Abstract
Although primarily regarded as a sex steroid, estrogen plays an important role in many other physiological processes including adipose development and disposition. Estrogen sulfotransferase (EST) regulates estrogen activity by catalyzing the sulfoconjugation and inactivation of estrogens. In the present study, we report the gender-specific expression of EST in adipose tissues of the mouse and describe contrasting mechanisms of EST regulation in the fat and liver. EST is expressed in the white adipose tissues of the male but not female mouse. Within the various fat depots of male mice, it is most abundantly expressed in the epididymal fat pad, with variable levels in other white fats and no expression in the brown fat. Fractionation of epididymal fat cells showed EST to be predominantly associated with stromal vascular cells (preadipocyte). EST expression in male mouse adipose tissues is dependent on testosterone as castration ablated, and administration of exogenous testosterone restored, EST expression. Furthermore, testosterone treatment induced abnormal EST expression in the parametrial fat of female mice. EST induction by testosterone in female mice is tissue specific because testosterone treatment had no effect on liver EST expression. Conversely, the liver X receptor agonist TO-901317 induced EST expression in female mouse liver but not in their adipose tissues. Finally, we demonstrate that male EST knockout mice developed increased epididymal fat accumulation with enlarged adipocyte size. We conclude that EST is expressed in adipose tissues in a sexually dimorphic manner, is regulated by testosterone, and plays a physiological role in regulating adipose tissue accumulation in male mice.
FAT DISTRIBUTION DIFFERS between men and women, with men displaying a central distribution, whereas women display a peripheral distribution (1). The development and disposition of adipose tissue has long been linked to sex steroids. In particular, estrogen has been shown to play an important role in regulating adipocyte metabolism and regional adipose distribution. Estrogen receptor (ER)-α and -β have been shown to be expressed in both human and rodent adipocytes (2,3,4). Estrogen has many effects on adipose tissues. Estrogen regulates lipoprotein lipase, decreases lipolysis, and increases preadipocyte proliferation (5,6,7,8). The effects of estrogen on fat tissues are not limited to direct stimulation of adipocytes. Estrogen signaling can occur in the hypothalamus, leading to a decrease in energy intake and an increase in energy expenditure (9). Estrogen signaling in adipose tissues has been examined in vivo as well. ER-α knockout mice express more white adipose tissue compared with wild-type mice (10). Aromatase-knockout mice showed an increase in gonadal fat pad weight (11). Both models demonstrate the importance of estrogen signaling in adipose tissue physiology.
The level of estrogen response in target tissues including the fat is influenced by many factors, most importantly by the presence of functional estrogen receptor and ligand availability. Estrogen ligand availability in a given tissue is in turn influenced by the expression of estrogen biosynthetic and metabolic enzymes. Whereas previous studies have established the presence of ERs and estrogen-synthesizing enzymes in adipose tissues (2,12,13), very little is known about the expression and regulation of estrogen metabolic enzymes. One important regulator of local estrogen activity in extrahepatic sites is estrogen sulfotransferase (EST), a cytosolic enzyme of the phase II drug metabolic system that inactivates estrogens by sulfoconjugation (14). EST has an unusually high affinity and specificity for estrogen substrates because mouse EST has a Michaelis constant of approximately 50 nm for both estrone and 17β-estradiol (15). Ectopic expression of EST in MCF-7 cells led to a decreased response to 17β-estradiol (16). Through the use of mouse models, previous studies in our laboratory investigated the role of EST in protecting the male reproductive system and the placenta from estrogen toxicity (17,18,19,20). In male mice, EST expression is found in the interstitial Leydig cells of the testis and the middle and distal sections of the epididymis (20). Targeted disruption of the EST gene led to age-related Leydig cell hyperplasia and hypertrophy, abnormal cholesterol accumulation, and reduced sperm motility (18). In the female mouse, EST is expressed in the placenta. Gene disruption in pregnant female mice led to placental thrombosis and subsequently spontaneous fetal loss (19).
In this study, we characterized and examined the role of EST in adipose tissues. We found that EST is expressed in the fat in a sexually dimorphic and fat depot-specific manner. EST is expressed in the white fat tissues of male mice and this expression requires the presence of testosterone. Furthermore, we demonstrate that testosterone treatment induced abnormal EST expression in the female mouse fat but not in their liver. Conversely, the liver X receptor (LXR) agonist TO-901317 (TO) induced EST expression in the female mouse liver but not their fat tissues. Finally, we found that mice deficient in EST accumulated more epididymal fat and had enlarged adipocyte size. We conclude that EST is expressed in adipose tissues in a sexually dimorphic manner, is regulated by testosterone, and plays a physiological role in regulating adipose tissue accumulation in male mice.
Materials and Methods
Animals and tissue collection
EST-deficient mice were generated by disruption of the estrogen sulfotransferase gene via gene targeting and backcrossed (for nine generations) to have a C57BL/6 background as previously described (17). Heterozygous males and females were bred to produce wild-type, heterozygous, and homozygous-null offspring for the adipose accumulation, growth curve, and food intake studies. Mice were screened by PCR using primers to the EST gene (5′-GCT-ATC-AGC-CAT-ACA-ACT-TC-3′ and 5′-GGT-CTT-CGT-TTC-TGC-ACT-C-3′) and the NEO cassette (5′-CTT-GGG-TGG-AGA-GGC-TAT-TC-3′ and 5′-AGG-TGA-GAT-GAC-AGG-AGA-TC-3′). Otherwise, wild-type mice used in the enzymatic and mRNA studies were purchased from The Jackson Laboratory (Bar Harbor, ME). Mature castrated C57BL/6 mice and db/db mice (strain name: BKS. Cg-m+/+ Leprdb/J) were obtained from The Jackson Laboratory. Immediately after the mice were killed, epididymal, inguinal, retroperitoneal, parametrial, and/or brown adipose depots were dissected and weighed. Epididymal fat was removed from areas surrounding the epididymis and testis. Inguinal fat was collected from the sc areas surrounding the torso by the hind leg. Retroperitoneal fat was collected from a depot lying under the kidney, next to the abdominal wall, and does not contain fat surrounding the kidney. Parametrial adipose was collected from areas surrounding the uterus. Brown adipose tissue was collected from the intrascapular region and cleared from any attached white adipose tissue. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Animal treatments
Castrated male and wild-type female mice were treated by sc injections with 100 μl of 10 mg/ml testosterone propionate (Sigma, St. Louis, MO; 50 mg/kg body weight) or vehicle (olive oil) for 5 consecutive days (20). Tissues were collected 6 h after the fifth injection and immediately placed in dry ice and stored at −80 C until use. LXR agonist, TO (Cayman Chemical, Ann Arbor, MI; catalog no. 71810), was initially prepared at 1 mg/μl in Tween-80 (Sigma). It was then diluted 1:100 into 1% methylcellulose (Sigma). Mice were administered 100 μl of the TO compound or vehicle (1% methylcellulose) by oral gavage (approximately 50 mg/kg·d) for 5 consecutive days (21), and tissues were collected 6 h after the final treatment and immediately placed in dry ice and stored at −80 C until use.
Purification of preadipocytes
Procedure was modified from elsewhere (22). Epididymal fat tissue was dissected under sterile conditions. The tissue was freed from blood capillaries as much as possible and bathed in general medium [DMEM (low glucose; Invitrogen, Carlsbad, CA; no. 11885-084) containing 3.7 g/liter NaHCO3, 33 μm biotin, 17 μm pantothenate, 10 U/ml penicillin, and 0.1 mg/ml streptomycin]. Tissue was digested in collegenase digesting medium (general medium with 2% BSA and 2 mg/ml collagenase) for 40 min at 37 C. Cell and tissue remnants were transferred to general medium containing 10% fetal bovine serum and filtered through a 40-μm nylon screen. The filtrate was centrifuged at 600 × g for 5 min to obtain the stromal vascular fraction (SVF). The low-speed centrifugation allowed for separation between the floating adipocytes and the SVF. The pellet was then treated with red cell lysis buffer for 2 min and washed in 10 × volume of general media containing 10% fetal bovine serum. The SVF suspension was centrifuged at 600 × g for 5 min and the pellet was collected. An aliquot of the SVF was examined microscopically, and the morphology of most cells was confirmed to resemble that of preadipocytes after culturing for 24 h.
Northern and Western blot analysis
Total mRNA was isolated from tissues by using Trizol reagent (Invitrogen). Total RNAs were separated on a 1% formaldehyde-agarose gel and capillary transferred onto a nylon membrane (Hybond-N; Amersham Pharmacia Biotech, Arlington Heights, IL). The membrane was cross-linked under UV and hybridized with a P32-labeled cDNA probe synthesized with random primers from the full-length mouse EST cDNA. Hybridization was carried out in QuickHyb solution (Stratagene, La Jolla, CA) at 68 C for 1 h. The membrane was washed two times in 2× saline sodium citrate/0.1% sodium dodecyl sulfate (SDS) at 60 C for 15 min, washed in 0.1× saline sodium citrate/0.1% SDS at 60 C for 30 min, and then exposed to x-ray film. For Western blot analysis, total proteins were isolated by homogenizing freshly isolated tissues at 4 C in PBS. The homogenate was centrifuged at 15,000 × g for 20 min and the resulting supernatant was used for Western blot analysis. Concentrations of the protein samples were determined by the Bradford method using a protein assay kit from Pierce Chemical Co. (Rockford, IL). Samples were electrophoresed on a 10% SDS polyacrylamide gel, blotted onto a nitrocellulose membrane (Bio-Rad Laboratories, Inc., Hercules, CA; 0.45 μm) and probed with a rabbit polyclonal antimouse Sult1E1 antibody (23), followed by reaction with horseradish peroxidase-conjugated antirabbit secondary antibody. Membranes were stripped and reprobed with a glyceraldehyde-3-phosphate dehydrogenase antibody (6C5; Santa Cruz Biotechnology, Santa Cruz, Ca) and a horseradish peroxidase-conjugated antimouse IgG antibody. Immunodetection was performed with the enhanced chemiluminescence Western blotting detection system (Amersham).
EST activity assays
Sulfotransferase activity was measured with 3H-labeled estradiol (estradiol-2,4–3H; Sigma; E-9767, final concentration 35 nm) in 200 μl PBS (pH 7.5), 0.625% Triton X-100, containing 100 mm 3′-phosphoadenosine 5′-phosphosulfate (Sigma; A-1651) and 200 μg protein lysate prepared from tissue homogenate (15,000 × g) in the same buffer. The reaction was initiated by the addition of substrate and continued for 30 min at 37 C. The reaction mixture was extracted with 2 volumes of dichloromethane, and an aliquot of the aqueous phase was counted for radioactivity using a scintillation counter. Enzyme activity was expressed as picomoles of estrogen sulfate formed per hour per mg of total protein.
Growth curve and food intake studies
EST+/+ and EST−/− male and female mice (n = 4–5) were weaned and housed individually at 4 wk of age. Mice were weighed weekly for 10 wk to determine the growth curve. Mice were on standard feed (PicoLab Rodent Diet 20, product no. 5053; LabDiet, Richmond, IN) with food consumption monitored. The food consumed per mouse was measured twice weekly. Before feeding, total food placed in food rack was weighed. After 3–4 d, food remaining in food rack was weighed. Food spilled from food rack was not collected for food measurements. Additional food was weighed before being added. Total food consumed over a 2-wk period was added up and divided by 14 to give a daily consumption rate.
Measurement of adipocyte size
Epididymal, inguinal, and retroperitoneal adipose tissues from 10-wk-old male EST+/+ (n = 4) and EST−/− mice (n = 5) were dissected, fixed in 4 C Bouin’s solution overnight, dehydrated, and paraffin embedded. Tissues were sectioned at 5 μm at −25 C. Sections were stained with hematoxylin and then counterstained with eosin. Images were taken randomly from four viewing fields per slide and two slides per fat depot per mouse. In each viewing field, 15 adipocytes were measured for cross-section area size using the National Institutes of Health’s ImageJ program (Bethesda, MD).
Results
EST is selectively expressed in male adipose tissues
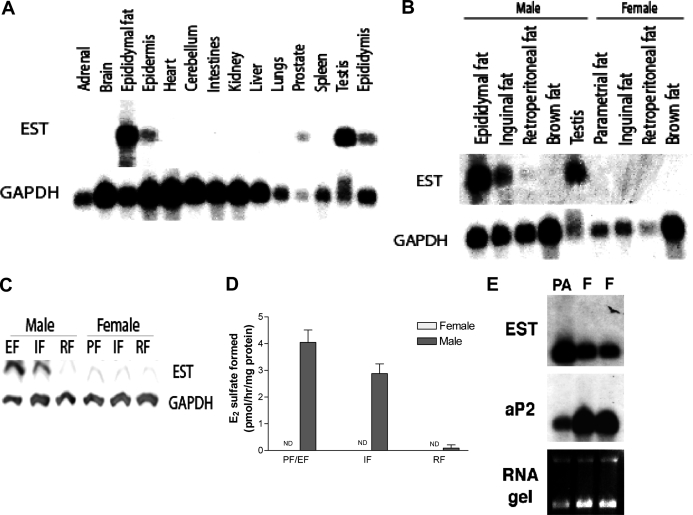
Earlier studies detected EST expression in the male reproductive system of the mouse (20,23,24). To obtain a more comprehensive profile of EST expression in the male mouse, we performed Northern blot analysis of 14 different tissues (Fig. 1). In addition to the previously reported EST-expressing sites such as testis and epididymis (23), we detected EST mRNA in the epididymal fat pad, epidermis, and prostate (Fig. 1A). Presence of EST mRNA in the prostate is consistent with estrogen-sulfating activity previously detected in this tissue (23). We also detected EST mRNA in the epidermis, although the possibility cannot be excluded that this was due to contamination with sc fat as described below. The high level EST expression in the epididymal fat pad (Fig. 1A) was striking and prompted us to investigate other fat depots in both male and female mice. By Northern blot, we observed a moderate level of EST mRNA in inguinal fat but very little in retroperitoneal fat and none in brown fat of the male mice (Fig. 1B). Interestingly, expression of EST in fat tissues appeared to be gender specific because no EST mRNA was detectible in any of the fat depots of the female mice examined (Fig. 1B). The gender-specific expression of EST was further confirmed by Western blot analysis and enzyme activity assays (Fig. 1, C and D). To determine whether EST is expressed in preadipocytes (stromal vascular cells) or fully differentiated adipocytes, we isolated preadipocytes from epididymal fat pad and compared their EST mRNA level with that of total epididymal fat pad. We performed Northern blot analysis and used adipocyte P2 (also called FABP4), a marker for mature adipocytes, as a control probe. Figure 1E shows, that compared with total epididymal fat, EST mRNA was significantly enriched in preadipocytes. However, we cannot exclude the possibility that other nonpreadipocytes, i.e. macrophages and lymphocytes, were in the stromal-vascular fraction and contributed to the EST signal. As expected, the opposite was observed for adipocyte P2, i.e. its mRNA was enriched in total epididymal fat compared with preadipocytes. These results suggested that EST is predominantly expressed in preadipocytes of fat tissues.
Figure 1.
EST is expressed in adipose tissues of male mice. A, Northern blot analysis of EST mRNA in C57BL/6 male mouse tissues. Tissues were collected and pooled from two mice, and 10 μg of RNA were loaded onto the gel. EST mRNA is found in epididymal fat, epidermis, prostate, testis, and epididymis. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase. B, EST mRNA is expressed in male epididymal and inguinal fat. Little or no EST mRNA is present in retroperitoneal fat or brown fat. None of the fat tissues examined in female mice expresses EST. C, Western blot analysis of EST protein in male and female adipose tissue. EST protein is found in male epididymal and inguinal adipose tissues only. D, Enzyme activity assay on WT male and female adipose tissues. EST activity is detected in male mouse epididymal and inguinal fat but not in retroperitoneal fat. E, Northern blot analysis showing EST is predominantly expressed in preadipocytes. IF, Inguinal fat; EF, epididymal fat; RF, retroperitoneal fat; PF, parametrial fat; ND, none detected; PA, preadipocytes; F, adipocytes.
Expression of EST in the epididymal fat pad is dependent on testosterone
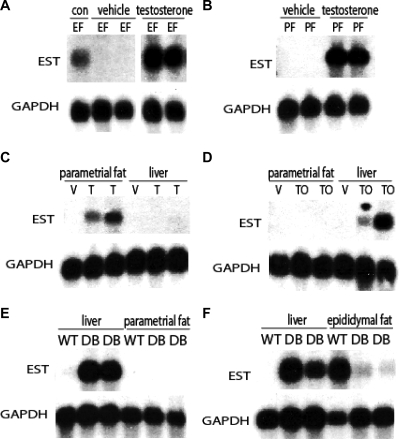
Given the sexually dimorphic expression of EST in adipose tissues, we hypothesized that EST expression in fat tissues is regulated by testosterone. To test this hypothesis, we examined EST expression in the epididymal fat of castrated male mice treated for 5 d with either testosterone propionate (T) or olive oil (vehicle control) by sc injection. The use of castrated mice allowed us to determine the role of testicular testosterone on EST. EST mRNA was not found in the epididymal fat pad of the castrated mice that were injected with vehicle (Fig. 2A), indicating that testicular testosterone is crucial for maintaining EST expression. Consistent with this conclusion, EST mRNA expression in the epididymal fat pad of the castrated mice was restored upon treatment with T (Fig. 2A). Because testosterone was all that was required to restore EST mRNA expression in the fat of castrated mice, we wondered whether testosterone treatment could lead to induction of EST expression in female mouse fat tissues. We treated female mice for 5 d with either T or olive oil. As shown in Fig. 2B, treatment of female mice with T but not vehicle led to EST expression in the parametrial adipose tissue. This result firmly established that adipose EST expression is directly under the control of testosterone.
Figure 2.
EST expression in adipose tissues is regulated by testosterone. A, Northern blot analysis of EST mRNA in epididymal fat (EF) of castrated male mice treated with T or vehicle (olive oil). The control (con) mouse was an intact male C57BL/6 mouse. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase. B, Northern blot analysis of EST mRNA in parametrial fat (PF) from female mice treated with either vehicle (olive oil) or T. C, Northern blot analysis of EST mRNA in parametrial fat and liver of female mice treated with T or vehicle (V). D, Northern blot analysis of EST mRNA in parametrial fat and liver of female mice treated with TO, an LXR agonist or vehicle (V). E, Female db/db (DB) mice had markedly induced EST expression in the liver but not in their parametrial fat. F, EST expression in the epididymal fat of db/db (DB) mice was dramatically reduced compared with normal (WT) mice, whereas EST expression was induced in their liver.
Differential regulation of EST in fat and liver
In a previous study, EST expression in the mouse liver was found to be induced by activation of the nuclear receptor LXRα (25). We next examined the tissue specificity of testosterone receptor-mediated and LXRα-mediated EST regulation. Whereas treatment of female mice with T led to EST induction in their parametrial fat, no EST expression in the liver of these mice was observed (Fig. 2C). Conversely, treatment of female mice with the LXR agonist, TO compound, led to EST expression in the liver but not in the adipose tissue (Fig. 2D). Thus, regulation of EST expression in the fat and liver appeared to be governed by different mechanisms. This finding prompted us to investigate EST expression in the fat and liver of db/db mice. The db/db mice are deficient in functional leptin receptor (26) and display a phenotype of increased adipose accumulation and metabolic syndrome that include steroid hormone imbalance (27,28,29,30). Previous studies have found dysregulated steroid sulfotransferase expression in the liver of db/db mice (23,31). Figure 2, E and F, shows that EST is robustly induced in the liver of both male and female db/db mice. Notably, EST is not induced in the parametrial fat of female db/db mice (Fig. 2E). Furthermore, compared with wild-type (WT) mice, EST expression in the epididymal fat pad of male db/db mice is dramatically suppressed (Fig. 2F). These data again highlight the differential mechanisms of EST regulation in the fat and liver of the mouse.
EST-deficient mice accumulate more adipose tissue
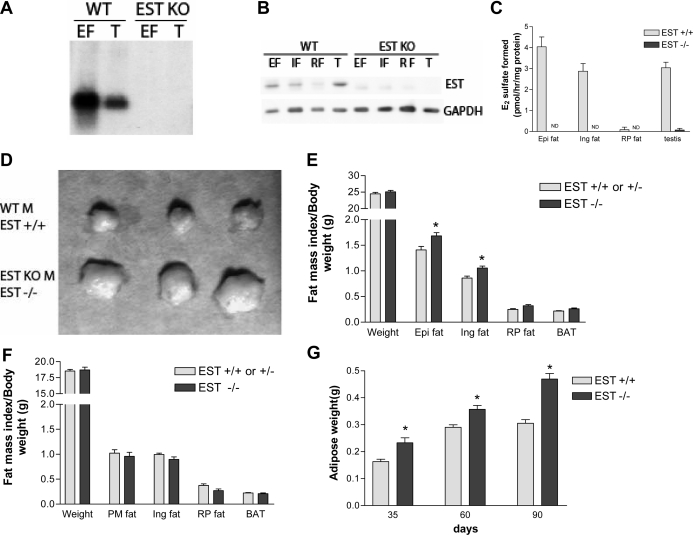
To determine the physiological role of EST in adipose tissues, we studied mice deficient in EST (EST−/−) that were previously generated in our laboratory by gene targeting (17). By Northern blot (Fig. 3A), Western blot (Fig. 3B), and enzyme activity assays (Fig. 3C), we confirmed that EST−/− mice lacked EST expression in their adipose tissues. To gain insight into the role EST plays in adipose tissues, we determined the adipose tissue weights and calculated the respective fat indices of WT and EST−/− mice. As illustrated in Fig. 3D, we found that epididymal fat pads were generally larger in male EST−/− mice than WT mice. This difference in epididymal fat pads could not be explained by differences in body weights (Fig. 3E). Indeed, the epididymal and inguinal fat indices (fat weight normalized to body weight) of male EST−/− mice were significantly higher than that of WT mice (Fig. 3E). There was no difference in retroperitoneal fat or brown fat indices between male WT and EST−/− mice (Fig. 3E), nor were there any differences in fat indices between female WT and EST−/− mice (Fig. 3F). An increase in total epididymal fat in EST−/− mice was observed in all three age groups examined (Fig. 3G).
Figure 3.
Increased adipogenesis in male EST-deficient mice. A, Northern blot analysis of EST expression in epididymal fat (EF) and testis (T) from WT and EST−/− male mice. B, Western blot analysis of EST expression in epididymal (EF), inguinal (IF), retroperitoneal (RF) fat, and testis of WT and EST−/− male mice. C, EST enzyme activity assay of various fat depots and testis of WT and EST−/− male mice. D, Representative epididymal fat masses collected from three WT and EST−/− male mice. KO, Knockout; M, male. E, Body weights and fat mass indices of 10-wk-old male EST WT/heterozygous (n = 23) and EST−/− mice (n = 16). Fat mass index = adipose weight/body weight × 100. Epi, Epididymal; Ing, inguinal; RP, retroperitoneal. F, Body weights and fat mass indices of 10-wk-old female EST WT/heterozygous (n = 18) and EST−/− mice (n = 7). PM, Parametrial. G, Increased epididymal fat mass in EST−/− mice was observed at all three ages examined (n = 8–12 for each group). Data presented are mean ± sem. *, P ≤ 0.05, Student’s t test.
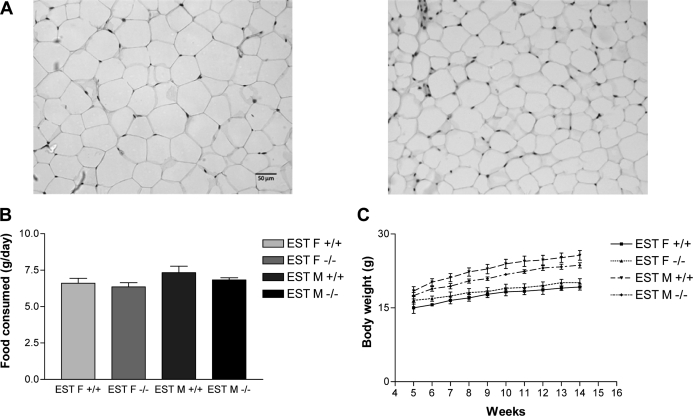
To further characterize this phenotype, we compared the adipocyte sizes in WT and EST−/− mice by measuring the cross-section areas of fat cells. Figure 4A and Table 1 show that adipocytes in EST−/− mouse epididymal and inguinal fats were markedly larger than that of WT mice (average adipocyte area in square micrometers: 1058 ± 15 vs. 1385 ± 12 for WT and EST−/− epididymal fat; 833 ± 9 vs. 1038 ± 11 for WT and EST−/− inguinal fat). A less dramatic although statistically significant increase in adipocyte area was also noted for the retroperitoneal fat of male EST−/− mice (938 ± 9 and 999 ± 10 μm2) (Table 1). The increases in epididymal and inguinal fat masses in EST−/− mice were not accompanied with metabolic abnormalities normally found in obese mice. We found no changes in glucose tolerance or systemic triglyceride levels in EST−/− mice (data not shown). There was also no difference in food intake between WT and EST−/− mice (Fig. 4B), and the growth curves as measured by body weight gains were similar between the WT and EST−/− mice over a 5- to 14-wk age span (Fig. 4C).
Figure 4.
Increased adipocyte size in male EST−/− mice. A, Epididymal adipocytes from EST−/− (left panel) mice are larger than those from WT (right panel). B, No difference in food intake was observed between WT and EST−/− mice over a 2-wk period (n = 3–5/group). F, Female; M, male. C, No difference in growth curve (weight gain) was observed between WT and EST−/− mice over a 12-wk period (n = 4–5/group).
Table 1.
Adipocyte size in WT and EST knockout (KO) male adipose tissues
| Fat pad | Genotype | Cell size (μm2) |
|---|---|---|
| Epididymal | WT | 1058 ± 15 |
| KO | 1385 ± 12a | |
| Inguinal | WT | 833 ± 9 |
| KO | 1038 ± 11a | |
| Retroperitoneal | WT | 937 ± 9 |
| KO | 1000 ± 10a |
Data represent mean ± sem.
A significant difference vs. WT at P < 0.05.
Discussion
EST inactivates estrogens by sulfoconjugation. Previous studies demonstrated that EST plays a role in regulating estrogen levels in tissues of the male reproductive system and in the female placenta (17,18,19,20). Targeted deletion of the EST gene led to age-dependent abnormalities and dysfunctions in testicular Leydig cells and the seminiferous tubules (18,20). These previous studies supported the idea that EST plays a role in regulating intracrine and paracrine estrogen activity in the male reproductive system.
In the present study, we demonstrate that EST also plays a role in adipose physiology. We show that the enzyme is expressed selectively in male adipose tissues and the expression is regulated by testosterone. Expression predominantly occurs in male epididymal fat and femoral inguinal fat. Of interest, EST is not expressed in the female mouse fat tissues. Castration of male mice abolished EST expression in epididymal fat, whereas replacement of testosterone restored its expression. Furthermore, EST expression in female mouse fat could be abnormally induced by testosterone treatment. Importantly, we found that mice engineered to be EST deficient had increased adipose accumulation in the epididymal and inguinal fat depots, which appeared to have resulted, at least partially, from enlarged adipocytes. We also showed that EST is highly expressed in preadipocytes, suggesting that it may regulate preadipocyte proliferation and/or differentiation.
Estrogen has long been linked to the development and distribution of fat (4,9). Along with the sexually dimorphic distribution of fat between males and females, physiological changes in the level of estrogen in women have been known to cause adipose shifts. For example, menopause in women causes their adipose tissue to shift to a central distribution, leading to an increase in cardiovascular risk compared with premenopausal women (32,33). However, women who receive treatment with estrogen replacement therapy do not display abdominal weight gain (34), suggesting that estrogen insufficiency may play a crucial role in post menopausal adiposity. In addition, female-like adipose distribution develops at the time of puberty (35,36). Estrogen treatment in males led to an adipose distribution shift as male to female transsexuals receiving ethinyl estradiol treatment developed an increase in sc and visceral fat (37). The development of genetically engineered mice lacking ER-α or aromatase further elucidated the role of estrogens in adipose physiology. ER-α knockout mice had increased adipose tissue, increased weight, insulin resistance, impaired glucose tolerance, and decreased energy expenditure (10). Changes in aromatase knockout mice include increased adipose tissue, elevated cholesterol and leptin circulating levels, reduced spontaneous activity levels, and reduced glucose oxidation (11). The role of estrogen in adipose tissue physiology, however, is not limited to the tissue itself. As highlighted by these two mouse models and other studies, estrogens affect energy intake and expenditure through central actions involving the hypothalamus. Estrogen deficiency has been shown to increase neuropeptide Y and decrease central leptin sensitivity (38,39), two important regulators of the lipostat system. Lazzarini and Wade (40) suggest estradiol may have two ways of effecting adipose tissue: acting on the brain, leading to stimulation of the sympathetic nerves enervating adipose tissue, and peripherally, leading to enhanced catecholamine response.
In our model, we found that estrogen sulfotransferase deficiency, which will lead to elevated local estrogen levels inside adipose tissue, resulted in an increase in adipose tissue accumulation in male mice and no differences in female adipose development. Increased adipose accumulation in EST-deficient mice may seem counterintuitive based on previous mouse models studying the role of estrogen in adipose tissues. However, it is important to point out that, whereas other models have decreased central and systemic levels of estrogen, our model affects only the local estrogen levels in tissues that express estrogen sulfotransferase, such as adipose tissue and the male reproductive system. Whereas insufficient systemic estrogen levels can lead to adiposity and metabolic deficiencies, our results suggest that hyperestrogen activity in adipose tissue in vivo results in an increase in adipose accumulation. Furthermore, previous reports suggested that estradiol can stimulate preadipocyte proliferation (41,42), the cell type in which EST is most abundantly expressed. Estrogen may regulate cell proliferation by multiple mechanisms. Rapid nongenomic activation of the MAPK pathway involved in cell proliferation has been described for rat white adipocytes (43). Additionally, ER-α has been shown to interact with the nuclear receptor peroxisome proliferator-activated receptor-γ (44), an adipogenesis regulator, and low doses of estradiol were shown to increase lipoprotein lipase in isolated human sc abdominal adipocytes (6). Taken together, these studies would suggest that EST inactivation could lead to increased preadipocyte proliferation and adipogenesis due to increased local estrogen levels.
An interesting finding in this study is that EST expression in fat is gender specific and expression of EST in the fat and liver is differentially regulated. We have shown that EST is expressed in male but not female mouse adipose tissue, whereas treating female mice with testosterone stimulated EST expression in the fat. EST induction appears to be specific to testosterone because treating females with either estrogen or dexamethasone did not induce EST expression (data not shown). Expression of EST in male adipose tissue is most likely due to the presence of basal testosterone levels and not due to androgen metabolizing enzymes in female adipose tissue as hydroxysteroid sulfotransferases, which metabolize dehydroepiandronesterone and other steroids, have not been reported to be expressed in adipose tissue (15,45,46). Testosterone treatment had no effect on EST expression in the liver, most likely due to the low testosterone receptor levels in adult rodent livers (47). EST expression in the male and female mouse liver has been shown to be induced by LXR agonists and the mouse EST gene promoter has also been found to be a transcriptional target of LXR (25). We confirmed that TO-901317, a LXR agonist, induced EST expression in the mouse liver. However, we found that TO-901317 had no effect on EST expression in the mouse adipose tissues despite reports of LXRα and -β being present in adipose tissue (48,49,50). This differential regulation of EST was also highlighted in db/db mice. EST expression was greatly reduced in the epididymal fat of the hypotestosteroneic male db/db mice but was heavily induced in their liver. Likewise, a high level EST expression was detected in the liver but not fat of female db/db mice. The mechanism of EST induction in the liver of db/db mice is not well understood, although previous studies suggested that elevated glucocorticoids might be responsible (51).
It is notable that among the various fat depots, EST is most abundantly expressed in epididymal and inguinal fats of the male mouse. Disparity in regional adipose EST expression is not unusual because similar variations have been described for other steroid hormone-related genes such as the glucocorticoid receptor and aromatase (52,53). Real-time PCR analysis revealed no correlation between EST and androgen receptor expression in different white fat depots of male and female mice (data not shown). It is possible that differences in local testosterone concentration and/or androgen receptor coactivators are responsible for the observed difference in EST expression among male and female mouse adipose tissues. Similar mechanisms could explain the lack of EST expression in brown adipose tissue (BAT). It is notable that although both ER-α and androgen receptor have been reported to be present in BAT (54), increased adipogenesis in ERα knockout mice was also limited to white adipose tissue and was not observed with BAT (10), possibly due to a differential regulation of fat cell proliferation between white adipose tissue and BAT (55).
The finding that EST knockout mice accumulated more adipose tissue mass than WT mice suggested that genetic or chemical inactivation of EST can lead to abnormal adipogenesis. Although EST expression has not been examined in human adipose tissues, our model allows us to speculate on the potential consequences of human EST inhibition. In this context, it is relevant to note previous enzymatic and crystallographic studies demonstrating potent inhibition of human EST by hydroxylated polychlorinated biphenyls (PCBs) (56,57). Although production of these chemicals ceased in the 1970s, the stable nature of PCBs has allowed them to persist in the environment (58). Concerns over PCBs have arisen due to their potential as endocrine disrupters in humans and wildlife. Given the tendency of these chemicals to accumulate in adipose tissues, the possibility exists that PCB metabolites disrupt normal adipogenesis in humans through inhibition of EST activity, and studies examining the expression and regulation of EST in human adipose tissues are therefore much warranted.
In summary, we have shown that EST is expressed in a sexually dimorphic and site-specific manner in adipose tissue. Expression occurs in the epididymal and the inguinal adipose tissues of the male mouse and is not seen in the female mouse. We demonstrate that EST expression is regulated by the sex hormone testosterone and castration of male mice led to a loss of expression, whereas replacement of the hormone restored expression. This expression can be abnormally induced in female by treating with exogenous testosterone. EST expression is highest in preadipocytes. Mice rendered deficient in EST accumulated more adipose and had larger adipocytes than WT littermates. Our results suggest that EST may play a role in regulating male adipose tissue accumulation.
Footnotes
This work was supported by National Institutes of Health Grant HD-042767 (to W.-C.S.) and a predoctoral fellowship (to V.K.K.) from the American Heart Association.
Disclosure Statement: The authors have nothing to disclose.
First Published Online July 31, 2008
Abbreviations: BAT, Brown adipose tissue; ER, estrogen receptor; EST, estrogen sulfotransferase; LXR, liver X receptor; PCB, polychlorinated biphenyl; SDS, sodium dodecyl sulfate; SVF, stromal vascular fraction; T, testosterone propionate; TO, TO-901317; WT, wild type.
References
- Bjorntorp P 1991 Adipose tissue distribution and function. Int J Obes 15(Suppl 2):67–81 [PubMed] [Google Scholar]
- Dieudonne MN, Leneveu MC, Giudicelli Y, Pecquery R 2004 Evidence for functional estrogen receptors α and β in human adipose cells: regional specificities and regulation by estrogens. Am J Physiol Cell Physiol 286:C655–C661 [DOI] [PubMed] [Google Scholar]
- Cooke PS, Naaz A 2004 Role of estrogens in adipocyte development and function. Exp Biol Med (Maywood) 229:1127–1135 [DOI] [PubMed] [Google Scholar]
- Wade GN, Gray JM 1978 Cytoplasmic 17β-[3H]estradiol binding in rat adipose tissues. Endocrinology 103:1695–1701 [DOI] [PubMed] [Google Scholar]
- Pedersen SB, Borglum JD, Moller-Pedersen T, Richelsen B 1992 Effects of in vivo estrogen treatment on adipose tissue metabolism and nuclear estrogen receptor binding in isolated rat adipocytes. Mol Cell Endocrinol 85:13–19 [DOI] [PubMed] [Google Scholar]
- Palin SL, McTernan PG, Anderson LA, Sturdee DW, Barnett AH, Kumar S 2003 17β-Estradiol and anti-estrogen ICI:compound 182,780 regulate expression of lipoprotein lipase and hormone-sensitive lipase in isolated subcutaneous abdominal adipocytes. Metabolism 52:383–388 [DOI] [PubMed] [Google Scholar]
- Pedersen SB, Kristensen K, Hermann PA, Katzenellenbogen JA, Richelsen B 2004 Estrogen controls lipolysis by up-regulating α2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor α. Implications for the female fat distribution. J Clin Endocrinol Metab 89:1869–1878 [DOI] [PubMed] [Google Scholar]
- Lacasa D, Garcia E, Henriot D, Agli B, Giudicelli Y 1997 Site-related specificities of the control by androgenic status of adipogenesis and mitogen-activated protein kinase cascade/c-fos signaling pathways in rat preadipocytes. Endocrinology 138:3181–3186 [DOI] [PubMed] [Google Scholar]
- Wade GN, Gray JM, Bartness TJ 1985 Gonadal influences on adiposity. Int J Obes 9(Suppl 1):83–92 [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS 2000 Increased adipose tissue in male and female estrogen receptor-α knockout mice. Proc Natl Acad Sci USA 97:12729–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, Simpson ER 2000 Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA 97:12735–12740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TM, O'Brien SN 1993 Determination of estrogen receptor messenger ribonucleic acid (mRNA) and cytochrome P450 aromatase mRNA levels in adipocytes and adipose stromal cells by competitive polymerase chain reaction amplification. J Clin Endocrinol Metab 77:1041–1045 [DOI] [PubMed] [Google Scholar]
- Pedersen SB, Borglum JD, Eriksen EF, Richelsen B 1991 Nuclear estradiol binding in rat adipocytes. Regional variations and regulatory influences of hormones. Biochim Biophys Acta 1093:80–86 [DOI] [PubMed] [Google Scholar]
- Falany CN 1991 Molecular enzymology of human liver cytosolic sulfotransferases. Trends Pharmacol Sci 12:255–259 [DOI] [PubMed] [Google Scholar]
- Strott CA 1996 Steroid sulfotransferases. Endocr Rev 17:670–697 [DOI] [PubMed] [Google Scholar]
- Qian Y, Deng C, Song WC 1998 Expression of estrogen sulfotransferase in MCF-7 cells by cDNA transfection suppresses the estrogen response: potential role of the enzyme in regulating estrogen-dependent growth of breast epithelial cells. J Pharmacol Exp Ther 286:555–560 [PubMed] [Google Scholar]
- Qian YM, Sun XJ, Tong MH, Li XP, Richa J, Song WC 2001 Targeted disruption of the mouse estrogen sulfotransferase gene reveals a role of estrogen metabolism in intracrine and paracrine estrogen regulation. Endocrinology 142:5342–5350 [DOI] [PubMed] [Google Scholar]
- Tong MH, Christenson LK, Song WC 2004 Aberrant cholesterol transport and impaired steroidogenesis in Leydig cells lacking estrogen sulfotransferase. Endocrinology 145:2487–2497 [DOI] [PubMed] [Google Scholar]
- Tong MH, Jiang H, Liu P, Lawson JA, Brass LF, Song WC 2005 Spontaneous fetal loss caused by placental thrombosis in estrogen sulfotransferase-deficient mice. Nat Med 11:153–159 [DOI] [PubMed] [Google Scholar]
- Tong MH, Song WC 2002 Estrogen sulfotransferase: discrete and androgen-dependent expression in the male reproductive tract and demonstration of an in vivo function in the mouse epididymis. Endocrinology 143:3144–3151 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Repa JJ, Gauthier K, Mangelsdorf DJ 2001 Regulation of lipoprotein lipase by the oxysterol receptors, LXRα and LXRβ. J Biol Chem 276:43018–43024 [DOI] [PubMed] [Google Scholar]
- Deslex S, Negrel R, Ailhaud G 1987 Development of a chemically defined serum-free medium for differentiation of rat adipose precursor cells. Exp Cell Res 168:15–30 [DOI] [PubMed] [Google Scholar]
- Song WC, Moore R, McLachlan JA, Negishi M 1995 Molecular characterization of a testis-specific estrogen sulfotransferase and aberrant liver expression in obese and diabetogenic C57BL/KsJ-db/db mice. Endocrinology 136:2477–2484 [DOI] [PubMed] [Google Scholar]
- Song WC, Qian Y, Sun X, Negishi M 1997 Cellular localization and regulation of expression of testicular estrogen sulfotransferase. Endocrinology 138:5006–5012 [DOI] [PubMed] [Google Scholar]
- Gong H, Guo P, Zhai Y, Zhou J, Uppal H, Jarzynka MJ, Song WC, Cheng SY, Xie W 2007 Estrogen deprivation and inhibition of breast cancer growth in vivo through activation of the orphan nuclear receptor liver X receptor. Mol Endocrinol 21:1781–1790 [DOI] [PubMed] [Google Scholar]
- Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM 1996 Abnormal splicing of the leptin receptor in diabetic mice. Nature 379:632–635 [DOI] [PubMed] [Google Scholar]
- Herberg L, Coleman DL 1977 Laboratory animals exhibiting obesity and diabetes syndromes. Metabolism 26:59–99 [DOI] [PubMed] [Google Scholar]
- Leiter EH, Chapman HD, Coleman DL 1989 The influence of genetic background on the expression of mutations at the diabetes locus in the mouse. V. Interaction between the db gene and hepatic sex steroid sulfotransferases correlates with gender-dependent susceptibility to hyperglycemia. Endocrinology 124:912–922 [DOI] [PubMed] [Google Scholar]
- Kuhn-Velten N, Codjambopoulo P, Herberg L, Kley HK, Staib W 1985 In vitro studies of the development of pituitary and testicular functions in diabetes (C57BL/KsJ-db/db) mutant mice. Horm Metab Res 17:576–579 [DOI] [PubMed] [Google Scholar]
- Nishina PM, Lowe S, Wang J, Paigen B 1994 Characterization of plasma lipids in genetically obese mice: the mutants obese, diabetes, fat, tubby, and lethal yellow. Metabolism 43:549–553 [DOI] [PubMed] [Google Scholar]
- Leiter EH, Chapman HD 1994 Obesity-induced diabetes (diabesity) in C57BL/KsJ mice produces aberrant trans-regulation of sex steroid sulfotransferase genes. J Clin Invest 93:2007–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB, Hjortland MC, McNamara PM, Gordon T 1976 Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med 85:447–452 [DOI] [PubMed] [Google Scholar]
- Ley CJ, Lees B, Stevenson JC 1992 Sex- and menopause-associated changes in body-fat distribution. Am J Clin Nutr 55:950–954 [DOI] [PubMed] [Google Scholar]
- Gambacciani M, Ciaponi M, Cappagli B, Piaggesi L, De Simone L, Orlandi R, Genazzani AR 1997 Body weight, body fat distribution, and hormonal replacement therapy in early postmenopausal women. J Clin Endocrinol Metab 82:414–417 [DOI] [PubMed] [Google Scholar]
- Sjostrom L, Smith U, Krotkiewski M, Bjorntorp P 1972 Cellularity in different regions of adipose tissue in young men and women. Metabolism 21:1143–1153 [DOI] [PubMed] [Google Scholar]
- Chumlea WC, Knittle JL, Roche AF, Siervogel RM, Webb P 1981 Size and number of adipocytes and measures of body fat in boys and girls 10 to 18 years of age. Am J Clin Nutr 34:1791–1797 [DOI] [PubMed] [Google Scholar]
- Elbers JM, Asscheman H, Seidell JC, Gooren LJ 1999 Effects of sex steroid hormones on regional fat depots as assessed by magnetic resonance imaging in transsexuals. Am J Physiol 276:E317–E325 [DOI] [PubMed] [Google Scholar]
- Ainslie DA, Morris MJ, Wittert G, Turnbull H, Proietto J, Thorburn AW 2001 Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int J Obes Relat Metab Disord 25:1680–1688 [DOI] [PubMed] [Google Scholar]
- Shimizu H, Ohtani K, Kato Y, Tanaka Y, Mori M 1996 Withdrawal of [corrected] estrogen increases hypothalamic neuropeptide Y (NPY) mRNA expression in ovariectomized obese rat. Neurosci Lett 204:81–84 [DOI] [PubMed] [Google Scholar]
- Lazzarini SJ, Wade GN 1991 Role of sympathetic nerves in effects of estradiol on rat white adipose tissue. Am J Physiol 260:R47–R51 [DOI] [PubMed] [Google Scholar]
- Lea-Currie YR, Monroe D, McIntosh MK 1999 Dehydroepiandrosterone and related steroids alter 3T3-L1 preadipocyte proliferation and differentiation. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 123:17–25 [DOI] [PubMed] [Google Scholar]
- Dieudonne MN, Pecquery R, Leneveu MC, Giudicelli Y 2000 Opposite effects of androgens and estrogens on adipogenesis in rat preadipocytes: evidence for sex and site-related specificities and possible involvement of insulin-like growth factor 1 receptor and peroxisome proliferator-activated receptor γ2. Endocrinology 141:649–656 [DOI] [PubMed] [Google Scholar]
- Dos Santos EG, Dieudonne MN, Pecquery R, Le Moal V, Giudicelli Y, Lacasa D 2002 Rapid nongenomic E2 effects on p42/p44 MAPK, activator protein-1, and cAMP response element binding protein in rat white adipocytes. Endocrinology 143:930–940 [DOI] [PubMed] [Google Scholar]
- Wang X, Kilgore MW 2002 Signal cross-talk between estrogen receptor α and β and the peroxisome proliferator-activated receptor γ1 in MDA-MB-231 and MCF-7 breast cancer cells. Mol Cell Endocrinol 194:123–133 [DOI] [PubMed] [Google Scholar]
- Geese WJ, Raftogianis RB 2001 Biochemical characterization and tissue distribution of human SULT2B1. Biochem Biophys Res Commun 288:280–289 [DOI] [PubMed] [Google Scholar]
- Javitt NB, Lee YC, Shimizu C, Fuda H, Strott CA 2001 Cholesterol and hydroxycholesterol sulfotransferases: identification, distinction from dehydroepiandrosterone sulfotransferase, and differential tissue expression. Endocrinology 142:2978–2984 [DOI] [PubMed] [Google Scholar]
- Song CS, Rao TR, Demyan WF, Mancini MA, Chatterjee B, Roy AK 1991 Androgen receptor messenger ribonucleic acid (mRNA) in the rat liver: changes in mRNA levels during maturation, aging, and calorie restriction. Endocrinology 128:349–356 [DOI] [PubMed] [Google Scholar]
- Apfel R, Benbrook D, Lernhardt E, Ortiz MA, Salbert G, Pfahl M 1994 A novel orphan receptor specific for a subset of thyroid hormone-responsive elements and its interaction with the retinoid/thyroid hormone receptor subfamily. Mol Cell Biol 14:7025–7035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa JJ, Mangelsdorf DJ 2000 The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu Rev Cell Dev Biol 16:459–481 [DOI] [PubMed] [Google Scholar]
- Teboul M, Enmark E, Li Q, Wikstrom AC, Pelto-Huikko M, Gustafsson JA 1995 OR-1, a member of the nuclear receptor superfamily that interacts with the 9-cis-retinoic acid receptor. Proc Natl Acad Sci USA 92:2096–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill AM, Leiter EH, Powell JG, Chapman HD, Yen TT 1994 Dexamethasone-induced hyperglycemia in obese Avy/a (viable yellow) female mice entails preferential induction of a hepatic estrogen sulfotransferase. Diabetes 43:999–1004 [DOI] [PubMed] [Google Scholar]
- Ackerman GE, Smith ME, Mendelson CR, MacDonald PC, Simpson ER 1981 Aromatization of androstenedione by human adipose tissue stromal cells in monolayer culture. J Clin Endocrinol Metab 53:412–417 [DOI] [PubMed] [Google Scholar]
- Joyner JM, Hutley LJ, Cameron DP 2000 Glucocorticoid receptors in human preadipocytes: regional and gender differences. J Endocrinol 166:145–152 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Cuenca S, Monjo M, Frontera M, Gianotti M, Proenza AM, Roca P 2007 Sex steroid receptor expression profile in brown adipose tissue. Effects of hormonal status. Cell Physiol Biochem 20:877–886 [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Song CK 2007 Thematic review series: adipocyte biology. Sympathetic and sensory innervation of white adipose tissue. J Lipid Res 48:1655–1672 [DOI] [PubMed] [Google Scholar]
- Kester MH, Bulduk S, Tibboel D, Meinl W, Glatt H, Falany CN, Coughtrie MW, Bergman A, Safe SH, Kuiper GG, Schuur AG, Brouwer A, Visser TJ 2000 Potent inhibition of estrogen sulfotransferase by hydroxylated PCB metabolites: a novel pathway explaining the estrogenic activity of PCBs. Endocrinology 141:1897–1900 [DOI] [PubMed] [Google Scholar]
- Shevtsov S, Petrotchenko EV, Pedersen LC, Negishi M 2003 Crystallographic analysis of a hydroxylated polychlorinated biphenyl (OH-PCB) bound to the catalytic estrogen binding site of human estrogen sulfotransferase. Environ Health Perspect 111:884–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe SH 1994 Polychlorinated biphenyls (PCBs): environmental impact, biochemical and toxic responses, and implications for risk assessment. Crit Rev Toxicol 24:87–149 [DOI] [PubMed] [Google Scholar]