Abstract
Previous work has implicated the forebrain glucocorticoid receptor (GR) in feedback regulation of the hypothalamic-pituitary-adrenocortical (HPA) axis. The present series of experiments used male mice with a targeted forebrain-specific GR knockout (in which forebrain includes the prefrontal cortex, hippocampus, and basolateral amygdala) to determine the role of forebrain GR in HPA axis regulation after stress. The data indicate that the forebrain GR is necessary for maintaining basal regulation of corticosterone secretion in the morning, confirming its role in HPA axis regulation. Our data further indicate that the forebrain GR is necessary for negative feedback after both mild and robust acute psychogenic stressors but not hypoxia, a systemic stressor. In contrast, forebrain-specific GR knockout and control mice exhibit equivalent HPA axis hyperactivity and facilitation after chronic variable stress, suggesting that changes in forebrain GR are not essential for chronic stress-induced pathology. These studies provide novel and definitive evidence that the forebrain GR selectively contributes negative feedback regulation of HPA axis responses to psychogenic stressors. Moreover, the data indicate that chronic stress-induced alterations in HPA axis function are mediated by mechanisms independent of the forebrain GR. Overall, the data are consistent with an essential role of the forebrain GR in coordinating endocrine responses to stimuli of a psychological origin.
ACTIVATION OF THE hypothalamic-pituitary-adrenocortical (HPA) axis mediates physiological responses that enable an organism to maintain or return to homeostasis. The end product of HPA axis activation is synthesis and secretion of glucocorticoids from the adrenal cortex (1). Glucocorticoid signaling fosters stress coping and is essential for survival. However, chronic activation of the system is associated with somatic dysregulation and stress-related affective disease states, underscoring the need to carefully regulate exposure to glucocorticoids (2,3,4,5). The magnitude and duration of stress responses are controlled in large part by glucocorticoid receptors (GR), which provide feedback signals that inhibit the release of ACTH via genomic and perhaps nongenomic mechanisms (6,7,8).
The forebrain, including the hippocampus, contains abundant GR mRNA, protein, and binding activity (9,10,11,12,13), and numerous studies suggest that glucocorticoid feedback inhibition of the HPA axis is mediated by this structure (14,15,16,17,18,19). However, these inhibitory effects are dependent on stressor modality. For example, hippocampal lesions cause a prolonged stress response to restraint or novelty (17,19,20), believed to be psychogenic in origin (i.e. requires higher order processing), but not to hypoxia or ether exposure (20), systemic stimuli that directly activate brain stem homeostatic relays (21). Neural processing of systemic and psychogenic stressors is handled by different circuits and can be considered in the light of direct or indirect pathways of feedback. The end result of any stress exposure is activation of homeostatic relays. What differentiates stressor subtypes are the brain regions required for stimulus processing. Systemic stress signaling is likely transduced via the direct pathway and signals constituent members of the HPA axis via brain stem relays. Psychogenic stress signaling requires comparison to past stimuli and other higher-order processing that relies on limbic circuitry (22).
Not surprisingly, GR regulation is affected by chronic stress. Previous studies indicate that chronic stress down-regulates GR mRNA in the hippocampus and frontoparietal cortex (23,24). Dexamethasone (DEX; a synthetic glucocorticoid with a high affinity for GR) binding in the hippocampus is decreased after chronic stress, an observation that is correlated with deficient DEX suppression of HPA axis activity (25). In addition, mice with a forebrain-specific GR deletion (FBGRKO) have higher basal corticosterone levels and display increased despair-like behaviors. Moreover, these animals fail to suppress corticosterone secretion after DEX administration (26). Together, these data suggest a role for the GR in the hippocampus, basolateral amygdala, and/or prefrontal cortex in glucocorticoid-negative feedback signaling.
Forebrain GR is clearly important in negative feedback regulation of the HPA axis. However, the nature of forebrain-negative feedback in modality-specific regulation remains to be determined. Furthermore, whereas a role for GR in feedback regulation of acute and chronic stress is hypothesized, its causality is not established. Therefore, the current study was designed to test the role of the forebrain GR in controlling HPA axis responses to acute psychological, acute systemic, and chronic variable stress (CVS). The current studies provide evidence that the forebrain GR is essential for glucocorticoid feedback inhibition of acute psychogenic but not systemic stress responses, whereas the integrity of forebrain GR is not required for development of chronic stress-induced changes in HPA axis function and behavior.
Materials and Methods
Subjects
FBGRKOs were generated by breeding knock-in mice engineered with loxP sites flanking exons 1C and 2 of the mouse GR gene (GRloxP) with mice expressing cAMP response element recombinase driven by the calmodulin-dependent protein kinase-IIα promoter (CamK-Cre) (27,28). Hemizygous mice were bred to produce GRloxP homozygous CamK-Cre− and GRloxP homozygous CamK-Cre+ offspring, of which only the latter have the GR gene deletion. Mice used in this study were on a mixed C57BL/6 × 129 × CBA background. In all cases, GRloxP homozygous CamK-Cre− and GRloxP homozygous CamK-Cre+ littermates were used, obviating possible problems related to strain. In addition, GRloxP CamK-Cre− dams were used for breeding, obviating any potential problems with maternal behavior that could occur consequent to GR gene deletion. Previous studies indicate that CamK-Cre expression is detectable approximately 3 wk after birth. By 6 months of age, there is a 90% reduction of GR immunoreactivity in a majority of the forebrain (prefrontal cortex, hippocampus, and basolateral amygdala), sparing GR expression in the hypothalamus and central amygdala (26). Therefore, animals used in this study were males aged between 6 and 12 months old.
All stressors were administered before noon (between 0800 and 1130 h). With the exception of afternoon basal blood collection, all blood collections took place before noon. Approximately 10–15 μl of blood were captured at each collection.
Animals were housed in a temperature- and humidity-controlled vivarium, with a12-h light, 12-h dark cycle, with lights on at 0600 h. Ad libitum access to food and water was provided throughout the study. All protocols were reviewed and approved by the Institutional Animal Care and Use Committee.
Basal and acute stress regulation of the HPA axis
This experiment included male control (n = 11) and FBGRKO (n = 12) mice. Basal blood samples were taken on two different occasions. Basal morning samples were collected by tail clip between 0630 and 0800 h. Basal afternoon samples were collected between 1630 and 1730 h. One week intervened between sampling.
One week after the afternoon tail bleed, animals were exposed to hypoxia to stimulate a systemic HPA axis response. Mice were placed in a clear Plexiglas chamber (with clean bedding) and exposed to 92% nitrogen and 8% oxygen for 30 min. Blood was collected by tail clip under light restraint at 30, 60, and 180 min after the onset of hypoxia.
Animals were allowed to recover for 1 wk, and anticipatory stress response was tested using a novel-environment exposure [elevated plus maze (EPM)]. Mice were placed in the center of a plus-shaped Plexiglas maze and allowed to explore for 5 min. The maze consisted of two closed arms bordered by dark Plexiglas (150 mm high) and two open arms that had a small (5 mm) rim. Each arm was 75 mm wide by 400 mm. Behavior was recorded for subsequent analysis. Blood was collected by tail clip at 30, 60, and 180 min after the onset of EPM exposure. After the blood collection at 180 min, animals were overdosed with sodium pentobarbital and perfused.
Regulation of the HPA axis response after a robust psychogenic stress exposure
The novel environment exposure paradigm typically produces lower corticosterone responses than hypoxia, raising the possibility that any observed changes in stress-induced secretion are related to intensity rather than modality. To control for this possibility, a subsequent experiment was performed to assess stress responses in a paradigm that produces peak corticosterone levels similar to those of hypoxia. This experiment included male control (n = 11) and FBGRKO (n = 10) mice. Animals were placed in plastic tube restrainers with adequate ventilation for 30 min. Blood was collected at 30 and 120 min after the onset of restraint. Immediately after the blood collection at 120 min, animals were overdosed with sodium-pentobarbital and perfused (see below).
Regulation of the HPA axis after chronic stress
This experiment included four groups of male mice: nonhandled controls (control no CVS; n = 7), nonhandled FBGRKO (FBGRKO no CVS; n = 7), control chronic variable stress (control CVS; n = 8), and FBGRKO CVS (FBGRKO CVS; n = 8). Non-CVS animals remained in their home cages for the duration of the experiment. Animals in the CVS groups were exposed to twice-daily randomly presented stressors for 14 d with occasional overnight stressors (see Table 1). Body weight was monitored throughout the experiment. Stressors included EPM (on the first and last days of CVS, with EPM serving as that morning’s stressor), rotation stress (orbital shaker at 100 rpm for 1 h), warm swim (29–32 C for 20 min), cold exposure (4 C for 15 min in clean cages without bedding), hypoxia (8% oxygen, 92% nitrogen for 30 min), and overnight isolation (one mouse/cage). On the day after CVS (d 15), animals were exposed to a 30-min novel restraint stress challenge, and blood was collected at 0, 30, and 120 min after the onset of restraint. After the blood collection at 120 min, animals were overdosed with sodium pentobarbital and perfused (see below). Adrenal and thymus glands were collected, cleaned, and weighed.
Table 1.
Chronic variable stress consisted of 14 d of twice-daily, randomly presented stressors
| Day | Time | Stressor |
|---|---|---|
| 1 | AM | EPM |
| PM | Warm swim | |
| 2 | AM | Shaker |
| PM | Cold room | |
| 3 | AM | Cold room |
| PM | Hypoxia + overnight single housed | |
| 4 | AM | Shaker |
| PM | Warm swim | |
| 5 | AM | Hypoxia |
| PM | Cold room | |
| 6 | AM | Shaker |
| PM | Warm swim + overnight single housed | |
| 7 | AM | Cold room |
| PM | Hypoxia | |
| 8 | AM | Shaker |
| PM | Warm swim + overnight single housed | |
| 9 | AM | Hypoxia |
| PM | Cold room + overnight single housed | |
| 10 | AM | Warm swim |
| PM | Hypoxia | |
| 11 | AM | Cold room |
| PM | Shaker | |
| 12 | AM | Hypoxia |
| PM | Shaker + overnight single housed | |
| 13 | AM | Shaker |
| PM | Hypoxia | |
| 14 | AM | EPM |
| PM | Warm swim | |
| 15 | AM | Novel restraint challenge |
Morning (AM) stressors occurred between 0700 and 1000 h. Afternoon (PM) stressors occurred between 1400 and 1700 h.
Immunohistochemistry
Animals were overdosed with sodium pentobarbital, transcardially flushed with 0.9% saline, and perfused with 3.7% formaldehyde in aqueous solution. Brains were extracted, postfixed in 3.7% formaldehyde solution for 24 h at 4 C, and then stored in 30% sucrose at 4 C. Brains were sectioned at 25 μm on a sliding microtome. Slices were washed in potassium PBS (KPBS) and endogenous peroxidases quenched with 1% H2O2. Slices were then washed in KPBS, blocked for 1 h (1% Triton X-100 and 2% BSA in KPBS), and incubated with primary antibody [GR (M-20) 1:1000; Santa Cruz Biotechnology, Santa Cruz, CA] at 4 C overnight. The next day, slices were rinsed with KPBS, incubated for 1 h with biotinylated antirabbit (1:500; Vector Laboratories, Burlingame, CA) secondary antibody, rinsed with KPBS, incubated with Vectastain ABC (1:1000; Vector Laboratories, Burlingame, CA) for 30 min, rinsed with KPBS, incubated with 0.4 mg/ml diaminobenzidine in 0.05% H2O2 in KPBS, rinsed with KPBS, and mounted onto Fisher Super stick glass slides (Fisher Scientific, Pittsburgh, PA). Slides were coverslipped with DPX mountant (Sigma-Aldrich, St. Louis, MO). Images were digitally captured at 20× magnification using a Zeiss Axioplan Microscope (Carl Zeiss, Thornwood, NY).
RIA
Plasma was separated by centrifugation (3300 × g, 15 min) and stored at −20 C until time of assay. Plasma corticosterone was measured using an 125I kit from MP Biomedicals (Orangeburg, NY). All samples were run in duplicate and analyzed in the same assay.
Statistical analysis
Data were analyzed using a student’s t test, ANOVA, and repeated-measures ANOVA. When the data were not homogeneously distributed, log or square root transformations were performed. Data are reported as original mean ± sem. Fisher’s least protected differences was used for post hoc testing when necessary. Significance was taken at P < 0.05.
Results
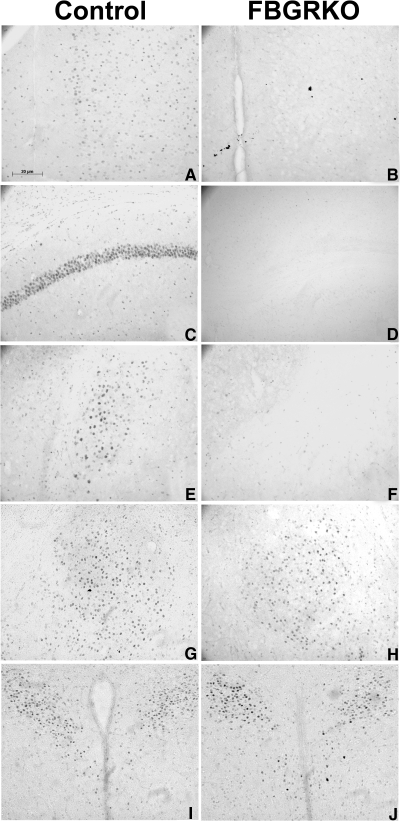
To verify genotyping and determine the specificity of the knockout, brain sections were processed for GR immunohistochemistry (Fig. 1). As can be observed in Fig. 1, A, C, and E, GR immunostained cell nuclei were present in the medial prefrontal cortex, hippocampal subfield CA1, dentate gyrus, and basolateral amygdala of control mice. In contrast, GR immunoreactivity was absent in corresponding brain regions of FBGRKO mice (Fig. 1, B, D, and F). The pattern of GR staining in the central amygdaloid nucleus was similar in both control and FBGRKO mice (Fig. 1, G and H), and importantly, both groups exhibited GR immunoreactivity in the hypothalamic paraventricular nucleus (PVN) (Fig. 1, I and J).
Figure 1.
Immunohistochemical verification. Immunostaining for GR in the medial prefrontal cortex (mPFC) (A and B), hippocampus (C and D), basolateral amygdala (BLA) (E and F), central amygdaloid nucleus (CeA) (G and H), and PVN (I and J) of control (A, C, E, G, and I) and FBGRKO (B, D, F, H, and J) mice. Note the absence of GR immunoreactivity in the mPFC, hippocampus, and BLA of FBGRKO mice. Importantly, the pattern and intensity of staining in the CeA and PVN is equivalent in FBGRKO mice and their littermate controls.
Basal HPA tone and acute stress responses
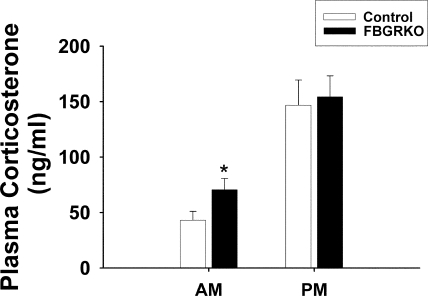
Mice were tested for basal and negative-feedback regulation after different subtypes of stressors. Because GR has been implicated in basal regulation of the HPA axis, plasma was collected in the morning and afternoon. Both FBGRKO and controls display an intact diurnal rhythm of basal corticosterone secretion (Fig. 2), with significantly increased plasma corticosterone in the afternoon (F 1,55 = 87.39, P < 0.05) as well as an interaction between genotype and time (F 1,55 = 0.15, P < 0.05), with the FBGRKO mice having elevated morning CORT levels.
Figure 2.
Basal corticosterone levels in control and FBGRKO mice. Both control and FBGRKO mice have intact diurnal regulation of plasma corticosterone. FBGRKO animals have significantly elevated levels in the morning (AM), compared with controls (*, P < 0.05). PM, Afternoon.
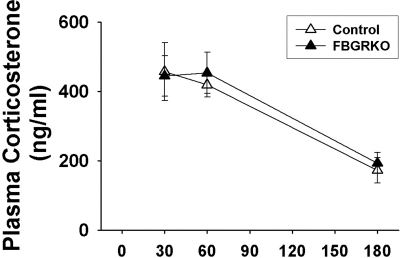
To determine whether GR is necessary for negative feedback after different types of stressors, animals were exposed to a systemic (hypoxia) and psychogenic (EPM) stressor, 1 wk apart. Because animals were tested at the same time as basal morning sampling, we did not obtain basal samples to obviate the stress of sample collection. Plasma corticosterone responses to a 30-min hypoxic challenge are shown in Fig. 3. All mice exhibited robust plasma corticosterone responses at 30 and 60 min after the onset of hypoxia as well as intact negative feedback with corticosterone values at 180 min, significantly different from those at 30 and 60 min (F 1,68 = 35.20, P < 0.05). There were no genotype effects (F 1,68 = 0.11, P = 0.74).
Figure 3.
Plasma corticosterone after hypoxia stress. Control and FBGRKO mice have a robust plasma corticosterone response to 30 min of hypoxia. There are no significant genotype differences in corticosterone levels at 180 min, indicative of intact negative feedback in both groups.
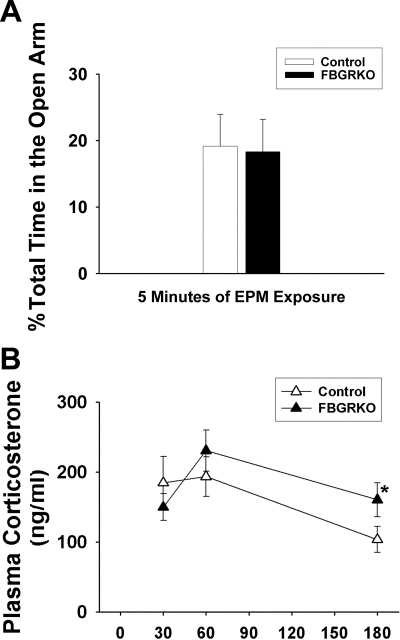
Figure 4, A and B, depicts behavioral (EPM) and neuroendocrine responses to novelty. There were no differences in time spent in the open arm of the EPM (P = 0.91) (Fig. 4A), indicating that the changes in corticosterone secretion are not accompanied by increased anxiety, at least in this design. There was a main effect of time (F 1,68 = 7.47, P < 0.05) but not genotype (F 1,68 = 1.78, P = 0.18) on plasma corticosterone responses (Fig. 4B). Additionally, FBGRKO mice have significantly higher levels of plasma corticosterone at 180 min after EPM (P < 0.05), suggesting impaired negative feedback regulation of corticosterone secretion after a mild psychogenic stressor.
Figure 4.
Behavior and plasma corticosterone after exposure to the EPM. A, There were no differences in time spent in the open arm of the EPM (P = 0.91). B, Both control and FBGRKO mice have a modest corticosterone response to a 5-min EPM exposure. FBGRKO mice have elevated plasma corticosterone levels at 180 min (*, P < 0.05), suggesting attenuated negative feedback.
HPA axis regulation after a robust psychogenic stressor
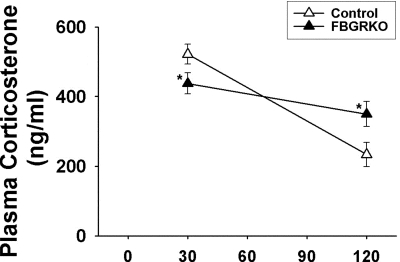
To determine whether the forebrain GR is necessary for appropriate feedback inhibition after a more robust psychogenic stress, mice were restrained in plastic tubes for 30 min and blood was collected. Although there was no main effect of genotype (F 1,41 = 0.15, P = 0.74), there was a main effect of time (F 1,41 = 28.31, P < 0.05) as well as an interaction between genotype and time (F 1,41 = 7.98, P < 0.05) (Fig. 5). Post hoc analysis revealed that FBGRKO plasma corticosterone was lower than controls at 30 min and elevated above controls at 120 min.
Figure 5.
Plasma corticosterone after restraint stress. There was no main effect of genotype. However, there was a main effect of time as well as an interaction between time and genotype. FBGRKO mice had lower corticosterone levels at 30 min and higher corticosterone levels at 120 min after the onset of restraint, compared with littermate controls (*, P < 0.05).
HPA axis regulation after chronic stress
Previous studies from our group show that male rats experiencing CVS have attenuated body weight gain. Therefore, body weight was measured throughout the experiment and is listed in grams on d 1 of CVS as well as d 15 body weight expressed as a percentage of d 1 in Table 2. There were no differences in raw body weight between the groups in regard to genotype (F 1,29 = 1.46, P = 0.24) or CVS assignment (F 1,29 = 0.03, P = 0.86) on d 1 of CVS. However, there was a main effect of 14 d of CVS exposure on percent body weight change over time (F 1,29 = 9.24, P < 0.05), with CVS mice presenting with lower body weight gain than nonhandled controls. There was no effect of genotype on body weight gain (F 1,29 = 3.94, P = 0.058). Thymic involution and adrenal hypertrophy are peripheral indications that the CVS paradigm was successful. Thymus and adrenal weights are expressed as a percentage of body weight in Table 2 (adjusted weights). There was a main effect of CVS (F 1,28 = 24.28, P < 0.05) and genotype (F 1,28 = 5.97, P < 0.05) on thymus weight when expressed as a percentage of body weight, with CVS animals experiencing thymic involution. Although there was a main effect of CVS (F 1,28 = 21.70, P < 0.05), the genotype effect failed to be significant when analyzing raw thymus weight (F 1,28 = 2.20, P = 0.151) (data not shown). Additionally, there was a main effect of both CVS (F 1,28 = 5.64, P < 0.05) and genotype (F 1,28 = 14.84, P < 0.05) on adrenal weights when expressed as a percentage of body weight as well as when expressed as raw weight (CVS: F 1,28 = 4.30, P < 0.05; genotype: F 1,28 = 11.96, P < 0.05; data not shown), with no interaction between the variables. Overall, CVS mice experienced thymic involution and adrenal hypertrophy, consistent with effective CVS. Also, FBGRKO mice had heavier adrenals than controls, regardless of CVS (P < 0.05).
Table 2.
Body weight (shown in grams on d 1 of CVS and on last day of CVS expressed as a percentage of body weight on d 1 of CVS) and adjusted thymus and adrenal weights (as a percentage of body weight) are peripheral indications of a successful chronic variable stress paradigm
| NO CVS
|
CVS
|
|||
|---|---|---|---|---|
| Control | FBGRKO | Control | FBGKRO | |
| Body weight (BW) (g) | 29.57 ± 2.14 | 26.47 ± 2.13 | 29.35 ± 2.00 | 27.45 ± 2.00 |
| % Initial BW | 106.4 ± 2.29 | 101.2 ± 2.34 | 98.87 ± 2.56a | 95.25 ± 2.14a |
| Adjusted thymus | 0.13 ± 0.02 | 0.11 ± 0.01 | 0.08 ± 0.01a | 0.06 ± 0.01a |
| Adjusted adrenal | 0.009 ± 0.001 | 0.015 ± 0.001b | 0.013 ± 0.001a | 0.018 ± 0.001b |
Data are listed as ± sem.
P < 0.05 vs. No CVS controls.
P < 0.05 vs. genotype controls.
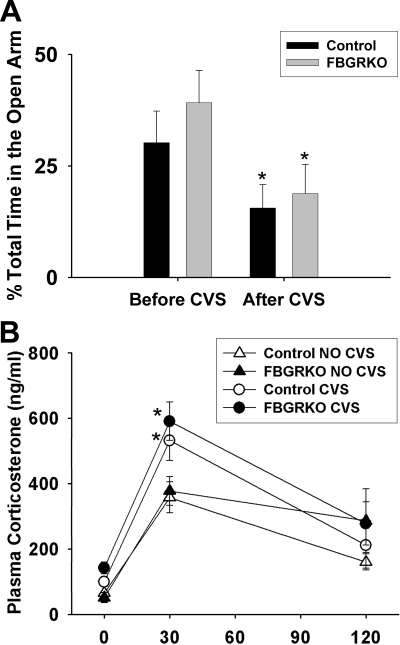
A previous study with the FBGRKO mice showed that these mice spend increased time in the open arm of the EPM, compared with controls (26). To determine CVS effects on this behavior, CVS mice were exposed to 5 min of EPM on d 1 and 14 of CVS. Figure 6A shows time spent in the open arm of the EPM as a percentage of total time, both before and after CVS. Both controls and FBGRKO mice spent less time in the open arm after 14 d of CVS (F 1,29 = 35.53, P < 0.05), but there was no effect of genotype on behavior (F 1,29 = 1.00, P = 0.33).
Figure 6.
Behavior before and after CVS exposure and plasma corticosterone after CVS exposure. A, There were no main effects of genotype on time spent in the open arm of the EPM, either before or after exposure to CVS. There was a main effect of time, with both groups spending less time in the open arm after CVS exposure. B, There was no main effect of genotype on plasma corticosterone responses to a 30-min restraint after CVS. However, there was a main effect of CVS and time, with CVS animals exhibiting facilitated corticosterone secretion at 30 min after the onset of novel stress exposure (*, P < 0.05).
After 14 d of CVS, mice were exposed to a novel 30-min restraint challenge. Plasma corticosterone at 0, 30, and 120 min after the onset of restraint is represented in Fig. 6B. There were main effects of CVS (F 1,86 = 7.36, P < 0.05) and time (F 1,86 = 66.02, P < 0.05) but no differences in genotype (F 1,86 = 2.13, P = 0.16). Additionally, there was as an interaction between CVS and time (F 2,86 = 3.74, P < 0.05). Specifically, CVS animals display a facilitated corticosterone response at 30 min regardless of genotype. There were no differences between groups in basal (measured at the onset of restraint) corticosterone secretion or resolution at 120 min.
Discussion
The current study demonstrates that the forebrain GR is required for normal termination of responses to mild and robust psychogenic stress but may not be required for normal shut-off of HPA axis responses to systemic stress (hypoxia). These data provide novel and definitive evidence that glucocorticoid feedback is a distributed rather than dedicated process, mediated by specific brain circuits responsible for processing salient aspects of threatening stimuli. Moreover, our data indicate that the forebrain GR is critical for feedback regulation of both basal HPA tone and HPA axis responses to psychogenic stressors but may not be essential for regulating responses to chronic stress. The results are consistent with a dissociation of the role of the forebrain GR in acute vs. chronic stress, implying that chronic stress-induced changes in HPA axis function are mediated by nonaffected GR-dependent circuits or GR-sensitive regions outside those affected by the GR deletion.
Chronic stress is clearly psychogenic in nature, and the lack of an effect of forebrain GR knockout suggests that the receptor is important in regulating responses to novel psychogenic stressors in an otherwise unstressed condition but less important against a backdrop of chronic drive. These data suggest that the normally inhibitory actions of the prefrontal cortex and hippocampus are taken off-line during chronic stress. However, we should also note that the loss of GR in the basolateral amygdala may contribute to these findings because this region is believed to be recruited during chronic stress (29,30,31). Thus, it is possible that the combinatorial effects of GR deficits in both stress-inhibitory and stress-excitatory circuits may underlie the absence of an effect of gene deletion on chronic stress processing.
Immunohistochemical analysis of tissue stained for GR indicates that the GR knockout is specific to the cortex, hippocampus, and basolateral amygdalar complex, leaving GR expression intact in the central amygdaloid nucleus and PVN. These findings are consistent with those of Boyle et al. (32), and indicate that our results are not associated with loss of GR function in other brain regions involved in HPA axis regulation.
In the present cohorts, FBGRKO mice maintained a diurnal rhythm of basal corticosterone secretion. However, FBGRKO mice hypersecreted corticosterone in the morning, suggesting that the forebrain GR may be important in modulating trough levels of glucocorticoid secretion. Additionally, our peripheral data support the finding of increased basal HPA drive in the form of increased adrenal weights, consistent with elevated adrenocortical stimulation by ACTH. Previous studies support a link between GR and basal HPA axis regulation. The forebrain richly expresses GR (33,34) and lesion of forebrain areas causes increased basal HPA axis drive (14,35,36). Expression of GR, DNA binding, and association with certain components of the chaperone complex follow a circadian rhythm that is linked to the diurnal variation in corticosterone secretion (37,38). In addition, studies using both GR-specific antagonists (35) and transgenic mouse models (26) show that blockade or absence of the GR causes basal HPA axis up-regulation. Importantly a pervious study using this same line of mice showed up-regulation of both morning and afternoon basal corticosterone secretion (26). In that study, the magnitude of difference between control and FBGRKO morning values is much greater than that of afternoon values. Our observed differences may be due to the fact that glucocorticoid levels show greater fluctuation in the afternoon, and it is possible that the time we selected for our corticosterone determinations may not be optimal for illustrating differences between the FBGRKO animals and littermate controls. Typically, mineralocorticoid receptor (MR) is associated with trough basal HPA axis tone and GR is associated with peak tone. As previously stated, in this study, only differences in morning regulation were detected. Although a previous study using these animals demonstrated no changes in basal MR levels (26), there may have been other compensatory changes that ultimately resulted in changes in trough regulation that were unrelated to MR expression or absence of forebrain GR. However, we are interpreting the data in combination and suggest that the data demonstrating increased basal corticosterone levels in FBGRKO mice are consistent with the forebrain GR playing a role in basal HPA axis regulation. These data contrast somewhat with data in rat indicating a preferential role for the MR in basal HPA regulation (39,40), differences that may be related to general adrenal hypertrophy causing enhanced corticosterone release to a given level of ACTH (41). Alternatively, it is possible that the role of GR and MR in regulation of basal HPA tone differ between rat and mouse.
The forebrain GR is of particular importance in limiting corticosterone responses to psychogenic stressors. Previous studies indicate that FBGRKO mice have impaired DEX suppression of the HPA axis (26). Together the data suggest that the deficit of forebrain GR causes a stressor-specific feedback impairment, which is not entirely surprising when one considers that the nature of the psychogenic stressor causes an organism to rely on stimulus comparison to past experiences (22). The stimulus comparison is dependent on brain regions such as the hippocampus and prefrontal cortex, the same regions in which GR expression has been disrupted. Moreover, our finding that forebrain GR is necessary for negative feedback after psychogenic but not systemic stress provides support for previous reports in the literature. For example, central administration of a glucocorticoid antagonist causes increased basal plasma ACTH and corticosterone as well as an increased response to novelty in rat (35), implicating GR as an agent of negative feedback. Additionally, lesion of the medial prefrontal cortex or hippocampus causes prolonged ACTH and corticosterone response to restraint but not ether exposure or hypoxia (17,20,42), further implicating these forebrain GR-containing regions in negative-feedback regulation after exposure to psychogenic but not systemic stressors. However, we should note that there is evidence for HPA axis responses that are generally insensitive to negative feedback effects of corticosteroids (7). It is possible that systemic stresses such as hypoxia may engage pathways that do not require corticosteroids for termination of stress responses.
Anxiety-like behavior was evaluated with the EPM test. The FBGRKO mice did not differ from controls in time spent in the open arm time of the EPM, indicating that deletion of the forebrain GR does not increase anxiety, at least as indicated by this test in our hands. It is possible that prolonged stress may be necessary to elicit an anxiety-like phenotype in these animals, so exposure to EPM before and after CVS was used to determine whether it affected this behavioral parameter. There were no genotype effects on time spent in the open arm either before or after CVS, which, again, may indicate that the forebrain GR does not play a major role in mediating anxiety-like behavior on the EPM. However, it should be noted that forebrain GR gene deletion elicits enhanced immobility in the forced swim test (26), consistent with alterations in affective processing. Our present results differ from previous studies conducted with these animals (26), demonstrating increased anxiety-like behavior in male FBGRKO mice. It is possible that environmental conditions under which these animals were tested differed from those used at the testing facility from previous reports. It is well known that detecting certain parameters of behavior can be very sensitive testing conditions. Although we were not able to detect genotype differences, it is important to note that after CVS, both groups of mice spent less time in the open arm of the EPM, consistent with increased anxiety-like behavior after chronic stress. Studies with rats report similar findings in the form of decreased open-field exploratory behavior after chronic restraint stress (43). Another study reported decreased time spent in the open arm of the EPM after chronic immobilization stress, but there were no detectable behavioral changes after chronic unpredictable stress in the same experiment (30). This suggests some degree of behavioral variability between tests and different stress exposures, and this degree may have come into play in the present experiments.
Limited numbers of subjects required us to perform repeated stress testing on individual animals. In rat experiments conducted in our laboratory, there is little contamination if tests are separated by 1 wk. For example, responses to hypoxia are not affected by exposure to restraint 1 wk earlier, or vice versa (our unpublished data). However, we need to acknowledge that we have not assessed the influence of repeated testing in mice. In addition, we should note that the current approach does not allow us to address issues of sensitization. Despite the fact that FBGRKO mice and controls showed similar responses to hypoxia, it remains possible that loss of forebrain GR may promote sensitization of future stress responses. There are some data to suggest that the neuronal GR plays a role in sensitization to drugs of abuse (44). However, sensitization of amphetamine-induced locomotor behavior is not observed in FBGRKO animals (our unpublished data), casting some doubt as to the role of the forebrain GR in sensitization-related processes.
Previous studies from our group (with rats) indicate that CVS induces thymic involution, adrenal hypertrophy, and attenuated weight gain (23,24,45,46,47,48). In the present study, CVS mice had thymic involution, adrenal hypertrophy and lower body weights, compared with nonhandled controls, regardless of genotype, suggesting an effective chronic stress paradigm. Furthermore, both control and FBGRKO CVS mice had a facilitated corticosterone response that was similar in magnitude at 30 min after the onset of restraint, indicating that absence of the forebrain GR did not exacerbate stress-induced facilitation of the HPA axis as predicted. Therefore, central and peripheral mechanisms involved in stress-induced facilitation do not seem to be affected by absence of the forebrain GR. It is possible that facilitation is dependent on GR in extraforebrain areas or that stress-induced facilitation occurs via other mechanisms such as different circuits or cell populations (49) or metabolic signaling (50). For example, injection of GR and MR antagonists (concurrently) into the posterior PVN of the thalamus (51) prevents HPA axis habituation to repeated restraint stress. Finally, it appears that absence of the forebrain GR does not influence the increase in adrenal sensitivity that is induced by chronic stress (41).
These data suggest that the HPA axis is a dynamic system that is regulated at several levels, likely by different receptors or circuits. The forebrain GR is required for normal basal corticosterone secretion and termination of stress responses to acute psychogenic stressors, thereby establishing a causal role for this receptor in negative feedback integration of daily glucocorticoid rhythms and in processing of psychological stressors. In contrast, the forebrain GR does not appear essential for integration of prolonged stress. Previous studies indicate that organisms recruit alternative brain pathways to maintain homeostatic integrity in the face of chronic insults (50), which may provide additional degrees of flexibility for adaptation in the face of adversity. It remains to be determined whether GR localized to nonforebrain circuits plays a role in modulating or controlling physiological and behavioral responses to chronic stressors.
Acknowledgments
The authors thank Dr. Louis J. Muglia (Washington University, St. Louis, MO) for generously contributing breeding pairs of FBGRKO mice. Special thanks go to Amanda (Jones) Robertson, Matia Solomon, and Benjamin Packard for expert technical assistance.
Footnotes
This work was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grants DK59803 (to A.R.F.), AG12962 National Institutes on Aging (to J.P.H.), MH 049698 National Institutes of Mental Health (to J.P.H.), and AG10836 National Institutes on Aging (to J.P.H.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online July 10, 2008
Abbreviations: CamK-Cre, cAMP response element recombinase driven by the calmodulin-dependent protein kinase-IIα promoter; CVS, chronic variable stress; DEX, dexamethasone; EPM, elevated plus maze; FBGRKO, forebrain-specific GR deletion; GR, glucocorticoid receptor; HPA, hypothalamic-pituitary-adrenocortical; KPBS, potassium PBS; MR, mineralocorticoid receptor; PVN, paraventricular nucleus.
References
- Herman JP, Prewitt CM, Cullinan WE 1996 Neuronal circuit regulation of the hypothalamo-pituitary-adrenocortical stress axis. Crit Rev Neurobiol 10:371–394 [DOI] [PubMed] [Google Scholar]
- Bale TL 2006 Stress sensitivity and the development of affective disorders. Horm Behav 50:529–533 [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, La Fleur SE, Warne JP, Ginsberg AB, Akana SF, Laugero KC, Houshyar H, Strack AM, Bhatnagar S, Bell ME 2006 Glucocorticoids, chronic stress, and obesity. Prog Brain Res 153:75–105 [DOI] [PubMed] [Google Scholar]
- Gaylord KM 2006 The psychosocial effects of combat: the frequently unseen injury. Crit Care Nurs Clin North Am 18:349–357 [DOI] [PubMed] [Google Scholar]
- Rojo L, Conesa L, Bermudez O, Livianos L 2006 Influence of stress in the onset of eating disorders: data from a two-stage epidemiologic controlled study. Psychosom Med 68:628–635 [DOI] [PubMed] [Google Scholar]
- Joels M, de Kloet ER 1994 Mineralocorticoid and glucocorticoid receptors in the brain. Implications for ion permeability and transmitter systems. Prog Neurobiol 43:1–36 [DOI] [PubMed] [Google Scholar]
- Keller-Wood ME, Dallman MF 1984 Corticosteroid inhibition of ACTH secretion. Endocr Rev 5:1–24 [DOI] [PubMed] [Google Scholar]
- Tasker JG, Di S, Malcher-Lopes R 2006 Minireview: rapid glucocorticoid signaling via membrane-associated receptors. Endocrinology 147:5549–5556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Patel PD, Akil H, Watson SJ 1989 Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol Endocrinol 3:1886–1894 [DOI] [PubMed] [Google Scholar]
- Ahima RS, Harlan RE 1990 Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience 39:579–604 [DOI] [PubMed] [Google Scholar]
- Chao HM, Choo PH, McEwen BS 1989 Glucocorticoid and mineralocorticoid receptor mRNA expression in rat brain. Neuroendocrinology 50:365–371 [DOI] [PubMed] [Google Scholar]
- Fuxe K, Wikstrom AC, Okret S, Agnati LF, Harfstrand A, Yu ZY, Granholm L, Zoli M, Vale W, Gustafsson JA 1985 Mapping of glucocorticoid receptor immunoreactive neurons in the rat tel- and diencephalon using a monoclonal antibody against rat liver glucocorticoid receptor. Endocrinology 117:1803–1812 [DOI] [PubMed] [Google Scholar]
- Spencer RL, Young EA, Choo PH, McEwen BS 1990 Adrenal steroid type I and type II receptor binding: estimates of in vivo receptor number, occupancy, and activation with varying level of steroid. Brain Res 514:37–48 [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R 1991 The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev 12:118–134 [DOI] [PubMed] [Google Scholar]
- van Haarst AD, Oitzl MS, de Kloet ER 1997 Facilitation of feedback inhibition through blockade of glucocorticoid receptors in the hippocampus. Neurochem Res 22:1323–1328 [DOI] [PubMed] [Google Scholar]
- Herman JP, Schafer MK, Young EA, Thompson R, Douglass J, Akil H, Watson SJ 1989 Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J Neurosci 9:3072–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Dolgas CM, Carlson SL 1998 Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors. Neuroscience 86:449–459 [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Young EA, Akil H, Watson SJ 1992 Selective forebrain fiber tract lesions implicate ventral hippocampal structures in tonic regulation of paraventricular nucleus corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) mRNA expression. Brain Res 592:228–238 [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Morano MI, Akil H, Watson SJ 1995 Contribution of the ventral subiculum to inhibitory regulation of the hypothalamo-pituitary-adrenocortical axis. J Neuroendocrinol 7:475–482 [DOI] [PubMed] [Google Scholar]
- Mueller NK, Dolgas CM, Herman JP 2004 Stressor-selective role of the ventral subiculum in regulation of neuroendocrine stress responses. Endocrinology 145:3763–3768 [DOI] [PubMed] [Google Scholar]
- Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C 1997 Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol 388:169–190 [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE 1997 Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci 20:78–84 [DOI] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewitt C 1995 Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology 61:180–190 [DOI] [PubMed] [Google Scholar]
- Paskitti ME, McCreary BJ, Herman JP 2000 Stress regulation of adrenocorticosteroid receptor gene transcription and mRNA expression in rat hippocampus: time-course analysis. Brain Res Mol Brain Res 80:142–152 [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Yuzurihara M, Ishige A, Sasaki H, Chui DH, Tabira T 2001 Chronic stress differentially regulates glucocorticoid negative feedback response in rats. Psychoneuroendocrinology 26:443–459 [DOI] [PubMed] [Google Scholar]
- Boyle MP, Brewer JA, Funatsu M, Wozniak DF, Tsien JZ, Izumi Y, Muglia LJ 2005 Acquired deficit of forebrain glucocorticoid receptor produces depression-like changes in adrenal axis regulation and behavior. Proc Natl Acad Sci USA 102:473–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle MP, Brewer JA, Vogt SK, Wozniak DF, Muglia LJ 2004 Genetic dissection of stress response pathways in vivo. Endocr Res 30:859–863 [DOI] [PubMed] [Google Scholar]
- Brewer JA, Khor B, Vogt SK, Muglia LM, Fujiwara H, Haegele KE, Sleckman BP, Muglia LJ 2003 T-cell glucocorticoid receptor is required to suppress COX-2-mediated lethal immune activation. Nat Med 9:1318–1322 [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C, Denski K 2004 Regulation of chronic stress-induced changes in hypothalamic-pituitary-adrenal activity by the basolateral amygdala. Ann NY Acad Sci 1032:315–319 [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S 2002 Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 22:6810–6818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Dallman M 1998 Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience 84:1025–1039 [DOI] [PubMed] [Google Scholar]
- Boyle MP, Kolber BJ, Vogt SK, Wozniak DF, Muglia LJ 2006 Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness. J Neurosci 26:1971–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Wallach G 1973 Corticosterone binding to hippocampus: nuclear and cytosol binding in vitro. Brain Res 57:373–386 [DOI] [PubMed] [Google Scholar]
- McEwen BS, Weiss JM, Schwartz LS 1969 Uptake of corticosterone by rat brain and its concentration by certain limbic structures. Brain Res 16:227–241 [DOI] [PubMed] [Google Scholar]
- van Haarst AD, Oitzl MS, Workel JO, de Kloet ER 1996 Chronic brain glucocorticoid receptor blockade enhances the rise in circadian and stress-induced pituitary-adrenal activity. Endocrinology 137:4935–4943 [DOI] [PubMed] [Google Scholar]
- Beier EV, Belik EV, Arushanian EB 1999 [Circadian fluctuations of the plasma corticosterone level and locomotion in rats subjected local hippocampectomy]. Ross Fiziol Zh Im I M Sechenova 85:616–620 [PubMed] [Google Scholar]
- Furay AR, Murphy EK, Mattson MP, Guo Z, Herman JP 2006 Region-specific regulation of glucocorticoid receptor/HSP90 expression and interaction in brain. J Neurochem 98:1176–1184 [DOI] [PubMed] [Google Scholar]
- Kitchener P, Di Blasi F, Borrelli E, Piazza PV 2004 Differences between brain structures in nuclear translocation and DNA binding of the glucocorticoid receptor during stress and the circadian cycle. Eur J Neurosci 19:1837–1846 [DOI] [PubMed] [Google Scholar]
- Ratka A, Sutanto W, Bloemers M, de Kloet ER 1989 On the role of brain mineralocorticoid (type I) and glucocorticoid (type II) receptors in neuroendocrine regulation. Neuroendocrinology 50:117–123 [DOI] [PubMed] [Google Scholar]
- Spencer RL, Kim PJ, Kalman BA, Cole MA 1998 Evidence for mineralocorticoid receptor facilitation of glucocorticoid receptor-dependent regulation of hypothalamic-pituitary-adrenal axis activity. Endocrinology 139:2718–2726 [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP 2006 Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab 291:E965–E973 [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ 1993 The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci 13:3839–3847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, LeDoux JE, Magarinos AM, McEwen BS 1999 Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci 113:902–913 [DOI] [PubMed] [Google Scholar]
- Izawa R, Jaber M, Deroche-Gamonet V, Sillaber I, Kellendonk C, Le Moal M, Tronche F, Piazza PV 2006 Gene expression regulation following behavioral sensitization to cocaine in transgenic mice lacking the glucocorticoid receptor in the brain. Neuroscience 137:915–924 [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ostrander MM, Thomas IM, Packard BA, Furay AR, Dolgas CM, Van Hooren DC, Figueiredo HF, Mueller NK, Choi DC, Herman JP 2007 Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology 148:1823–1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrander MM, Ulrich-Lai YM, Choi DC, Richtand NM, Herman JP 2006 Hypoactivity of the hypothalamo-pituitary-adrenocortical axis during recovery from chronic variable stress. Endocrinology 147:2008–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Furay AR, Evanson NK, Ulrich-Lai YM, Nguyen MM, Ostrander MM, Herman JP 2008 The role of the posterior medial bed nucleus of the stria terminalis in modulating hypothalamic-pituitary-adrenocortical axis responsiveness to acute and chronic stress. Psychoneuroendocrinology 33:659–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauchi M, Zhang R, D'Alessio DA, Seeley RJ, Herman JP 2008 Role of central glucagon-like peptide-1 in hypothalamo-pituitary-adrenocortical facilitation following chronic stress. Exp Neurol 210:458–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon MS, Seo YJ, Shim EJ, Choi SS, Lee JY, Suh HW 2006 The effect of single or repeated restraint stress on several signal molecules in paraventricular nucleus, arcuate nucleus and locus caeruleus. Neuroscience 142:1281–1292 [DOI] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Strack AM, Scribner KS, Pecoraro N, La Fleur SE, Houshyar H, Gomez F 2004 Chronic stress-induced effects of corticosterone on brain: direct and indirect. Ann NY Acad Sci 1018:141–150 [DOI] [PubMed] [Google Scholar]
- Jaferi A, Bhatnagar S 2006 Corticosterone can act at the posterior paraventricular thalamus to inhibit hypothalamic-pituitary-adrenal activity in animals that habituate to repeated stress. Endocrinology 147:4917–4930 [DOI] [PubMed] [Google Scholar]