Abstract
Suppressor of cytokine signaling 3 (Socs3) has been identified as a mediator of central leptin resistance, but the identity of specific neurons in which Socs3 acts to suppress leptin signaling remains elusive. The ventromedial hypothalamus (VMH) was recently shown to be an important site for leptin action because deleting leptin receptor within VMH neurons causes obesity. To examine the role of VMH Socs3 in leptin resistance and energy homeostasis, we generated mice lacking Socs3 specifically in neurons positive for steroidogenic factor 1 (SF1), which is expressed abundantly in the VMH. These mice had increased phosphorylation of signal transducer and activator of transcription-3 in VMH neurons, suggesting improved leptin signaling, and consistently, food intake and weight-reducing effects of exogenous leptin were enhanced. Furthermore, on either chow or high-fat diets, these mice had reduced food intake. Unexpectedly, energy expenditure was reduced as well. Mice lacking Socs3 in SF1 neurons, despite no change in body weight, had improved glucose homeostasis and were partially protected from hyperglycemia and hyperinsulinemia induced by high-fat diets. These results suggest that Socs3 in SF1 neurons negatively regulates leptin signaling and plays important roles in mediating leptin sensitivity, glucose homeostasis, and energy expenditure.
LEPTIN, THE PRODUCT of the obese (Lep) gene (1), reverses obesity in leptin-deficient mice (2,3,4) and humans (5,6), and it causes leanness in wild-type mice, by decreasing food intake and increasing energy expenditure (7,8). Obese mice and humans, however, develop high leptin levels and do not fully respond to leptin treatment, suggesting a state of leptin resistance (9,10,11,12). Several mechanisms mediating leptin resistance have been proposed (13,14,15,16,17,18,19).
Suppressor of cytokine signaling 3 (Socs3) was previously identified in our laboratory as a potential mediator of central leptin resistance (18), and this was confirmed by subsequent genetic experiments (20,21,22). Mice with Socs3 haploinsufficiency (21) or neuronal deletion (20) had attenuated diet-induced obesity, reduced food intake, and improved leptin and insulin sensitivity. Furthermore, mice with Socs3 deficiency specifically in proopiomelanocortin (POMC) neurons, which are within the arcuate nucleus of the hypothalamus, showed significant but mild improvement in diet-induced obesity and associated metabolic complications (22). Clearly, in addition to POMC neurons, other neurons must be involved in Socs3-mediated leptin resistance. Moreover, recent evidence suggests that distinct hypothalamic regions can control food intake and energy expenditure separately (23). We then posed the following questions: what additional neurons are involved in Socs3-mediated leptin resistance and what roles does Socs3 in these neurons play in regulation of energy and metabolic homeostasis.
One candidate region is the ventromedial hypothalamus (VMH), which has long been implicated in regulation of energy balance. For instance, VMH lesions cause obesity (24) and leptin receptors are abundantly expressed in the VMH (25). Steroidogenic factor 1 (SF1) is a transcription factor that is expressed in the VMH (26). Deletion of the leptin receptor gene specifically in SF1 neurons resulted in obesity (27,28). These results suggest a potential role of Socs3 in the VMH in mediating leptin sensitivity. To test this hypothesis, we generated mice lacking Socs3 specifically in SF1 neurons and then examined the effect of Socs3 deficiency in these neurons on leptin sensitivity and other metabolic parameters, such as food intake, energy expenditure, and glucose homeostasis.
Materials and Methods
Animal care and high-fat diet studies
All animal protocols were approved by the Animal Care and Use Committee of the Beth Israel Deaconess Medical Center. Mice were housed at 22–24 C with a 14-h light, 10-h dark cycle and provided with ad libitum water and a chow diet (6% calories from fat, 8664; Harlan Teklad, Indianapolis, IN). Four-week-old male mice were placed on either a chow diet or a high-fat, high-sucrose diet (58% kcal from fat, 26% kcal from sucrose, D-12331; Research Diets, New Brunswick, NJ). All mice on diets were housed individually, and food intake and body weight were measured weekly.
Generation of Socs3flox/flox, Sf1-Cre mice
Mice harboring loxP sites flanking the second exon of the Socs3 gene (Socs3flox/flox) were described previously (29). Mice in which the expression of Cre recombinase is driven by regulatory elements of Sf1 (Sf1-Cre) were described elsewhere (27). The official symbol for Sf1 is Nr5a1. Socs3flox/flox mice were mated with Sf1-Cre mice to generate offspring carrying both the Cre gene and one allele of loxP-flanked Socs3 (Socs3flox/+, Sf1-Cre). These mice were then mated with Socs3flox/flox mice to generate mice homozygous for the floxed Socs3 alleles, either with (Socs3flox/flox, Sf1-Cre) or without Cre (Socs3flox/flox).
Leptin infusion study
Twenty-week-old male Socs3flox/flox, Sf1-Cre (n = 6) and Socs3flox/flox littermates (n = 6) were anesthetized with isoflurane and implanted with sc osmotic minipumps (model 1002; Durect, Cupertino, CA) that delivered 0.5 μg/h murine leptin (National Hormone and Peptide Program, Torrance, CA). Another group of wild-type C57B6 mice (n = 5) were infused with saline as a control. The mice were then singly housed and body weight and food intake were measured daily.
RNA extraction and real-time PCR
To compare the expression of Socs3 in the VMH of Socs3flox/flox, Sf1-Cre and Socs3flox/flox mice, 12-wk-old male mice (three in each group) were fasted overnight to suppress leptin levels and were then injected with 1 μg/g body weight of murine leptin. One hour later, the mice were killed and brains were then rapidly removed and placed on a cooled mouse brain matrix with 1-mm section dividers (ASI Instruments Inc., Warren, MI). Two sagittal sections in the middle were made, and landmarks, such as fornix, optic tracts, and mammillary nuclei, were used to dissect VMH/dorsomedial hypothalamus (DMH) regions, which were immediately put into RNAlater solution for subsequent RNA extraction.
Total RNA was isolated from tissues with RNeasy tissue minikit with deoxyribonuclease treatment (QIAGEN, Valencia, CA). One microgram of RNA was reverse transcribed to cDNA using random hexamers (Superscript; Ambion, Austin, TX). Real-time PCR was performed on a MX3000 Stratagene system, using Taqman based probes and Taqman universal PCR master mix (Applied Biosystems, Foster City, CA). Primer and probe sets for Cyclophillin were from Applied Biosystems, and those for Socs3 were described previously (21). Each gene assay was run in a singleplex reaction in duplicate. Relative expression levels were calculated by standard curve method, and Cyclophillin was used as an internal control.
Glucose and insulin tolerance tests
Glucose and insulin tolerance tests were performed on Socs3flox/flox, Sf1-Cre and Socs3flox/flox mice. For the glucose tolerance test, mice were fasted overnight and then injected ip with glucose (1.5 mg/g body weight), and glucose levels were measured using blood collected through tail-nicks at 0, 15, 30, 60, and 120 min. For the insulin tolerance test, mice were fasted for 3 h and then insulin (Humulin R; Eli Lilly, Indianapolis, IN) was injected ip (1 mU/g body weight), and blood glucose levels were then measured as described above.
Assays
Leptin and insulin concentrations were measured by ELISA kits (Crystal Chem, Chicago, IL), and corticosterone levels were measured by a competitive enzyme immunoassay kit (Immunodiagnostic Systems, Fountain Hills, AZ). Blood glucose levels were measured by using a glucose monitor (Glucometer; Bayer, Indianapolis, IN).
Hypothalamic signal transducer and activator of transcription (Stat)-3 activation and immunohistochemistry
Sixteen-week-old male Socs3flox/flox, Sf1-Cre and Socs3flox/flox littermates (three in each group) were fasted overnight and then injected ip with murine leptin (3 μg/g body weight). Control mice from each genotype were injected with PBS. The mice were perfused with formaldehyde 1 h after either leptin or PBS injection. Hypothalamic sections from all mice were immunostained with a rabbit polyclonal antibody for phospho-Stat3 (Cell Signaling, Beverly, MA) using the protocol previous described (30). Antibody for Sf1 was a gift from Dr. Ken Morohashi (National Institute for Basic Biology, Okazaki, Japan).
Energy expenditure
The comprehensive laboratory animal monitoring system (Columbus Instruments, Columbus, OH) was used to examine various parameters of energy expenditure. Oxygen (O2) and carbon dioxide (CO2) contents were determined by O2 and CO2 sensors by which sample air passed through. Heat production on per animal basis was calculated from the following equation: (3.82 + 1.23 × RER) × VO2, where RER is the respiratory exchange ratio (volume of CO2 produced/volume of O2 consumed per hour). Ambulatory activity was determined using an eight-cage rack OPTO-M3 sensor system. Consecutive photobeam breaks occurring in adjacent photobeams were scored as an ambulatory movement. Cumulative ambulatory activity counts were recorded every 30 min throughout the light and dark cycles. Mice were housed individually in specially built Plexiglas cages maintained at 22 C under an alternating 12-h light, 12-h dark cycle (light period 0800–2000 h). Mice were weighed before each trial and were acclimatized to monitoring cages for 24 h before data collection.
Statistical analysis
Data are expressed as the mean ± sem. Statistical significance was tested with unpaired two-tailed Student’s t tests. The differences were considered significant if P < 0.05.
Results
Enhanced phosphorylation of Stat3 in the VMH in mice lacking Socs3 in SF1 neurons
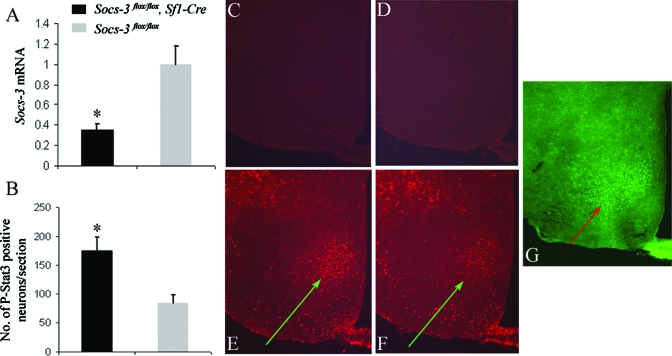
To examine the functions of VMH Socs3, we generated mice lacking Socs3 in SF1 neurons by crossing mice expressing Cre recombinase under the control of SF1 regulatory elements (Sf1-Cre) with those homozygous for Socs3 floxed alleles (Socs3flox/flox). Hypothalamic expression of Socs3 was shown to be highly induced by exogenous leptin (31). To confirm the loss of Socs3 expression in the VMH, we performed real-time PCR to examine the Socs3 expression in dissected VMH/DMH regions following ip injection of leptin. Indeed, there was approximately a 3-fold reduction in Socs3 mRNA levels in Socs3flox/flox, Sf1-Cre mice (Fig. 1A).
Figure 1.
Reduced Socs3 expression and enhanced Stat3 phosphorylation in the VMH of Socs3flox/flox, Sf1-Cre mice. A, Socs3 mRNA expression in the VMH/DMH (n = 3 per group). B, Average numbers of leptin-induced phosphorylated Stat3 (P-Stat3)-positive neurons within the VMH per section (n = 3 per group). Data are represented as mean ± sem. P-Stat3 immunohistochemistry from representative saline-injected Socs3flox/flox (C), Sf1-Cre and Socs3flox/flox (D) mice. P-Stat3 immunohistochemistry from representative leptin-injected Socs3flox/flox, Sf1-Cre (E) and Socs3flox/flox (F) mice. G, SF1 immunohistochemistry for Socs3flox/flox mice. Arrows indicate VMH regions.
Previous studies in mice with either Socs3 haploinsufficiency or selective Socs3 deletion in neurons suggested that Socs3 deficiency enhances leptin induced phosphorylation of hypothalamic Stat3 (20,21). We therefore performed immunohistochemistry using phospho-specific antibody for Stat3 to examine the phosphorylation of Stat3 in the hypothalamus after leptin injection. We also performed immunohistochemistry for Sf1 to confirm the location of the VMH (Fig. 1G). Phosphorylation of Stat3 was increased in the VMH of Socs3flox/flox, Sf1-Cre mice, compared with that in Socs3flox/flox mice (Fig. 1, C and D), suggesting that Socs3 in the VMH negatively regulates leptin signaling. Quantitative analysis revealed that there was an approximately 2-fold increase in the number of neurons containing phosphorylated Stat3 in the VMH of Socs3flox/flox, Sf1-Cre mice (Fig. 1B), whereas no difference was observed within the arcuate nucleus of the hypothalamus (data not shown).
Enhanced effect of exogenous leptin to reduce weight and food intake in mice lacking Socs3 in SF1 neurons
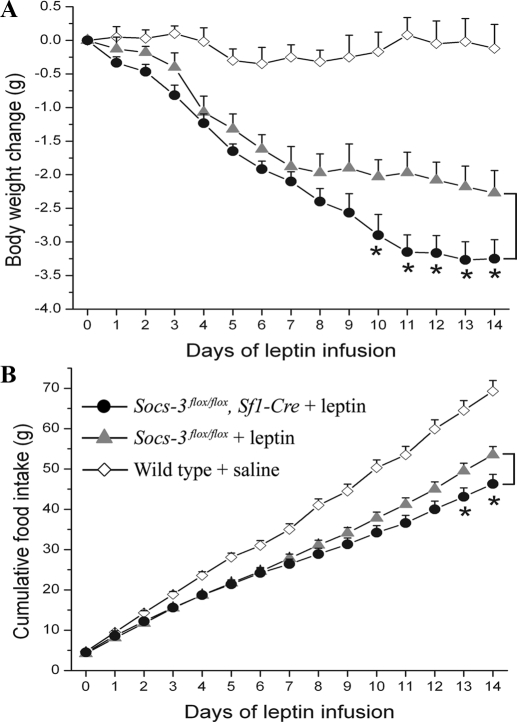
Mice lose weight in response to exogenous leptin, and this effect is enhanced by Socs3 haploinsufficiency or neuronal deletion (20,21). To determine whether reduced expression of Socs3 in SF1 neurons affects sensitivity to exogenous leptin in vivo, we performed leptin infusion studies in Socs3flox/flox, Sf1-Cre mice (n = 6) and Socs3flox/flox littermates (n = 6) by implanting minipumps that delivered recombinant leptin. Wild-type C57B6 mice were infused with saline as a control, and these mice showed no weight loss after minipump implantation and anesthetization procedures. In contrast, mice infused with leptin showed weight loss. After 10 d of leptin infusion, Socs3flox/flox, Sf1-Cre mice showed significantly greater weight loss than that of Socs3flox/flox littermates (Fig. 2A). Although daily food intake showed much variation, cumulative food intake showed a consistent pattern such that, after leptin infusion, Socs3flox/flox, Sf1-Cre mice ate less than Socs3flox/flox littermates. The difference became statistically significant after 13 d of leptin infusion (Fig. 2B). These results demonstrate that mice lacking Socs3 in SF1 neurons had enhanced sensitivity to exogenous leptin.
Figure 2.
Enhanced weight-reducing and food intake-reducing effects of exogenous leptin in Socs3flox/flox, Sf1-Cre mice. A, Body weight change of 20-wk-old Socs3flox/flox, Sf1-Cre mice and Socs3flox/flox mice after leptin infusion. B, Cumulative food intake of 20-wk-old Socs3flox/flox, Sf1-Cre mice and Socs3flox/flox mice after leptin infusion. Wild-type C57B6 mice were infused with saline as a control. Mouse numbers were six and six for Socs3flox/flox, Sf1-Cre and Socs3flox/flox mice, respectively. Data are expressed as mean ± sem. *, P < 0.05.
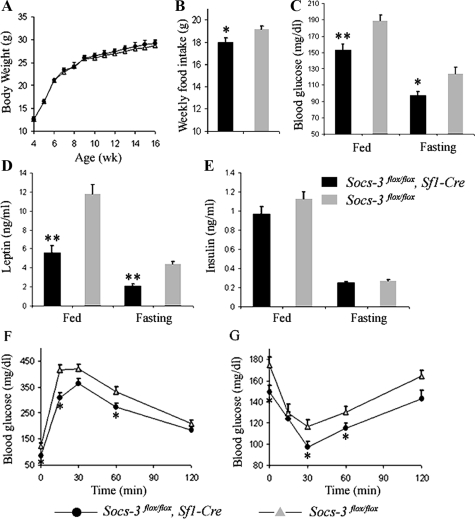
On chow diets, mice lacking Socs3 in SF1 neurons had reduced food intake, glucose levels, leptin levels, and improved glucose homeostasis
The VMH is involved in body weight control, and indeed, deletion of leptin receptor specifically in SF1 neurons resulted in weight gain (27,28). Because mice lacking Socs3 in SF1 neurons had increased leptin signaling reflected by enhanced Stat3 phosphorylation, we measured the body weight of these mice. Surprisingly, there was no difference in body weight between Socs3flox/flox, Sf1-Cre mice (n = 6) and Socs3flox/flox littermates (n = 7) on chow diets (Fig. 3A). However, food intake was reduced in Socs3flox/flox, Sf1-Cre mice, consistent with the hypothesis that leptin signaling is increased in these mice (Fig. 3B). This reduction in food intake persisted despite lower circulating leptin levels in Socs3flox/flox, Sf1-Cre mice in both fed and fasted states (Fig. 3D).
Figure 3.
Reduced food intake, blood glucose levels, leptin levels, and improved glucose homeostasis in Socs3flox/flox, Sf1-Cre mice. Body weight (A) and average weekly food intake (B) of Socs3flox/flox, Sf1-Cre and Socs3flox/flox mice are shown. Fed and fasting blood glucose levels at wk 10 (C), leptin levels at wk 12 (D), and insulin levels at wk 12 (E) for Socs3flox/flox, Sf1-Cre and Socs3flox/flox mice are shown. F, Glucose tolerance test at wk 13 (1.5 mg glucose per gram body weight). G, Insulin tolerance test at wk 15 (1 mU human Insulin R per gram body weight; n = 6 and 7 for Socs3flox/flox, Sf1-Cre and Socs3flox/flox mice, respectively). Data are expressed as mean ± sem. *, P < 0.05; **, P < 0.01.
Interestingly, blood glucose levels in both fed and fasted states were lower in Socs3flox/flox, Sf1-Cre mice, compared with controls (Fig. 3C), suggesting improved glucose homeostasis. Insulin levels in both fed and fasted states tended to be lower in Socs3flox/flox, Sf1-Cre mice, although not statistically significant (Fig. 3E). To further examine glucose homeostasis, we performed glucose tolerance tests (GTT) and insulin tolerance tests (ITT). Compared with Socs3flox/flox littermates, Socs3flox/flox, Sf1-Cre mice more rapidly cleared glucose in GTTs (Fig. 3F) and were also more sensitive to exogenous insulin in ITTs (Fig. 3G). These results suggest that mice lacking Socs3 in SF1 neurons had improved glucose homeostasis without changes in body weight on chow diets.
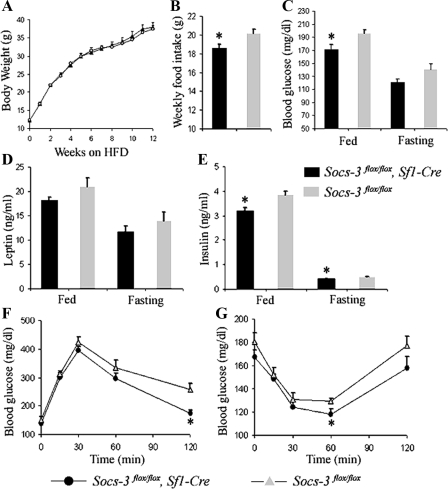
On HFD, mice lacking Socs3 in SF1 neurons had reduced food intake, glucose levels, insulin levels, and improved glucose homeostasis
High-fat diet (HFD) results in obesity and hyperleptinemia, inducing leptin resistance in mice. To examine the role of Socs3 in the VMH on obesity-associated phenotypes, mice were placed on HFD starting at 4 wk of age. Although body weights were not significantly different between Socs3flox/flox, Sf1-Cre mice (n = 6) and Socs3flox/flox littermates (n = 9) (Fig. 4A), the former had reduced food intake (Fig. 4B). On HFD, Socs3flox/flox, Sf1-Cre mice tended to have lower leptin levels, in both fed and fasted states, compared with those in Socs3flox/flox mice (Fig. 4D); however, this decrease was not statistically significant.
Figure 4.
On HFD, Socs3flox/flox, Sf1-Cre mice had reduced food intake, blood glucose levels, insulin levels, and improved glucose homeostasis. Body weight (A) and average weekly food intake (B) of Socs3flox/flox, Sf1-Cre and Socs3flox/flox mice are shown. Fed and fasting blood glucose levels at wk 10 (C), leptin levels at wk 12 (D), and insulin levels at wk12 (E) for Socs3flox/flox, Sf1-Cre and Socs3flox/flox mice are shown. F, Glucose tolerance test at wk 13 (1.5 mg glucose per gram body weight). G, ITT at wk 16 (1 mU human Insulin R per gram body weight; n = 6 and 9 for Socs3flox/flox, Sf1-Cre and Socs3flox/flox mice, respectively). Data are expressed as mean ± sem. *, P <0.05.
Experimental results for mice on HFD suggested that Socs3flox/flox, Sf1-Cre mice have improved glucose homeostasis. Insulin levels of Socs3flox/flox, Sf1-Cre mice in both fed and fasting states were lower than those of Socs3flox/flox littermates (Fig. 4E). Glucose levels of Socs3flox/flox, Sf1-Cre mice in the fed state were also significantly lower (Fig. 4C). We then did GTTs and ITTs to further examine glucose homeostasis in these mice. During GTTs, there was a tendency for Socs3flox/flox, Sf1-Cre mice to more rapidly clear glucose because at 120 min after glucose injection, glucose levels were significantly lower in Socs3flox/flox, Sf1-Cre mice compared with those of controls (Fig. 4F). Likewise, during ITTs, there was a tendency for Socs3flox/flox, Sf1-Cre mice to be more sensitive to insulin because at 60 min after insulin injection, blood glucose levels were lower in Socs3flox/flox, Sf1-Cre mice (Fig. 4G). These results support the conclusion that on HFD, mice lacking Socs3 in SF1 neurons have improved glucose homeostasis, independent of body weight.
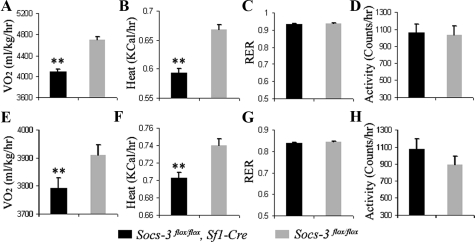
Reduced energy expenditure in mice lacking Socs3 in SF1 neurons
Energy homeostasis plays an important role in determining body weight. Leptin reduces body weight by both decreasing food intake and increasing energy expenditure. To examine the effect of Socs3 deficiency in SF1 neurons on energy expenditure, we used the comprehensive laboratory animal monitoring system (CLAMS) to measure oxygen consumption (VO2), the RER, heat production, and locomotor activity. Unexpectedly, on both regular chow and HFD (n = 6 and 6, respectively), Socs3flox/flox, Sf1-Cre mice had reduced oxygen consumption compared with that of Socs3flox/flox littermates (n = 7 and 9, respectively) (Fig. 5A and E); Socs3flox/flox, Sf1-Cre mice also had a reduced heat production on both regular chow and HFD (Fig. 5, B and F). There was no significant change between mice of the two genotypes in RER and locomotor activity on both regular chow and HFD (Fig. 5, C, D, G, and H). Collectively, these results suggest that removal of Socs3 in SF1 neurons decreased energy expenditure.
Figure 5.
Reduced energy expenditure of Socs3flox/flox, Sf1-Cre mice on chow and HFD. Oxygen consumption (A), heat production (B), RER (VO2 to VCO2) (C), and locomotor activity (D), of mice on chow diets are shown (n = 6 and 7 for Socs3flox/flox, Sf1-Cre and Socs3flox/flox mice, respectively). Oxygen consumption (E), heat production (F), RER (VO2 to VCO2) (G), and locomotor activity (H) of mice on HFD are shown (n = 6 and 9 for Socs3flox/flox, Sf1-Cre and Socs3flox/flox mice, respectively). Data are expressed as mean ± sem. **, P <0.01.
Discussion
The central nervous system (CNS) has a complex and highly orchestrated neuronal network that mediates regulation of body weight, food intake, energy expenditure, and glucose homeostasis. Although much has been learned in recent years, we have an incomplete understanding of the details of this network. Leptin, among many other important hormones, is a key regulator of this system. After the discovery of leptin, whose deficiency causes massive obesity that is responsive to replacement therapy (5,6), the response to leptin replacement in nonleptin-deficient obese humans has been disappointing (12,32,33) because leptin therapy was relatively unsuccessful in this group due to leptin resistance. Elucidating the mechanisms underlying leptin resistance has therefore become a critical question in the field (for some reviews, see Refs. 34,35,36,37,38).
Leptin exerts many of its effects through the CNS, which, therefore, is a key site for the mechanism of leptin resistance. Indeed, in mice with obesity induced by HFD, the ability of both peripheral and intracerebroventricular leptin to activate Stat3 phosphorylation in the hypothalamus was much reduced, suggesting a signaling defect upstream of Stat3 in leptin-responsive hypothalamic neurons (7,39). The level of neuronal Socs3 expression has been identified as being responsive to leptin (18), and genetic studies demonstrated Socs3 as a mechanism that can regulate central leptin sensitivity (20,21,22).
Because of the complexity of the CNS, it is critical to modify distinct populations of neurons to fully understand their functions. The Cre/loxP system is available to generate genetically modified mice with inactivation of genes in specific neuronal populations. In the current study, we selectively deleted the Socs3 gene in SF1 neurons by crossing mice harboring a Socs3 gene flanked by loxP sites with mice expressing Cre recombinase under the control of SF1 regulatory sequences.
Interestingly, mice with genetic deletion of Socs3 in SF1 neurons had increased leptin-induced Stat3 phosphorylation specifically in the VMH. This result shows that Socs3 negatively regulates leptin signaling in the VMH in vivo. Consistent with this result, and demonstrating its physiological relevance, mice lacking Socs3 in SF1 neurons lost more weight and had greater suppression in food intake in response to exogenous leptin. Thus, SF1 Socs3 regulates leptin signaling and leptin-mediated physiological effects on food intake and body weight.
When mice were placed on either regular chow or HFDs, Socs3flox/flox, Sf1-Cre mice showed reduced food intake, consistent with the aforementioned increased leptin signaling in the VMH. Unexpectedly, however, energy expenditure, as assessed by VO2 and heat production, was also decreased. To examine whether Socs3flox/flox, Sf1-Cre mice had functional changes in the adrenal cortex, in which Sf1 is also expressed (40), we measured serum corticosterone levels, which did not show significant differences (data not shown), suggesting that the adrenal cortex is not involved in the observed phenotypes in these mice. Whatever the mechanism for the discordant effects of VMH Socs3 on food intake (decreased) and energy expenditure (reduced), these changes likely explain the fact that the body weights of these mice were not different. Although body weights were similar, Socs3flox/flox, Sf1-Cre mice had lower leptin levels than Socs3flox/flox mice on chow diets, consistent with increased leptin sensitivity. We then examined weights of individual fat pads, which did not show significant differences (data not shown). It is possible that the reduced food intake played a role in the reduced leptin levels in Socs3flox/flox, Sf1-Cre mice.
An important phenotype of Socs3flox/flox, Sf1-Cre mice was their improved glucose homeostasis. On both chow diets and HFDs, glucose levels were lower in Socs3flox/flox, Sf1-Cre mice than in littermate controls. Furthermore, GTTs and ITTs also suggested that Socs3flox/flox, Sf1-Cre mice were more sensitive to insulin. It is noteworthy that improved insulin sensitivity usually follows amelioration of obesity; mice lacking Socs3 in SF1 neurons, however, had improved glucose homeostasis, with no body weight loss and were partially protected from hyperglycemia and hyperinsulinemia induced by HFD. These results are also consistent with increased central leptin signaling independent of weight loss, which may be associated with improved glucose homeostasis. For instance, central leptin administration restores decreased insulin sensitivity in overfed rats (41). It is also possible that Socs3 deficiency in SF1 neurons improves glucose homeostasis by mechanisms independent of leptin. For example, Socs3 also suppresses insulin signaling (42,43,44). Because central insulin administration can inhibit peripheral glucose production (45), it is possible that removal of Socs3 in SF1 neurons enhanced VMH insulin signaling, which might then have lowered glucose level and increased insulin sensitivity. Consistently, mice lacking Socs3 in SF1 neurons indeed had lower glucose levels (Figs. 3C and 4C).
Mice with Socs3 haploinsufficiency (21), Socs3 neuronal deletion (20), and Socs3 deletion in POMC neurons (22) have previously been generated. It is interesting to compare and contrast the phenotypes of these three mouse models with the mice generated here (Table 1). For mice on HFD, in all four models, leptin sensitivity was increased in relevant cells, and levels of leptin, glucose, and insulin were decreased. There are also striking differences. In mice lacking Socs3 in SF1 neurons, food intake was decreased and body weight was not changed, whereas in those lacking Socs3 in POMC neurons, food intake was unchanged and body weight was decreased. Furthermore, in the former, energy expenditure was decreased, whereas in the latter, it was increased. On HFD, mice lacking Socs3 in POMC neurons have more pronounced glucose homeostasis phenotype than those lacking Socs3 in SF1 neurons, and this is probably due to reduced energy expenditure in the latter and the different contributions of Socs3 in these two populations of neurons.
Table 1.
Comparison of genetic models of Socs3 and Lepr deficiency in micea
| Phenotypesb | Sites of Socs3 deletion
|
Sites of Lepr deletion
|
||||
|---|---|---|---|---|---|---|
| VMH (SF1 neurons) | POMC neurons | All neurons | Haploinsufficiency | VMH (SF1 neurons) | POMC neurons | |
| Body weight | – | ↓ | ↓ | ↓ | ↑ | ↑ |
| Food intake | ↓ | – | ↓ | ↓ | ↑ | – |
| Energy expenditure | ↓ | ↑ | NA | NA | ↓ | – |
| Glucose | ↓ | ↓ | ↓ | ↓ | – | – |
| Leptin | ↓ | ↓ | ↓ | ↓ | ↑ | ↑ |
| Insulin | ↓ | ↓ | ↓ | ↓ | ↑ | – |
| Leptin sensitivity | ↑ | ↑ | ↑ | ↑ | NA | NA |
The references (22,20,21) are for the Socs3 deletion in POMC neurons, Socs3 deletion in the brain, and Socs3 haploinsufficiency, respectively. The references (27,28) are for Lepr deletion in SF1 neurons and the reference (46) is for Lepr deletion in POMC neurons. –, No significant change is observed.
The phenotypes are for mice placed on HFDs, except those of mice with Lepr deletion in POMC neurons.
It is also interesting to compare the phenotypes of mice with Socs3 vs. Lepr deletions in SF1 neurons. Because Socs3 in SF1 neurons is shown here to suppress leptin signaling, we might expect mice from the two genetic models to have opposite phenotypes in terms of leptin-related metabolic parameters. Indeed, in mice with Socs3 deletion in SF1 neurons, levels of leptin and insulin were decreased, but in those with Lepr deletion in the same cells, these hormone levels were increased. Similarly, in the former, food intake was decreased, whereas in the latter, it was increased. Unexpectedly, however, energy expenditure was decreased in both models. This suggests that Socs3 in SF1 neurons may have effects on energy expenditure that are independent of leptin signaling.
In summary, our results show that Socs3 is an active regulator of leptin signaling and physiological effects in SF1 neurons. Removal of Socs3 in SF1 neurons enhanced phosphorylation of Stat3 in the VMH, and food intake and weight-reducing effects of exogenous leptin were enhanced in these mice. Body weight was not changed on either chow or HFDs, and this is likely due to the fact that energy expenditure was, surprisingly, reduced as well. Despite no difference in body weights, mice lacking Socs3 in SF1 neurons had lower glucose levels and improved glucose homeostasis. This suggests a metabolic action via the VMH that is independent of weight, either through leptin or insulin pathway in these neurons. The clear difference in energy expenditure between mice lacking Socs3 in SF1 and POMC neurons suggests that Socs3 in different brain regions has diverse roles in regulating energy expenditure. Collectively, these results suggests that Socs3 in SF1 neurons negatively regulates leptin signaling and plays important roles in leptin sensitivity, glucose homeostasis, and energy expenditure.
Acknowledgments
We thank Christian Bjorbaek for stimulating discussions and Fenfen Liu for technical assistance in CLAMS studies.
Footnotes
This work was supported by National Institutes of Health Grants DK R37 28082 (to J.S.F.), RO1 DK56113 (to E.M.-F.), and RO1 DK0710151 (to B.B.L.). The CLAMS support was provided by the physiology core of PO1 DK56116 (to E.M.-F.)
Disclosure Statement: The authors have nothing to disclose.
First Published Online July 31, 2008
Abbreviations: CLAMS, comprehensive laboratory animal monitoring system; CNS, central nervous system; DMH, dorsomedial hypothalamus; GTT, glucose tolerance test; HFD, high-fat diet; ITT, insulin tolerance test; Lepr, leptin receptor; POMC, proopiomelanocortin; RER, respiratory exchange ratio; SF1, steroidogenic factor 1; Socs, suppressor of cytokine signaling; Stat, signal transducer and activator of transcription; VMH, ventromedial hypothalamus; VO2, oxygen consumption.
References
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM 1994 Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432 [DOI] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM 1995 Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269:543–546 [DOI] [PubMed] [Google Scholar]
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F 1995 Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269:540–543 [DOI] [PubMed] [Google Scholar]
- Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P 1995 Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269:546–549 [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O'Rahilly S 1999 Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 341:879–884 [DOI] [PubMed] [Google Scholar]
- Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S 1997 Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387:903–908 [DOI] [PubMed] [Google Scholar]
- Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM 1997 Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA 94:8878–8883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell BB, Spiegelman BM 2000 Toward a molecular understanding of adaptive thermogenesis. Nature 404:652–660 [DOI] [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S et al 1995 Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1:1155–1161 [DOI] [PubMed] [Google Scholar]
- Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS 1995 Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med 1:1311–1314 [DOI] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL et al 1996 Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334:292–295 [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, McCamish M 1999 Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282:1568–1575 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte Jr D 1996 Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med 2:589–593 [DOI] [PubMed] [Google Scholar]
- Carpenter LR, Farruggella TJ, Symes A, Karow ML, Yancopoulos GD, Stahl N 1998 Enhancing leptin response by preventing SH2-containing phosphatase 2 interaction with Ob receptor. Proc Natl Acad Sci USA 95:6061–6066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP 1999 Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283:1544–1548 [DOI] [PubMed] [Google Scholar]
- Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, Kim YB, Elmquist JK, Tartaglia LA, Kahn BB, Neel BG 2002 PTP1B regulates leptin signal transduction in vivo. Dev Cell 2:489–495 [DOI] [PubMed] [Google Scholar]
- Cheng A, Uetani N, Simoncic PD, Chaubey VP, Lee-Loy A, McGlade CJ, Kennedy BP, Tremblay ML 2002 Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev Cell 2:497–503 [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS 1998 Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell 1:619–625 [DOI] [PubMed] [Google Scholar]
- Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL, Sinha MK, Considine RV 1996 Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet 348:159–161 [DOI] [PubMed] [Google Scholar]
- Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A 2004 Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med 10:739–743 [DOI] [PubMed] [Google Scholar]
- Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjorbaek C, Flier JS 2004 Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med 10:734–738 [DOI] [PubMed] [Google Scholar]
- Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, Lee CE, Elmquist JK, Yoshimura A, Flier JS 2006 Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab 4:123–132 [DOI] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB 2005 Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123:493–505 [DOI] [PubMed] [Google Scholar]
- Hetherington A, Ranson S 1940 Hypothalamic lesions and adiposity in the rat. Anat Rec 78:149–172 [Google Scholar]
- Elmquist JK, Ahima RS, Elias CF, Flier JS, Saper CB 1998 Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc Natl Acad Sci USA 95:741–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellovade TL, Young M, Ross EP, Henderson R, Caron K, Parker K, Tobet SA 2000 Disruption of the gene encoding SF-1 alters the distribution of hypothalamic neuronal phenotypes. J Comp Neurol 423:579–589 [DOI] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua Jr S, Elmquist JK, Lowell BB 2006 Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49:191–203 [DOI] [PubMed] [Google Scholar]
- Bingham NC, Anderson KK, Reuter AL, Stallings NR, Parker KL 2008 Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology 149:2138–2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, Hanada T, Takeda K, Akira S, Hoshijima M, Hirano T, Chien KR, Yoshimura A 2003 IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol 4:551–556 [DOI] [PubMed] [Google Scholar]
- Munzberg H, Huo L, Nillni EA, Hollenberg AN, Bjorbaek C 2003 Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology 144:2121–2131 [DOI] [PubMed] [Google Scholar]
- Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK 1999 Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron 23:775–786 [DOI] [PubMed] [Google Scholar]
- Mantzoros CS, Flier JS 2000 Editorial: leptin as a therapeutic agent—trials and tribulations. J Clin Endocrinol Metab 85:4000–4002 [DOI] [PubMed] [Google Scholar]
- Hukshorn CJ, Saris WH, Westerterp-Plantenga MS, Farid AR, Smith FJ, Campfield LA 2000 Weekly subcutaneous pegylated recombinant native human leptin (PEG-OB) administration in obese men. J Clin Endocrinol Metab 85:4003–4009 [DOI] [PubMed] [Google Scholar]
- Flier JS 2004 Obesity wars: molecular progress confronts an expanding epidemic. Cell 116:337–350 [DOI] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL 1998 Leptin and the regulation of body weight in mammals. Nature 395:763–770 [DOI] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS 1996 Adipogenesis and obesity: rounding out the big picture. Cell 87:377–389 [DOI] [PubMed] [Google Scholar]
- Friedman JM 2000 Obesity in the new millennium. Nature 404:632–634 [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW 2006 Central nervous system control of food intake and body weight. Nature 443:289–295 [DOI] [PubMed] [Google Scholar]
- El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS 2000 Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 105:1827–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallings NR, Hanley NA, Majdic G, Zhao L, Bakke M, Parker KL 2002 Development of a transgenic green fluorescent protein lineage marker for steroidogenic factor 1. Mol Endocrinol 16:2360–2370 [DOI] [PubMed] [Google Scholar]
- Pocai A, Morgan K, Buettner C, Gutierrez-Juarez R, Obici S, Rossetti L 2005 Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes 54:3182–3189 [DOI] [PubMed] [Google Scholar]
- Shi H, Tzameli I, Bjorbaek C, Flier JS 2004 Suppressor of cytokine signaling 3 is a physiological regulator of adipocyte insulin signaling. J Biol Chem 279:34733–34740 [DOI] [PubMed] [Google Scholar]
- Senn JJ, Klover PJ, Nowak IA, Zimmers TA, Koniaris LG, Furlanetto RW, Mooney RA 2003 Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem 278:13740–13746 [DOI] [PubMed] [Google Scholar]
- Ueki K, Kondo T, Kahn CR 2004 Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol 24:5434–5446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obici S, Zhang BB, Karkanias G, Rossetti L 2002 Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med 8:1376–1382 [DOI] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua Jr SC, Elmquist JK, Lowell BB 2004 Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42:983–991 [DOI] [PubMed] [Google Scholar]