involved in energy homeostasis
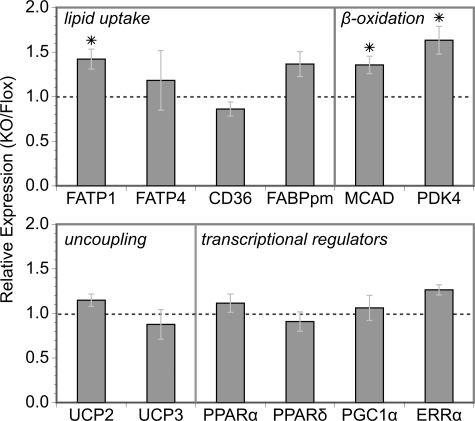
Given that PPARγ and/or TZDs up-regulate the expression of several genes involved in fatty acid uptake (13,16,34), we surveyed the expression of candidate energy homeostasis genes in MuPPARγKO vs. Flox control muscle. Interestingly, of these, only the expression of fatty acid transport protein (FATP)-1 was significantly perturbed, showing increased expression in MuPPARγKO mice (Fig. 6), confirming earlier findings from our laboratory (10). Likewise, the expression of medium-chain Acyl-CoA dehydrogenase and pyruvate dehydrogenase kinase-4, two enzymes that participate in or promote fatty acid oxidation, were both also up-regulated by the absence of PPARγ (Fig. 6). The expression of UCP2 and UCP3 were unchanged in MuPPARγKO mice (Fig. 6). The expression of UCP1 could not be reliably detected, presumably due to very low expression as expected in this tissue. Importantly, there was no change in the expression of transcription regulators related to PPARγ and/or energy homeostasis in skeletal muscle (Fig. 6).
Figure 6.
The fold change in expression of selected genes between MuPPARγKO and Flox control mice was measured using real-time RT-PCR on RNA isolated from skeletal muscle. FABPpm, Fatty acid binding protein plasma membrane; MCAD, medium-chain acyl-CoA dehydrogenase; PDK4, pyruvate dehydrogenase kinase-4; PGC1α, PPARγ-coactivator 1α; ERRα, estrogen-related receptor α. *, P < 0.05 MuPPARγKO vs. Flox.
Discussion
PPARγ is the nuclear receptor for TZD pharmaceuticals and likewise presumably responds to endogenous lipophilic ligands produced under selected conditions (35). Whereas the main actions of PPARγ are in adipose tissue, previous studies from our laboratory (10) and others (9) have disclosed an important role of PPARγ in skeletal muscle. Specifically, mice that lack PPARγ in muscle develop mild obesity, circulating lipid abnormalities, insulin resistance, and under certain conditions impaired glucose tolerance. In the present study, we sought to understand the mechanism by which these mice develop obesity and altered lipid metabolism. This undertaking is complicated by the relatively mild obesity phenotype of MuPPARγKO mice, which exhibit an excess weight gain of about 0.06 g/d per mouse, although this amounts over 7 wk to an excess 2.8 g of stored triglyceride (10) or 8% of the baseline weight, compared with controls. This finding of mild obesity is more striking if one considers the finding herein that MuPPARγKO mice exhibit normal to increased levels of activity and that MuPPARγKO mice accumulate excess fat mass despite reduced dietary intake (10). Taken together, these findings are indicative of a significant defect in energy metabolism (36) induced by loss of muscle PPARγ. This, combined with known functions of PPARγ in other tissues, led to our hypothesis that muscle PPARγ regulates cellular lipid metabolism, such that the MuPPARγKO mice have obesity resulting from an impairment of lipid clearance in the skeletal muscle. This hypothesis is supported by findings that: 1) the obesity of these mice occurs at a young age before the onset of hyperinsulinemia and impaired glucose tolerance (10), 2) the obesity of these mice is exacerbated by high-fat diet, 3) serum lipid levels are elevated in a similar model (9), and 4) a classically described direct action of PPARγ is to direct cellular transport and accumulation of lipid (1).
Consistent with this hypothesis, we found that MuPPARγKO mice, compared with controls, exhibit a 25% reduction in fatty acid uptake into the soleus, an increase in RER, and elevated serum fatty acids. Soleus, a predominantly oxidative muscle, is normally characterized by its ability to uptake, retain, and oxidize lipid, compared with glycolytic muscle types such as EDL. However, in MuPPARγKO mice, the uptake capacity of soleus was reduced to midway between that of normal soleus and EDL, as a measure of the significance of the effect. This defect is likely a primary component of the pathogenesis in MuPPARγKO mice because it is present before the mice develop obesity, hyperglycemia, or hyperinsulinemia. The defect in fatty acid disposal would reduce overall clearance of fatty acid, resulting in unspent fatty acids, which are likely to instead partition into adipose tissue. The overall importance of this is heightened in that oxidative muscle represents a primary site of fatty acid disposal (37). The extent of unspent lipids might be expected to be more prominent on a high-fat diet, leading to the observed excess adiposity, increased fat pad size, and elevated serum lipids. Indeed, such a mechanism of impaired muscle disposal of fatty acids is thought to contribute to obesity and type 2 diabetes in humans (38). Serum fatty acids were similar between MuPPARγKO and Flox mice on high-fat diet, whereas fatty acids were higher in MuPPARγKO vs. Flox mice on normal chow. One interpretation of this result is that on normal chow MuPPARγKO mice develop a fatty-acid overload due to reduced muscle clearance of fatty acids. However, on high-fat diet, both genotypes experience fatty acid overload with elevated circulating lipids, but because of the defect in muscle clearance of lipid energy, the MuPPARγKO mice over time become more adipose than controls.
Energetically the magnitude of the fatty acid disposal defect we observed in MuPPARγKO mice is in order with their rate of excess weight gain and with the magnitude of the measured elevation in RER (see supplementary discussion). The fact that there was no parallel decrease in the calculated heat production of MuPPARγKO mice probably reflects the modest nature of the energetic defect in MuPPARγKO animals, which amounts to merely an extra 0.02 kcal/h of unspent energy. It is likely that such a small energetic defect would be difficult to detect by the indirect calorimetric methods employed (compare 0.02 kcal/h to the error bars in Fig. 2B). Not surprisingly, identifying energetic defects in obesity by indirect calorimetry in rodent models has been notoriously difficult (39). The increased nocturnal activity in fasted MuPPARγKO mice was unexpected. One possibility for this increased activity may be due to the systemic lipid overload that occurs in MuPPARγKO mice because lipid overload increases activity levels in various rodents models (40,41).
Taken together, these findings add muscle as another tissue in which PPARγ coordinates the uptake of fatty acids. Other tissues in which PPARγ induces lipid uptake and/or accumulation include liver (42,43), adipose tissue (44), macrophage (45,46), β-islets (47), and placenta (15). An implication of these findings is that the muscle of MuPPARγKO mice are expected to be protected from excess lipid exposure, possibly explaining why the skeletal muscles of these animals retain normal insulin sensitivity despite the overall obesity and reduced insulin sensitivity in liver (10). An analogous mechanism exists for the liver-specific PPARγ knockout mouse, which is protected from hepatic steatosis, retains hepatic insulin sensitivity, but develops insulin resistance in other organs (42).
The effect of PPARγ on fatty acid uptake in some tissues is mediated in part by up-regulation of the expression of several genes that enhance fatty acid transport into the cell, including FATP1, which has an active PPAR response elements in its promoter (13), and FATP4, fatty acid translocase (CD36), and fatty acid binding protein plasma membrane, which are induced by TZDs (15,16,48). However, in MuPPARγKO mice, there was no observed decrease in the expression of these genes and in fact an increase in the expression of FATP1. The elevated expression of this lipid transport gene in MuPPARγKO mice suggests an up-regulation via non-PPARγ-dependent pathways to compensate for the defect in lipid transport in skeletal muscle.
Fatty acid oxidation was not diminished in MuPPARγKO mice. This finding is consistent with several studies finding no effect of TZDs to stimulate fatty acid oxidation in skeletal muscle (49,50), although several studies found contrasting results with enhanced fatty acid oxidation in response to TZDs (51,52,53). It is interesting that, although fatty acid uptake was diminished in MuPPARγKO soleus, fatty acid oxidation was not. However, the increased expression in medium-chain Acyl-CoA dehydrogenase, an enzyme involved in β-oxidation, and pyruvate dehydrogenase kinase-4, a kinase that promotes β-oxidation, may have been compensatory responses, which helped prevent oxidation rates from diminishing. In the long term, presumably, decreased fatty acid uptake should ultimately lead to decreased fatty acid oxidation. In muscle, fatty acids may first be stored as triglyceride before becoming available for oxidation (54,55). Our experimental approach, using a short-term fatty acid incubation, thus may not be well suited to uncover defects in fatty acid oxidation due to diminished cellular uptake.
In various tissues, PPARγ up-regulates uncoupling proteins, either directly via a PPAR-response element in the cases of UCP1 (14) and UCP3 (31) or via an unknown mechanism in the case of UCP2 (17). Although loss of muscle PPARγ could thus be envisioned to impair mitochondrial uncoupling, we found that mitochondria isolated from skeletal muscle exhibited a small increase in uncoupling. This unexpected increase in uncoupling demonstrates that alterations in uncoupling are not responsible for the obesity of MuPPARγKO mice. One possible explanation for the increased uncoupling would be that it was a secondary phenomena, related to the adiposity of the MuPPARγKO mice because obesity has been suggested to increase mitochondrial uncoupling in cardiac muscle (56). Alternatively, the increased uncoupling may be due to an alteration in exposure of mitochondria to fatty acids, a circumstance that might be expected to alter the tissues uncoupling properties (57), followed by compensatory uncoupling mechanisms manifest ex vivo under conditions in which the mitochondria are incubated in medium that doses not differ between the two genotypes. The uncoupling studies were performed in mice older than those used in our other studies. Mitochondrial uncoupling increases with age in skeletal muscle (58). Likewise, insulin stimulated fatty acid uptake and oxidation are increased in aged rat muscle (59) whereas basal fatty acid oxidation is decreased (60). Whether these age-dependent phenomena interact with muscle PPARγ is unclear. However, the metabolic derangement in MuPPARγKO mice is progressive because our MuPPARγKO mice exhibit a normal insulin tolerance test until 20 months of age, at which time they develop a blunted response to insulin (data not shown). Progressive insulin resistance has likewise been reported in independently created MuPPARγKO mice (9). Thus, it is possible that the increased mitochondrial uncoupling observed in the MuPPARγKO mice is not a primary phenomenon but rather due to an interaction between muscle PPARγ and aging.
We found that expression of AMPKα1 was reduced in MuPPARγKO mice. It is not clear from this experiment alone whether PPARγ directly controls the expression of AMPKα1 because the observed change in expression might be due to secondary effects. In general, little has been published on the transcriptional regulation of AMPKα1. Despite the decrease in AMPKα1 expression, there was no defect in AMPKα1 activity in MuPPARγKO mice. This suggests the presence of PPARγ-independent compensatory mechanisms that increase per-molecule AMPKα1 activity, although firm conclusions are not possible because the AMPKα1 expression and activity levels were measured under differing conditions (see Fig. 4).
It has been postulated that TZDs may act in part via AMPK (32,33); thus, some of our findings are unexpected. In particular, we found that TZD treatment reduced AMPKα2 activity and phosphorylation of AMPK and furthermore that this effect was dependent on muscle PPARγ. In contrast, enhancement of AMPK signaling, including phosphorylation of AMPK and ACC, have been observed in vivo upon treatment of Zucker obese or high-fat diet-treated rats with 3 mg/kg·d ROSI (33). Several groups demonstrated increases in AMPK activity upon treatment of cultured myocytes with TZDs; however, high concentrations of TZDs are required (11–50 μm troglitazone or 5–200 μm rosiglitazone) (32,33,61,62), and thus, the effect has been postulated to be PPARγ independent. In contrast, other groups have found that TZDs have no effect on muscle AMPK activity, i.e. in humans with polycystic ovary syndrome (63). Our results suggest that the PPARγ-dependent effects of TZDs in muscle may inherently reduce AMPKα2 activity and AMPK phosphorylation. However, caution must be used when interpreting the effects of TZDs and PPARγ on AMPK activity because these agents induce potent changes in circulating fuel levels and in intracellular fuel handling, which could directly affect AMPK activity. For example, TZD use in type 2 diabetes will decrease circulating glucose and lipid levels, an event that may alone stimulate AMPK. However, TZDs also improve cellular uptake of fuel, which could be envisioned to reduce AMPK activity. Indeed, in directly comparing TZD responses in insulin-resistant vs. normal rats, it was found that only the insulin-resistant rats demonstrated an increase in AMPK activity (64). As a further confounder, fatty acids may stimulate AMPK activity independent of AMP levels (65), so any changes in cellular fatty acid handling induced by TZDs might also affect AMPK activity. Our results suggest that muscle PPARγ alone under basal conditions has little inherent effect to alter AMPK activity in the skeletal muscle of nondiabetic rodents and highlight the need for further careful dissection of the effects of TZDs on AMPK.
Interestingly, the findings of decreased muscle fatty acid uptake and progressive obesity in MuPPARγKO mice imply that muscle PPARγ has innate activity in the absence of pharmacological activation. Innate activity of PPARγ in muscle could arise in at least two manners. One possibility would be via the production of an endogenous ligand. However, it is difficult to speculate on this possibility due to uncertainty in the identity of the endogenous ligand(s) that activate PPARγ in vivo (35). A second mechanism that could produce innate activity of PPARγ in muscle is ligand-independent activation. The PPARγ gene produces two splice isoforms that differ in ligand dependence and tissue localization. PPARγ1 is found at low levels in many tissues, whereas PPARγ2 is localized primarily to adipose tissue in which it is highly expressed (66,67). PPARγ2 contains an additional 30 N-terminal amino acids, which confer a higher degree of ligand-independent activation than exhibited by PPARγ1 (68). Both PPARγ1 and PPARγ2 are expressed in skeletal muscle, in both humans (66) and rodents (67). MuPPARγKO mice have a loss of both PPARγ isoforms, and it is therefore conceivable that the innate activity of PPARγ lost in MuPPARγKO mice represents the low levels of PPARγ2 expressed in skeletal muscle. In such a scenario, one might expected that pharmacological activation would, in addition, stimulate PPARγ1 activity and thus possibly produce a different spectrum of actions.
Clinically, PPARγ is activated through the use of pharmacological agonists, namely the TZDs. The resultant effects on muscle are expected to be more complex than that of pure PPARγ action on this tissue due to tissue cross talk and due to the complexities of nuclear receptor effects in the basal vs. liganded state (69). Our ex vivo data suggest that TZDs alone do not strongly increase or decrease fatty acid uptake or oxidation in soleus or EDL in mice under normal conditions. It is possible that TZDs may have more pronounced effects on lipid handling in mice under abnormal conditions such as diabetes or insulin resistance. Indeed, there have been conflicting findings as to whether TZDs increase in vivo fatty acid partitioning into skeletal muscle. For example, decreased muscle fatty acid uptake was observed in normal rats treated with 4 mg/kg·d ROSI (70), whereas increased uptake was observed in high-fat-fed rats treated with a high dose of troglitazone (1.6% food admixture) (71). Findings in primary myocytes taken from humans with type 2 diabetes are similar to the latter result, in that PPARγ agonists increase fatty acid uptake and oxidation (53), associated with increased CD36 expression (72).
Fatty acid uptake must balance myocyte lipid usage/oxidation to prevent the accumulation of excess intramyocellular lipid, which is considered one important component of insulin resistance. There have been conflicting results as to the effects of TZDs on intramyocellular lipid levels, with reductions (73,74,75) or increases having been noted (71). The reasons for these discrepancies are unclear. However, our data add clarity by providing evidence that muscle PPARγ acts intrinsically to increase fatty acid uptake in skeletal muscle.
PPARδ (22,23,24) is well described to up-regulate fatty oxidation in skeletal muscle, a role that PPARα may contribute to under certain conditions (20,21). In contrast, our results suggest that PPARγ coordinates skeletal muscle fatty acid uptake. The implication is that PPARγ is necessary for skeletal muscle to have full access to lipid for downstream purposes.
In summary, these data clearly demonstrate that muscle PPARγ regulates fatty acid uptake in skeletal muscle. This capability of PPARγ explains much of the MuPPARγKO phenotype, in that when muscle PPARγ is absent, there is a loss of lipid disposal capacity in skeletal muscle. This leads to lipid overload of other tissues, creating obesity and insulin resistance in tissues other than muscle. As novel PPAR activators with selective tissue and isoform properties are created, their action on muscle lipid handling should be examined.
Supplementary Material
Acknowledgments
The authors thank Brian Fink, Shanming Hu, Laureen Mazzola, and Maria Petruzzelli for technical assistance and Dr. Terry Maratos-Flier for her direction of the indirect calorimetry facility.
Footnotes
This work was supported by National Institutes of Health Grants K08-DK064906 (to A.W.N.), R01-AR45670 and R01-DK068626 (to L.J.G.), Veterans Affairs Medical Research Funds and Grant DK25295 (to W.I.S.), Grant R01-DK60837 (to C.R.K.), and Grant DK36836 to the Joslin Diabetes Center Diabetes and Endocrinology Research Center.
Disclosure Statement: A.W.N., M.F.H., J.Y., N.J., N.M., W.I.S., L.J.G., and C.R.K. have nothing to declare. L.C. is currently employed by and has equity interest in GlaxoSmithKline.
First Published Online July 24, 2008
Abbreviations: ACC, Acetyl-CoA carboxylase; AMPK, AMP kinase; EDL, extensor digitorum longus; FATP, fatty acid transport protein; MuPPARγKO, muscle-specific knockout of PPARγ; NEFA, nonesterified fatty acid; PPAR, peroxisome proliferator-activated receptor; RER, respiratory exchange ratio; ROSI, rosiglitazone; TZD, thiazolidinedione; UCP, uncoupling protein; VO2, oxygen consumption.
References
- Spiegelman BM 1998 PPARγ: adipogenic regulator and thiazolidinedione receptor. Diabetes 47:507–514 [DOI] [PubMed] [Google Scholar]
- Gerstein HC, Yusuf S, Bosch J, Pogue J, Sheridan P, Dinccag N, Hanefeld M, Hoogwerf B, Laakso M, Mohan V, Shaw J, Zinman B, Holman RR 2006 Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. Lancet 368:1096–1105 [DOI] [PubMed] [Google Scholar]
- Semple RK, Chatterjee VKK, O'Rahilly S 2006 PPARγ and human metabolic disease. J Clin Invest 116:581–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierath JR, Ryder JW, Doebber T, Woods J, Wu M, Ventre J, Li Z, McCrary C, Berger J, Zhang B, Moller DE 1998 Role of skeletal muscle in thiazolidinedione insulin sensitizer (PPARγ agonist) action. Endocrinology 139:5034–5041 [DOI] [PubMed] [Google Scholar]
- Petersen KF, Krssak M, Inzucchi S, Cline GW, Dufour S, Shulman GI 2000 Mechanism of troglitazone action in type 2 diabetes. Diabetes 49:827–831 [DOI] [PubMed] [Google Scholar]
- Kraegen EW, James DE, Jenkins AB, Chisholm DJ, Storlien LH 1989 A potent in vivo effect of ciglitazone on muscle insulin resistance induced by high fat feeding of rats. Metabolism 38:1089–1093 [DOI] [PubMed] [Google Scholar]
- Chao L, Marcus-Samuels B, Mason MM, Moitra J, Vinson C, Arioglu E, Gavrilova O, Reitman ML 2000 Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones. J Clin Invest 106:1221–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruszynska YT, Mukherjee R, Jow L, Dana S, Paterniti JR, Olefsky JM 1998 Skeletal muscle peroxisome proliferator-activated receptor-γ expression in obesity and non-insulin-dependent diabetes mellitus. J Clin Invest 101:543–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevener AL, He W, Barak Y, Le J, Bandyopadhyay G, Olson P, Wilkes J, Evans RM, Olefsky J 2003 Muscle-specific PPARγ deletion causes insulin resistance. Nat Med 9:1491–1497 [DOI] [PubMed] [Google Scholar]
- Norris AW, Chen L, Fisher SJ, Szanto I, Ristow M, Jozsi AC, Hirshman MF, Rosen ED, Goodyear LJ, Gonzalez FJ, Spiegelman BM, Kahn CR 2003 Muscle-specific PPARγ-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J Clin Invest 112:608–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann J, Neumann-Haefelin C, Belz U, Kalisch J, Juretschke H, Stein M, Kleinschmidt E, Kramer W, Herling AW 2003 Intramyocellular lipid and insulin resistance: a longitudinal in vivo 1H-spectroscopic study in Zucker diabetic fatty rats. Diabetes 52:138–144 [DOI] [PubMed] [Google Scholar]
- Rasouli N, Raue U, Miles LM, Lu T, Di Gregorio GB, Elbein SC, Kern PA 2005 Pioglitazone improves insulin sensitivity through reduction in muscle lipid and redistribution of lipid into adipose tissue. Am J Physiol Endocrinol Metab 288:E930–E934 [DOI] [PubMed] [Google Scholar]
- Frohnert BI, Hui TY, Bernlohr DA 1999 Identification of a functional peroxisome proliferator-responsive element in the murine fatty acid transport protein gene. J Biol Chem 274:3970–3977 [DOI] [PubMed] [Google Scholar]
- Sears IB, MacGinnitie MA, Kovacs LG, Graves RA 1996 Differentiation-dependent expression of the brown adipocyte uncoupling protein gene: regulation by peroxisome proliferator-activated receptor γ. Mol Cell Biol 16:3410–3419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaiff WT, Bildirici I, Cheong M, Chern PL, Nelson DM, Sadovsky Y 2005 Peroxisome proliferator-activated receptor-γ and retinoid X receptor signaling regulate fatty acid uptake by primary human placental trophoblasts. J Clin Endocrinol Metab 90:4267–4275 [DOI] [PubMed] [Google Scholar]
- Sato O, Kuriki C, Fukui Y, Motojima K 2002 Dual promoter structure of mouse and human fatty acid translocase/CD36 genes and unique transcriptional activation by peroxisome proliferator-activated receptor α and γ ligands. J Biol Chem 277:15703–15711 [DOI] [PubMed] [Google Scholar]
- Strobel A, Siquier K, Zilberfarb V, Strosberg AD, Issad T 1999 Effect of thiazolidinediones on expression of UCP2 and adipocyte markers in human PAZ6 adipocytes. Diabetologia 42:527–533 [DOI] [PubMed] [Google Scholar]
- Leone TC, Weinheimer CJ, Kelly DP 1999 A critical role for the peroxisome proliferator-activated receptor α (PPARα) in the cellular fasting response: the PPARα-null mouse as a model of fatty acid oxidation disorders. Proc Natl Acad Sci USA 96:7473–7478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ 1998 Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor α (PPARα). J Biol Chem 273:5678–5684 [DOI] [PubMed] [Google Scholar]
- Finck BN, Bernal-Mizrachi C, Han DH, Coleman T, Sambandam N, LaRiviere LL, Holloszy JO, Semenkovich CF, Kelly DP 2005 A potential link between muscle peroxisome proliferator-activated receptor-α signaling and obesity-related diabetes. Cell Metab 1:133–144 [DOI] [PubMed] [Google Scholar]
- Muoio DM, MacLean PS, Lang DB, Li S, Houmard JA, Way JM, Winegar DA, Corton JC, Dohm GL, Kraus WE 2002 Fatty acid homeostasis and induction of lipid regulatory genes in skeletal muscles of peroxisome proliferator-activated receptor (PPAR) α knock-out mice: evidence for compensatory regulation by PPARδ. J Biol Chem 277:26089–26097 [DOI] [PubMed] [Google Scholar]
- Luquet S, Lopez-Soriano J, Holst D, Fredenrich A, Melki J, Rassoulzadegan M, Grimaldi PA 2003 Peroxisome proliferator-activated receptor δ controls muscle development and oxidative capability. FASEB J 17:2299–2301 [DOI] [PubMed] [Google Scholar]
- Lee C, Olson P, Hevener A, Mehl I, Chong L, Olefsky JM, Gonzalez FJ, Ham J, Kang H, Peters JM, Evans RM 2006 PPARδ regulates glucose metabolism and insulin sensitivity. Proc Natl Acad Sci USA 103:3444–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunmair B, Staniek K, Dorig J, Szocs Z, Stadlbauer K, Marian V, Gras F, Anderwald C, Nohl H, Waldhausl W, Furnsinn C 2006 Activation of PPAR-δ in isolated rat skeletal muscle switches fuel preference from glucose to fatty acids. Diabetologia 49:2713–2722 [DOI] [PubMed] [Google Scholar]
- Bates SH, Dundon TA, Seifert M, Carlson M, Maratos-Flier E, Myers MGJ 2004 LRb-STAT3 signaling is required for the neuroendocrine regulation of energy expenditure by leptin. Diabetes 53:3067–3073 [DOI] [PubMed] [Google Scholar]
- Albarado DC, McClaine J, Stephens JM, Mynatt RL, Ye J, Bannon AW, Richards WG, Butler AA 2004 Impaired coordination of nutrient intake and substrate oxidation in melanocortin-4 receptor knockout mice. Endocrinology 145:243–252 [DOI] [PubMed] [Google Scholar]
- Fink BD, Hong Y, Mathahs MM, Scholz TD, Dillon JS, Sivitz WI 2002 UCP2-dependent proton leak in isolated mammalian mitochondria. J Biol Chem 277:3918–3925 [DOI] [PubMed] [Google Scholar]
- Musi N, Fujii N, Hirshman MF, Ekberg I, Froberg S, Ljungqvist O, Thorell A, Goodyear LJ 2001 AMP-activated protein kinase (AMPK) is activated in muscle of subjects with type 2 diabetes during exercise. Diabetes 50:921–927 [DOI] [PubMed] [Google Scholar]
- Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic S, Moller DE, Thorell A, Goodyear LJ 2002 Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 51:2074–2081 [DOI] [PubMed] [Google Scholar]
- Kokkotou E, Jeon JY, Wang X, Marino FE, Carlson M, Trombly DJ, Maratos-Flier E 2005 Mice with MCH ablation resist diet-induced obesity through strain-specific mechanisms. Am J Physiol Regul Integr Comp Physiol 289:R117–R124 [DOI] [PubMed] [Google Scholar]
- Solanes G, Pedraza N, Iglesias R, Giralt M, Villarroya F 2003 Functional relationship between MyoD and peroxisome proliferator-activated receptor-dependent regulatory pathways in the control of the human uncoupling protein-3 gene transcription. Mol Endocrinol 17:1944–1958 [DOI] [PubMed] [Google Scholar]
- Fryer LGD, Parbu-Patel A, Carling D 2002 The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem 277:25226–25232 [DOI] [PubMed] [Google Scholar]
- Lessard SJ, Chen Z, Watt MJ, Hashem M, Reid JJ, Febbraio MA, Kemp BE, Hawley JA 2006 Chronic rosiglitazone treatment restores AMPKα2 activity in insulin-resistant rat skeletal muscle. Am J Physiol Endocrinol Metab 290:E251–E257 [DOI] [PubMed] [Google Scholar]
- Schaiff WT, Knapp FFRJ, Barak Y, Biron-Shental T, Nelson DM, Sadovsky Y 2007 Ligand-activated peroxisome proliferator activated receptor γ alters placental morphology and placental fatty acid uptake in mice. Endocrinology 148:3625–3634 [DOI] [PubMed] [Google Scholar]
- Tzameli I, Fang H, Ollero M, Shi H, Hamm JK, Kievit P, Hollenberg AN, Flier JS 2004 Regulated production of a peroxisome proliferator-activated receptor-γ ligand during an early phase of adipocyte differentiation in 3T3-L1 adipocytes. J Biol Chem 279:36093–36102 [DOI] [PubMed] [Google Scholar]
- Arch JRS 2002 Lessons in obesity from transgenic animals. J Endocrinol Invest 25:867–875 [DOI] [PubMed] [Google Scholar]
- Andres R, Cader G, Zierler KL 1956 The quantitatively minor role of carbohydrate in oxidative metabolism by skeletal muscle in intact man in the basal state: measurements of oxygen and glucose uptake and carbon dioxide and lactate production in the forearm. J Clin Invest 35:671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaak EE, van Aggel-Leijssen DP, Wagenmakers AJ, Saris WH, van Baak MA 2000 Impaired oxidation of plasma-derived fatty acids in type 2 diabetic subjects during moderate-intensity exercise. Diabetes 49:2102–2107 [DOI] [PubMed] [Google Scholar]
- Arch JRS, Hislop D, Wang SJY, Speakman JR 2006 Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int J Obes (Lond) 30:1322–1331 [DOI] [PubMed] [Google Scholar]
- Simoncic M, Horvat S, Stevenson PL, Bünger L, Holmes MC, Kenyon CJ, Speakman JR, Morton NM 2008 Divergent physical activity and novel alternative responses to high fat feeding in polygenic fat and lean mice. Behav Genet 38:292–300 [DOI] [PubMed] [Google Scholar]
- Buwalda B, Blom WA, Koolhaas JM, van Dijk G 2001 Behavioral and physiological responses to stress are affected by high-fat feeding in male rats. Physiol Behav 73:371–377 [DOI] [PubMed] [Google Scholar]
- Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR, Nicol CJ, Vinson C, Gonzalez FJ, Reitman ML 2003 Liver peroxisome proliferator-activated receptor γ contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem 278:34268–34276 [DOI] [PubMed] [Google Scholar]
- Yu S, Matsusue K, Kashireddy P, Cao W, Yeldandi V, Yeldandi AV, Rao MS, Gonzalez FJ, Reddy JK 2003 Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor γ1 (PPARγ1) overexpression. J Biol Chem 278:498–505 [DOI] [PubMed] [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM 1999 PPAR γ is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 4:611–617 [DOI] [PubMed] [Google Scholar]
- Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM 2001 PPAR-γ dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med 7:48–52 [DOI] [PubMed] [Google Scholar]
- Moore KJ, Rosen ED, Fitzgerald ML, Randow F, Andersson LP, Altshuler D, Milstone DS, Mortensen RM, Spiegelman BM, Freeman MW 2001 The role of PPAR-γ in macrophage differentiation and cholesterol uptake. Nat Med 7:41–47 [DOI] [PubMed] [Google Scholar]
- Parton LE, Diraison F, Neill SE, Ghosh SK, Rubino MA, Bisi JE, Briscoe CP, Rutter GA 2004 Impact of PPARγ overexpression and activation on pancreatic islet gene expression profile analyzed with oligonucleotide microarrays. Am J Physiol Endocrinol Metab 287:E390–E404 [DOI] [PubMed] [Google Scholar]
- Benton CR, Koonen DPY, Calles-Escandon J, Tandon NN, Glatz JFC, Luiken JJFP, Heikkila JJ, Bonen A 2006 Differential effects of contraction and PPAR agonists on the expression of fatty acid transporters in rat skeletal muscle. J Physiol 573:199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard SJ, Rivas DA, Chen Z, Bonen A, Febbraio MA, Reeder DW, Kemp BE, Yaspelkis BB3, Hawley JA 2007 Tissue-specific effects of rosiglitazone and exercise in the treatment of lipid-induced insulin resistance. Diabetes 56:1856–1864 [DOI] [PubMed] [Google Scholar]
- Sreenan S, Keck S, Fuller T, Cockburn B, Burant CF 1999 Effects of troglitazone on substrate storage and utilization in insulin-resistant rats. Am J Physiol 276:E1119–E1129 [DOI] [PubMed] [Google Scholar]
- Benton CR, Holloway GP, Campbell SE, Yoshida Y, Tandon NN, Glatz JFC, Luiken JJJFP, Spriet LL, Bonen A 2008 Rosiglitazone increases fatty acid oxidation and fatty acid translocase (FAT/CD36) but not carnitine palmitoyltransferase I in rat muscle mitochondria. J Physiol 586:1755–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay GK, Yu JG, Ofrecio J, Olefsky JM 2006 Increased malonyl-CoA levels in muscle from obese and type 2 diabetic subjects lead to decreased fatty acid oxidation and increased lipogenesis; thiazolidinedione treatment reverses these defects. Diabetes 55:2277–2285 [DOI] [PubMed] [Google Scholar]
- Cha B, Ciaraldi TP, Park K, Carter L, Mudaliar SR, Henry RR 2005 Impaired fatty acid metabolism in type 2 diabetic skeletal muscle cells is reversed by PPARγ agonists. Am J Physiol Endocrinol Metab 289:E151–E159 [DOI] [PubMed] [Google Scholar]
- Dagenais GR, Tancredi RG, Zierler KL 1976 Free fatty acid oxidation by forearm muscle at rest, and evidence for an intramuscular lipid pool in the human forearm. J Clin Invest 58:421–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro C, Bajpeyi S, Smith SR 2008 Determinants of intramyocellular triglyceride turnover: implications for insulin sensitivity. Am J Physiol Endocrinol Metab 294:E203–E213 [DOI] [PubMed] [Google Scholar]
- Boudina S, Sena S, O'Neill BT, Tathireddy P, Young ME, Abel ED 2005 Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation 112:2686–2695 [DOI] [PubMed] [Google Scholar]
- Garvey WT 2003 The role of uncoupling protein 3 in human physiology. J Clin Invest 111:438–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal SB, Ramsey JJ, Monemdjou S, Weindruch R, Harper ME 2001 Effects of caloric restriction on skeletal muscle mitochondrial proton leak in aging rats. J Gerontol A Biol Sci Med Sci. 56:B116–B122 [DOI] [PubMed] [Google Scholar]
- Tucker MZ, Turcotte LP 2003 Aging is associated with elevated muscle triglyceride content and increased insulin-stimulated fatty acid uptake. Am J Physiol Endocrinol Metab 285:E827–E835 [DOI] [PubMed] [Google Scholar]
- Tucker MZ, Turcotte LP 2002 Impaired fatty acid oxidation in muscle of aging rats perfused under basal conditions. Am J Physiol Endocrinol Metab 282:E1102–E1109 [DOI] [PubMed] [Google Scholar]
- Fediuc S, Pimenta AS, Gaidhu MP, Ceddia RB 2008 Activation of AMP-activated protein kinase, inhibition of pyruvate dehydrogenase activity, and redistribution of substrate partitioning mediate the acute insulin-sensitizing effects of troglitazone in skeletal muscle cells. J Cell Physiol 215:392–400 [DOI] [PubMed] [Google Scholar]
- Konrad D, Rudich A, Bilan PJ, Patel N, Richardson C, Witters LA, Klip A 2005 Troglitazone causes acute mitochondrial membrane depolarisation and an AMPK-mediated increase in glucose phosphorylation in muscle cells. Diabetologia 48:954–966 [DOI] [PubMed] [Google Scholar]
- Højlund K, Glintborg D, Andersen NR, Birk JB, Treebak JT, Frøsig C, Beck-Nielsen H, Wojtaszewski JFP 2008 Impaired insulin-stimulated phosphorylation of AKT and AS160 in skeletal muscle of women with polycystic ovary syndrome is reversed by pioglitazone treatment. Diabetes 57:357–366 [DOI] [PubMed] [Google Scholar]
- Ye J, Dzamko N, Hoy AJ, Iglesias MA, Kemp B, Kraegen E 2006 Rosiglitazone treatment enhances acute amp-activated protein kinase-mediated muscle and adipose tissue glucose uptake in high-fat-fed rats. Diabetes 55:2797–2804 [DOI] [PubMed] [Google Scholar]
- Watt MJ, Steinberg GR, Chen Z, Kemp BE, Febbraio MA 2006 Fatty acids stimulate AMP-activated protein kinase and enhance fatty acid oxidation in L6 myotubes. J Physiol 574:139–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Puig AJ, Considine RV, Jimenez-Liñan M, Werman A, Pories WJ, Caro JF, Flier JS 1997 Peroxisome proliferator-activated receptor gene expression in human tissues: effects of obesity, weight loss, and regulation by insulin and glucocorticoids. J Clin Invest 99:2416–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Puig A, Jimenez-Liñan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Flier JS, Moller DE 1996 Regulation of PPAR γ gene expression by nutrition and obesity in rodents. J Clin Invest 97:2553–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werman A, Hollenberg A, Solanes G, Bjorbaek C, Vidal-Puig AJ, Flier JS 1997 Ligand-independent activation domain in the n terminus of peroxisome proliferator-activated receptor γ (PPARγ): differential activity of PPARγ1 and -2 isoforms and influence of insulin. J Biol Chem 272:20230–20235 [DOI] [PubMed] [Google Scholar]
- Rochette-Egly C 2005 Dynamic combinatorial networks in nuclear receptor-mediated transcription. J Biol Chem 280:32565–32568 [DOI] [PubMed] [Google Scholar]
- Ye J, Dzamko N, Cleasby ME, Hegarty BD, Furler SM, Cooney GJ, Kraegen EW 2004 Direct demonstration of lipid sequestration as a mechanism by which rosiglitazone prevents fatty-acid-induced insulin resistance in the rat: comparison with metformin. Diabetologia 47:1306–1313 [DOI] [PubMed] [Google Scholar]
- Todd MK, Watt MJ, Le J, Hevener AL, Turcotte LP 2007 Thiazolidinediones enhance skeletal muscle triacylglycerol synthesis while protecting against fatty acid-induced inflammation and insulin resistance. Am J Physiol Endocrinol Metab 292:E485–E493 [DOI] [PubMed] [Google Scholar]
- Wilmsen HM, Ciaraldi TP, Carter L, Reehman N, Mudaliar SR, Henry RR 2003 Thiazolidinediones upregulate impaired fatty acid uptake in skeletal muscle of type 2 diabetic subjects. Am J Physiol Endocrinol Metab 285:E354–E362 [DOI] [PubMed] [Google Scholar]
- Hockings PD, Changani KK, Saeed N, Reid DG, Birmingham J, O'Brien P, Osborne J, Toseland CN, Buckingham RE 2003 Rapid reversal of hepatic steatosis, and reduction of muscle triglyceride, by rosiglitazone: MRI/S studies in Zucker fatty rats. Diabetes Obes Metab 5:234–243 [DOI] [PubMed] [Google Scholar]
- Jucker BM, Schaeffer TR, Haimbach RE, Mayer ME, Ohlstein DH, Smith SA, Cobitz AR, Sarkar SK 2003 Reduction of intramyocellular lipid following short-term rosiglitazone treatment in Zucker fatty rats: an in vivo nuclear magnetic resonance study. Metabolism 52:218–225 [DOI] [PubMed] [Google Scholar]
- Kim JK, Fillmore JJ, Gavrilova O, Chao L, Higashimori T, Choi H, Kim H, Yu C, Chen Y, Qu X, Haluzik M, Reitman ML, Shulman GI 2003 Differential effects of rosiglitazone on skeletal muscle and liver insulin resistance in A-ZIP/F-1 fatless mice. Diabetes 52:1311–1318 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.