Abstract
GnRH1 stimulates the synthesis and secretion of FSH and LH from the anterior pituitary gland. The molecular mechanisms through which GnRH1 produces these effects in humans have not been determined. Here, we examined transcriptional regulation of the human FSHβ (FSHB) subunit using reporter assays in immortalized murine gonadotrope cells. GnRH1 dose and time dependently stimulated FSHB promoter activity, with peak stimulation occurring at 8 h. GnRH1 rapidly stimulated various MAPK cascades, though the ERK1/2 and p38 pathways appeared to be most critical for FSHB induction. Indeed, constitutively active forms of both Raf1 kinase and MAP2K6 (MKK6) were sufficient to stimulate reporter activity. GnRH1 stimulated activator protein-1 (AP-1) (FosB, c-fos, JunB, and cJun) synthesis and complex formation, the latter of which bound to a conserved cis-element within −120 bp of the transcription start site. A second, lower affinity, site was mapped more proximally. Mutations of both cis-elements diminished GnRH1-stimulated promoter activity, though disruption of the higher affinity site had a more dramatic effect. A dominant-negative Fos protein dose dependently inhibited GnRH1-stimulated FSHB transcription, confirming a role for endogenous AP-1 proteins. MAPK kinase 1 (MEK1) and p38 inhibitors significantly attenuated GnRH1-stimulated c-fos, FosB, and JunB synthesis, suggesting a mechanism whereby the ERK1/2 and p38 signaling pathways regulate FSHB transcription. Activins and inhibins potently regulate FSH synthesis in rodents, but their roles in FSH regulation in humans are less clear. Activin A, though weak on its own, synergized with GnRH1 to stimulate human FSHB promoter activity. In contrast, activin A partially inhibited GnRH1-stimulated LHβ subunit (LHB) transcription. The GnRH1 and activin A signaling pathways appear to converge at the level of the high-affinity AP-1 site. Fos and Jun proteins synergistically regulate reporter activity through this element, and their effects are potentiated by coexpression of either Smad2 or Smad3, effectors in the activin signaling cascade. In summary, GnRH1 and activin A synergistically regulate human FSHB subunit transcription. The combined actions of AP-1 and Smad proteins acting through a conserved AP-1 element provide a candidate mechanism for this effect. The ability of activins to potentiate selectively the effects of GnRH1 on FSHB expression suggests a model for preferential increases in FSH secretion at the luteal-follicular transition of the menstrual cycle.
THE GONADOTROPINS, FSH and LH, are coordinately and differentially regulated across the human menstrual cycle. The two are secreted together before ovulation at the end of the follicular phase, and both are suppressed during the luteal phase. At the luteal to follicular phase transition, there is a selective increase in FSH that drives dominant follicle selection and maturation. The mechanisms controlling this singular FSH elevation have not been definitively established and, in fact, are a subject of some debate (e.g. Refs. 1 and 2).
The follicular phase FSH increase in humans is analogous to the secondary FSH surge in rodents. In the latter case, declines in inhibin A and inhibin B after the primary gonadotropin surges, as well as decreases intrapituitary follistatin expression, provide a permissive endocrine/paracrine environment for pituitary activins to stimulate FSH synthesis and secretion (3,4). That is, in the absence (or reduction) of the antagonistic effects of inhibins (competition for activin receptors) (5,6) and follistatins (bioneutralization of activins through irreversible binding) (7), activins can stimulate expression of the FSHβ (Fshb) subunit gene, the rate-limiting step in hormone synthesis (8,9,10,11,12,13). A role for inhibins and activins in FSH regulation in humans is controversial.
How activins and endocrine hormones such as GnRH1 and sex steroids regulate gonadotropin synthesis has been actively investigated. In many cases these hormones and paracrine factors act, either directly or indirectly, to regulate transcription of the unique FSH and LH β (LHB) subunits (14). Interestingly, the regulation of these subunit genes in humans has received considerably less attention than in popular model organisms such as rodents, sheep, cows, and pigs. This likely derives from the perceived paucity of adequate homologous cell model systems in which to perform traditional transcriptional assays. Indeed, there are currently no clonal human gonadotrope cell lines. Nonetheless, both the human FSHB gene (15,16) and gonadotropin α-subunit promoters (CGA) (17,18) are functional and appropriately regulated in gonadotrope cells of transgenic mice. These observations suggest that murine gonadotrope cells, and by extension cell lines, may provide useful and valid models for investigations of transcriptional regulation of the human gonadotropin subunit genes.
We have used the murine gonadotrope cell line, LβT2 (19), to examine regulation of the murine and human Fshb/FSHB promoters (8,20,21,22,23). Others have similarly used this cell model for examination of the Cga, Lhb, and Fshb subunit promoters from a host of species (e.g. Refs. 11 and 24,25,26,27). We and others have delineated a signaling cascade through which activins directly regulate the rat and murine Fshb subunit genes (8,11,22,28). We have further argued that this mechanism might explain the rapid synthesis of FSH necessary for generation of the secondary surge in these animals (22). At the same time, we observed that the human FSHB promoter lacks at least one cis-element critical for rapid activation by activins and is largely insensitive to activin A even with prolonged ligand treatment. Nonetheless, activin A stimulates FSH expression and secretion in rhesus monkeys, both in vitro and in vivo (29,30,31), suggesting that the FSHB gene might be activin responsive in primates.
A recent report showed that sequence flanking the 3′ end of exon 3 of the human FSHB gene is necessary for gonadotrope-restricted expression in transgenic mice (32). Therefore, it is possible that our previous investigations using only promoter (or 5′ flanking region) sequence lacked critical regulatory elements and, therefore, underestimated the role of activins in FSHB regulation. Alternatively, given the different dynamics of FSH across human and rodent reproductive cycles, activins might play an indirect role in FSHB regulation, perhaps by modulating the actions of other hormones.
GnRH1’s function as an FSH secretagogue in humans is indisputable. Whether GnRH1 regulates FSHB transcription in humans, as it does the orthologous promoters in other species, is not known. Therefore, we examined regulation of the human FSHB promoter by GnRH1 in LβT2 cells. We observed that GnRH1 potently stimulated FSHB promoter-reporter activity and that this response was potentiated by activin A. In contrast, GnRH1-stimulated human LHB promoter activity was partially inhibited by activin A. The opposing effects of activin A on GnRH1-regulated FSHB and LHB transcription may contribute to differential regulation of the gonadotropins at the luteal-follicular phase transition of the menstrual cycle.
Materials and Methods
Reagents and constructs
DMEM with 4.5 g/liter glucose, l-glutamine, and sodium pyruvate was from Wisent (St. Bruno, Quebec, Canada). Fetal bovine serum, Lipofectamine/Plus, Lipofectamine 2000, gentamycin, and NuPAGE gels were purchased from Invitrogen Canada (Burlington, Ontario, Canada). Human recombinant activin A was purchased from R&D Systems, Inc. (Minneapolis, MN). GnRH1 (LHRH), anti-β-actin, cycloheximide, aprotinin, leupeptin, pepstatin, phenylmethylsulfonylfluoride, SB431542, SB203580, and SP600125 were from Sigma-Aldrich Corp. (St. Louis, MO). SB202190 was from Calbiochem (La Jolla, CA). Deoxynucleotide triphosphates, Taq polymerase, U0126, and 5× Passive Lysis Buffer were from Promega Corp. (Madison, WI). Protease inhibitor tablets (Complete Mini) were purchased from Roche Applied Science (Laval, Québec, Canada). Oligonucleotides were synthesized by Integrated DNA Technologies (Coralville, IA). Poly(dI).poly(dC), ECL-plus reagent, and protein markers were purchased from GE Healthcare (Piscataway, NJ). [γ-32P]ATP was from PerkinElmer (Boston, MA). Phospho-ERK1/2 (T202/Y204; no. 9101), phospho-p38 (T180/Y182; no. 9211), phospho-SAPK/JNK (T183/Y185; no. 9251), and phospho-c-Jun (Ser63; no. 9261) rabbit polyclonal antibodies were purchased from Cell Signaling Technology, Inc. (Danvers, MA). c-fos (sc-52X), FosB (sc-48X), and JunB (SC-73X) rabbit polyclonals were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-c-Jun (mouse IgG2A) was from BD Biosciences (Mississauga, Ontario, Canada).
The −1195/+1 murine Fshb-luc, various human FSHB-luc reporters in pGL3-Basic, GAL4-Elk1, and 5xGAL4-E1B-luc were described previously (22,33,34,35). Here, we subcloned the human −1028/+7 and −126/+7 FSHB promoter fragments into the KpnI/HindIII (blunted) sites of pA3-luc (36) because we observed that the empty pGL3-Basic vector was GnRH1 responsive in preliminary analyses (data not shown). Mutations were introduced into the indicated reporters using the primers in Table 1 and the QuikChange site-directed mutagenesis protocol (Stratagene, La Jolla, CA). A human LHB luciferase reporter was produced by PCR amplifying approximately 0.2 kb of the 5′ flanking region of the LHB gene from genomic DNA from one of the investigators (D.J.B.) using the primers in Table 1 and ligating it into the KpnI/HindIII sites of pA3-luc. The McGill University Institutional Review Board approved the use of the DNA for this purpose. Constitutively active (ca) MAPK kinase kinase (MEKK) 1 (38) and GAL4-c-Jun (39) were generously provided by Drs. Carol Lange (University of Minnesota, Minneapolis, MN) and Michael Karin (University of California San Diego, San Diego, CA), respectively. caMKK6 (MKK6EE) in pcDNA3 was from Dr. David Engelberg (Hebrew University, Jerusalem, Israel) (40). Raf-CAAX was from Dr. Linda Van Aelst (Cold Spring Harbor Laboratory, Cold Spring Harbor NY). The FosB expression vector was from Dr. Paula Ulery (University of Texas-Southwestern Medical Center, Dallas, TX). c-Jun and JunB expression vectors were from Alain Mauviel (HÃ′pital Saint-Louis, Vellefaux, Paris, France). The c-fos expression vector was from Dr. Paul Dobner (University of Massachusetts, Worcester, MA), and A-Fos (41) was from Dr. Charles Vinson (National Cancer Institute, Bethesda, MD). Constructs were verified by DNA sequencing (GenomeQuébec, Montréal, Quebec, Canada, or Genewiz, South Plainfield, NJ).
Table 1.
Primer and probe sequences
| Cloninga | |
| hLHB.for | CGGGGTACCCTCACCTCTGGCGCTAGACC |
| hLHB.rev | CGGAAGCTTCTTGGTGCATCCCCTGCCT |
| Mutagenesisb | |
| hFSHB.−115/−113mut.for | CTTACGCGTCTAAACACTGcggCACTTACAGCAAGCTTCAG |
| hFSHB.−80/−79.for | CTTCAGGCTAGCATTGGTCccATTAATACCCAACAAATCC |
| hFSHB.−81/−80mut.for | GCTTCAGGCTAGCATTGGTacTATTAATACCCAACAAATC |
| Gel shiftsb | |
| hFSHB.−126/−94 | TCTAAACACTGATTCACTTACAGCAAGCTTCAG |
| hFSHB.−126(−117/−112) | TCTAAACACaGATctACTTACAGCAAGCTTCAG |
| hFSHB.−126(−115/−113) | TCTAAACACTGcggCACTTACAGCAAGCTTCAG |
| hFSHB.−93/−61 | GCTAGCATTGGTCATATTAATACCCAACAAATC |
| hFSHB.−93(−80/−79) | GCTAGCATTGGTCccATTAATACCCAACAAATC |
| hFSHB.−93(−81/−80) | GCTAGCATTGGTacTATTAATACCCAACAAATC |
| hFSHB.−60/−27 | CACAAGGTGTTAGTTGCACATGATTTTGTATAAA |
| hFSHB.−60(−38/−36) | CACAAGGTGTTAGTTGCACATGcggTTGTATAAA |
| hFSHB.−26/+7 | AGGTGAACTGAGATTTCATTCAGTCTACAGCTC |
for, Forward; mut, mutation; rev, reverse.
Restriction sites are underlined.
Only the sense strand is shown. Mutations are in lowercase.
Cell culture, transfection, and reporter assay
LβT2 and αT3-1 cells were generously provided by Dr. Pamela Mellon (University of California, San Diego, CA) and were cultured as described previously (8,22). For reporter assays, cells were plated in 24-well plates at a density of 2.5 × 105 cells per well 2–3 d before transfection. Cells were transfected overnight with Lipofectamine 2000. Reporter and expression plasmids were transfected at the indicated concentrations, and total DNA transfected was balanced across conditions. Cells were washed in 1× PBS before treatment with the indicated ligands at the indicated concentrations and times in serum-free DMEM. Inhibitors were applied at the indicated concentrations 30 min before ligand treatments. Whole cell lysates were prepared in 1× Passive Lysis Buffer, and luciferase activity was measured on an Orion II microplate luminometer (Berthold, Pforzheim, Germany) using standard reagents. In our experience, standard vectors used to control for transfection efficiency are regulated by activins and various overexpressed proteins, and, therefore, could not be used here. Measurements of protein content did not indicate any effects of the treatments on cell viability. All experiments were performed a minimum of three times and all treatments performed in duplicate or triplicate. For Western blot, DNA pull-down, and gel shift analyses, cells were plated in either six-well or 10-cm plates.
EMSA, Western blot, and DNA pull-down assays
Nuclear extracts were collected and gel shift experiments performed as previously described (22,33) using the probes described in Table 1. Western blots were performed on nuclear extracts or whole cell extracts prepared in radioimmunoprecipitation assay (RIPA) buffer containing protease inhibitors (Roche Applied Science) as described previously (8,22,33). DNA pull-down assays were performed on whole cell extracts from control or GnRH1 treated cells using biotinylated wild-type (WT) or mutant −126/−94 human FSHB probes (Table 1) as previously described (22,33).
Statistics
The data presented were from representative experiments. Luciferase reporter data are presented as fold change from the control condition (set to one) in each experiment. Differences between means were compared using one-, two-, or three-way ANOVA, followed by post hoc tests (Tukey) where appropriate (Systat 10.2; Systat Software, Inc., Richmond, CA). Data were log transformed before analysis when the variances were unequal between groups. Statistical significance was assessed relative to P < 0.05.
Results
Human FSHB promoter-reporter activity is stimulated by GnRH1
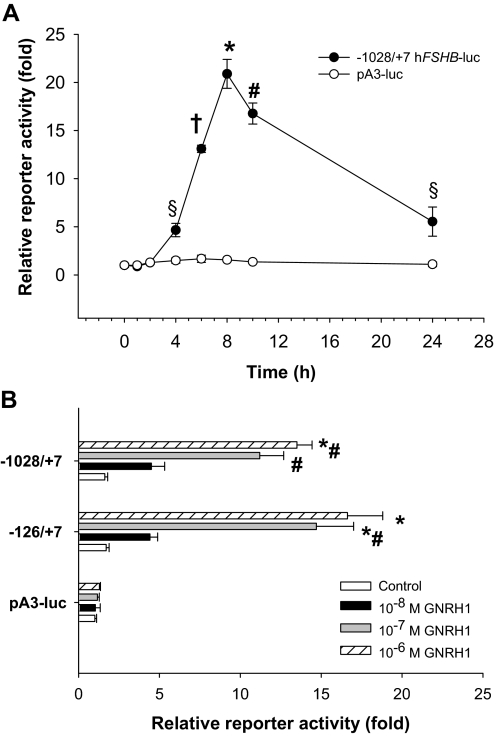
A human FSHB promoter-reporter construct in which −1028/+7 of the 5′ flanking region (+1 = start of transcription) was ligated upstream of the luciferase coding sequence in pA3-luc was transfected into LβT2 cells, followed by treatment with 10−7 m GnRH1 for 0, 1, 2, 4, 6, 8, 10, or 24 h. GnRH1 time dependently stimulated reporter activity (Fig. 1A), with peak activation occurring after 8 h. Reporter activity returned to near baseline levels by 24 h, consistent with previous reports of GnRH1 receptor desensitization/adaptation with continuous ligand treatment both in vivo and in immortalized gonadotropes (e.g. Refs. 42 and 43). The empty reporter vector was insensitive to GnRH1 treatment. In subsequent analyses we used 6-h GnRH1 to ensure that measurements were made before ligand-induced receptor desensitization.
Figure 1.
A, LβT2 cells were transfected with 450 ng/well −1028/+7 hFSHB-luc or empty pA3-luc reporters and then treated in serum-free medium with 10−7 m GnRH1 for the indicated times (in hours). The data reflect the mean of triplicate (±sd) samples and are presented relative to the luciferase activity measured in control cells not exposed to ligand. Data were analyzed by one-way ANOVA (Tukey post hoc test). B, Cells were transfected as in A with the indicated human FSHB promoter-reporters in pA3-luc or with the empty vector. Cells were then treated for 6 h with the indicated concentrations of GnRH1. All treatments were performed in duplicate. Significant differences between treatments are indicated with symbols.
To identify GnRH1 responsive promoter elements, we transfected LβT2 cells with 5′ deletions of the human reporter, followed by treatment with 10−6 to 10−8 m GnRH1 for 6 h. Deletions to −126/+7 did not significantly alter basal or GnRH1-stimulated reporter activity, indicating that the critical cis-elements mapped proximally (Fig. 1B) (data not shown). Therefore, in subsequent analyses, we used the −126/+7 (minimal) reporter. Notably, the GnRH1 response was cell type specific. When the −126/+7 hFSHB-luc construct was transfected into the “immature” gonadotrope cell line, αT3-1, which expresses the GnRH1 receptor and is GnRH1 responsive, we observed little or no change in reporter activity after 6 h of 10−7 m GnRH1 treatment (data not shown). Similar results were reported with an ovine Fshb promoter-reporter in this cell line (e.g. Ref. 10).
MAPK pathways mediate GnRH1-stimulated FSHB promoter activity
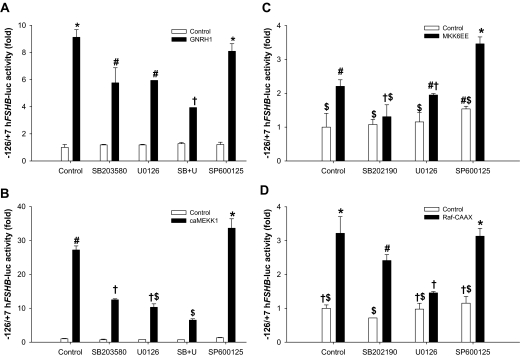
Previous analyses showed that GnRH1 stimulates activation of the ERK1/2, p38, and c-Jun N-terminal kinase (JNK) signaling cascades in LβT2 cells (e.g. Refs. 45 and 46). We confirmed rapid activation of these pathways by 10−7 m GnRH1 using Western blots of whole cell extracts and antibodies directed against phosphorylated forms of ERK1/2, p38, and JNK (data not shown). To assess the roles of these pathways in human FSHB regulation, we transfected cells with the −126/+7 reporter and incubated them with MAPK kinase (MEK) 1 (U0126, 5 μm), p38 (SB203580, 5 μm; or SB202190, 10 μm), or JNK (SP600125, 25 μm) inhibitors 30 min before treatment with GnRH1 for 6 h. The MEK1 and p38 inhibitors significantly attenuated ligand-stimulated, but not basal, reporter activity (Fig. 2A). The JNK inhibitor had inconsistent effects but generally suppressed the GnRH1 response little or not at all. Nonetheless, at the concentration used (25 μm), SP600125 consistently attenuated both GnRH1-stimulated c-Jun phosphorylation (See Fig. 6A, lane 6) and c-Jun-mediated trans-activation in a heterologous reporter assay (data not shown). The effects of the MEK1 and p38 inhibitors together were more pronounced than either alone (Fig. 2A). The residual GnRH1 response in the presence of p38 and MEK1 inhibitors could reflect the use of subsaturating concentrations. Indeed, when used at 50 μm, both U0126 and SB203580 abrogated the GnRH1 response (data not shown). However, at these high concentrations, there is likely a loss of specificity in the actions of the inhibitors (47,48). In fact, we observed that at both 5 and 10 μm, the p38 inhibitors nonspecifically antagonized activin type I receptor signaling (data not shown). For U0126, we confirmed that that the 5 μm dose was effective and selective, inhibiting GnRH1-stimulated ERK1/2 phosphorylation and Elk1-dependent transcription without affecting p38 or JNK phosphorylation (e.g. Fig. 6A, lane 5) (data not shown).
Figure 2.
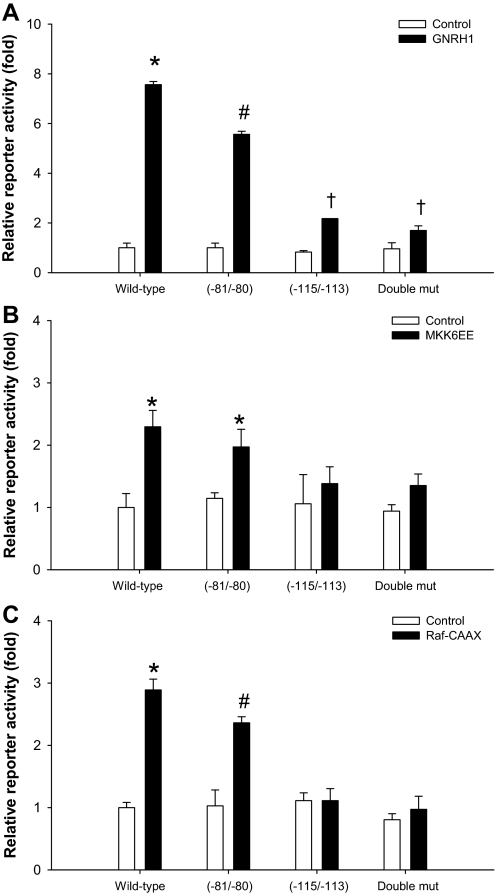
A, LβT2 cells were transfected with 450 ng/well of the −126/+7 hFSHB-luc reporter overnight. Cells were serum starved overnight and then incubated for 30 min with the indicated inhibitors, followed by 10−7 m GnRH for 6 h. B, Cells were cotransfected with the reporter as in A plus 50 ng/well caMEKK1 or pcDNA3 (control). The following day, cells were treated with the inhibitors in serum-free medium for 24 h. In both panels, data represent the mean reporter activity from duplicate samples per treatment. In panels C and D, cells were transfected and treated as in B, except 400 (MKK6EE) or 500 ng/well (Raf-CAAX) expression vector was used, and treatments were performed in triplicate. Symbols show differences as assessed by post hoc analysis of the significant interactions.
Figure 6.
A, LβT2 cells were treated with 10−7 m GnRH1 for 2 h (lanes 4–6) after 30 min preincubation with 5 μm U0126 (lanes 2 and 5) or 25 μm SP600125 (lanes 3 and 6). Nuclear extracts were used in gel shifts with the −126/−94 probe as described (top panel, EMSA) or used in Western blot analyses and probed with the indicated antibodies. B, The analyses were performed as described in A, except cells were preincubated in 10 μm SB202190 for 30 min (lanes 5 and 6) or overnight (o/n) (lanes 3 and 4) before 10−7 m GnRH1 treatment for 2 h (lanes 2, 4, and 6).
To demonstrate the sufficiency of MAPK signaling pathways in FSHB regulation, we cotransfected LβT2 cells with a ca form of MAP3K1 (caMEKK1) (38) and the −126/+7 reporter. This kinase can activate ERK1/2, p38, and JNK signaling (49,50). caMEKK1 robustly and dose dependently stimulated reporter activity (Fig. 2B) (data not shown). This effect was inhibited approximately 50% by the p38 and MEK1 inhibitors alone and by approximately 75% when used in combination. The JNK inhibitor failed to block caMEKK1-stimulated reporter activity. We next transfected cells with ca forms of kinases selective to the ERK1/2 [Raf1 kinase, Raf-CAAX (51)] and p38 pathways [MAP2K6, MKK6EE (40)]. Both kinases stimulated reporter activity, and their specificities for the indicated MAPK pathways were confirmed with the inhibitors (Fig. 2, C and D). Raf-CAAX and MKK6EE had an additive effect when used in combination (data not shown). These data confirm that p38 and ERK1/2 signaling pathways can regulate human FSHB transcription in transfected cells. The data with MKK6EE were particularly important in light of concerns about the specificity of the p38 small molecule inhibitors.
GnRH1 stimulates the formation of a protein complex that can bind to the proximal promoter
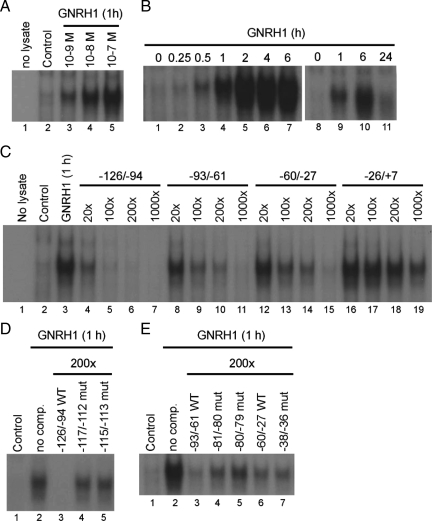
Having defined the proximal promoter as necessary and sufficient for the GnRH1 effect, we next turned to identifying specific cis-elements and trans-acting factors mediating the response. We generated four, nonoverlapping double-stranded oligonucleotide probes corresponding to −126 through +7 of the human FSHB promoter (Fig. 3A and Table 1). The probes were end labeled and incubated with nuclear extracts from LβT2 cells treated with GnRH1. Specific, ligand-induced protein complexes dose (Fig. 4A) and time dependently (Fig. 4B) bound to the −126/−94 probe, but not to the other promoter regions examined (data not shown). Figure 5 provides a clearer picture of the presence of multiple protein complexes. Additional probes, which bridged the junctions between these nonoverlapping probes, did not reveal additional GnRH1-stimulated complexes (data not shown). The complexes binding the −126/−94 probe were first detected within 30 min (Fig. 4B, lane 3) of 10−7 m GnRH1 treatment, were abundantly expressed through at least 6 h (lanes 4–7), and then declined to near baseline levels by 24 h (lane 11). The complexes were specific and could be competed by 20- to 1000-fold excess unlabeled homologous probe (Fig. 4C, lanes 4–7), but not by an unlabeled probe corresponding to −26/+7 (lanes 16–19). Interestingly, probes corresponding to −93/−61 (lanes 8–11) and −60/−27 (lanes 12–15) could also compete for binding, suggesting the presence of lower affinity sites therein. This was confirmed by titrating the amount of competitor probes and showing that, although 5- to 10-fold less effective than the homologous probe, the −94/−61 and −60/−27 probes could compete for binding, even at 100× concentrations (Fig. 4C).
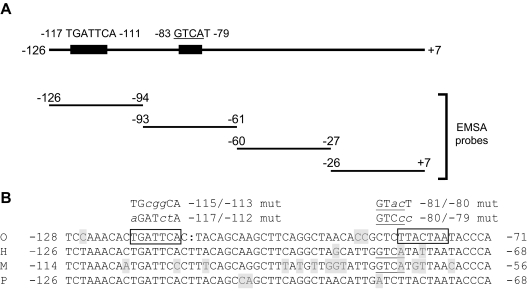
Figure 3.
A, Schematic representation of the proximal human FSHB promoter (−126 to +7) showing the sequences and relative position of two AP-1 cis-elements. The positions of the double-stranded DNA probes used in gel shift analyses are shown in the lower portion (see Table 1 for probe sequences). B, Alignment of FSHB/Fshb proximal promoter sequences, including putative AP-1 elements from sheep (O), human (H), mouse (M), and pig (P). AP-1 sites in the ovine promoter are boxed, and the putative AP-1 half-site in the murine and human promoters is underlined. Bases differing from the consensus are shaded. Mutations introduced into gel shift competitor probes and/or reporter constructs are shown at the top in lowercase/italics.
Figure 4.
Nuclear extracts from LβT2 cells treated with the indicated concentrations of GnRH1 for 1 h (A) or 10−7 m GnRH1 for the indicated periods of time (in hours) (B) were incubated with a radiolabeled double-stranded oligo-probe corresponding to −126/−94 of the human FSHB promoter. DNA-protein complexes were resolved by native PAGE and visualized by autoradiography. C, Gel shifts were performed as described in A and B with nuclear extracts from LβT2 cells treated with 10−7 m GnRH1 for 1 h and the radiolabeled −126/−94 probe. Binding was competed with 20, 100, 200, or 1000-fold excess unlabeled probes corresponding to −126/−94 (lanes 4–7), −93/−61 (lanes 8–11), −60/−27 (lanes 12–15), or −26/+7 (lanes 16–19). D, Competition (comp) experiments in gel shifts were performed as described in C with the indicated WT (lane 3) and mutant −126/−94 probes (lanes 4 and 5). E, Competition experiments were performed as in D with WT (lanes 3 and 6) and mutant −93/−61 or −60/−27 probes (lanes 4, 5, or 7).
Figure 5.
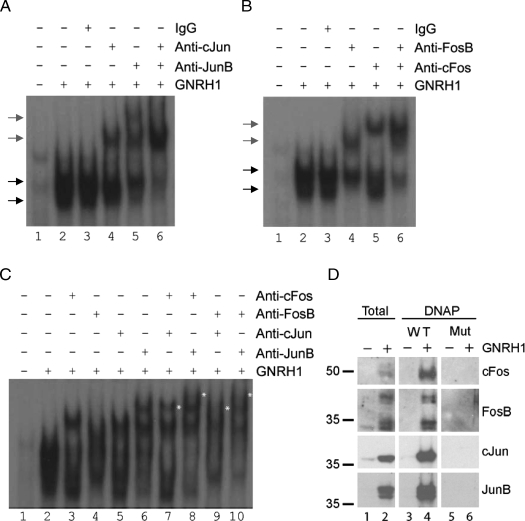
Gel shifts were performed with the −126/−94 probe and nuclear extracts from GnRH1 treated LβT2 cells (10−7 m for 2 h) as in Fig. 4, except here supershifts were performed with Jun (A), Fos (B), or Fos and Jun (C) antibodies. The black arrows in A and B indicate the two main complexes observed in these analyses, whereas the gray arrows show supershifted complexes. In C, the asterisks are used to denote the positions of super-supershifted complexes. D, Extracts from control (lane 1) or GnRH1-stimulated cells (2 h) (lane 2) were run on SDS-PAGE directly or after incubation with biotinylated WT (lanes 3 and 4) or −115/−113 mutant (Mut) (lanes 5 and 6) −126/−94 double-stranded probes and subjected to streptavidin paramagnetic bead mediated pull down. Proteins were transferred to nitrocellulose and sequentially immunoblotted with the indicated antibodies. Numbers at the left reflect molecular mass in kDa.
Previously, GnRH1 was shown to stimulate ovine Fshb promoter activity in heterologous cells overexpressing the GnRH1 receptor through activator protein-1 (AP-1) factors binding to two cis-elements in the proximal promoter (52,53) (boxed sequences in Fig. 3B). One of these two elements, −120/−114 in sheep, is perfectly conserved in human within the −126/−94 interval (at −117/−111) (Fig. 3B). To determine whether the analogous site might mediate the GnRH1-induced protein complex binding, we introduced the 3-bp mutation shown previously to block AP-1 mediated transcription of the ovine promoter (52) (−117/−112; TGATTCA→ aGATctA; Fig. 3B) into a competitor probe and showed that it failed to inhibit complex binding to the WT human −126/−94 probe (Fig. 4D, lane 4). Similarly, a novel 3-bp mutation (−115/−113; TGATTCA→ TGcggCA; Fig. 3B) in the same element also prevented competition for binding (Fig. 4D, lane 5). These data showed that GnRH1 stimulated the formation of protein complexes that could bind to an AP-1-like element at −117/−112.
GnRH1 stimulates AP-1 complex formation
To determine whether or not AP-1 proteins were actually contained with the GnRH1-stimulated complexes, we first performed supershift experiments with Fos and Jun specific antibodies. GnRH1 was shown previously to stimulate the FosB, c-fos, JunB, and cJun in LβT2 cells (54,55), and we confirmed this here (Figs. 5D and 6). When we included antibodies for cJun or JunB in the gel shift analyses, we observed clear supershifts (Fig. 5A, compare lanes 2, 4, and 5). When both antibodies were used together, the GnRH1-induced complexes were almost completely supershifted (lane 6). No new complexes relative to those seen with the antibodies alone were observed. These data indicated that the majority of the complexes contained cJun or JunB but that heterodimers of the two were likely rare.
Similar results were observed when we included antibodies against FosB and c-fos (Fig. 5B, compare lanes 2, 4, and 5). When the two antibodies were used in combination, there were no super-supershifted complexes noted (lane 6), and there was only a small amount of residual binding activity. These data suggested that the majority of the binding was accounted for by different combinations of Fos and Jun proteins.
We hypothesized that the complexes were predominantly heterodimers of the different GnRH1-induced Fos and Jun proteins. To address this possibility, we repeated the supershift analyses, but here used the Fos and Jun antibodies in combination (Fig. 5C). When used alone, the antibodies produced the same supershifted complexes as seen in Fig. 5, A and B. However, all combinations of Fos and Jun antibodies produced super-supershifted complexes, consistent with the presence of Fos/Jun heterodimeric pairs (Fig. 5C, see asterisks in lanes 7–10). With the exception of the c-fos/cJun combination (lane 7), all of the antibody pairs almost completely shifted the GnRH1-stimulated complexes. None of the supershifted complexes was observed when the antibodies were used with lysates from control cells (data not shown).
Finally, we used DNA pull-down analyses (DNAP) to corroborate the findings with gel shifts (Fig. 5D). Here, 2-h GnRH1 treatment stimulated increases in c-fos, FosB, cJun, and JunB protein levels. In the case of FosB, we observed two sets of bands, the lower of which may represent ΔFosB. A WT biotinylated −126/−94 probe could bind and pull down all of the AP-1 proteins examined (Fig. 5D, lanes 3 and 4), whereas a probe containing the 3-bp mutation at −115/−113 (Mut) could not. Collectively, these data showed that GnRH1 stimulated the synthesis of Fos and Jun proteins, which formed heterodimers capable of binding the human FSHB promoter.
Two low-affinity AP-1 sites are present within the proximal FSHB promoter
The data in Fig. 4C suggested that there may be lower affinity AP-1-like elements in the −93/−61 and −60/−27 regions of the promoter. Within −93/−61 resides an AP-1 half site (GTCA) previously characterized in the murine Fshb promoter at −71/−68 (−83/−80 in human) (54) (underlined in Fig. 3B). This element is also conserved in rat, but not ovine, porcine, or bovine Fshb; however, there is some (spatial) overlap between this element and the second AP-1 site identified in sheep (52,53) (compare boxed and underlined sequences at right of Fig. 3B). A 2-bp mutation in the AP-1 half site of the murine promoter (mut I in Ref. 54) decreased the GnRH1 response by about 30%. We introduced the corresponding (−80/−79, AT→ cc) or a second mutation (−81/−80, CA→ ac) into the −93/−61 competitor probe. The −81/−80 mutant was included because it contained 2 bp in the cis-element, whereas the −80/−79 mutant only affected 1 bp of the GTCA sequence. Neither mutation would be expected to impact the adjacent NF-Y site (54). Both mutations partially inhibited the ability of the −93/−61 probe to compete for binding to the radiolabeled −126/−94 probe (Fig. 4E, compare lanes 4 and 5 with 3).
Database searches of the sequence in the −60/−27 probe did not identify any candidate AP-1-like elements. However, a visual scan revealed the sequence TGATT (−40/−36), which is equivalent to the first 5 bp of the AP-1 site at −117/−111. The TGATTCA to TGcggCA (−115/−113) mutation blocked AP-1 binding to the −126/−94 probe (Fig. 4D, lane 5). Therefore, we introduced the comparable mutation, TGATT to TGcgg at −38/−36, into the −60/−27 probe, but this did not alter competition for AP-1 protein complex binding to the −126/−94 probe (Fig. 4E, compare lanes 6 and 7). We have not yet determined what sequence within −60/−27 competes for AP-1 binding.
GnRH1-stimulated AP-1 complex synthesis is MEK1 and p38 dependent
Given the observations that MEK1 and p38 inhibitors attenuated both GnRH1- and caMEKK1-stimulated reporter activities (Fig. 2, A and B), we examined whether disruption of signaling via one or both pathways might impact AP-1 complex binding to the −126/−94 probe. Cells were treated with the MEK1, p38, or JNK inhibitors for 30 min before 2 h of 10−7 m GnRH1 treatment. The MEK1 inhibitor significantly impaired DNA/protein complex formation (Fig. 6A, top panel, lane 5), whereas JNK inhibitor (lane 6) had no effect, despite its clear inhibition of cJun phosphorylation (second panel from bottom, lane 6). The MEK1 inhibitor significantly inhibited GnRH1-stimulated ERK1/2 phosphorylation, as well as FosB, c-fos, and JunB protein expression, while having little to no effect on cJun expression.
Both p38 inhibitors, SB202190 or SB203580, only marginally inhibited AP-1 binding activity when applied to cells 30 min before GnRH1 treatment (Fig. 6B, top panel, compare lanes 2 and 6) (data not shown). This was associated with decreases in both FosB and c-fos expression. When applied overnight, SB202190 significantly impaired GnRH1-stimulated AP-1 complex binding and FosB/c-fos, but not JunB or cJun, expression (lane 4). This overnight treatment was also significantly more effective than 30 min pretreatment in impairing GnRH1-stimulated reporter activity (data not shown).
Collectively, these data showed that GnRH1 signals via both MEK1 and p38-dependent pathways to stimulate FosB and c-fos (and JunB in the case of MEK1) production, which are required for AP-1 binding activity to the human FSHB promoter. Pretreatment with cycloheximide completely blocked GnRH1-induced AP-1 complex binding (data not shown), indicating that these proteins (and likely cJun) are synthesized de novo.
GnRH1, Raf1, and MKK6 regulation of human FSHB promoter activity requires both high and low-affinity AP-1 elements
We next determined the relative contribution of the defined AP-1 sites to GnRH1-regulated reporter activity. When introduced into the −126/+7 hFSHB-luc reporter, the −115/−113 mutation greatly inhibited GnRH1-stimulated, but not basal, reporter activity (Fig. 7A). Mutations at −80/−79 and −81/−80 also inhibited GnRH1-stimulated reporter activity, though the effect of the −80/−79 mutant was more variable (i.e. on some occasions there was no inhibition) (data not shown). Therefore, we focused on the −81/−80 mutation, which consistently inhibited the GnRH1 response, though significantly less so than the −115/−113 mutation (Fig. 7A). The two mutations together were statistically equivalent to the −115/−113 mutation alone, suggesting that GnRH1 signaling through the −81/−80 site may also require the higher affinity site at −117/−111. The mutations similarly affected reporter activity induced by caMKK6 (Fig. 7B), Raf1 (Fig. 7C), and MEKK1 (data not shown). These data indicated that the bulk of GnRH1, ERK1/2, and p38 regulated activity required an intact high-affinity AP-1 site.
Figure 7.
A, LβT2 cells were transfected with WT or the indicated mutant −126/+7 hFSHB-luc reporters and then treated with 10−7 m GnRH1 for 6 h. All treatments were performed in duplicate and the data analyzed by three-way ANOVA (−81/−80 × −115/−113 × GnRH1). Results of the Tukey post hoc analysis of the significant three-way interaction are indicated with symbols. B and C, Cells were cotransfected in triplicate with the indicated hFSHB-luc reporters (450 ng/well) and 400 ng/well (MKK6EE) or 500 ng/well (Raf-CAAX) expression vectors. Empty vector was transfected at the same concentration as a control in each experiment. After overnight transfection, cells were cultured for 24 h in serum-free medium before lysates were collected for luciferase assays.
AP-1 proteins are necessary and sufficient to stimulate FSHB transcription
Thus far, we demonstrated that GnRH1 stimulated the formation of AP-1 complexes, which bound to a conserved cis-element, and that GnRH1 required the high-affinity AP-1 site to exert its effects on transcription. We next addressed the necessity and sufficiency of AP-1 proteins in FSHB transcription. First, we transfected cells with the −126/+7 hFSHB-luc reporter along with expression vectors for FosB, c-fos, JunB, and cJun alone and in combination. Both Jun proteins stimulated reporter activity but did not act in synergy. In contrast, neither Fos protein had an effect on its own, but both significantly potentiated the effects of JunB or cJun (Fig. 8, A and B) (data not shown). The effects with the different Fos and Jun pairs were similar (Fig. 8B). In subsequent analyses we used the combination of c-fos/JunB and/or FosB/cJun, both of which were confirmed to be in GnRH1-stimulated complexes (Fig. 5C, lanes 8 and 9).
Figure 8.
A, Cells were transfected in triplicate with the indicated hFSHB-luc reporters (450 ng/well) and FosB/cJun (100 ng each/well) expression vector(s). The data were analyzed by three-way ANOVA as described in Fig. 7A. B, Cells were transfected in triplicate with the −126/+7 hFSHB-luc reporter (450 ng/well), and the indicated Fos/Jun pairs (25 ng each/well) with and without A-Fos (200 ng/well). Cells were transfected with human FSHB (C) or LHB (D) reporters (450 ng/well) and the indicated concentrations of A-Fos. Cells were then treated with 10−7 m GnRH1 for 6 h before luciferase assays. Data in B–D were analyzed by two-way ANOVA and post hoc tests (Tukey) performed on the significant interactions. Symbols show differences as assessed by post hoc analysis.
The −115/−113 and, to a lesser extent, −81/80 mutations significantly impaired the effects of FosB/cJun and c-fos/JunB (Fig. 8A) (data not shown), confirming that AP-1 heterodimers are sufficient to stimulate the human FSHB promoter and that they do so primarily through the defined AP-1 elements.
To demonstrate the necessity of AP-1 proteins in mediating the GnRH1 response, we used a dominant-negative Fos protein, A-Fos, which dimerizes with Jun proteins with high affinity and inhibits their DNA binding (41). When cotransfected with different Fos/Jun pairs, A-Fos completely inhibited AP-1 mediated trans-activation of FSHB reporter activity (Fig. 8B). This confirmed the efficacy of A-Fos in our assay system. Next, we transfected increasing amounts of A-Fos into cells and examined the effects on GnRH1-stimulated FSHB reporter activity. As predicted, A-Fos dose dependently attenuated the GnRH1 effect (Fig. 8C). We did not observe complete inhibition, which might reflect insufficient A-Fos expression and/or AP-1 independent activation of transcription by GnRH1. To show the specificity of the effect, we repeated the analysis with a human LHB promoter-reporter, which we observed in preliminary analyses was regulated by GnRH1, but not AP-1 proteins (Figs. 9C and 10F). Here, A-Fos did not inhibit the GnRH1 response but, rather, potentiated it (Fig. 8D). These data suggest that GnRH1 signals via AP-1 proteins to positively regulate human FSHB, but not LHB, transcription through defined AP-1 cis-elements. Whereas we cannot completely exclude the possibility that A-Fos overexpression is working through a dominant-negative mechanism involving bZIP proteins beyond Fos and Jun family members, the DNA binding data (gel shift and DNA affinity studies) strongly implicate Fos/Jun heterodimers in the regulation of human FSHB by GnRH.
Figure 9.
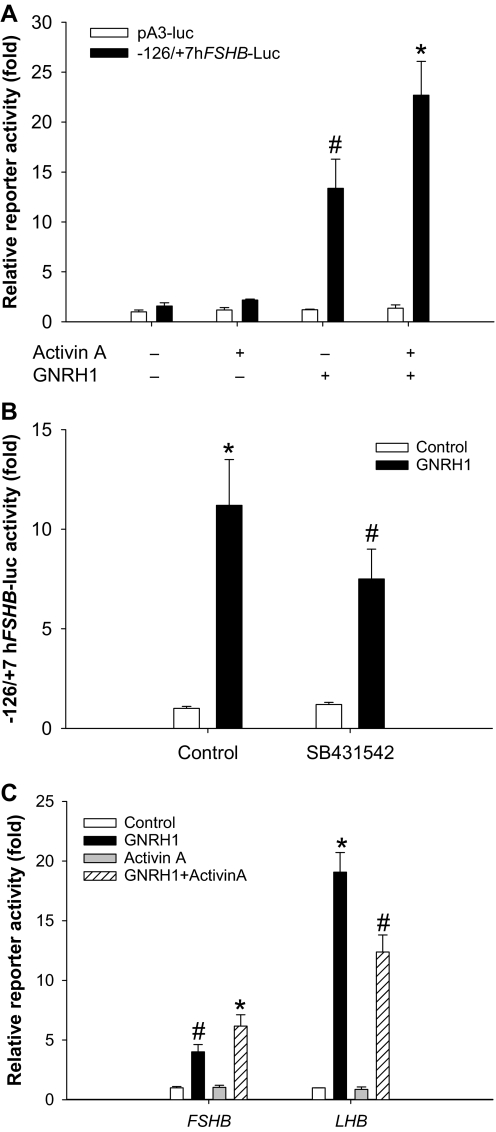
A, LβT2 cells were transfected with the human FSHB reporter (black bars) or empty vector (white bars) and treated with 10−9 m activin A in serum-free medium. After 18 h, 10−7 m GnRH1 was added for an additional 6-h treatment period. Treatments were performed in triplicate. Data were analyzed by two-way ANOVAs (activin A × GnRH1). Post hoc analysis (Tukey) was performed on the significant interaction. B, Cells transfected with −126/+7 hFSHB-luc were treated with GnRH1 for 6 h after 30 min incubation in the presence or absence of 10 μm SB431542. Treatments were performed in triplicate. Symbols reflect the results of the post hoc analysis of the significant GnRH1 × SB431542 interaction. C, Cells were transfected with either −126/+7 hFSHB-luc (left) or −197/+8 hLHB-luc (right). The day after, cells were treated with 10−7 m GnRH1 ± 10−9 m activin A for 3 h. All treatments were performed in triplicate and the data for the two reporters analyzed separately in two-way ANOVAs. Symbols show differences as assessed by post hoc analysis of the significant interactions.
Figure 10.
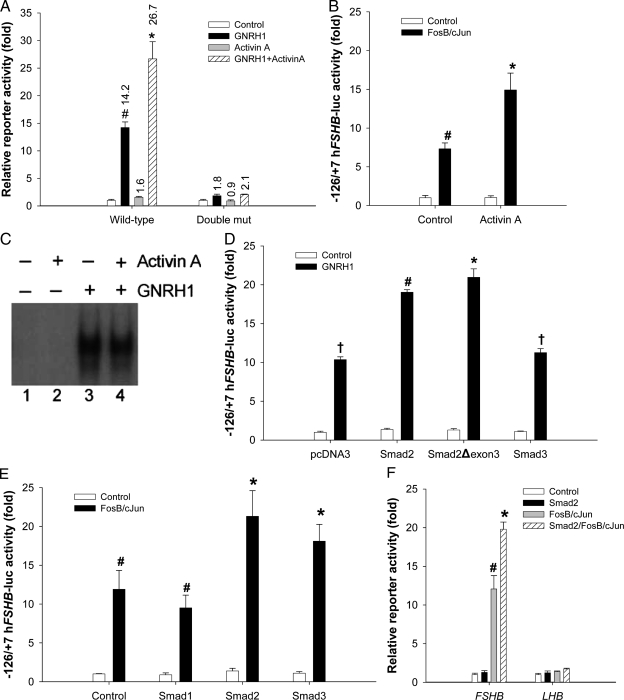
A, LβT2 cells were transected with the indicated reporters and then treated with 10−7 m GnRH1 ± 10−9 m activin A for 6 h (n = 3 per treatment). Fold stimulation by the ligands (relative to no treatment for the same reporter) is shown above each bar. Results of the two-way ANOVAs are shown with symbols. B, Cells were transfected with the WT human FSHB reporter and FosB/cJun as described in Fig. 8A. Cells were treated with 10−9 m activin A for 6 h before collection of lysates for luciferase assays. Treatments were performed in triplicate and data analyzed by two-way ANOVA (activin A × FosB/cJun). C, Cells were treated with nothing (lane 2), 10−9 m activin A for 1 h (lane 3), 10−7 m GnRH1 for 1 h (lane 4), or activin A and GnRH1 for 1 h. Nuclear extracts were subjected to gel shift analysis with the −126/−94 probe as described in Fig. 4. D, Cells were transfected with −126/+7 hFSHB-luc along with 100 ng/well of the indicated Smad constructs or pcDNA3 (n = 3 per treatment). Cells were incubated overnight in serum-free medium and then treated with GnRH1 for 6 h before collection of lysates. Data were analyzed by two-way ANOVA. E, Cells were transfected with −126/+7 hFSHB-luc along with 100 ng each/well FosB and cJun plus 100 ng/well Smad1, Smad2, or Smad3. pcDNA3 (control) was used to balance DNA transfected across conditions. Lysates were collected after overnight incubation in serum-free medium and data analyzed by two-way ANOVA. F, WT FSHB and LHB reporters were cotransfected into LβT2 cells with combinations of FosB, cJun, and Smad2 as described in E. Data were analyzed separately for the two reporters.
GnRH1 and activin A synergistically regulate human FSHB, but not LHB, reporter activity
Although our previous data suggested that the human FSHB promoter may not be a direct target of the activin signaling cascade (22), they did not exclude the possibility that activins modulate GnRH1 effects. Indeed, the two ligands synergistically regulate rat, murine, and ovine Fshb promoter activities and mRNA expression (e.g. Refs. 55,56,57). We treated LβT2 cells transfected with the −126/+7 human reporter with 10−9 m activin A for 24 h. During the last 6 h of this interval, we administered 10−7 m GnRH1. GnRH1 stimulated reporter activity on its own, whereas the effect of activin A was negligible (Fig. 9A). When the two ligands were used in combination, the GnRH1 effect was significantly enhanced. The empty reporter (pA3-luc) was unaffected by ligand treatment. LβT2 cells appear to make activin B endogenously (10,23). Therefore, we asked whether endogenous activin signaling might contribute to GnRH1-stimulated reporter activity. We treated cells with the activin type I receptor inhibitor, SB431542, which we showed previously abolishes activin signaling in these cells (23), but does not impair GnRH1-stimulated ERK1/2, p38, or JNK phosphorylation (data not shown). The compound significantly inhibited the GnRH1 effect (Fig. 9B), suggesting a role for endogenous activin B as a modulator of GnRH1 signaling. Others have reported similar effects with the activin antagonist, follistatin-288, on GnRH1-stimulated ovine or murine Fshb reporter activities (10,55).
Activin A stimulates murine GnRH1 receptor (Gnrhr) expression (e.g. Refs. 58,59,60), providing a candidate mechanism for the GnRH1/activin A synergism observed here. We confirmed that activin A stimulated a murine Gnrhr promoter-reporter (gift of Colin Clay, Colorado State University, Fort Collins, CO) in LβT2 cells, with increases observed as early as 4 h (data not shown). However, an up-regulation of Gnrhr expression would be predicted, based on data in the literature, to favor enhanced LHB relative to FSHB expression (e.g. Refs. 61 and 62). In addition, we observed that activin A and GnRH1 synergistically activated −126/+7 hFSHB-luc within 3 h cotreatment (Fig. 9C). It is not clear that receptors would be sufficiently up-regulated within this brief time interval to mediate this response. Nevertheless, we examined the relative effects of activin A cotreatment on human FSHB vs. LHB reporters to assess whether Gnrhr up-regulation might account for the observed synergism.
GnRH1 dose- and time-dependently stimulated the LHB reporter with similar, though not identical kinetics to FSHB (data not shown). We compared the effects of GnRH1 (3 h) alone and in combination with activin A (3 h) on the two human gonadotropin β-subunit reporters. Again, activin A had no effect on its own but potentiated the GnRH1 effect on FSHB-luc (Fig. 9C). In contrast, activin A partially inhibited GnRH1-stimulated human LHB-luc reporter activity. These data failed to support a role for increased GnRH1 receptor expression in activin A/GnRH1 synergism.
AP-1 and Smad proteins synergistically regulate FSHB transcription
Having demonstrated that GnRH1 signals at least in part via AP-1 proteins to regulate human FSHB promoter activity (Fig. 8C), we examined whether the defined AP-1 cis-elements were required for GnRH1/activin A synergism. Indeed, mutation of these binding sites completely abrogated the combined actions of the two ligands, though there was a small residual effect of GnRH1 (Fig. 10A). The −115/−113 mutation alone produced the same effect (data not shown). These data suggested that the AP-1 sites might comprise a point of convergence for the activin A and GnRH1 signaling pathways. To determine the nature of this interaction, we measured whether activin A could modulate AP-1-mediated reporter activity. Indeed, activin A significantly potentiated the effects of transfected FosB/cJun (Fig. 10B). The activin type I receptor inhibitor, SB431542, inhibited the effects of FosB/cJun, suggesting synergism with endogenous activin B signaling as well (data not shown).
A previous report suggested that activin A might potentiate GnRH1-stimulated c-fos and FosB production via enhancement of p38-mediated signaling (55). However, in our hands, activin A did not potentiate, and may have slightly inhibited, GnRH1-stimulated AP-1 factor binding to the −117/−111 cis-element (Fig. 10C, compare lanes 3 and 4).
Smads 2 and 3 are the best-known effectors of activin A signaling. Therefore, we investigated whether Smads might mediate activin A’s effects. We cotransfected cells with the FSHB reporter and Smads 2 or 3, followed by 6 h GnRH1 treatment. Smad2, but not Smad3, potentiated the GnRH1 response (Fig. 10D). A previous report indicated synergistic actions of Smad3 and GnRH1 on the murine Fshb promoter (55), and we replicated this result (data not shown). These observations highlight both the functionality of our Smad3 expression vector and potential interspecies variation in underlying regulatory mechanisms. Smad2 does not bind DNA, suggesting that DNA binding is not necessary for the effect with the human promoter. When we transfected cells with a splice variant of Smad2, Smad2Δexon3, which can bind DNA like Smad3, we observed the same synergism as with full-length Smad2 (Fig. 10D). These data suggest that differences between Smads 2 and 3 independent of their DNA binding abilities contributed to their differential abilities to act in synergy with GnRH1.
Previous studies have established both antagonistic and cooperative actions of Smads and AP-1 proteins (e.g. Refs. 63 and 64), mediated by direct physical interactions between Smad3 and cJun or JunB (65,66). One group reported that in the context of a promoter containing an AP-1 element, but no Smad binding element (SBE), Smad3 and JunB or cJun synergistically activated promoter activity (63). Because the −126/+7 human FSHB promoter contains at least two AP-1 sites, but no obvious SBEs (22), we asked whether Smads might potentiate the synergistic actions of Fos/Jun dimers on reporter activity. Smads 2 and 3 alone had no effect on human FSHB promoter activity, as we reported previously (22), but significantly augmented the effects of FosB/cJun and c-fos/JunB (Fig. 10E) (data not shown). In contrast, the bone morphogenetic protein-regulated Smad, Smad1, had no effect. The similarity in actions of Smads 2 and 3 suggested that direct DNA binding of Smads was not required for the observed synergism. Why Smad3 synergized with overexpressed AP-1 proteins, but not GnRH1, to regulate FSHB transcription is not yet clear. Nonetheless, Smad2 was consistent in synergizing with both GnRH1 and AP-1 proteins to regulate the human gene. In contrast to FSHB, the human LHB promoter was insensitive to FosB/cJun alone and in combination with Smads 2 or 3 (Fig. 10F) (data not shown). Collectively, these data are consistent with the hypothesis that activin A and GnRH1 stimulate the formation Smad2/AP-1 complexes that cooperatively regulate FSHB transcription through the AP-1 element at −117/−111 and perhaps −83/−80.
Discussion
Here, we present the first systematic analysis of transcriptional regulation of the human FSHB subunit promoter by GnRH1. Our results suggest that GnRH1 stimulates the de novo synthesis of AP-1 protein complexes, which bind to both high and low-affinity cis-elements in the proximal promoter to drive transcription. GnRH1 signals through both p38 and ERK1/2-dependent pathways to stimulate c-fos and FosB production. ERK1/2 signaling also partially mediates GnRH1 effects on JunB expression (54). Therefore, inhibition of these pathways attenuates AP-1 complex formation and DNA binding, and, hence, FSHB transcription. Interestingly, these two MAPK pathways contribute minimally to GnRH1-stimulated cJun synthesis. The JNK pathway, though clearly involved in cJun phosphorylation, is not required for Fos or Jun synthesis, nor is cJun phosphorylation required for DNA binding or promoter trans-activation.
Our analysis defines at least two cis-elements mediating GnRH1 responsiveness of the human FSHB promoter, and both correspond to sites previously identified in GnRH1 regulation of Fshb promoters from other species. Interestingly, humans are the only species examined thus far that possesses the combination of both of these elements. The high-affinity site at −117/−111 corresponds to the AP-1 element at −120/−114 in the ovine promoter (52,53) and is similarly conserved in pig. Therefore, this element may contribute to GnRH1 responsiveness in all three species. The 3′ most base pair in this site diverges in rodents, and this may explain its apparent inability to mediate GnRH1 signaling in mice (54). Instead, an alternative element, described as an AP-1 half-site, mediates part of the GnRH1 response in the murine promoter. This element corresponds to the second AP-1-like site at −83/−80 in human FSHB. Although we did not directly demonstrate AP-1 factor binding to this element, the sequences between human and mouse are perfectly conserved, and a probe containing this element competes for AP-1 factor binding to the higher affinity site at −117/−111. Moreover, mutation of this element significantly inhibits GnRH1 and AP-1 responsiveness, but less so than mutation of the higher affinity site. Given that there is residual GnRH1-stimulated activity in promoter reporters with mutations in both elements, there are likely additional sites mediating the GnRH1 response of the human promoter. Although we have not yet mapped these elements, our gel shift data suggest the presence of an additional low-affinity AP-1-like element within −60/−27. Whether this element contributes to the GnRH1 response remains to be determined. In addition, our observation that the dominant-negative Fos protein failed to block completely the GnRH1 effect suggests that GnRH1 may also use AP-1-independent mechanisms to regulate the human FSHB gene.
Although activin A does not appear to regulate human FSHB reporter activity on its own, it does potentiate GnRH1’s actions. Similar results have been reported for Fshb promoters in other species (9,55,56,57). However, a major difference is that the promoters in these other species are directly regulated by activins. In fact, it was recently suggested that the consensus 8-bp SBE in the murine Fshb promoter is necessary for the synergism between GnRH1 and activin A (55). The human FSHB promoter lacks this SBE, and yet we still observe the cooperative actions of the two ligands, indicating that this element is not required for the effect. In fact, when we abolish this element in a murine Fshb reporter, both the GnRH1 and activin A/GnRH1 responses are enhanced (data not shown) (see also Fig. 7 in Ref. 55). Similarly, the ovine promoter lacks this SBE and is synergistically regulated by the two ligands (57). Thus, the consensus SBE is not required for activin A/GnRH1 synergism, though it clearly contributes to the overall activin responsiveness of the murine promoter (22).
Our data implicate a functional interaction between AP-1 proteins and Smad2 (and perhaps Smad3) as part of the mechanism through which GnRH1 and activin A synergistically regulate FSHB transcription. The ability of Smads to physically interact directly with JunB and cJun is well established (e.g. Refs. 65 and 66). Here, we show that AP-1 heterodimers stimulate FSHB transcription through the two defined AP-1 sites and that coexpression of Smad2 or Smad3, effectors in the activin signaling cascade, potentiate this effect. Smad2 potentiates the GnRH1 effect on promoter activity, as does activin A on AP-1-dependent transcription. Binding of Smads to DNA may not be required because full-length Smad2 does not bind DNA directly. Therefore, these data suggest that Smads may associate with the human FSHB promoter indirectly through their interaction with DNA bound AP-1 proteins, though we have not yet demonstrated this directly.
In contrast to the results with FSHB, AP-1 proteins do not stimulate human LHB transcription, nor do they functionally synergize with Smads 2 or 3 to regulate the LHB promoter. We do not yet know how Smads and AP-1 proteins function together to regulate FSHB; however, in other promoter contexts where AP-1 and Smad proteins antagonize one another’s actions, there appears to be competition for limiting coactivators, such as p300 (67). Therefore, it is possible that when AP-1 and Smads work together, their interaction may facilitate cofactor recruitment (68).
The data reported here may contribute to a mechanistic understanding of differential gonadotropin regulation during the luteal-follicular phase of the menstrual cycle. At the end of the luteal phase, circulating estradiol and progesterone levels decline markedly. The loss of these negative feedback signals leads to increases in GnRH1 pulsatility, as reflected by increased LH pulses observed at this stage of the cycle (e.g. Ref. 69). Rapid GnRH1 pulse frequencies are argued to favor LH rather than FSH secretion (e.g. Ref. 37); however, it is the preferential elevation of FSH that is the hallmark of the luteal-follicular transition. Although it is possible that the particular pulse frequency at this stage of the cycle might favor FSH release, LH pulses at this time occur approximately every 90 min, indirectly demonstrating relatively rapid GnRH1 pulsatility. At this rate, how then is FSH preferentially secreted? Whereas declining steroid levels undoubtedly account for increased GnRH1 pulse frequency, the concurrent loss of inhibin A at the end of the luteal phase could contribute to the selective FSH elevation. How this is manifested in humans has not been established.
In rodents, activin regulation of Fshb is robust and occurs independently of GnRH1 during the secondary FSH surge of the estrous cycle (44). In contrast, we suggest that in humans, activins likely require underlying GnRH1 signaling for their effects to be manifested. Therefore, when GnRH1 pulsatility increases at the luteal-follicular phase transition, it stimulates both FSH and LH. However, in the face of reduced inhibin A negative feedback, pituitary activins are disinhibited at the level of the gonadotrope. Our data suggest that increased activin signaling will potentiate GnRH1-stimulated FSHB transcription via AP-1/Smad protein interactions, leading to the observed increases in FSH. In contrast, GnRH1-stimulated LHB transcription is AP-1-independent and, therefore, is not potentiated by activin-stimulated Smad activation. Instead, activins partially inhibit GnRH1-regulated LHB expression, through an as yet to be determined mechanism, which may restrain LH synthesis and secretion in the face of relatively rapid GnRH1 pulses.
Acknowledgments
We thank Drs. C. Clay, P. Dobner, D. Engelberg, M. Karin, C. Lange, A. Mauviel, P. Mellon, P. Ulery, L. Van Aelst, and C. Vinson for their generous contribution of reagents. We also thank Vishal Khivansara and Michelle Santos for their valuable technical assistance during the initial phases of the study.
Footnotes
This work was supported by National Institutes of Health R01 Grants HD34772 (to M.S.R.) and HD47794 (to D.J.B.), and by Cariplo Foundation Grant 1055/104878-2005 (to L.P.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online July 24, 2008
Abbreviations: AP-1, Activator protein-1; ca, constitutively active; JNK, c-Jun N-terminal kinase; MEK, MAPK kinase; MEKK, MAPK kinase kinase; SBE, Smad binding element; WT, wild type.
References
- Lahlou N, Chabbert-Buffet N, Christin-Maitre S, Le Nestour E, Roger M, Bouchard P Main inhibitor of follicle stimulating hormone in the luteal-follicular transition: inhibin A, oestradiol, or inhibin B? Hum Reprod 14:1190–1193 [DOI] [PubMed] [Google Scholar]
- Welt CK, Pagan YL, Smith PC, Rado KB, Hall JE 2003 Control of follicle-stimulating hormone by estradiol and the inhibins: critical role of estradiol at the hypothalamus during the luteal-follicular transition. J Clin Endocrinol Metab 88:1766–1771 [DOI] [PubMed] [Google Scholar]
- Besecke LM, Guendner MJ, Sluss PA, Polak AG, Woodruff TK, Jameson JL, Bauer-Dantoin AC, Weiss J 1997 Pituitary follistatin regulates activin-mediated production of follicle-stimulating hormone during the rat estrous cycle. Endocrinology 138:2841–2848 [DOI] [PubMed] [Google Scholar]
- Woodruff TK, Besecke LM, Groome N, Draper LB, Schwartz NB, Weiss J 1996 Inhibin A and inhibin B are inversely correlated to follicle-stimulating hormone, yet are discordant during the follicular phase of the rat estrous cycle, and inhibin A is expressed in a sexually dimorphic manner. Endocrinology 137:5463–5467 [DOI] [PubMed] [Google Scholar]
- Cook RW, Thompson TB, Jardetzky TS, Woodruff TK 2004 Molecular biology of inhibin action. Semin Reprod Med 22:269–276 [DOI] [PubMed] [Google Scholar]
- Harrison CA, Gray PC, Vale WW, Robertson DM 2005 Antagonists of activin signaling: mechanisms and potential biological applications. Trends Endocrinol Metab 16:73–78 [DOI] [PubMed] [Google Scholar]
- Thompson TB, Lerch TF, Cook RW, Woodruff TK, Jardetzky TS 2005 The structure of the follistatin:activin complex reveals antagonism of both type I and type II receptor binding. Dev Cell 9:535–543 [DOI] [PubMed] [Google Scholar]
- Bernard DJ 2004 Both SMAD2 and SMAD3 mediate activin-stimulated expression of the follicle-stimulating hormone β subunit in mouse gonadotrope cells. Mol Endocrinol 18:606–623 [DOI] [PubMed] [Google Scholar]
- Graham KE, Nusser KD, Low MJ 1999 LβT2 gonadotroph cells secrete follicle stimulating hormone (FSH) in response to active A. J Endocrinol 162:R1–R5 [DOI] [PubMed] [Google Scholar]
- Pernasetti F, Vasilyev VV, Rosenberg SB, Bailey JS, Huang HJ, Miller WL, Mellon PL 2001 Cell-specific transcriptional regulation of follicle-stimulating hormone-β by activin and gonadotropin-releasing hormone in the LβT2 pituitary gonadotrope cell model. Endocrinology 142:2284–2295 [DOI] [PubMed] [Google Scholar]
- Suszko MI, Lo DJ, Suh H, Camper SA, Woodruff TK 2003 Regulation of the rat follicle-stimulating hormone β-subunit promoter by activin. Mol Endocrinol 17:318–332 [DOI] [PubMed] [Google Scholar]
- Attardi B, Miklos J 1990 Rapid stimulatory effect of activin-A on messenger RNA encoding the follicle-stimulating hormone β-subunit in rat pituitary cell cultures. Mol Endocrinol 4:721–726 [DOI] [PubMed] [Google Scholar]
- Weiss J, Guendner MJ, Halvorson LM, Jameson JL 1995 Transcriptional activation of the follicle-stimulating hormone β-subunit gene by activin. Endocrinology 136:1885–1891 [DOI] [PubMed] [Google Scholar]
- Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC 2004 Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol 33:559–584 [DOI] [PubMed] [Google Scholar]
- Kumar TR, Fairchild-Huntress V, Low MJ 1992 Gonadotrope-specific expression of the human follicle-stimulating hormone β-subunit gene in pituitaries of transgenic mice. Mol Endocrinol 6:81–90 [DOI] [PubMed] [Google Scholar]
- Kumar TR, Low MJ, Matzuk MM 1998 Genetic rescue of follicle-stimulating hormone β-deficient mice. Endocrinology 139:3289–3295 [DOI] [PubMed] [Google Scholar]
- Bokar JA, Keri RA, Farmerie TA, Fenstermaker RA, Andersen B, Hamernik DL, Yun J, Wagner T, Nilson JH 1989 Expression of the glycoprotein hormone α-subunit gene in the placenta requires a functional cyclic AMP response element, whereas a different cis-acting element mediates pituitary-specific expression. Mol Cell Biol 9:5113–5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamernik DL, Keri RA, Clay CM, Clay JN, Sherman GB, Sawyer Jr HR, Nett TM, Nilson JH 1992 Gonadotrope- and thyrotrope-specific expression of the human and bovine glycoprotein hormone α-subunit genes is regulated by distinct cis-acting elements. Mol Endocrinol 6:1745–1755 [DOI] [PubMed] [Google Scholar]
- Alarid ET, Windle JJ, Whyte DB, Mellon PL 1996 Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development 122:3319–3329 [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Lee KB, Santos MM 2006 Activin B can signal through both ALK4 and ALK7 in gonadotrope cells. Reprod Biol Endocrinol 4:52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AJ, Philips DP, Miller WL, Bernard DJ 2005 Differential regulation of follicle stimulating hormone by activin A and TGFB1 in murine gonadotropes. Reprod Biol Endocrinol 3:73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamba P, Santos MM, Philips DP, Bernard DJ 2006 Acute regulation of murine follicle-stimulating hormone β subunit transcription by activin A. J Mol Endocrinol 36:201–220 [DOI] [PubMed] [Google Scholar]
- Lee KB, Khivansara V, Santos MM, Lamba P, Yuen T, Sealfon SC, Bernard DJ 2007 Bone morphogenetic protein 2 and activin A synergistically stimulate follicle-stimulating hormone β subunit transcription. J Mol Endocrinol 38:315–330 [DOI] [PubMed] [Google Scholar]
- Aikawa S, Susa T, Sato T, Kitahara K, Kato T, Kato Y 2005 Transcriptional activity of the 5′ upstream region of the porcine glycoprotein hormone α subunit gene. J Reprod Dev 51:117–121 [DOI] [PubMed] [Google Scholar]
- Fowkes RC, King P, Burrin JM 2002 Regulation of human glycoprotein hormone α-subunit gene transcription in LβT2 gonadotropes by protein kinase C and extracellular signal-regulated kinase 1/2. Biol Reprod 67:725–734 [DOI] [PubMed] [Google Scholar]
- Quirk CC, Lozada KL, Keri RA, Nilson JH 2001 A single Pitx1 binding site is essential for activity of the LHβ promoter in transgenic mice. Mol Endocrinol 15:734–746 [DOI] [PubMed] [Google Scholar]
- Wolfe MW 1999 The equine luteinizing hormone β-subunit promoter contains two functional steroidogenic factor-1 response elements. Mol Endocrinol 13:1497–1510 [DOI] [PubMed] [Google Scholar]
- Suszko MI, Balkin DM, Chen Y, Woodruff TK 2005 Smad3 mediates activin-induced transcription of follicle-stimulating hormone β-subunit gene. Mol Endocrinol 19:1849–1858 [DOI] [PubMed] [Google Scholar]
- Kawakami S, Fujii Y, Okada Y, Winters SJ 2002 Paracrine regulation of FSH by follistatin in folliculostellate cell-enriched primate pituitary cell cultures. Endocrinology 143:2250–2258 [DOI] [PubMed] [Google Scholar]
- Stouffer RL, Dahl KD, Hess DL, Woodruff TK, Mather JP, Molskness TA 1994 Systemic and intraluteal infusion of inhibin A or activin A in rhesus monkeys during the luteal phase of the menstrual cycle. Biol Reprod 50:888–895 [DOI] [PubMed] [Google Scholar]
- Stouffer RL, Woodruff TK, Dahl KD, Hess DL, Mather JP, Molskness TA 1993 Human recombinant activin-A alters pituitary luteinizing hormone and follicle-stimulating hormone secretion, follicular development, and steroidogenesis, during the menstrual cycle in rhesus monkeys. J Clin Endocrinol Metab 77:241–248 [DOI] [PubMed] [Google Scholar]
- Kumar TR, Schuff KG, Nusser KD, Low MJ 2006 Gonadotroph-specific expression of the human follicle stimulating hormone β gene in transgenic mice. Mol Cell Endocrinol 247:103–115 [DOI] [PubMed] [Google Scholar]
- Lamba P, Khivansara V, D'Alessio AC, Santos MM, Bernard DJ 2008 Paired-like homeodomain transcription factors 1 and 2 regulate follicle stimulating hormone β-subunit transcription through a conserved cis-element. Endocrinology 149:3095–3108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson MS, Misra-Press A, Laurance ME, Stork PJ, Maurer RA 1995 A role for mitogen-activated protein kinase in mediating activation of the glycoprotein hormone α-subunit promoter by gonadotropin-releasing hormone. Mol Cell Biol 15:3531–3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P, Enslen H, Myung PS, Maurer RA 1994 Differential activation of CREB by Ca2+/calmodulin-dependent protein kinases type II and type IV involves phosphorylation of a site that negatively regulates activity. Genes Dev 8:2527–2539 [DOI] [PubMed] [Google Scholar]
- Maxwell IH, Harrison GS, Wood WM, Maxwell F 1989 A DNA cassette containing a trimerized SV40 polyadenylation signal which efficiently blocks spurious plasmid-initiated transcription. Biotechniques 7:276–280 [PubMed] [Google Scholar]
- Marshall JC, Dalkin AC, Haisenleder DJ, Paul SJ, Ortolano GA, Kelch RP 1991 Gonadotropin-releasing hormone pulses: regulators of gonadotropin synthesis and ovulatory cycles. Recent Prog Horm Res 47:155–187 [DOI] [PubMed] [Google Scholar]
- Blumer KJ, Johnson GL, Lange-Carter CA 1994 Mammalian mitogen-activated protein kinase kinase kinase (MEKK) can function in a yeast mitogen-activated protein kinase pathway downstream of protein kinase C. Proc Natl Acad Sci USA 91:4925–4929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Karin M 1999 Stimulation of Elk1 transcriptional activity by mitogen-activated protein kinases is negatively regulated by protein phosphatase 2B (calcineurin). J Biol Chem 274:15173–15180 [DOI] [PubMed] [Google Scholar]
- Askari N, Diskin R, Avitzour M, Capone R, Livnah O, Engelberg D 2007 Hyperactive variants of p38α induce, whereas hyperactive variants of p38γ suppress, activating protein 1-mediated transcription. J Biol Chem 282:91–99 [DOI] [PubMed] [Google Scholar]
- Olive M, Krylov D, Echlin DR, Gardner K, Taparowsky E, Vinson C 1997 A dominant negative to activation protein-1 (AP1) that abolishes DNA binding and inhibits oncogenesis. J Biol Chem 272:18586–18594 [DOI] [PubMed] [Google Scholar]
- McArdle CA, Forrest-Owen W, Willars G, Davidson J, Poch A, Kratzmeier M 1995 Desensitization of gonadotropin-releasing hormone action in the gonadotrope-derived α T3-1 cell line. Endocrinology 136:4864–4871 [DOI] [PubMed] [Google Scholar]
- McArdle CA, Willars GB, Fowkes RC, Nahorski SR, Davidson JS, Forrest-Owen W 1996 Desensitization of gonadotropin-releasing hormone action in αT3-1 cells due to uncoupling of inositol 1,4,5-trisphosphate generation and Ca2+ mobilization. J Biol Chem 271:23711–23717 [DOI] [PubMed] [Google Scholar]
- Rivier C, Schwall R, Mason A, Burton L, Vale W 1991 Effect of recombinant inhibin on gonadotropin secretion during proestrus and estrus in the rat. Endocrinology 128:2223–2228 [DOI] [PubMed] [Google Scholar]
- Kanasaki H, Bedecarrats GY, Kam KY, Xu S, Kaiser UB 2005 Gonadotropin-releasing hormone pulse frequency-dependent activation of extracellular signal-regulated kinase pathways in perifused LβT2 cells. Endocrinology 146:5503–5513 [DOI] [PubMed] [Google Scholar]
- Vasilyev VV, Pernasetti F, Rosenberg SB, Barsoum MJ, Austin DA, Webster NJ, Mellon PL 2002 Transcriptional activation of the ovine follicle-stimulating hormone-β gene by gonadotropin-releasing hormone involves multiple signal transduction pathways. Endocrinology 143:1651–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM 1998 Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem 273:18623–18632 [DOI] [PubMed] [Google Scholar]
- Laping NJ, Grygielko E, Mathur A, Butter S, Bomberger J, Tweed C, Martin W, Fornwald J, Lehr R, Harling J, Gaster L, Callahan JF, Olson BA 2002 Inhibition of transforming growth factor (TGF)-β1-induced extracellular matrix with a novel inhibitor of the TGF-β type I receptor kinase activity: SB-431542. Mol Pharmacol 62:58–64 [DOI] [PubMed] [Google Scholar]
- Uhlik MT, Abell AN, Cuevas BD, Nakamura K, Johnson GL 2004 Wiring diagrams of MAPK regulation by MEKK1, 2, and 3. Biochem Cell Biol 82:658–663 [DOI] [PubMed] [Google Scholar]
- Bogoyevitch MA, Gillespie-Brown J, Ketterman AJ, Fuller SJ, Ben-Levy R, Ashworth A, Marshall CJ, Sugden PH 1996 Stimulation of the stress-activated mitogen-activated protein kinase subfamilies in perfused heart. p38/RK mitogen-activated protein kinases and c-Jun N-terminal kinases are activated by ischemia/reperfusion. Circ Res 79:162–173 [DOI] [PubMed] [Google Scholar]
- Leevers SJ, Paterson HF, Marshall CJ 1994 Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature 369:411–414 [DOI] [PubMed] [Google Scholar]
- Strahl BD, Huang HJ, Pedersen NR, Wu JC, Ghosh BR, Miller WL 1997 Two proximal activating protein-1-binding sites are sufficient to stimulate transcription of the ovine follicle-stimulating hormone-β gene. Endocrinology 138:2621–2631 [DOI] [PubMed] [Google Scholar]
- Strahl BD, Huang HJ, Sebastian J, Ghosh BR, Miller WL 1998 Transcriptional activation of the ovine follicle-stimulating hormone β-subunit gene by gonadotropin-releasing hormone: involvement of two activating protein-1-binding sites and protein kinase C. Endocrinology 139:4455–4465 [DOI] [PubMed] [Google Scholar]
- Coss D, Jacobs SB, Bender CE, Mellon PL 2004 A novel AP-1 site is critical for maximal induction of the follicle-stimulating hormone β gene by gonadotropin-releasing hormone. J Biol Chem 279:152–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coss D, Hand CM, Yaphockun KK, Ely HA, Mellon PL 2007 p38 mitogen-activated protein kinase is critical for synergistic induction of the FSHβ gene by gonadotropin-releasing hormone and activin through augmentation of c-fos induction and Smad phosphorylation. Mol Endocrinol 21:3071–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory SJ, Lacza CT, Detz AA, Xu S, Petrillo LA, Kaiser UB 2005 Synergy between activin A and gonadotropin-releasing hormone in transcriptional activation of the rat follicle-stimulating hormone-β gene. Mol Endocrinol 19:237–254 [DOI] [PubMed] [Google Scholar]
- Huang HJ, Sebastian J, Strahl BD, Wu JC, Miller WL 2001 Transcriptional regulation of the ovine follicle-stimulating hormone-β gene by activin and gonadotropin-releasing hormone (GnRH): involvement of two proximal activator protein-1 sites for GnRH stimulation. Endocrinology 142:2267–2274 [DOI] [PubMed] [Google Scholar]
- Cherrington BD, Farmerie TA, Lents CA, Cantlon JD, Roberson MS, Clay CM 2005 Activin responsiveness of the murine gonadotropin-releasing hormone receptor gene is mediated by a composite enhancer containing spatially distinct regulatory elements. Mol Endocrinol 19:898–912 [DOI] [PubMed] [Google Scholar]
- Ellsworth BS, Burns AT, Escudero KW, Duval DL, Nelson SE, Clay CM 2003 The gonadotropin releasing hormone (GnRH) receptor activating sequence (GRAS) is a composite regulatory element that interacts with multiple classes of transcription factors including Smads, AP-1 and a forkhead DNA binding protein. Mol Cell Endocrinol 206:93–111 [DOI] [PubMed] [Google Scholar]
- Fernandez-Vazquez G, Kaiser UB, Albarracin CT, Chin WW 1996 Transcriptional activation of the gonadotropin-releasing hormone receptor gene by activin A. Mol Endocrinol 10:356–366 [DOI] [PubMed] [Google Scholar]
- Bedecarrats GY, Kaiser UB 2003 Differential regulation of gonadotropin subunit gene promoter activity by pulsatile gonadotropin-releasing hormone (GnRH) in perifused L β T2 cells: role of GnRH receptor concentration. Endocrinology 144:1802–1811 [DOI] [PubMed] [Google Scholar]
- Kaiser UB, Conn PM, Chin WW 1997 Studies of gonadotropin-releasing hormone (GnRH) action using GnRH receptor-expressing pituitary cell lines. Endocr Rev 18:46–70 [DOI] [PubMed] [Google Scholar]
- Verrecchia F, Vindevoghel L, Lechleider RJ, Uitto J, Roberts AB, Mauviel A 2001 Smad3/AP-1 interactions control transcriptional responses to TGF-β in a promoter-specific manner. Oncogene 20:3332–3340 [DOI] [PubMed] [Google Scholar]
- Wong C, Rougier-Chapman EM, Frederick JP, Datto MB, Liberati NT, Li JM, Wang XF 1999 Smad3-Smad4 and AP-1 complexes synergize in transcriptional activation of the c-Jun promoter by transforming growth factor β. Mol Cell Biol 19:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati NT, Datto MB, Frederick JP, Shen X, Wong C, Rougier-Chapman EM, Wang XF 1999 Smads bind directly to the Jun family of AP-1 transcription factors. Proc Natl Acad Sci USA 96:4844–4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Feng XH, Derynck R 1998 Smad3 and Smad4 cooperate with c-Jun/c-Fos to mediate TGF-β-induced transcription. Nature 394:909–913 [DOI] [PubMed] [Google Scholar]
- Verrecchia F, Pessah M, Atfi A, Mauviel A 2000 Tumor necrosis factor-α inhibits transforming growth factor-β/Smad signaling in human dermal fibroblasts via AP-1 activation. J Biol Chem 275:30226–30231 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pascual F, Redondo-Horcajo M, Lamas S 2003 Functional cooperation between Smad proteins and activator protein-1 regulates transforming growth factor-β-mediated induction of endothelin-1 expression. Circ Res 92:1288–1295 [DOI] [PubMed] [Google Scholar]
- Hall JE, Schoenfeld DA, Martin KA, Crowley Jr WF 1992 Hypothalamic gonadotropin-releasing hormone secretion and follicle-stimulating hormone dynamics during the luteal-follicular transition. J Clin Endocrinol Metab 74:600–607 [DOI] [PubMed] [Google Scholar]