Abstract
Prostaglandins in the corpus luteum (CL) reportedly serve as luteotropic and luteolytic agents. Based mainly on studies conducted in domesticated animals and rodents, prostaglandin E2 (PGE2) is generally considered a luteotropic factor, whereas uterine-derived prostaglandin F2α (PGF2α) initiates luteolysis. However, the role of prostaglandins in regulating primate luteal structure-function is poorly understood. Therefore, a comprehensive analysis of individual mRNA or proteins that are involved in PGE2 and PGF2α biosynthesis, metabolism, and signaling was performed using CL obtained at distinct stages of the luteal life span during the menstrual cycle in rhesus monkeys. Peak levels of proteins involved in PGE2 synthesis (prostaglandin-endoperoxide synthase 2, microsomal PGE2 synthase-1) and signaling (PGE2 receptor 3) occurred during periods corresponding to development and maintenance of the primate CL. Immunohistochemistry studies indicated that large luteal cells express PGE2 synthesizing and signaling proteins. Expression of PGE2 synthesizing and signaling proteins significantly decreased preceding the period of functional regression of the CL, which also coincided with increasing levels of PGF2α receptor protein expression within the large luteal cells. Moreover, significant levels of mRNA expression for several aldoketo reductase family members that synthesize PGF2α from other prostaglandins were observed throughout the rhesus macaque luteal phase, thus supporting the possibility of intraluteal PGF2α production. Collectively, our results indicate that there may be intraluteal synthesis and signaling of PGE2 during development and maintenance of the primate CL, followed by a shift to intraluteal PGF2α synthesis and signaling as the CL nears the time of luteolysis.
THE CORPUS LUTEUM (CL) is a transient endocrine gland that forms from the retained granulosa/theca cells of the ruptured ovulatory follicle. The CL is the primary source of progesterone during the menstrual cycle and early pregnancy in primates, which in turn is absolutely required for initiation of pregnancy and controlling menstrual cycle length. During nonfertile cycles, the lifespan of the CL has three general phases: luteinization, maintenance, and luteolysis. Luteinization is the process whereby cells of the ovulatory follicle undergo dramatic morphologic and biochemical changes to form the highly steroidogenic CL (1,2,3). This is followed by a period of peak progesterone production in the fully developed CL (maintenance). In nonconception cycles, luteolysis occurs and can be subdivided into two phases: cessation of progesterone secretion (functional regression) and major structural remodeling and apoptosis of luteal cells (structural regression) (4,5,6,7). Whereas the factors that regulate the structure and function of the CL throughout its life span are poorly defined, there is evidence mainly from nonprimate species that support a role for prostaglandins in luteal development and regression.
Prostaglandin (PG) E2 is generally considered to be a luteotropic prostaglandin. Administration of PGE2 prevents spontaneous or induced luteolysis in ewes (8,9,10,11), and PGE2 is believed to be involved in maintaining luteal function during early pregnancy in domestic animals (12). Similar luteotrophic/antiluteolytic effects have been reported in primates. Intraluteal infusion of PGE2 to monkeys prevents PGF2α-induced luteolysis (13). Also, PGE2 stimulates progesterone and/or cAMP synthesis in primate luteal cells or tissue slices obtained during the luteal phase or early pregnancy (14,15,16). Furthermore, intraluteal infusion of the prostaglandin synthesis inhibitor sodium meclofenamate during the mid luteal phase reduced serum progesterone levels and resulted in premature menses in monkeys (17), an effect not observed with systemic administration indicating a role for local prostaglandins in maintaining the primate CL. The identity of the prostaglandin as well as the specific receptor signaling pathway responsible for this in vivo effect remains unknown.
In contrast to the luteotropic effects of PGE2, PGF2α released from the uterus is the signal that initiates luteolysis in many nonprimate species (1,3). Hysterectomy in rabbits, rats, cattle, sheep, pigs, guinea pigs, and horses results in an extension of CL life span (1,3), indicating that the signal initiating luteolysis is of uterine origin. Moreover, the prostaglandin synthesis inhibitor indomethacin prevented luteolysis in pseudopregnant rodents (18,19). It was subsequently determined that PGF2α was the luteolysin because it caused premature luteolysis in pseudopregnant rats, an effect not observed with other prostaglandins (20), and PGF2α immunization prevented spontaneous luteolysis in the ewe and cow (21,22). However, the mechanism of luteolysis in primates is presently unknown. Hysterectomy of primates does not extend the life span of the CL (23,24), indicating that the luteolytic signal is not of uterine origin. PGF2α may still have a luteolytic role in primates because intraluteal infusion of PGF2α analogs causes luteolysis in monkeys and women (13,25,26,27,28,29). Thus, the origin and role of PGF2α in spontaneous luteolysis of the primate CL remains to be determined.
Previously we reported that genes encoding prostaglandin-endoperoxide synthase 2 (PTGS2), 15-(NAD)-hydroxyprostaglandin dehydrogenase (HPGD), solute carrier organic anion transporter family member 2A1 (prostaglandin transporter), microsomal PGE2 synthase-1 (PTGES), PGE2 receptor 3 (PTGER3), and PGF2α receptor (PTGFR) are dynamically regulated through the stages of the luteal phase that correspond to luteinization, maintenance, and functional regression of the rhesus macaque CL (30). In the current study, these findings are extended by performing a comprehensive analysis of the PGE2/PGF2α synthesis, metabolism, and signaling components in the primate CL during its normal life span. The objectives were: 1) to determine whether there are corresponding changes in PTGS2, PTGES, HPGD, PTGER3, and PTGFR protein levels through the luteal phase; 2) determine their cellular localization of expression using immunohistochemistry (IHC); and 3) to evaluate mRNA expression of aldoketo reductase (AKR) family members that may be involved in PGE2/PGF2α synthesis and metabolism, as well as the additional subtypes of PGE2 receptors (PTGER1, PTGER2, PTGER4) during the luteal phase of the primate menstrual cycle.
Materials and Methods
CL collection
All protocols involving animals were approved by the Oregon National Primate Research Center’s Institutional Animal Care and Use Committee. The care and handling of rhesus macaques was performed as described previously (30). The CL were collected (n = 4 CL/stage) between d 3 and 5 (early stage, developing CL), 7 and 8 (midstage, fully functional CL), 10 and 12 (mid-late stage, functional CL on the verge of regression), 14 and 16 (late stage, functionally regressing CL), or 18 and 19 (very late stage, menses) after the LH surge as previously detailed (31,32,33,34,35,36). The early CL are undergoing luteinization, the mid-CL are fully functional CL that are at their peak progesterone-producing capacity, the mid-late stage is a transitional period whereby CL are still producing significant quantities of progesterone just before the initiation of luteolysis in nonconception cycles, the late stage corresponds to CL undergoing functional regression (cessation of progesterone secretion), and the very late stage (menses) is when the structural remodeling and apoptosis associated with luteolysis is occurring.
Microarray analysis
Microarray data corresponding to the AKR family were obtained from our previously published microarray results (30) that is accessible through the National Center for Biotechnology Information GEO database (accession no. GSE10367; http://www.ncbi.nlm.nih.gov/geo/). Probe sets corresponding to genes of interest were identified (http://www.affymetrix.com/analysis/index.affx) and located within the microarray expression database that covers the entire rhesus macaque genome (Affymetrix, Santa Clara, CA). The microarray expression data were normalized using the robust multichip analysis (RMA) algorithm, and log (base 2) transformed (30). Data were analyzed using one-way ANOVA followed by pairwise comparisons with the Student-Newman-Keuls (Student-Newman-Keuls) test, and differences were considered statistically significant at P < 0.05.
TaqMan quantitative real-time PCR (Q-PCR)
RNA was isolated and reverse transcribed to cDNA as described previously (30). The complete cDNA sequence for each gene of interest was obtained from the National Center for Biotechnology Information rhesus macaque genome database and was subsequently used to design the Q-PCR primers and TaqMan MGB probes using PrimerExpress software (Applied Biosystems, Foster City, CA). Primers and probes were purchased from Invitrogen (Carlsbad, CA) and Applied Biosystems, respectively. Sequences of primers and probes used are provided in Table 1. Q-PCR was performed as described previously (30).
Table 1.
Forward primer, reverse primer, and MGB probe sequences used for Q-PCR analysis
| Gene symbol | Forward primer (5′–3′) | Reverse primer (5′–3′) | Probe (5′–3′) 6FAM-sequence-MGBNFQ |
|---|---|---|---|
| PTGS1 | TGTGGATGTCATCAGGGAGTCT | GAAGGAGGTGTAGGGCTTCATG | TCAATGAGTACCGCAAGAG |
| AKR1C1 | CCTTGGAAAGGTCACTGAAAAATC | GATCAGTTCCTCACCTGGCTTTAG | ACTATGTTGACCTCTATCTTAT |
| PTGER1 | CAGCCACGACGTGGAGATG | GCCACCAACACCAGCATTG | TGCACGACACCACCATGATA |
| PTGER2 | AGACCTGCTGACAAGGCACTTC | TGTTCCTCCAAAGGCCAAGTA | AGGAGCTACAAAACCTACCCT |
| PTGER4 | TAACATCCAGAGCTTCGTCGTATT | TCATCCATAAGGCAGTCCTCATAG | TTAGAAAGGCTCTATTCCAG |
Relative expression of the target genes were normalized to 18s rRNA levels, and the ratios were log transformed before statistical analysis. Data were analyzed using one-way ANOVA followed by pairwise comparisons with the Student-Newman-Keuls (Student-Newman-Keuls) test, and differences were considered statistically significant at P < 0.05.
Western blot analysis
Tissue homogenates were prepared from frozen (−80 C) CL as described previously (30). Antibodies produced from human antigens corresponding to PTGES (catalog no. 160140), HPGD (catalog no. 160615), and PTGER3 (catalog no. 101760) were purchased from Cayman Chemical Co. (Ann Arbor, MI). The PTGS2 polyclonal antibody (catalog no. ab15191) used in Western blot analysis was produced using rat protein and was purchased from Abcam (Cambridge, MA). The PTGFR (catalog no. sc-33364) and β-tubulin (TUBB) (catalog no. sc-9104) antibodies against human proteins were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Twenty micrograms (PTGES), 25 μg (PTGER3), 40 μg (PTGS2 and HPGD), or 50 μg (PTGFR) of total protein were loaded. Western blot procedures were similar to those described previously (30). Antibodies were used at the following concentrations: anti-PTGES, anti-PTGER3, and anti-PTGS2, 0.4 μg/ml; anti-PTGFR, 1 μg/ml; anti-HPGD, 2 μg/ml; and anti-TUBB, 0.2 μg/ml. Specificity was determined by estimating molecular weight via comparison of bands with molecular weight markers, preabsorbing the primary antibody with immunizing peptide if available (PTGES, PTGER3, HPGD, and PTGFR), or excluding the primary antibody if no peptide was available (PTGS2).
Densitometry was performed using Quantity One version 4.3.1 software (Bio-Rad, Hercules, CA). The background-adjusted volume of each band was normalized to TUBB for each sample, and data were log transformed if necessary for statistical normalization. The ratio of target protein to TUBB for each stage was analyzed by one-way ANOVA followed by pairwise comparison using the Student-Newman-Keuls test with differences considered significant at P < 0.05.
IHC
Antibodies used in IHC were the same as for Western blot analysis except for PTGS2, which used a mouse monoclonal anti-PTGS2 antibody from Cayman Chemical (catalog no. 160112) that was produced by immunization with a human antigen. Specificity of all antibodies was determined by probing a CL section from the stage of the luteal phase in which expression of the target protein was highest (as determined by Western blot analysis) with primary antibody that had been preabsorbed with its immunizing peptide. Concentrations of primary antibodies were: PTGS2, 3 μg/ml; PTGES and PTGER3, 0.8 μg/ml; and PTGFR, 0.7 μg/ml.
Formalin-fixed luteal sections (5 μm) were deparaffinized using three washes for 5 min each in xylene. Sections were rehydrated in a graded series of ethanol (down to 70%), and washed in PBS for 20 min. Antigen retrieval occurred by boiling sections for 15 min in 100 mm sodium citrate buffer (pH 6.0). Slides were briefly washed in water, and incubated with 3% hydrogen peroxide in methanol for 10 min to quench endogenous peroxidase activity. Slides were washed three times for 5 min each in PBS, and sections were covered with blocking buffer (PBS with 1:70 diluted normal rabbit or goat antiserum) and incubated in a humidified box for 1 h at room temperature. Primary antibodies were diluted in blocking buffer, added to sections, and incubated overnight at 4 C in a humidified box. Slides were washed as before, incubated with the appropriate biotinylated secondary antibody (Vector Laboratories, Inc., Burlingame, CA; diluted 1:200 in blocking buffer) for 1 h at room temperature. Slides were washed and incubated an additional hour at room temperature with avidin and biotinylated horseradish peroxidase macromolecular complex reagent (Vector Laboratories). After a final wash, slides were developed for 1–5 min using diaminobenzidine (Vector Laboratories). The diaminobenzidine reaction was stopped by placing slides in water. The slides were counterstained in hematoxylin, washed in water, and dehydrated using a graded series of alcohols from 70 up to 100% followed by a final wash in xylene. Coverslips were added using nonaqueous mounting medium.
Digital photomicrographs were captured using a DEI-750 CCD camera (Optronics, Goleta, CA) through planapochromatic lenses (Carl Zeiss, Thornwood, NY). Images from all slides that were processed with the same antibody (representing two to three different animals for each stage of the luteal phase analyzed) were packaged into a digital contact sheet using Photoshop 7 (Adobe, San Jose, CA). Corrections to white balance and other adjustments were made on the contact sheet so that changes were applied uniformly across all images for the same antibody.
Results
Protein levels of PTGS2 and PTGES
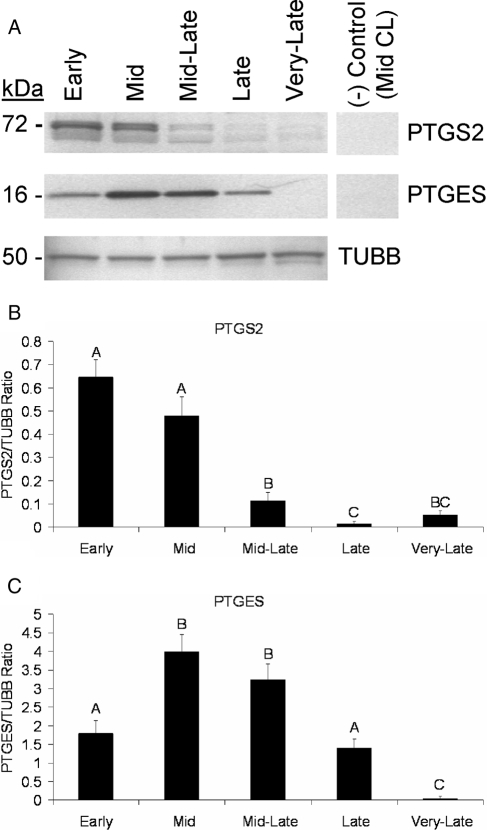
PTGS2 was detected as a 72- to 74-kDa doublet (Fig. 1A) as previously reported (37,38), with both bands being combined for quantification. Peak expression of PTGS2 occurred in the early to midstages, followed by a greater than 4-fold decrease (P < 0.05) from the mid- to mid-late stages of the macaque luteal phase (Fig. 1, A and B). Another decrease (P < 0.05) occurred between the mid-late and late stages when levels of PTGS2 became nearly nondetectable.
Figure 1.
PTGS2 and PTGES protein levels in rhesus macaque CL obtained throughout the luteal phase. A, Representative Western blots for PTGS2, PTGES, and TUBB using samples pooled from CL collected at each stage of the macaque luteal phase. The negative control is pooled midstage CL in which the primary antibody was omitted (PTGS2) or probed with preabsorbed primary antibody (PTGES). B and C Quantitative assessment of PTGS2 and PTGES expression, respectively, as determined by densitometry. Levels of PTGS2 and PTGES expression from individual CL (n = 4 CL/stage) were normalized to TUBB, and the resultant ratio was analyzed by ANOVA followed by comparison between groups using the Student-Newman-Keuls test. Error bars, 1 sem. Columns with different letters are significantly different (P < 0.05).
There was a greater than 2-fold increase (P < 0.05) in PTGES concentrations from the early to mid stages of the luteal phase, followed by a greater than 2.5-fold decrease (P < 0.05) in levels from the mid- to late CL (Fig. 1, A and C). PTGES was almost nondetectable in very late CL, with levels lower (P < 0.05) than all other stages.
Localization of PTGS2 and PTGES expression
Strong staining for PTGS2 was observed in tissue sections from early (Fig. 2A) and midstage CL (data not shown). PTGS2 was expressed predominantly in large luteal cells because there was minimal staining observed in small luteal/stromal or endothelial cells. PTGS2 was concentrated in the perinuclear region of large luteal cells during the early and midstages. In contrast, PTGS2 protein was still detected primarily in large luteal cells of the late stage CL, although its distribution was more diffuse throughout the cells and not localized in the perinuclear region (Fig. 2A).
Figure 2.
Localization of PTGS2 and PTGES proteins in rhesus macaque CL. Representative photomicrographs of PTGS2 (A) and PTGES (B) IHC. The insets in the lower left corner of the first image for A and B are adjacent sections that were processed with primary antibody preabsorbed with immunizing peptide. Approximate locations of various cell types are indicated. L, Large luteal cells; S, small luteal or stromal cells; V, blood vessel. Scale bar in the lower right hand corner of the higher-magnification images, 50 μm.
PTGES immunoreactivity was strongest in sections from mid stage CL (Fig. 2B) compared with all other stages (early, mid-late, and late; data not shown). PTGES protein was also observed predominantly in large luteal cells. By the very late stage, PTGES staining was virtually absent. Interestingly, a small number of luteal cells located proximal to blood vessels retained strong PTGES immunoreactivity in the very late stage. Luteal cells that were more distal to blood vessels were mostly devoid of PTGES expression (Fig. 2B).
PGE2 receptor subtype mRNA levels
Our previous analysis of PGE2 receptors (30), which includes four subtypes designated PTGER1–4 (39), focused only on PTGER3. Therefore, to extend these findings, we determined mRNA levels of PTGER1, PTGER2, and PTGER4 during the luteal phase using Q-PCR (Fig. 3). There was a steady decrease in PTGER1 mRNA from the early through late stages with the early CL having greater than 2-fold higher (P < 0.05) mRNA levels compared with late CL. Levels of PTGER2 and PTGER4 did not change from the early through late stages. Concentrations of mRNA for PTGER2 and PTGER4 were significantly higher (P < 0.05) in the very-late stage compared with all other stages, with PTGER2 having greater than 2.5-fold, and PTGER4 having greater than 4.5-fold higher mRNA levels in the very late CL compared with the highest concentration detected in the early through late stages (Fig. 3).
Figure 3.
PTGER1, PTGER2, and PTGER4 mRNA levels in rhesus macaque CL obtained throughout the luteal phase. Levels of mRNA were determined by Q-PCR and normalized to 18s rRNA in individual CL (n = 4 CL/stage). Data were analyzed by ANOVA followed by comparison between groups using the Student-Newman-Keuls test. Error bars, 1 sem. Columns with different letters or numbers are significantly different (P < 0.05), with uppercase letters, lowercase letters, and numerals corresponding to PTGER1, PTGER2, and PTGER4, respectively.
Absolute levels of mRNA for PTGER1, PTGER2, and PTGER4 from the early through late stages of the macaque luteal phase were low compared with PTGER3 as determined by microarray analysis (30). The absolute expression levels detected by microarray from the early through late CL are PTGER3≫PTGER1>PTGER4>PTGER2. This ordering of PGE2 receptor subtype expression was confirmed by Q-PCR (data not shown). PTGER4 had the highest relative expression value of the PGE2 receptor subtypes in the very-late CL as determined by microarray and Q-PCR.
PTGER3 protein expression and localization
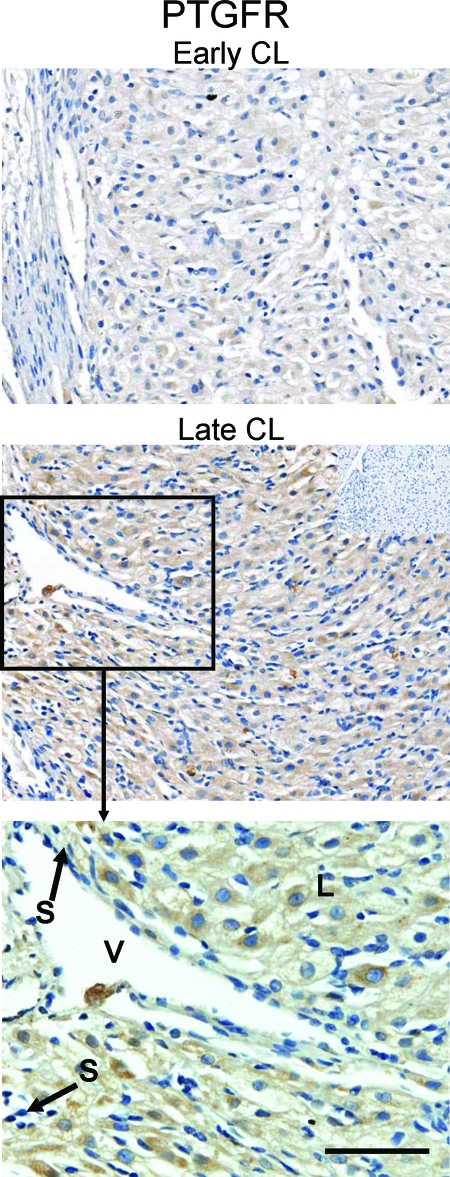
Concentrations of PTGER3 protein were significantly (P < 0.05) higher in the early and very-late stages compared with all other stages (Fig. 4, A and B). Staining for PTGER3 in sections of early CL (Fig. 5) was apparent in multiple cell types. PTGER3 was localized to large and small luteal/stromal cells as well as vascular endothelial cells. Interestingly, whereas PTGER3 staining was observed in large luteal cells throughout the luteal phase (data not shown), PTGER3 immunoreactivity in the vascular endothelial cells appeared to be virtually absent in very late CL (Fig. 5, bottom panel).
Figure 4.
PTGER3 protein levels in rhesus macaque CL obtained throughout the luteal phase. A, Representative Western blot for PTGER3 using pooled CL samples for each stage of the luteal phase. The upper image is PTGER3 and the lower corresponds to TUBB levels, which served as a loading control. The negative control is pooled early-stage CL probed with primary antibody that had been preabsorbed with its immunizing peptide. B, Mean level of expression as determined by densitometry. Levels of PTGER3 from individual CL (n = 4 CL/stage) were normalized to TUBB, and the resultant ratio was analyzed by ANOVA followed by comparison between groups using the Student-Newman-Keuls test. Error bars, 1 sem. Columns with different letters are significantly different (P < 0.05).
Figure 5.
Localization of PTGER3 protein in rhesus macaque CL. The first two images are from an early-stage CL section. The inset in the top image is an adjacent section that was probed with primary antibody that had been preabsorbed with immunizing peptide. The bottom image is PTGER3 staining in very late stage CL. Approximate locations of various cell types are indicated. L, Large luteal cells; S, small luteal or stromal cells; and V, blood vessel. Note the apparent decrease in staining of vascular endothelial cells (indicated by arrows) between the early and very late stages. The scale bar in the lower right hand corner of the higher magnification images, 50 μm.
Protein levels of HPGD
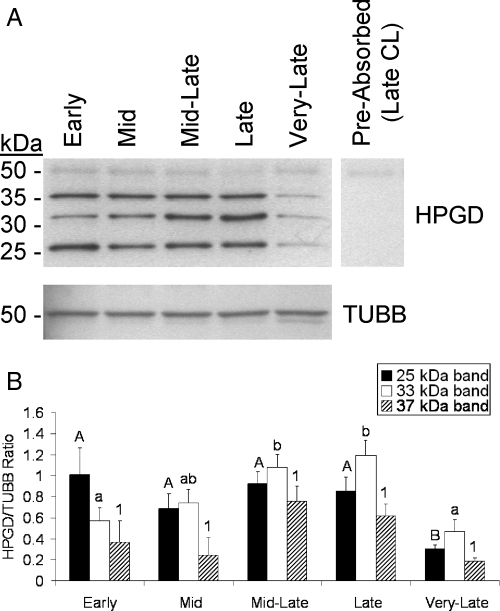
To consider whether prostaglandin degradation also takes place in the macaque CL, HPGD protein levels were determined by Western blot analysis. Three bands were detected at approximately 25, 33, and 37 kDa that likely correspond to various isoforms of HPGD (predicted molecular mass = 29 kDa, Fig. 6A). The detection of all three bands was prevented by preabsorption of the primary antibody with the immunizing peptide (Fig. 6A). Also, the intensity of all three bands was lower in the very-late stage compared with the mid-late or late stages, consistent with our previously reported mRNA levels of HPGD (30). Densitometry performed on the 33-kDa band revealed a steady increase from the early through late stages, with mid-late and late CL having about 2-fold higher (P < 0.05) levels than early or very late stage CL. Very late CL had lower (>2-fold, P < 0.05) levels of the 25 kDa isoform compared with all other stages, and the 37-kDa isoform displayed a similar pattern as the 25-kDa band although these changes were not statistically significant (Fig. 6B).
Figure 6.
HPGD protein levels throughout the rhesus macaque luteal phase. A, Representative Western blot for HPGD using pooled CL samples corresponding to each stage of the macaque luteal phase. The upper image is HPGD and the lower corresponds to TUBB, whereas the negative control is the pooled late CL sample probed with primary antibody that had been preabsorbed with its immunizing peptide. B, Densitometry data for each of the detected HPGD isoforms normalized to TUBB (n = 4 CL/stage). The resultant ratio was analyzed by ANOVA followed by comparison between groups using the Student-Newman-Keuls test. Error bars, 1 sem. Columns with different letters are significantly different (P < 0.05).
PTGFR protein expression and localization
Levels of PTGFR protein increased from the early through late stages with the late CL having higher (>3-fold, P < 0.05) PTGFR levels than either early or midstage CL (Fig. 7). Also, the mid-late and very late stages had higher (P < 0.05) PTGFR levels than the early stage. Immunohistochemical analysis revealed PTGFR expression in large luteal cells, with some staining evident in vascular endothelial cells (Fig. 8).
Figure 7.
PTGFR protein levels throughout the rhesus macaque luteal phase. A, Representative Western blot for PTGFR using pooled CL samples for each stage of the macaque luteal phase. PTGFR levels are shown in the upper image, whereas the levels of the internal control TUBB are shown in the lower image. The negative control is the pooled late CL sample probed with primary antibody that had been preabsorbed with its immunizing peptide. B, Mean level of PTGFR expression as determined by densitometry. Levels of PTGFR from individual CL (n = 4 CL/stage) were normalized to TUBB, and the resultant ratio was analyzed by ANOVA followed by comparison between groups using the Student-Newman-Keuls test. Error bars, 1 sem. Columns with different letters are significantly different (P < 0.05).
Figure 8.
Localization of PTGFR protein in rhesus macaque CL. Staining for PTGFR in early-stage (top panel) and late-stage (middle panel) CL as well as a higher magnification (bottom panel) for the late-stage CL. The inset in the middle panel is the negative control from an adjacent late stage section. Approximate locations of various cell types are indicated:. L, Large luteal cells; S, small luteal or stromal cells; V, blood vessel. The scale bar in the lower right hand corner of the bottom panel, 50 μm.
PGF2α synthase and PGE2/PGF2α reductase
The gene(s) representing PGF2α synthase (PGFS) in the primate is unclear. AKR1C3 was reported to possess PGFS activity in humans (40). AKR1B5, for which the human counterpart is AKR1B1, was reported as the PGFS in bovine endometrium (41). Also, some members of the AKR family (e.g. AKR1C1 and AKR1C2) have 9-keto prostaglandin reductase (9K-PGR) activity, which catalyzes the conversion of PGE2 into PGF2α (41,42). Therefore, mRNA expression of key members of the AKR family that may produce PGF2α via PGFS or 9K-PGR activity was determined in CL obtained throughout the luteal phase.
Messenger RNA levels of AKR1B1 and AKR1C3 (Fig. 9A) were determined through the analysis of a previously described DNA microarray database (30). There were no significant changes in mRNA levels of either gene from the early through late stages. Very late CL had higher (P < 0.05) mRNA levels of AKR1C3 and lower (P < 0.05) AKR1B1 mRNA levels compared with all other stages. Importantly, the relative expression values of AKR1B1 were 100- to 120-fold higher than AKR1C3 from the early through late stages, and were still approximately 17-fold higher in the very late stage (Fig. 9A).
Figure 9.
Intraluteal AKR family member 1B1, 1C1, 1C2, and 1C3 mRNA levels throughout the rhesus macaque luteal phase. A, Microarray expression data of two genes that possess PGFS activity, AKR1B1 and AKR1C3. Microarray data were analyzed by ANOVA followed by comparison between groups using the Student-Newman-Keuls test. Columns with different letters are significantly different (P < 0.05) with upper-case letters representing AKR1B1 mRNA levels and lower-case letters representing AKR1C3 mRNA levels. B, Microarray expression data for AKR1C1 and AKR1C2. C, Q-PCR results for AKR1C1 throughout the rhesus macaque luteal phase. Q-PCR data were analyzed by ANOVA followed by comparison between groups using the Student-Newman-Keuls test. Columns with different letters are significantly different (P < 0.05). The error bars (A–C), 1 sem.
Levels of mRNA for AKR1C1 and the closely related AKR1C2, which are likely responsible for 9K-PGR activity in the primate CL, did not change significantly during the luteal phase as determined by microarray (Fig. 9B). However, both genes displayed a similar pattern with a greater than 2-fold drop from the early to midstage (Fig. 9B). Q-PCR revealed a similar pattern of AKR1C1 expression to that observed by microarray, whereby the midstage CL had significantly (P < 0.05) less AKR1C1 mRNA than all other stages (Fig. 9C).
Discussion
This report details for the first time the expression of mRNA or proteins in the primate CL that are critical for the synthesis, metabolism, and signaling of prostaglandins. Arachidonic acid is converted to PGH2, the common intermediate for production of PGE2 and PGF2α, by the actions of PTGS1 and PTGS2. By microarray (30) and Q-PCR (data not shown) analysis, PTGS1 mRNA levels were minimal and did not change throughout the macaque luteal phase. In contrast, PTGS2 expression was dependent on the stage of the luteal phase, with peak mRNA (30) and protein levels (current study) occurring during the early to midstages of the macaque luteal phase. These results are consistent with reports from other species in terms of PTGS1 being constitutively expressed, whereas PTGS2 represents the inducible form (42). However, our results indicate there may be a significant discrepancy between primate and nonprimate species in the timing of PTGS2 expression during the luteal phase. In a similar study performed in the bovine (43), peak mRNA and protein levels of PTGS2 occurred in mature and regressing CL, which were significantly higher than in the developing CL. Thus, in the primate, it appears that the capacity for luteal PGH2 synthesis may be highest during luteinization and maintenance of the CL, but in nonprimate species luteal PGH2 production may be highest during maintenance and luteolysis.
Once PGH2 is formed, it can be converted to PGE2 by the actions of PTGES. PTGES mRNA (30) and protein (current study) was highest in mid CL, with moderately high levels of expression occurring in early CL as well, approximately coinciding with peak expression of PTGS2. These results regarding the timing of PTGES expression during the rhesus macaque luteal phase are similar to a report detailing its expression in the bovine CL (43). It has been estimated that PTGES is 150-fold more efficient at converting PGH2 to PGE2 compared with its conversion to PGF2α by PGFS (41,44). These results indicate the primate CL may preferentially produce PGE2 during the early to midstages of the luteal phase.
Expression of PTGS2 and PTGES was found primarily in the large luteal cells as determined by IHC analysis. These findings are similar to those involving nonprimate species in that expression of PTGS2 and PTGES have been found to be selectively expressed in bovine large luteal cells (43). The small number of cells located proximally to blood vessels, which retained strong PTGES staining in the very late stage, appear to be large luteal cells due to the high cytoplasmic to nuclear ratio, although other cell types (e.g. immune cells) may be present. Dual-labeling experiments would be needed to confirm the cell types represented. Collectively, these studies indicate that large luteal cells may be responsible for intraluteal PGE2 production.
There are four subtypes of PGE2 receptors that regulate different intracellular signaling pathways (39). PGE2 receptors belong to the G protein-coupled receptor superfamily. PTGER2 and PTGER4 are adenylate cyclase-coupled receptors that increase cAMP and protein kinase A signaling. PTGER1 activates phospholipase C, which ultimately causes protein kinase C activation. PTGER3 has multiple isoforms arising from alternative splicing that affect different signaling pathways including: reduced cAMP synthesis (Gi-coupled), protein kinase C activation, or protein kinase A activation (39). Because PTGER3 had the highest relative mRNA expression levels as determined by microarray and Q-PCR, as well as the finding that PTGER3 had the greatest fold-change in mRNA expression of all PGE2 receptor subtypes analyzed (30), it may be an important mediator of PGE2 effects in the primate CL. Thus, we focused on PTGER3 protein localization and levels in the rhesus macaque CL through the luteal phase.
PTGER3 protein levels were highest in the early CL, consistent with a potential role of PGE2 in luteinization. Staining for PTGER3 was present in large and small luteal/stromal cells as well as vascular endothelial cells. Thus, effects of PGE2 may include modulation of steroidogenesis (large and small luteal cells), and the regulation of angiogenesis and/or vascular stability (vascular endothelial cells). Identifying the signaling pathway activated by PTGER3 within the primate CL will require further research. High PTGER3 expression in the early CL of the primate is different from what was reported in the bovine CL in which PTGER3 mRNA was highest during periods of maintenance and luteolysis (43). Also, whereas PTGER2 mRNA and protein was significantly higher during development and maintenance of the bovine CL compared with luteolysis (43), the present study found that PTGER2 mRNA levels in the primate CL did not change from the early through late stages, and its absolute mRNA levels were the lowest of all the PGE2 receptor subtypes. As such, PGE2 receptor subtype expression in the CL during the luteal phase may vary between primate and nonprimate species.
Metabolism of PGE2 and PGF2α into inactive PGE and PGF metabolites, respectively, occurs via HPGD (45). Previously we reported that mRNA for HPGD was highest in the mid-late and late CL and lowest in very late CL (30). In the current study, multiple potential isoforms of HPGD were detected. All potential protein isoforms had their lowest expression in the very late stage, consistent with HPGD mRNA expression levels. One isoform (33 kDa) increased in expression from the early through late stages, which is consistent with the mRNA results. All isoforms appeared to have significant levels of expression during the mid-late to late stages of the luteal phase, which also coincides with peak mRNA levels. Thus, HPGD activity may serve as an important determinant of prostaglandin levels by counteracting their synthesis within the primate CL.
Intraluteal infusion of PGF2α analogs causes luteolysis in monkeys and women as indicated by decreased circulating progesterone concentrations and premature menses (13,25,26,27,28,29). Additionally, exogenous estradiol administration, which inhibits progesterone production by the primate CL, increases concentrations of PGF2α in the ovarian vein and cotreatment with the prostaglandin synthesis inhibitor indomethacin blocks the antisteroidogenic effect of estradiol indicating that PGF2α mediates estradiol-induced luteolysis (46). Collectively these studies indicate that the receptor and signaling systems necessary for PGF2α-mediated luteolysis are both present in the primate CL. This is consistent with our findings that mRNA (30) and protein (current study) levels of PTGFR are highest in late CL just before or during functional regression. The large luteal cells may be the target of PGF2α actions as indicated by IHC analysis, which is consistent with the reported selectivity of PGF2α binding to large luteal cells in the ovine (47). However, there is no uterine signal initiating luteolysis in primates (23,24). If PGF2α is the signal initiating luteolysis in primates, as occurs in other species, this raises the question as to what is the source of PGF2α during spontaneous luteolysis? One possibility is that intraluteal production of PGF2α results in luteolysis of the primate CL through autocrine/paracrine activities (1,3).
Earlier studies have quantified PGF2α levels in the primate CL, with higher concentrations measured in the late luteal phase relative to the early luteal phase in both women (48,49,50) and monkeys (51). Thus, it appears that PGF2α is present in the late luteal phase, even though PTGS2 mRNA and protein levels are low, whereas HPGD mRNA and protein expression is high in the CL during this time. A mechanism whereby PGF2α could be produced in the primate CL may involve the 9K-PGR and/or PGFS activity of various AKR family members. For example, 9K-PGR can convert PGE2 to PGF2α (41,42) and is likely the same enzyme as 20α-hydroxysteroid dehydrogenase (52,53). Genes whose corresponding proteins have been identified as possessing 9K-PGR activity include AKR1C1 and AKR1C2 (41,42), and those with PGFS activity include AKR1C3 (40) and AKR1B1 (the homolog of AKR1B5 that is the PGFS in bovine endometrium) (41). mRNA for these genes was measured throughout the luteal phase, with relatively high levels of AKR1C1, AKR1C2, and AKR1B1 detected in the late luteal phase. Therefore, further research is needed to determine whether their mRNA levels can be extrapolated to protein levels and enzyme activity, which may result in intraluteal production of PGF2α. Interestingly, it has previously been reported that in dispersed luteal cells obtained from rhesus macaque CL, PGF2α production decreased from the early to midluteal phase, with late luteal phase PGF2α production returning to early luteal phase levels (54). This pattern of PGF2α synthesis is similar to results from the current study in that AKR1B1, AKR1C1, and AKR1C2 mRNA levels were lower in midstage CL compared with early, mid-late, or late stage CL (P < 0.05 for AKR1C1 as determined by Q-PCR). Additionally, early luteal phase CL may not be responsive to PGF2α due to low PTGFR levels, whereas increasing levels of PTGFR in the late luteal phase may make the CL more sensitive to intraluteal PGF2α synthesis.
This report provides the first comprehensive analysis of the expression of genes involved in PGE2 and PGF2α synthesis, metabolism, and signaling in the primate CL throughout the luteal phase of the natural menstrual cycle. Based on data reported herein and previously (30), we hypothesize that during development and maintenance of the primate CL, the CL will synthesize (PTGS2 and PTGES) and respond (PTGER3) to PGE2, indicating that PGE2 may be important for development and maintenance of the primate CL. Furthermore, we hypothesize that there is a switch in the primate CL from an environment that favors PGE2 synthesis and signaling during the early and midluteal phase to one that supports PGF2α synthesis and signaling as the CL nears the time of luteolysis, indicating a role for PGF2α in the regression of the primate CL.
It has previously been reported that PGE2 stimulated progesterone production from primate luteal cells isolated between 4 and 7 d (early to midstages of present study) after the LH surge, and PGE2 was 10-fold more potent than PGF2α in stimulating P4 synthesis at this stage (16). However, by d 8–10 of the luteal phase (mid to mid-late stages of present study), neither prostaglandin stimulated progesterone production and both PGE2 and PGF2α inhibited human chorionic gonadotropin (hCG)-stimulated progesterone secretion (16). Similar results were reported from a study involving human CL slices incubated in the presence of PGE2 or PGF2α, in which PGE2 was found to stimulate cAMP formation in early but not midluteal phase CL. In contrast, PGF2α inhibited hCG-stimulated cAMP and progesterone accumulation in mid but not early luteal phase CL (15).
These in vitro results are consistent with findings in the present study as the stimulatory effect of PGE2 on steroidogenesis occurred approximately when the highest expression of proteins necessary for PGE2 synthesis (PTGS2, PTGES) and signaling (PTGER3) was observed, and the loss of PGE2-stimulated progesterone production occurred when the levels of these proteins were decreasing. Additionally, the anti-steroidogenic effects of either PGE2 or PGF2α did not occur until the approximate time when significantly increasing levels of PTGFR were detected. The ability of PGE2 to inhibit hCG-stimulated steroidogenesis in luteal cells obtained from CL isolated 8–10 d after the midcycle surge, which is in contrast to its stimulatory effect on luteal cells obtained earlier in the luteal phase, could be due to a change in PGE2 receptor subtype expression. Alternatively, such effects may be the result of PGE2 conversion to PGF2α and subsequent activation of PTGFR. Such possibilities will be tested directly in future studies.
These data complement previous in vitro studies and support the possibility of a physiological role for prostaglandins to either promote or inhibit luteal function at different stages of the primate luteal life span. These data are also important for designing future studies that will elucidate the role of PGE2 and PGF2α in regulating the structure and function of the primate CL.
Acknowledgments
The authors thank the following core facilities at the Oregon National Primate Research Center: Molecular and Cellular Biology Core (Drs. Eliot Spindel and Yibing Ja) and the Imaging and Morphology Core (Dr. Anda Cornea). Thanks also to Dr. Ov Slayden, who provided the microscope and camera setup for capturing digital photomicrographs as well as technical assistance on their use.
Footnotes
This research was supported by National Institutes of Health Grants R01 HD20869 (to R.L.S.), U54 HD18185 (to R.L.S.), R01 HD42000 (to J.D.H.), U54 HD55744 (to J.D.H., R.L.S.), RR00163 (to J.D.H., R.L.S.), and T32 Training Grant HD007133 (to R.L.B.).
First Published Online July 17, 2008
Abbreviations: AKR, Aldoketo reductase; CL, corpus luteum; hCG, human chorionic gonadotropin; HPGD, 15-(NAD)-hydroxyprostaglandin dehydrogenase; IHC, immunohistochemistry; 9K-PGR, 9-keto prostaglandin reductase; PG, prostaglandin; PGFS, PGF2α synthase; PTGER3, PGE2 receptor 3; PTGES, PGE2 synthase-1; PTGFR, PGF2α receptor; PTGS2, prostaglandin-endoperoxide synthase 2; Q-PCR, quantitative real-time PCR; RMA, robust multichip analysis; TUBB, β-tubulin.
References
- Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW 2000 Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev 80:1–29 [DOI] [PubMed] [Google Scholar]
- Smith MF, McIntush EW, Smith GW 1994 Mechanisms associated with corpus luteum development. J Anim Sci 72:1857–1872 [DOI] [PubMed] [Google Scholar]
- Stouffer RL 2006 Structure, function, and regulation of the corpus luteum. In: Knobil E, Neill JD, eds. The physiology of reproduction. New York: Raven; 475–526 [Google Scholar]
- Stouffer RL 2003 Progesterone as a mediator of gonadotrophin action in the corpus luteum: beyond steroidogenesis. Hum Reprod Update 9:99–117 [DOI] [PubMed] [Google Scholar]
- Hoyer PB 1998 Regulation of luteal regression: the ewe as a model. J Soc Gynecol Investig 5:49–57 [DOI] [PubMed] [Google Scholar]
- Davis JS, Rueda BR 2002 The corpus luteum: an ovarian structure with maternal instincts and suicidal tendencies. Front Biosci 7:d1949–d1978 [DOI] [PubMed] [Google Scholar]
- McCracken JA, Custer EE, Lamsa JC 1999 Luteolysis: a neuroendocrine-mediated event. Physiol Rev 79:263–323 [DOI] [PubMed] [Google Scholar]
- Pratt BR, Butcher RL, Inskeep EK 1977 Antiluteolytic effect of the conceptus and of PGE2 in ewes. J Anim Sci 45:784–791 [DOI] [PubMed] [Google Scholar]
- Magness RR, Huie JM, Hoyer GL, Huecksteadt TP, Reynolds LP, Seperich GJ, Whysong G, Weems CW 1981 Effect of chronic ipsilateral or contralateral intrauterine infusion of prostaglandin E2 (PGE2) on luteal function of unilaterally ovariectomized ewes. Prostaglandins Med 6:389–401 [DOI] [PubMed] [Google Scholar]
- Henderson KM, Scaramuzzi RJ, Baird DT 1977 Simultaneous infusion of prostaglandin E2 antagonizes the luteolytic action of prostaglandin F2α in vivo. J Endocrinol 72:379–383 [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Stigler J, Hoyer GL, Magness RR, Huie JM, Huecksteadt TP, Whysong GL, Behrman HR, Weems CW 1981 Effect of PGE1 on PGF2 alpha-induced luteolysis in nonbred ewes. Prostaglandins 21:957–972 [DOI] [PubMed] [Google Scholar]
- Pate JL 2003 Lives in the balance: responsiveness of the corpus luteum to uterine and embryonic signals. Reprod Suppl 61:207–217 [PubMed] [Google Scholar]
- Zelinski-Wooten MB, Stouffer RL 1990 Intraluteal infusions of prostaglandins of the E, D, I, and A series prevent PGF2α-induced, but not spontaneous, luteal regression in rhesus monkeys. Biol Reprod 43:507–516 [DOI] [PubMed] [Google Scholar]
- Hahlin M, Dennefors B, Johanson C, Hamberger L 1988 Luteotropic effects of prostaglandin E2 on the human corpus luteum of the menstrual cycle and early pregnancy. J Clin Endocrinol Metab 66:909–914 [DOI] [PubMed] [Google Scholar]
- Dennefors BL, Sjogren A, Hamberger L 1982 Progesterone and adenosine 3′,5′-monophosphate formation by isolated human corpora lutea of different ages: influences of human chorionic gonadotropin and prostaglandins. J Clin Endocrinol Metab 55:102–107 [DOI] [PubMed] [Google Scholar]
- Stouffer RL, Nixon WE, Hodgen GD 1979 Disparate effects of prostaglandins on basal and gonadotropin-stimulated progesterone production by luteal cells isolated from rhesus monkeys during the menstrual cycle and pregnancy. Biol Reprod 20:897–903 [DOI] [PubMed] [Google Scholar]
- Sargent EL, Baughman WL, Novy MJ, Stouffer RL 1988 Intraluteal infusion of a prostaglandin synthesis inhibitor, sodium meclofenamate, causes premature luteolysis in rhesus monkeys. Endocrinology 123:2261–2269 [DOI] [PubMed] [Google Scholar]
- Critser ES, Rutledge JJ, French LR 1981 Effect of indomethacin on the interestrous interval of intact and hysterectomized pseudopregnant mice. Biol Reprod 24:1000–1005 [DOI] [PubMed] [Google Scholar]
- Lau IF, Saksena SK, Chang MC 1975 Effect of indomethacin, an inhibitor of prostaglandin biosynthesis on the length of pseudopregnancy in rats and hamsters. Acta Endocrinol (Copenh) 78:343–348 [DOI] [PubMed] [Google Scholar]
- Pharriss BB, Wyngarden LJ 1969 The effect of prostaglandin F2α on the progestogen content of ovaries from pseudopregnant rats. Proc Soc Exp Biol Med 130:92–94 [DOI] [PubMed] [Google Scholar]
- Scaramuzzi RJ, Baird DT 1976 The oestrous cycle of the ewe after active immunization against prostaglandin F-2α. J Reprod Fertil 46:39–47 [DOI] [PubMed] [Google Scholar]
- Fairclough RJ, Smith JF, McGowan LT 1981 Prolongation of the oestrous cycle in cows and ewes after passive immunization with PGF antibodies. J Reprod Fertil 62:213–219 [DOI] [PubMed] [Google Scholar]
- Castracane VD, Moore GT, Shaikh AA 1979 Ovarian function of hysterectomized Macaca fascicularis. Biol Reprod 20:462–472 [DOI] [PubMed] [Google Scholar]
- Ranney B, Abu-Ghazaleh S 1977 The future function and fortune of ovarian tissue which is retained in vivo during hysterectomy. Am J Obstet Gynecol 128:626–634 [DOI] [PubMed] [Google Scholar]
- Auletta FJ, Speroff L, Caldwell BV 1973 Prostaglandin F-2 induced steroidogenesis and luteolysis in the primate corpus luteum. J Clin Endocrinol Metab 36:405–407 [DOI] [PubMed] [Google Scholar]
- Wentz AC, Jones GS 1973 Transient luteolytic effect of prostaglandin F2α in the human. Obstet Gynecol 42:172–181 [PubMed] [Google Scholar]
- Kirton KT, Pharriss BB, Forbes AD 1970 Luteolytic effects of prostaglandin F2α in primates. Proc Soc Exp Biol Med 133:314–316 [DOI] [PubMed] [Google Scholar]
- Auletta FJ, Kamps DL, Pories S, Bisset J, Gibson M 1984 An intra-corpus luteum site for the luteolytic action of prostaglandin F2α in the rhesus monkey. Prostaglandins 27:285–298 [DOI] [PubMed] [Google Scholar]
- Bennegard B, Hahlin M, Wennberg E, Noren H 1991 Local luteolytic effect of prostaglandin F2α in the human corpus luteum. Fertil Steril 56:1070–1076 [PubMed] [Google Scholar]
- Bogan RL, Murphy MJ, Stouffer RL, Hennebold JD 2008 Systematic determination of differential gene expression in the primate corpus luteum during the luteal phase of the menstrual cycle. Mol Endocrinol 22:1260–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy DM, Wells TR, Haluska GJ, Stouffer RL 1997 The ratio of progesterone receptor isoforms changes in the monkey corpus luteum during the luteal phase of the menstrual cycle. Biol Reprod 57:693–699 [DOI] [PubMed] [Google Scholar]
- Duffy DM, Chaffin CL, Stouffer RL 2000 Expression of estrogen receptor α and β in the rhesus monkey corpus luteum during the menstrual cycle: regulation by luteinizing hormone and progesterone. Endocrinology 141:1711–1717 [DOI] [PubMed] [Google Scholar]
- Hazzard TM, Christenson LK, Stouffer RL 2000 Changes in expression of vascular endothelial growth factor and angiopoietin-1 and -2 in the macaque corpus luteum during the menstrual cycle. Mol Hum Reprod 6:993–998 [DOI] [PubMed] [Google Scholar]
- Tesone M, Stouffer RL, Borman SM, Hennebold JD, Molskness TA 2005 Vascular endothelial growth factor (VEGF) production by the monkey corpus luteum during the menstrual cycle: isoform-selective messenger RNA expression in vivo and hypoxia-regulated protein secretion in vitro. Biol Reprod 73:927–934 [DOI] [PubMed] [Google Scholar]
- Xu J, Hennebold JD, Stouffer RL 2006 Dynamic expression and regulation of the corticotropin-releasing hormone/urocortin-receptor-binding protein system in the primate ovary during the menstrual cycle. J Clin Endocrinol Metab 91:1544–1553 [DOI] [PubMed] [Google Scholar]
- Young KA, Tumlinson B, Stouffer RL 2004 ADAMTS-1/METH-1 and TIMP-3 expression in the primate corpus luteum: divergent patterns and stage-dependent regulation during the natural menstrual cycle. Mol Hum Reprod 10:559–565 [DOI] [PubMed] [Google Scholar]
- Rogge CE, Liu W, Wu G, Wang LH, Kulmacz RJ, Tsai AL 2004 Identification of Tyr504 as an alternative tyrosyl radical site in human prostaglandin H synthase-2. Biochemistry 43:1560–1568 [DOI] [PubMed] [Google Scholar]
- Pestili de Almeida EM, Piche C, Sirois J, Dore M 2001 Expression of cyclo-oxygenase-2 in naturally occurring squamous cell carcinomas in dogs. J Histochem Cytochem 49:867–875 [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Narumiya S 2007 Prostaglandin E receptors. J Biol Chem 282:11613–11617 [DOI] [PubMed] [Google Scholar]
- Jez JM, Flynn TG, Penning TM 1997 A new nomenclature for the aldo-keto reductase superfamily. Biochem Pharmacol 54:639–647 [DOI] [PubMed] [Google Scholar]
- Madore E, Harvey N, Parent J, Chapdelaine P, Arosh JA, Fortier MA 2003 An aldose reductase with 20α-hydroxysteroid dehydrogenase activity is most likely the enzyme responsible for the production of prostaglandin F2α in the bovine endometrium. J Biol Chem 278:11205–11212 [DOI] [PubMed] [Google Scholar]
- Wiltbank MC, Ottobre JS 2003 Regulation of intraluteal production of prostaglandins. Reprod Biol Endocrinol 1:91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arosh JA, Banu SK, Chapdelaine P, Madore E, Sirois J, Fortier MA 2004 Prostaglandin biosynthesis, transport, and signaling in corpus luteum: a basis for autoregulation of luteal function. Endocrinology 145:2551–2560 [DOI] [PubMed] [Google Scholar]
- Thoren S, Weinander R, Saha S, Jegerschold C, Pettersson PL, Samuelsson B, Hebert H, Hamberg M, Morgenstern R, Jakobsson PJ 2003 Human microsomal prostaglandin E synthase-1: purification, functional characterization, and projection structure determination. J Biol Chem 278:22199–22209 [DOI] [PubMed] [Google Scholar]
- Tai HH, Ensor CM, Tong M, Zhou H, Yan F 2002 Prostaglandin catabolizing enzymes. Prostaglandins Other Lipid Mediat 68–69:483–493 [DOI] [PubMed] [Google Scholar]
- Auletta FJ, Flint AP 1988 Mechanisms controlling corpus luteum function in sheep, cows, nonhuman primates, and women especially in relation to the time of luteolysis. Endocr Rev 9:88–105 [DOI] [PubMed] [Google Scholar]
- Fitz TA, Mayan MH, Sawyer HR, Niswender GD 1982 Characterization of two steroidogenic cell types in the ovine corpus luteum. Biol Reprod 27:703–711 [DOI] [PubMed] [Google Scholar]
- Patwardhan VV, Lanthier A 1980 Concentration of prostaglandins PGE and PGF, estrone, estradiol, and progesterone in human corpora lutea. Prostaglandins 20:963–969 [DOI] [PubMed] [Google Scholar]
- Vijayakumar R, Walters WA 1983 Human luteal tissue prostaglandins, 17β-estradiol, and progesterone in relation to the growth and senescence of the corpus luteum. Fertil Steril 39:298–303 [DOI] [PubMed] [Google Scholar]
- Shutt DA, Clarke AH, Fraser IS, Goh P, McMahon GR, Saunders DM, Shearman RP 1976 Changes in concentration of prostaglandin F and steroids in human corpora lutea in relation to growth of the corpus luteum and luteolysis. J Endocrinol 71:453–454 [DOI] [PubMed] [Google Scholar]
- Balmaceda J, Asch RH, Fernandez EO, Valenzuela G, Eddy CA, Pauerstein CJ 1979 Prostaglandin production by rhesus monkey corpora lutea in vitro. Fertil Steril 31:214–216 [PubMed] [Google Scholar]
- Wintergalen N, Thole HH, Galla HJ, Schlegel W 1995 Prostaglandin-E2 9-reductase from corpus luteum of pseudopregnant rabbit is a member of the aldo-keto reductase superfamily featuring 20α-hydroxysteroid dehydrogenase activity. Eur J Biochem 234:264–270 [DOI] [PubMed] [Google Scholar]
- Asselin E, Fortier MA 2000 Detection and regulation of the messenger for a putative bovine endometrial 9-keto-prostaglandin E(2) reductase: effect of oxytocin and interferon-τ. Biol Reprod 62:125–131 [DOI] [PubMed] [Google Scholar]
- Houmard BS, Ottobre JS 1989 Progesterone and prostaglandin production by primate luteal cells collected at various stages of the luteal phase: modulation by calcium ionophore. Biol Reprod 41:401–408 [DOI] [PubMed] [Google Scholar]