Abstract
17β-Estradiol (E2) activates the estrogen receptor (ER) through multiple genomic and nongenomic pathways in various tissues/organs. ERα/specificity protein-dependent activation of E2-responsive genes containing GC-rich promoters has been identified in breast and other cancer cell lines, and in this study, we describe transgenic animals overexpressing a transgene containing three tandem GC-rich sites linked to a minimal TATA or thymidine kinase promoter and a luciferase gene. Several mouse lines expressing the transgenes were characterized and, in line 15, E2 induced a 9-fold increase in luciferase activity in the female mouse uterus, and the synthetic estrogens bisphenol A and nonylphenol also induced uterine luciferase activity. The pure antiestrogen ICI 182,780 induced luciferase activity in the mouse uterus, and similar results were observed for ICI 182,780 in breast cancer cells transfected with this construct. Differences in the ER agonist and antagonist activities of E2, nonylphenol, bisphenol A, and ICI 182,780 were investigated in the male testis and penis and the male and female stomach in line 15 transgenic mice. All of these tissues were hormone responsive; however, the patterns of induced or repressed luciferase activity were ligand structure, tissue, and sex dependent. These results demonstrate for the first time hormonal activation or repression of a GC-rich promoter in vivo, and the results suggest that the ERα/specificity protein pathway may contribute to E2-dependent induction and repression of genes.
17β-ESTRADIOL (E2) AND related steroidal estrogens play a critical role in development of the male and female reproductive tract, vascular physiology, skeletal development and growth, and neuronal function (1,2,3,4). Estrogens also play a role in hormone-dependent diseases, and epidemiological studies show that lifetime exposure to estrogen is a risk factor for development of breast cancer in women (5,6,7,8,9). Because many early-stage mammary tumors are estrogen receptor (ER) positive, these tumors respond to endocrine therapy and treatment with antiestrogens such as tamoxifen and raloxifene or with aromatase inhibitors (10,11). Moreover, recent epidemiological studies arising from the Women’s Health Initiative demonstrate that hormone replacement therapy for postmenopausal women can also lead to adverse health effects, including decreased cognitive function, possible dementia, and increased risk for venous thrombosis and strokes (12,13,14).
The classical mechanism of ER-mediated transactivation requires interaction of the ligand bound nuclear ER dimer with estrogen-responsive elements (EREs) in target gene promoters (15,16,17,18). However, genomic mechanisms of ER-mediated transactivation are complex and involve interactions of ER with multiple ERE half-sites and with other DNA-bound transcription factors such as nuclear factor-κB (NFκB), GATA, activation protein-1 (AP1), and specificity protein 1 (Sp1) (19,20,21,22). In addition to these genomic pathways of ER action, there is evidence that cytosolic/membrane-bound ER interacts with many other proteins to mediate hormone-dependent activation of several kinase pathways that in turn modulate gene expression (23,24,25,26). Research in this laboratory first observed that the proximal GC-rich site alone in the Hsp27 promoter was estrogen responsive, and similar results have been obtained using pSp1 and pSp13, which are constructs containing one or three consensus GC-rich Sp1 binding sites linked to luciferase (27,28,29). Subsequent studies have identified a large number of hormone-responsive genes in breast cancer cells and other cell lines that are regulated by ERα/Sp1 (22,30,31,32,33,34,35,36,37,38,39,40,41,42). RNA interference using a small inhibitory RNA for Sp1 (iSp1) showed that iSp1 inhibited basal and E2-induced G0/G1→S phase cell cycle progression, indicating that ERα/Sp1-dependent genes play an important role in ER-positive breast cancer cell proliferation (43).
Mechanistic studies in breast cancer cell lines show that ligand-dependent activation of GC-rich promoter constructs and their corresponding genes is complex (29,44,45). For example, a recent study shows that VEGF receptor 2 (VEGFR2, KDR) is expressed in ZR-75 breast cancer cells and induced by E2 (46). Promoter analysis showed that two proximal GC-rich sites were required for transactivation, and RNA interference with iSp1, iSp3, and iSp4 shows that Sp3 and Sp4 but not Sp1 are required for hormone-dependent activation of VEGFR2. In this study, we have generated transgenic mice expressing a construct containing three tandem GC-rich sites linked to a luciferase reporter gene, and we have used this animal model to investigate tissue-specific modulation of luciferase activity by estrogenic compounds. E2, the antiestrogen ICI 182,780, and the synthetic industrial estrogens bisphenol A (BPA) and nonylphenol (NP) exhibit structure-dependent, sex- and tissue-specific effects on increased or repressed luciferase activity and confirms that the ERα/Sp pathway is functional in vivo.
Materials and Methods
Reagents and plasmids
Fetal bovine serum (FBS) was obtained from JRH Biosciences (Lenexa, KS). Antibiotic antimycotic solution (×100) was obtained from Sigma-Aldrich (St. Louis, MO). The following test chemicals (and purities) were purchased from Sigma-Aldrich: p-NP (98%) and BPA (>99%), 4-hydroxytamoxifen (≥98%), E2 (≥98%), resveratrol (>99%). ICI 182,780 was provided by Dr. Alan Wakeling (Astra-Zeneca, Macclesfield, UK). Plasmid preparation kits were purchased from Sigma. Human ERα expression plasmid was kindly provided by Dr. Ming-Jer Tsai (Baylor College of Medicine, Houston, TX).
Cells and transient transfection assays
ZR-75 human breast cancer cell were obtained from American Type Culture Collection (Manassas, VA) and were maintained in RPMI 1640 media (Sigma-Aldrich) supplemented with 1.5 g/liter sodium bicarbonate, 2.38 g/liter HEPES, 0.11 g/liter sodium pyruvate, 4.5 g/liter glucose, 10% FBS, and 5 ml/liter antibiotic antimycotic solution at pH 7.4. Cells were cultured and grown in a 37 C incubator with humidified 5% CO2, 95% air. For transient transfection studies, ZR-75 cells were seeded in 12-well plates in DMEM/F-12 medium without phenol red supplemented with 2.2 g/liter sodium bicarbonate and 2.5% charcoal-stripped FBS. After 24 h, cells were transfected using the calcium phosphate method with 350 ng luciferase reporter construct, 100 ng pcDNA3/His/lacZ (Invitrogen, Carlsbad, CA) as a standard reference for transfection efficiency, and increasing amounts of the appropriate ERα expression plasmid. Six hours after transfection, cells were shocked with 25% glycerol/PBS for 1 min, washed with PBS, and then treated with dimethylsulfoxide (DMSO) solvent or 10 nm E2 in DMSO for another 30–48 h. Cells were then washed twice in PBS and harvested with 100 μl reporter lysis buffer (Promega Corp., Madison, WI). After one freeze-thaw cycle, cell lysates were centrifuged for 1 min at 16,000 × g, and the supernatant was used for determination of protein activity. Luciferase (Promega) and β-galactosidase activities were determined using the Tropix Galacto-Light Plus assay system (Tropix, Bedford, MA). Light emission was detected on a LumiCount micro-well plate reader (Packard Instruments, Meriden, CT), and luciferase activity was calculated by normalizing against β-galactosidase activity obtained from the same sample and compared with the DMSO control group (set at 100%) for each set of experiments.
Experiment animals
Animal care and experiments were approved by Texas A&M University Laboratory Animal Care Committee. All animals were housed on a 12-h light, 12-h dark cycle at 22–25 C and supplied with water and rodent chow diet ad libitum. Transgenic animals were generated by microinjection into single-cell-stage C57BL/6 embryos, and the embryos were implanted into pseudopregnant mice. Transgene-positive animals were used as founders to breed with wild-type C57BL/6 mice, and the offspring were used as experimental animals.
Transgenes construction
The (GC)3-TA-CAT reporter with (GC)3-TA-luc digest was made by inserting (GC)3-TA into pGL3-Basic vector (Promega) containing the firefly luciferase gene sequence. The pGL3-(GC)3-TA plasmid was digested with NheI and BamHI, and a 2-kb DNA fragment containing a 72-bp GC-rich site, a TATA box, and the firefly luciferase cDNA were purified by agarose gel electrophoresis. The DNA fragment was extracted using a gel purification kit (QIAGEN, Valencia, CA) and dissolved in the transgene buffer [10 mm Tris, 0.1 nm EDTA (pH 7.4)]. The thymidine kinase (TK) promoter was amplified by PCR from a TK-ERE-CAT plasmid using forward primer (5′-AAT AAG ATC TCC TAG GAT CCG GCC CC-3′) and reverse primer (5′-ATA CAA GCT TAT CTG CGG CAC GCT GT-3′), the TATA box of (GC)3-TA-Luc was replaced by digesting with the BglII and HindIII. The construct was linearized with NheI and SalI to release the TK-(GC)3-luc transgene cassette used to generate transgenic mice.
Genotyping
Mouse tail DNA for PCR analysis was prepared by the HotSHOT method (47). Briefly, a 2-mm tail clip is taken from 15-d-old mice and is placed in a PCR tube. DNA is released from the tissue by adding 75 μl base solution [25 mm NaOH, 0.2 mm EDTA (pH 12)] and heated at 95 C for 1 h, after which 75 μl neutralization solution (40 mm Tris-HCl, pH 5) was then added. PCR was used according to a universal PCR genotyping assay (48), which is sufficiently sensitive to detect a single copy of a transgene. Basically, a master mix was made, and for a single reaction, the mixture contained 2.545 μl water, 2.6 μl 5 m betaine (Sigma), 1 μl 10× buffer, 0.025 μl 20 mm deoxynucleotide triphosphate mix, 0.05 μl 1 mm Cresol Red (Sigma), 0.06 μl 5′-Fabpi primer (20 μm) (TGG ACA GGA CTG GAC CTC TGC TTT CCT AGA), 0.06 μl 3′-Fabpi primer (20 μm) (TAG AGC TTT GCC ACA TCA CAG GTC ATT CAG), 0.5 μl 5′-luciferase primer (20 μm) (5′-AGA CGC CAA AAA CAT AAA GAA AGG CCC GGC-3′), 0.5 μl 3′-luciferase primer (20 μm) (5′-TAT AAA TGT CGT TCG CGG GCG CAA CTG CAA-3′), 0.02 μl Klentaq LA (Clontech, Mountain View, CA), and 2 μl genomic DNA template. The total reaction volume is 10 μl. Primers for Fabpi were used as the loading control to monitor the quality of the genomic DNA and amplify a sequence from the intestinal fatty acid binding protein gene. PCRs are as follows: step 1, 93 C for 1 min; step 2, 93 C for 2 min; step 3, 68 C for 3 min; steps 2 and 3 repeated for a total of 30 cycles; and step 4, 4 C until samples are retrieved. After the PCR is finished, PCR samples are analyzed on 1% agarose gel at 120 V for 14 min in 0.8 mm Tris-acetate/ethylenediaminetetraacetic acid buffer.
Uterine wet weight assay
The 21-d-old heterozygous females were injected with 100 μl corn oil (vehicle control), or 50 μg/kg body weight (bw) E2, or 250 μg/kg ICI 182, 780, or 250 mg/kg BPA, or 250 mg/kg nonylphenol, or cotreated with ICI 182, 780 plus E2 for 3 d consecutively, and on d 4, animals were killed by CO2 asphyxiation. A relatively high dose of bisphenol A was selected due the insensitivity of this compound in the rodent uterine assay (48), and nonylphenol was administered at the same dose. After making an incision in the skin and the abdominal muscle, the uterine cervix was cut away from the vaginal fornix. Because fluid imbibition is an estrogen response, care was taken to retain all the uterine luminal fluid. The uterus was then removed by gently lifting tissue anteriorly and trimming away the mesometrium. A cut was made at the uterotubal junction, thus preserving the integrity of both uterine horns and avoiding loss of uterine fluid. The uterus was immediately weighed, and placed on absorbent cardboard to maintain the original in vivo orientation.
Luciferase assay
The 21-d-old heterozygous mice were treated on 3 d consecutively with sc injections of the different compounds dissolved in vehicle (corn oil). On d 4, animals were killed by CO2 asphyxiation, and organs were immediately removed. Tissue extracts were homogenized and sonicated using a Pro200 micro-homogenizer (Pro Scientific, Oxford, CT) in 500 μl lysis buffer [1 mm dithiothreitol, 4 mm EGTA, 4 mm EDTA, 0.7 mm phenylmethylsulfonyl fluoride, 100 mm potassium phosphate (pH 7.8)]. After three freeze-thaw cycles by liquid nitrogen, cell lysates were centrifuged for 30 min at 16,000 × g, and the supernatant was used for determining protein activity. Luciferase activity was determined by measuring light emission using a LumiCount micro-well plate reader (Packard Instruments) over 10 sec, and luciferase activity was calculated by normalizing against protein concentration obtained from the same sample measured using the Bradford reagent (Bio-Rad, Hercules, CA).
In vivo luminescence imaging
The 21-d-old transgenic mice were visualized with a NightOwl imaging unit (Berthold Technologies, Bad Wildbad, Germany). The images were generated by a NightOwl image processor and analyzed by WinLight softerware (Berthold Technologies). Because the dark skin of C57BL/6 mice prevents detection of the luminescence signal, hybrid mice with a gray coat color resulting from breeding the C57BL/6 transgenic animals with FVB mice were used for in vivo imaging. Mice were anesthetized with 2% isoflurane using the anesthesia unit from Berthold and received ip injection of an aqueous solution of d-luciferin (beetle luciferin potassium salt; Promega; 25 mg/kg bw). After 20 min, the animals were placed in a dark imaging chamber under isoflurane anesthesia. A gray-scale image of the animals was first taken, and then photon emission was detected with a sensitive CCD camera. The image setting was exposure time 5 min and pixel binning 8 × 8 with cosmic suppression. For colocalization of the bioluminescent photon emission, gray-scale and pseudocolor images were overlaid using WinLight32 imaging software. Fifty micrograms per kilogram bw was injected sc for 3 d consecutively. Luminescence measurements are expressed as the average brightness (picowatts)/area (square millimeter) with sd for at least four animals per treatment group.
Statistical analysis
For transient transfection studies, results are expressed as means ± sd for at least three separate experiments for each treatment group. Statistical differences (P < 0.05) between control (DMSO) and treatment groups were determined by ANOVA and Scheffé’s test. For the luciferase assay of activity in various mouse tissues, results are expressed as the means of changes in compound-induced activity relative to the corn oil control (±sd) for at least seven animals per treatment group.
Results
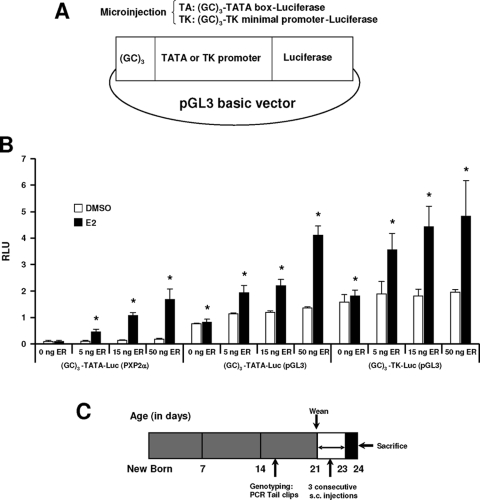
Previous reports show that E2 activates constructs containing GC-rich promoter inserts from multiple E2-responsive genes (22,30), and the pSp13 construct containing three tandem consensus GC-rich Sp1 binding sites has been used as a prototype for mechanistic studies (44,45). In this study, we generated two constructs containing three tandem GC-rich sites linked to a minimal TATA box (GC3-TATA-Luc) or TK promoter (GC3-TK-Luc) in the pGL3-Basic vector (Fig. 1A) and compared the E2 responsiveness of these plasmids to a comparable (GC)3-TATA-Luc (PXP2) construct (Fig. 1B). This latter construct in a PXP2 vector has been extensively used in previous in vitro studies (44,45). In ZR-75 breast cancer cells transfected with these constructs, treatment with 10 nm E2 did not induce luciferase activity. However, after cotransfection with 5, 15, or 50 ng ERα expression plasmid, significant induction by E2 was observed (Fig. 1B). These results are consistent with results of previous studies with GC-rich constructs in ZR-75 or MCF-7 cells, and the lack of hormonal activation in the absence of transfected ERα is due to limiting levels of endogenous ERα in these cells and overexpression of the constructs (22,30). Maximal induced activities were observed with the constructs in pGL3; however, fold induction was higher using (GC)3-TATA-Luc (PXP2) due to the low basal activity in the solvent (DMSO)-treated group.
Figure 1.
Transgene constructs and experimental time line. A, Two transgene constructs were generated by inserting three tandem GC-rich sequences with a TATA box or minimal TK promoter into the pGL3 basic luciferase vector. B, Transgene constructs are expressed and induced by E2 in breast cancer cells. ZR-75 cells were transfected with different reporter genes and increasing amount of ERα. (GC)3-TATA-Luc (PXP2), which has been extensively used in in vitro studies, was included as a control. Cells were treated with DMSO or 10 nm E2 and luciferase activity determined as described in Materials and Methods. *, Significant (P < 0.05) induction. C, Experimental time line. The 21-d-old animals were injected with different compounds sc for 3 d consecutively and killed 24 h after the last injection. Tissues were separated and homogenized, and luciferase activity was measured as outlined in Materials and Methods.
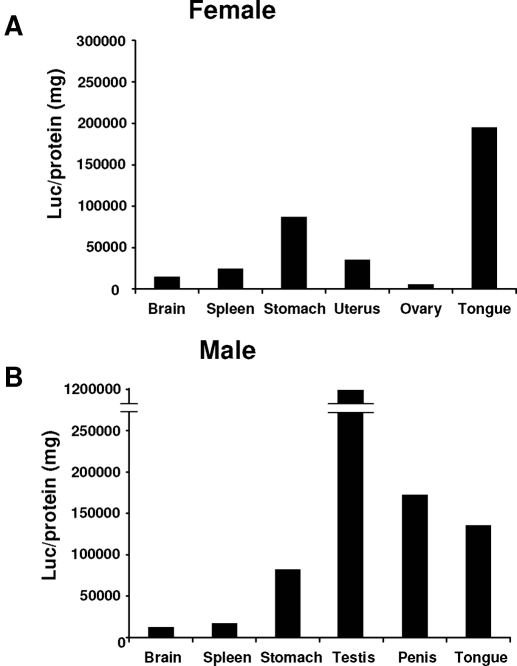
The linearized (GC)3-TATA-Luc and (GC)3-TK-Luc vectors were injected into oocytes, eight lines were isolated, and with the exception of line 17, all of the remaining lines were fertile. Luciferase expression in various tissues/organs were determined in 24-d-old mice using the experimental protocol illustrated in Fig. 1C. At 15 d of age, the mice were genotyped and then weaned at 21 d. Luciferase activity in both males and females from line 41 and line 42 was not detected, and activity was detected only in male testis of line 35. For estrogen responsiveness, the 21-d-old female and male mice were administered corn oil (10 ml/kg bw) or E2 (50 μg/kg bw) sc for 3 d consecutively and then killed 24 h after last treatment (Fig. 1C). Table 1 summarizes results of the initial screening studies for basal and E2-inducible expression of luciferase activity in brain, thymus, heart, lung, liver, spleen, pancreas, stomach, bladder, tongue, eye, bone, uterus/ovary (females only), and testis/penis (male only). In these studies, we used 21-d-old female and male mice, and at this age, endogenous E2 levels are low in females and they are not cycling. The results were highly variable not only among tissues/organs but also among the various mouse lines. Figure 2A illustrates luciferase activity in line 15 female mice where the highest expression was observed in tongue and stomach, and comparable responses were observed in male mice from this line (Fig. 2B). Brain and spleen luciferase activities were low in males and females. Uterine and ovarian luciferase activities were also low in females. In contrast, basal luciferase expression in line 15 penis and testis was high. Among the male mice in lines 11, 15, 16, and 21, basal luciferase activity was detected, and after treatment with E2, there was a decrease in activity. Similar results were observed in the penis in lines 11, 15, and 21. Luciferase activity was also observed in the brain and stomach in lines 11, 15, 16, and 21, and treatment with E2 did not affect brain luciferase activity in any of the animal lines, whereas in stomach, E2 decreased luciferase activity in females (lines 11, 15, and 16) or had no effects (line 21) and both increased (lines 15 and 21) and decreased (line 11 and 16) luciferase activity in male mice. In female mice, luciferase activity (basal and inducible) was not detected in the ovaries, whereas E2 induced activity in the uterus in lines 15, 16, and 21 but not in line 11. Basal or induced luciferase activity was not observed in the mammary gland using the Berthold NightOwl instrument (data not shown), and we are planning to target the mammary gland using a GC-luc construct with an MMTV promoter. Based on the magnitude of the basal and E2-induced uterine luciferase activity in line 15, we further used this transgenic animal for quantitatively determining ligand-dependent activation of the (GC)3-TATA-Luc construct in vivo.
Table 1.
Luciferase expression in transgenic lines
| Tissues | (GC)3-TATA-luciferase
|
(GC)3-TK-luciferase | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Line 11
|
Line 15
|
Line 16
|
Line 21
|
Line 35 | |||||
| ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | |
| Brain | (+)→ | (+)→ | (+)→ | (+)→ | (+)→ | (+)→ | (+)→ | (+)→ | (−) |
| Thymus | (−) | (−) | (−) | (−) | (+)→ | (+)→ | (+)↓ | (+)→ | (−) |
| Heart | (−) | (−) | (−) | (−) | (−) | (−) | (+)→ | (+)→ | (−) |
| Lung | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| Liver | (+)→ | (+)→ | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| Spleen | (−) | (−) | (+)→ | (+)→ | (−) | (−) | (−) | (−) | (−) |
| Kidney | (−) | (−) | (−) | (−) | (−) | (−) | (+)↓ | (+)↑ | (−) |
| Pancreas | (−) | (−) | (−) | (−) | (−) | (−) | (+)→ | (+)↑ | (−) |
| Stomach | (+)↓ | (+)↓ | (+)↑ | (+)↓ | (+)↓ | (+)↓ | (+)↑ | (+)→ | (−) |
| Bladder | (−) | (−) | (−) | (−) | (−) | (−) | (+)↑ | (+)↑ | (−) |
| Tongue | (−) | (−) | (+)→ | (+)→ | (+)→ | (+)↓ | (+)↑ | (+)→ | (−) |
| Eye | (−) | (−) | (−) | (−) | (−) | (−) | (+)↑ | (+)↓ | (−) |
| Bone | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) | (−) |
| Uterus | (+)→ | (+)↑ | (+)↑ | (+)↑ | |||||
| Ovary | (−) | (−) | (−) | (−) | |||||
| Testis | (+)↓ | (+)↓ | (+)↓ | (+)↓ | (+)↓ | ||||
| Penis | (+)↓ | (+)↓ | (+)→ | (+)↓ | (−) | ||||
For the (GC)3-TATA-luciferase construct, there are no data for line 17 because the founder female failed to successfully feed the pups. For the (GC)3-TK-luciferase construct, there was no protein expression in any tissue in line 35 females, the transgene could not be inherited to F2 in line 41, and there was no protein expression in any tissue in line 42. Activity designations are shown as follows: (−), no detectable activity; (+), detectable activity; (+)↑, activity induced by E2; (+)↓, activity decreased by E2; (+)→, activity unchanged by E2 (50 μg/kg).
Figure 2.
Basal luciferase activity is comparable in male and female mice. The 21-d-old animals were killed by CO2 asphyxiation, and organs were immediately removed. Luciferase activity was determined as described in Materials and Methods. At least seven animals are included for male and female groups.
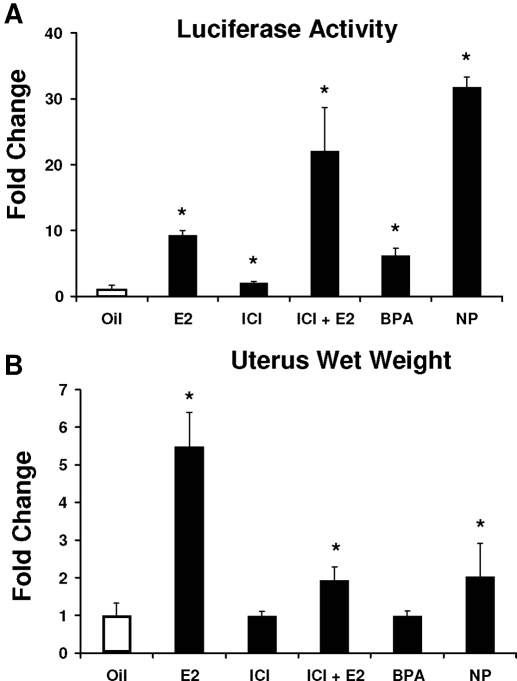
Results in Fig. 3A show that E2 (50 μg/kg bw) significantly induces mouse uterine luciferase activity by approximately 10-fold. The estrogenic activity of two synthetic estrogens, BPA (250 mg/kg bw) and NP (250 mg/kg bw), was also determined using the same protocol, and these treatments resulted in a 7.2-fold and 38.4-fold increase in luciferase activity, which is consistent with their activation of GC-rich constructs in breast cancer cells (22,30). The pure antiestrogen ICI 182, 780 also induced mouse uterine luciferase activity, and these results are consistent with the ERα agonist activity of ICI 182,780 observed in breast cancer cells transfected with the same GC-rich constructs (44,45). Not surprisingly, cotreatment of the animals with E2 plus ICI 182,780 also induced mouse uterine luciferase activity. We compared the activation of mouse uterine luciferase (Fig. 3A) with the well-characterized mouse uterine wet weight response in which treatment with E2 increased uterine wet weight (Fig. 3B). In addition, NP also induced a uterine wet weight increase at a dose of 250 mg/kg bw, whereas the same dose of BPA is inactive, which is consistent with the relatively low potency of BPA for this response (49). ICI 182,780 alone did not affect uterine wet weight, but in combination with E2, the antiestrogen significantly inhibited hormone-induced uterine wet weight increase. Results obtained in the mouse uterine luciferase and uterine wet weight assays were complementary except for the estrogenic effects of ICI 182,780 on activation of the GC-rich promoter in the mouse uterus, which was previously observed in breast cancer cells (44,45).
Figure 3.
Uterotrophic responses in transgenic mice. Luciferase (A) and uterine wet weight responses (B) of 21-d-old female mice were treated by sc injection with corn oil, 50 μg/kg bw E2, 250 μg/kg bw ICI 182,780 alone or in combination with E2, 250 mg/kg bw BPA, or 250 mg/kg bw NP alone for 3 d consecutively. On d 4, animals were killed, and the uterus was obtained and weighed (B) and then homogenized in the lysis buffer for determining luciferase activity. Luciferase activity (A) was determined as described in Materials and Methods. At least seven animals are included in each treatment group. *, Significant (P < 0.05) induction; **, significant inhibition of E2-induced activity by ICI 182,780.
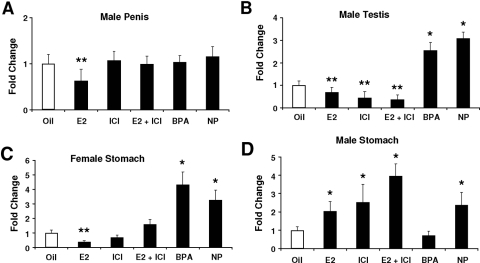
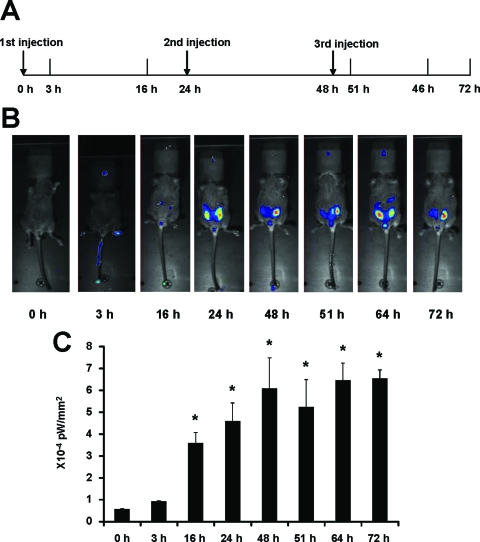
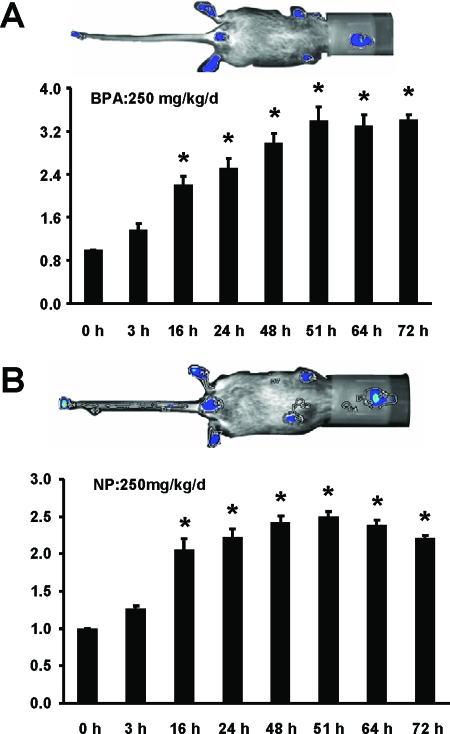
We also examined activation of luciferase activity in two male-specific tissues, the penis and testis, in line 15 (Fig. 4, A and B). In the penis, treatment with E2 alone significantly decreased activity, whereas ICI 182,780, BPA, and NP did not induce activity. However, ICI 182,780 exhibited antiestrogenic activity in the mouse penis and inhibited E2-induced luciferase activity. ERα agonist/antagonist activities were determined in the male testis (line 15) treated with the same compounds. The results showed that both E2 and ICI 182,780 alone and in combination decreased luciferase activity, whereas the synthetic estrogens BPA and NP caused a more than 2.5-fold increase in activity. These results suggest that ER/Sp-mediated up- or down-regulation of luciferase activity was dependent on ligand structure. Sex-specific activation of luciferase activity was investigated in the male and female stomach of line 15 (Fig. 4, C and D). In females, E2 decreased luciferase activity and ICI 182,780 exhibited antiestrogenic activity and blocked the effects of the hormone (Fig. 4C). ICI 182,780 alone was inactive, whereas both BPA and NP induced luciferase activity. In contrast, E2, ICI 182,780, and their combination and NP induced activity in the male stomach of line 15 (Fig. 4B), and this resembled the responses observed in the female mouse uterus (Fig. 3A). BPA was inactive in the male stomach, which was in contrast to the induction response observed in females (Fig. 4A). Results in Fig. 5 show that E2 also induces uterine luciferase activity in vivo in the offspring of C57BL/6 (line 15) mice crossed with white FVB mice. These lighter colored mice can be imaged in vivo. E2 significantly induced luciferase activity within 16 h after treatment, and induction was maintained for 72 h after multiple (3) treatments every 24 h with E2. Figure 6 illustrates the induction of uterine luciferase activity in the transgenic mice treated with NP (250 mg/kg·d) and BPA (250 mg/kg·d), and significantly induced activity was observed within 3–16 h after treatment with both compounds.
Figure 4.
Effects of estrogenic compounds are tissue and sex specific. Effects of compounds on the male penis (A), testis (B), female stomach (C), and male stomach (D). The 21-d-old mice were treated by sc injection of corn oil, 50 μg/kg bw E2, 250 μg/kg bw ICI 182,780 alone or in combination with E2, 250 mg/kg bw BPA, or 250 mg/kg bw NP for 3 d consecutively. On d 4, animals were killed by CO2 asphyxiation, and organs were immediately removed. Luciferase activity was determined as described in Materials and Methods. At least seven animals are included in each treatment group. *, Significant (P < 0.05) induction; **, significant inhibition.
Figure 5.
In vivo activation of the reporter construct by estrogen. A, Experiment design as described in Materials and Methods. The first injection time was set as time 0. B, In vivo activation of the luciferase construct by 50 μg/kg bw E2 in 21-d-old female mice measured with the NightOwl image unit. Time-dependent activation of the luciferase expression was measured. Number of photons is depicted in a pseudocolor image superimposed on a gray-scale picture. Only one animal is shown as an example. C, Quantification of the photon signal produced in the uterus of the transgenic mice after E2 treatment. The data are expressed as the average of brightness (picowatts)/area (square millimeters) with sd for four animals per treatment group. *, Significant (P < 0.05) induction.
Figure 6.
Induction of luciferase activity by BPA (A) and NP (B). The 21-d-old mice (as described in Fig. 5) were treated with BPA (250 mg/kg·d) or NP (250 mg/kg·d) for 72 h, and uterine luciferase activity was determined at different time points using the Berthold NightOwl imaging system as described in Materials and Methods. *, Significant (P < 0.05) induction. Results are expressed as means ± sd for at least four mice per treatment group. Time (h) is measured on the horizontal axis and relative activity represented on the vertical axis.
These results demonstrate the estrogenic or antiestrogenic activity of various ligands that bind the ER in transgenic mice expressing a GC-rich promoter-luciferase construct. Activation or repression of activity was tissue/organ, sex, and ligand structure dependent, suggesting that these compounds are selective ER modulators (SERMs).
Discussion
Estrogens induce responses in multiple tissues/organs and play a critical role in normal physiology and in hormone-dependent cancers (1,2,3,4,5,6,7,8,9). The classical mechanism of estrogen action involves hormone-induced formation of ER, the dimer of which interacts with ERE motifs in target gene promoters, and depending on the subset of nuclear proteins recruited to the E2-responsive promoters, genes can be induced or repressed. The precise identity of ERE-dependent genes and their hormone-dependent regulation in various tissues is not well understood; however, computational and microarray approaches have identified multiple ERE sites, particularly in breast cancer cells and the rodent uterus (50,51,52,53,54,55,56).
An in vivo approach for investigating activation of ERE promoters has been reported using transgenic animals expressing ERE-luciferase constructs. Although this method does not identify specific genes activated or repressed by ER-ERE interactions, luciferase activity is induced or repressed in E2-responsive tissue of ovariectomized or 21-d-old mice treated with E2 (57,58,59). Tissue-specific basal and E2-dependent expression of luciferase activity in the transgenic animal models was highly variable between the different transgenic lines with the same transgene construct, and variability was also observed using different transgene constructs (57,58,59). In most of these studies, E2 induced luciferase activity in various tissues; however, fold inducibility was variable and dependent, in part, on basal activity in untreated control mice. For example, Ciana and co-workers (57) observed a 5-fold or higher induction of luciferase activity in liver, lung, bone marrow, spleen, brain, and thymus and a 2.5- to 4.9-fold induction in skin, bladder, uterus, eye, spinal cord, and adipocytes. In other organs, such as esophagus, aorta, thyroid, tail, pancreas, stomach, heart, skeletal, muscle, and blood, E2 did not induce luciferase activity in mice expressing the ERE-luc transgene.
There is also evidence that E2 can act through other genomic and nongenomic pathways, and research in this laboratory has focused on ER/Sp-dependent activation of GC-rich promoter constructs. This pathway is important for E2-dependent activation of multiple genes in breast cancer and other cell lines associated with cell proliferation, survival, nucleotide metabolism, angiogenesis, and receptors involved in nuclear and membrane associated signaling (27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42). In addition, there is also evidence for ERα/Sp-dependent down-regulation of VEGF in HEC1A1 endometrial cancer cells (60).
We have extensively used (GC)3-TATA-Luc (44,45) as a model for investigating ligand-dependent activation of wild-type and variant ERα/Sp-mediated transactivation, and the properties of this artificial construct resemble those of GC-rich promoter from E2-responsive genes (22,30). The major exception is that the antiestrogen ICI 182,780 activates (GC)3-TATA-Luc (45) but acts as a typical antiestrogen in cells transfected with GC-rich promoters from E2-responsive genes (61,62), and the reasons for these promoter-specific differences in response to ICI 182,780 are unknown and are currently being investigated.
We used the consensus GC-rich constructs (Fig. 1A) to generate transgenic animals expressing luciferase activity in various mouse tissues. The expression patterns and luciferase activity observed in these animals differed from the corresponding ERE-Luc transgenic animals, and this was due to differences in the constructs used. In this study, the transgene expresses a luciferase gene regulated by a (GC)3-TATA or (GC)3-TK promoter, whereas in studies with mice expressing ERE-luc transgenes, the constructs also contained TATA or TK promoters and insulator sequences that prevent inappropriate interactions between adjacent chromatin domains (63). In a previous report that compared ERE constructs with or without insulators, luciferase activity was lower without the insulator, but inducibility by E2 was comparable (59). In this study, the GC-rich constructs did not contain an insulator, and there may be some influence of the surrounding sequences on both basal and E2-inducible activity. Table 1 illustrates differences in basal and E2-dependent luciferase activity in the various transgenic mouse lines, and we primarily focused on line 15 in which E2 significantly induced luciferase activity in the mouse uterus, which is a highly estrogen-responsive organ. E2 and the synthetic estrogen BPA and NP all induced uterine luciferase activity in the transgenic animals, and these same compounds induce ERα/Sp-dependent transactivation in breast cancer cells (44,45). Interestingly, ICI 182,780 also induces luciferase activity in the mouse uterus (Fig. 3A) as previously reported for the same transfected promoter-reporter construct in breast cancer cells (44,45). In contrast, using the uterine wet weight assay for estrogenicity, ICI 182,780 exhibits antiestrogenic activity. NP is also estrogenic in the uterus, whereas BPA is inactive at a dose of 250 mg/kg bw, which is consistent with previous reports showing the relatively weak ER agonist and partial antagonist activity of this compound in the rodent uterotrophic assay (49).
We also investigated E2 responsiveness in other tissues and compared the effects of E2 with ICI 182,780 and the synthetic estrogens BPA and NP. Previous in vitro studies suggest that NP and BPA differentially activate wild-type and variant ERα in breast cancer cells transfected with ERE and GC-rich promoters, suggesting that these compounds exhibit SERM-like activity, which assumes tissue-specific ER agonist and antagonist activities (64,65). Differences in the hormonal activities of BPA/NP compared with E2 were investigated in the male penis and testis (Fig. 4, A and B) and female and male stomach (Fig. 4, C and D), and the results demonstrated sex- and tissue-specific differences in activation of the GC-rich promoter by various compounds. E2 decreased luciferase activity in the male penis and testis and female stomach but induced activity in the male stomach. This pattern of estrogenic activity was not observed in mice treated with ICI 182,780, NP, or BPA. For example, ICI 182,780 alone did not affect luciferase activity in the penis (male) and stomach (female) of line 15 mice and both decreased and increased activity in the testis and stomach (male) and therefore acted as an E2 mimic (male testis and male stomach) or as an antiestrogen (male penis and female stomach). NP and BPA exhibited a similar pattern of induced luciferase activity except for the male stomach where NP induced and BPA did not affect activity (Fig. 4, C and D). BPA and NP did not affect luciferase activity in the penis; however, in two tissues where E2 significantly decreased activity (testis and female stomach), both NP and BPA induced activity. Tissue-specific differences in the estrogenic activity of o,p′- and p,p′-DDT isomers and E2 have also been observed in the ERE-reporter mouse (66), confirming the SERM-like activity even among these structurally similar isomers.
Our initial experiments were carried out in C57BL/6 mice because this model has been widely used for studying estrogenic uterine responses. In vivo imaging with these black mice is problematic due to signal quenching; however, the mice resulting from the cross of transgenic C57BL/6 (line 15) and FVB mice can be directly imaged (Figs. 5 and 6), and luciferase activity is induced in the uterus by E2, NP, and BPA within 16 h after treatment. Multiple (3) dosing maintains this activity over 72 h. Future studies will take advantage of backcrossed mice to investigate the tissue-specific estrogenic activity of synthetic and phytoestrogenic compounds and their activation of ERα/Sp-dependent gene expression.
In summary, our studies report for the first time that mice expressing a GC-rich luciferase construct in multiple tissues are responsive to E2 and other estrogenic/antiestrogenic compounds. The patterns of induced or repressed luciferase activity are tissue and sex specific and structure dependent. These results are consistent with the SERM-like activity of estrogenic compounds, which is dictated by the complex pharmacology of SERMs, which is dependent on ligand structure, promoter, and cell context (10,11,57,58,59). In contrast to previous studies with ERE-transgenic mice (66), we observed that in some tissues, E2 decreased luciferase activity, suggesting that ERα/Sp-dependent regulation of E2-responsive genes may be an important pathway for hormone-dependent gene repression, and ongoing studies in breast cancer cells show that knockdown of Sp proteins abrogates hormone activation or repression of several genes. Current studies are focused on development and application of transgenic mice expressing other GC-rich promoters and on identifying critical E2-responsive genes regulated by ERα/Sp complexes.
Footnotes
This work was supported by the National Institutes of Health (ES04917, CA104116, and ES09106) and the Texas Agricultural Experiment Station.
Disclosure Statement: The authors have nothing to disclose.
First Published Online July 17, 2008
Abbreviations: AP1, Activation protein 1; BPA, bisphenol A; bw, body weight; DMSO, dimethylsulfoxide; E2, 17β-estradiol; ER, estrogen receptor; ERE, estrogen-responsive element; FBS, fetal bovine serum; iSp1, small inhibitory RNA for Sp1; NP, nonylphenol; NFκB, nuclear factor κB; SERM, selective ER modulator; Sp, specificity protein; TK, thymidine kinase.
References
- Clark JH 1998 Female reproduction and toxicology of estrogens. In: Korach KS, ed. Reproductive and developmental toxicology. New York: Marcel Dekker; 259–275 [Google Scholar]
- Chow J, Tobias JH, Colston KW 1992 Estrogen maintains trabecular bone volume in rats not only by suppression of bone resorption but also by stimulation of bone formation. J Clin Invest 89:74–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat MY, Lavigne MC, Ramwell PW 1996 The vascular protective effects of estrogen. FASEB J 10:615–624 [PubMed] [Google Scholar]
- Turner RT, Riggs BL, Spelsberg TC 1994 Skeletal effects of estrogen. Endocr Rev 15:275–300 [DOI] [PubMed] [Google Scholar]
- Beatson GT 1986 On the treatment of inoperable cases of carcinoma of the mamma; suggestions for a new method of treatment with illustrative cases. Lancet 2:104–107 [PMC free article] [PubMed] [Google Scholar]
- Kuschel B, Betz B, Niederacher D, Beckmann MW 2000 Hereditary breast cancer: molecular and chemical differences from sporadic breast cancer. J Women’s Cancer 2:93–100 [Google Scholar]
- Hulka BS 1997 Epidemiologic analysis of breast and gynecologic cancers. In: Aldaz M, Gould MN, McLachlan J, Slaga TJ, eds. Etiology of breast and gynecological cancers. New York: Wiley-Liss; 17–29 [PubMed] [Google Scholar]
- Hulka BS, Liu ET, Lininger RA 1994 Steroid hormones and risk of breast cancer. Cancer 74:1111–1124 [DOI] [PubMed] [Google Scholar]
- Hulka BS 1995 Breast cancer: cause and prevention. Lancet 346:883–887 [DOI] [PubMed] [Google Scholar]
- Jordan VC 2003 Antiestrogens and selective estrogen receptor modulators as multifunctional medicines. 2. Clinical considerations and new agents. J Med Chem 46:1081–1111 [DOI] [PubMed] [Google Scholar]
- Jordan VC 2003 Antiestrogens and selective estrogen receptor modulators as multifunctional medicines. 1. Receptor interactions. J Med Chem 46:883–908 [DOI] [PubMed] [Google Scholar]
- Cushman M, Kuller LH, Prentice R, Rodabough RJ, Psaty BM, Stafford RS, Sidney S, Rosendaal FR 2004 Estrogen plus progestin and risk of venous thrombosis. JAMA 292:1573–1580 [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J 2002 Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333 [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH 2004 Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 291:2947–2958 [DOI] [PubMed] [Google Scholar]
- Cowley SM, Hoare S, Mosselman S, Parker MG 1997 Estrogen receptors α and β form heterodimers on DNA. J Biol Chem 272:19858–19862 [DOI] [PubMed] [Google Scholar]
- Hyder SM, Chiappetta C, Stancel GM 1999 Interaction of human estrogen receptors α and β with the same naturally occurring estrogen response elements. Biochem Pharmacol 57:597–601 [DOI] [PubMed] [Google Scholar]
- Klein-Hitpass L, Schorpp M, Wagner U, Ryffel GU 1986 An estrogen-responsive element derived from the 5′-flanking region of the Xenopus vitellogenin A2 gene functions in transfected human cells. Cell 46:1053–1061 [DOI] [PubMed] [Google Scholar]
- Pace P, Taylor J, Suntharalingam S, Coombes RC, Ali S 1997 Human estrogen receptor β binds DNA in a manner similar to and dimerizes with estrogen receptor α. J Biol Chem 272:25832–25838 [DOI] [PubMed] [Google Scholar]
- Blobel GA, Orkin SH 1996 Estrogen-induced apoptosis by inhibition of the erythroid transcription factor GATA-1. Mol Cell Biol 16:1687–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino F, Walker WH 1999 Hormonal regulation of the NF-κB signaling pathway. Mol Cell Endocrinol 157:1–9 [DOI] [PubMed] [Google Scholar]
- Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P 2000 Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol 74:311–317 [DOI] [PubMed] [Google Scholar]
- Safe S, Kim K 2004 Nuclear receptor-mediated transactivation through interaction with Sp proteins. Prog Nucleic Acids Res Mol Biol 77:1–36 [DOI] [PubMed] [Google Scholar]
- Kelly MJ, Qiu J, Ronnekleiv OK 2003 Estrogen modulation of G-protein-coupled receptor activation of potassium channels in the central nervous system. Ann NY Acad Sci 1007:6–16 [DOI] [PubMed] [Google Scholar]
- Levin ER 2005 Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol 19:1951–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER 2004 Plasma membrane estrogen receptors exist and functions as dimers. Mol Endocrinol 18:2854–2865 [DOI] [PubMed] [Google Scholar]
- Watson CS, Campbell CH, Gametchu B 2002 The dynamic and elusive membrane estrogen receptor-α. Steroids 67:429–437 [DOI] [PubMed] [Google Scholar]
- Porter W, Saville B, Hoivik D, Safe S 1997 Functional synergy between the transcription factor Sp1 and the estrogen receptor. Mol Endocrinol 11:1569–1580 [DOI] [PubMed] [Google Scholar]
- Porter W, Wang F, Wang W, Duan R, Safe S 1996 Role of estrogen receptor/Sp1 complexes in estrogen-induced heat shock protein 27 gene expression. Mol Endocrinol 10:1371–1378 [DOI] [PubMed] [Google Scholar]
- Saville B, Wormke M, Wang F, Nguyen T, Enmark E, Kuiper G, Gustafsson J-A, Safe S 2000 Ligand-, cell- and estrogen receptor subtype (α/β)-dependent activation at GC-rich (Sp1) promoter elements. J Biol Chem 275:5379–5387 [DOI] [PubMed] [Google Scholar]
- Safe S, Abdelrahim M 2005 Sp transcription factor family and its role in cancer. Eur J Cancer 41:2438–2448 [DOI] [PubMed] [Google Scholar]
- Bardin A, Moll F, Margueron R, Delfour C, Chu ML, Maudelonde T, Cavailles V, Pujol P 2005 Transcriptional and posttranscriptional regulation of fibulin-1 by estrogens leads to differential induction of messenger ribonucleic acid variants in ovarian and breast cancer cells. Endocrinology 146:760–768 [DOI] [PubMed] [Google Scholar]
- Bruning JC, Lingohr P, Gillette J, Hanstein B, Avci H, Krone W, Muller-Wieland D, Kotzka J 2003 Estrogen receptor-α and Sp1 interact in the induction of the low density lipoprotein-receptor. J Steroid Biochem Mol Biol 86:113–121 [DOI] [PubMed] [Google Scholar]
- Byrne IM, Flanagan L, Tenniswood MP, Welsh J 2000 Identification of a hormone-responsive promoter immediately upstream of exon 1c in the human vitamin D receptor gene. Endocrinology 141:2829–2836 [DOI] [PubMed] [Google Scholar]
- Fujita N, Kajita M, Taysavang P, Wade PA 2004 Hormonal regulation of metastasis-associated protein 3 transcription in breast cancer cells. Mol Endocrinol 18:2937–2949 [DOI] [PubMed] [Google Scholar]
- Schultz JR, Petz LN, Nardulli AM 2005 Cell- and ligand-specific regulation of promoters containing activator protein-1 and Sp1 sites by estrogen receptors α and β. J Biol Chem 280:347–354 [DOI] [PubMed] [Google Scholar]
- Sun JM, Spencer VA, Li L, Yu CH, Yu J, Davie JR 2005 Estrogen regulation of trefoil factor 1 expression by estrogen receptor α and Sp proteins. Exp Cell Res 302:96–107 [DOI] [PubMed] [Google Scholar]
- Zhao YL, Han WD, Li Q, Mu YM, Lu XC, Yu L, Song HJ, Li X, Lu JM, Pan CY 2005 Mechanism of transcriptional regulation of LRP16 gene expression by 17-β estradiol in MCF-7 human breast cancer cells. J Mol Endocrinol 34:77–89 [DOI] [PubMed] [Google Scholar]
- Jacobson D, Pribnow D, Herson PS, Maylie J, Adelman JP 2003 Determinants contributing to estrogen-regulated expression of SK3. Biochem Biophys Res Commun 303:660–668 [DOI] [PubMed] [Google Scholar]
- Li C, Briggs MR, Ahlborn TE, Kraemer FB, Liu J 2001 Requirement of Sp1 and estrogen receptor α interaction in 17β-estradiol-mediated transcriptional activation of the low density lipoprotein receptor gene expression. Endocrinology 142:1546–1553 [DOI] [PubMed] [Google Scholar]
- Salvatori L, Ravenna L, Felli MP, Cardillo MR, Russo MA, Frati L, Gulino A, Petrangeli E 2000 Identification of an estrogen-mediated deoxyribonucleic acid-binding independent transactivation pathway on the epidermal growth factor receptor gene promoter. Endocrinology 141:2266–2274 [DOI] [PubMed] [Google Scholar]
- Schultz JR, Petz LN, Nardulli AM 2003 Estrogen receptor α and Sp1 regulate progesterone receptor gene expression. Mol Cell Endocrinol 201:165–175 [DOI] [PubMed] [Google Scholar]
- Tanaka N, Yonekura H, Yamagishi S, Fujimori H, Yamamoto Y, Yamamoto H 2000 The receptor for advanced glycation end products is induced by the glycation products themselves and tumor necrosis factor-α through nuclear factor-κB, and by 17β-estradiol through Sp-1 in human vascular endothelial cells. J Biol Chem 275:25781–25790 [DOI] [PubMed] [Google Scholar]
- Abdelrahim M, Samudio I, Smith R, Burghardt R, Safe S 2002 Small inhibitory RNA duplexes for Sp1 mRNA block basal and estrogen-induced gene expression and cell cycle progression in MCF-7 breast cancer cells. J Biol Chem 277:28815–28822 [DOI] [PubMed] [Google Scholar]
- Kim K, Barhoumi R, Burghardt R, Safe S 2005 Analysis of estrogen receptor α-Sp1 interactions in breast cancer cells by fluorescence resonance energy transfer. Mol Endocrinol 19:843–854 [DOI] [PubMed] [Google Scholar]
- Kim K, Nguyen T, Saville B, Safe S 2003 Domains of estrogen receptor α (ERα) required for ERα/Sp1-mediated activation of GC-rich promoters by estrogens and antiestrogens in breast cancer cells. Mol Endocrinol 17:804–817 [DOI] [PubMed] [Google Scholar]
- Higgins KJ, Liu S, Abdelrahim M, Yoon K, Vanderlaag K, Porter W, Metz RP, Safe S 2006 Vascular endothelial growth factor receptor-2 expression is induced by 17β-estradiol in ZR-75 breast cancer cells by estrogen receptor α/Sp proteins. Endocrinology 147:3285–3295 [DOI] [PubMed] [Google Scholar]
- Truett GE, Heeger P, Mynatt RL, Truett AA, Walker JA, Warman ML 2000 Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 29:52, 54 [DOI] [PubMed] [Google Scholar]
- Stratman JL, Barnes WM, Simon TC 2003 Universal PCR genotyping assay that achieves single copy sensitivity with any primer pair. Transgenic Res 12:521–522 [DOI] [PubMed] [Google Scholar]
- Ashby J 2001 Increasing the sensitivity of the rodent uterotrophic assay to estrogens, with particular reference to bisphenol A. Environ Health Perspect 109:1091–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JS, Brown M 2006 Estrogen receptor target gene: an evolving concept. Mol Endocrinol 20:1707–1714 [DOI] [PubMed] [Google Scholar]
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M 2005 Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122:33–43 [DOI] [PubMed] [Google Scholar]
- Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M 2006 Genome-wide analysis of estrogen receptor binding sites. Nat Genet 38:1289–1297 [DOI] [PubMed] [Google Scholar]
- Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS 2003 Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinology 144:4562–4574 [DOI] [PubMed] [Google Scholar]
- Klinge CM 2001 Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res 29:2905–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishidate T, Katagiri T, Lin ML, Mano Y, Miki Y, Kasumi F, Yoshimoto M, Tsunoda T, Hirata K, Nakamura Y 2004 Genome-wide gene-expression profiles of breast-cancer cells purified with laser microbeam microdissection: identification of genes associated with progression and metastasis. Int J Oncol 25:797–819 [PubMed] [Google Scholar]
- O'Lone R, Frith MC, Karlsson EK, Hansen U 2004 Genomic targets of nuclear estrogen receptors. Mol Endocrinol 18:1859–1875 [DOI] [PubMed] [Google Scholar]
- Ciana P, Di Luccio G, Belcredito S, Pollio G, Vegeto E, Tatangelo L, Tiveron C, Maggi A 2001 Engineering of a mouse for the in vivo profiling of estrogen receptor activity. Mol Endocrinol 15:1104–1113 [DOI] [PubMed] [Google Scholar]
- Ciana P, Raviscioni M, Mussi P, Vegeto E, Que I, Parker MG, Lowik C, Maggi A 2003 In vivo imaging of transcriptionally active estrogen receptors. Nat Med 9:82–86 [DOI] [PubMed] [Google Scholar]
- Lemmen JG, Arends RJ, van Boxtel AL, Van der Saag PT, van der BB 2004 Tissue- and time-dependent estrogen receptor activation in estrogen reporter mice. J Mol Endocrinol 32:689–701 [DOI] [PubMed] [Google Scholar]
- Stoner M, Wang F, Wormke M, Nguyen T, Samudio I, Vyhlidal C, Marme D, Finkenzeller G, Safe S 2000 Inhibition of vascular endothelial growth factor expression in HEC1A endometrial cancer cells through interactions of estrogen receptor α and Sp3 proteins. J Biol Chem 275:22769–22779 [DOI] [PubMed] [Google Scholar]
- Ngwenya S, Safe S 2003 Cell context-dependent differences in the induction of E2F-1 gene expression by 17β-estradiol in MCF-7 and ZR-75 cells. Endocrinology 144:1675–1685 [DOI] [PubMed] [Google Scholar]
- Khan S, Abdelrahim M, Samudio I, Safe S 2003 Estrogen receptor/Sp1 complexes are required for induction of cad gene expression by 17β-estradiol in breast cancer cells. Endocrinology 144:2325–2335 [DOI] [PubMed] [Google Scholar]
- Gaszner M, Felsenfeld G 2006 Insulators: exploiting transcriptional and epigenetic mechanisms. Nat Rev Genet 7:703–713 [DOI] [PubMed] [Google Scholar]
- Yoon K, Pallaroni L, Stoner M, Gaido K, Safe S 2001 Differential activation of wild-type and variant forms of estrogen receptor α by synthetic and natural estrogenic compounds using a promoter containing three tandem estrogen-responsive elements. J Steroid Biochem Mol Biol 78:25–32 [DOI] [PubMed] [Google Scholar]
- Wu F, Safe S 2007 Differential activation of wild-type estrogen receptor α and C-terminal deletion mutants by estrogens, antiestrogens and xenoestrogens in breast cancer cells. J Steroid Biochem Mol Biol 103:1–9 [DOI] [PubMed] [Google Scholar]
- Di Lorenzo D, Villa R, Biasiotto G, Belloli S, Ruggeri G, Albertini A, Apostoli P, Raviscioni M, Ciana P, Maggi A 2002 Isomer-specific activity of dichlorodyphenyltrichloroethane with estrogen receptor in adult and suckling estrogen reporter mice. Endocrinology 143:4544–4551 [DOI] [PubMed] [Google Scholar]