Abstract
The modulation of gene regulation by progesterone (P) and its classical intracellular regulation by progestin receptors in the brain, resulting in alterations in physiology and behavior has been well studied. The mechanisms mediating the short latency effects of P are less well understood. Recent studies have revealed rapid nonclassical signaling action of P involving the activation of intracellular signaling pathways. We explored the involvement of protein kinase C (PKC) in P-induced rapid signaling in the ventromedial nucleus of the hypothalamus (VMN) and preoptic area (POA) of the rat brain. Both the Ca2+-independent (basal) PKC activity representing the activation of PKC by the in vivo treatments and the Ca+2-dependent (total) PKC activity assayed in the presence of exogenous cofactors in vitro were determined. A comparison of the two activities demonstrated the strength and temporal status of PKC regulation by steroid hormones in vivo. P treatment resulted in a rapid increase in basal PKC activity in the VMN but not the POA. Estradiol benzoate priming augmented P-initiated increase in PKC basal activity in both the VMN and POA. These increases were inhibited by intracerebroventricular administration of a PKC inhibitor administered 30 min prior to P. The total PKC activity remained unchanged demonstrating maximal PKC activation within 30 min in the VMN. In contrast, P regulation in the POA significantly attenuated total PKC activity ± estradiol benzoate priming. These rapid changes in P-initiated PKC activity were not due to changes in PKC protein levels or phosphorylation status.
OVARIAN STEROID HORMONES, estradiol (E2) and progesterone (P), have profound modulatory influences on the neuronal networks in the brain, resulting in alterations in reproductive physiology and behavior (1,2,3,4,5). P effects on the reproductive behavior are dependent on the prior conditioning of neural tissues with E2. The regulatory action of E2 is thought to primarily involve the activation of estrogen receptors in the ventromedial nucleus of the hypothalamus (VMN) and preoptic area (POA), which in turn, act as ligand-dependent transcription factors altering the expression of genes, including the progestin receptor (PR) (1,4). E2 priming results in an increase in PR mRNA in the VMN and POA of the rat brain, followed by induction of PR synthesis (1). Numerous studies using intracranial implants and localized brain lesions suggest that the VMN and POA are the key sites of action of E2 and P for facilitating female receptive behavior in rats, displayed by the arching of the back termed lordosis response (5).
P facilitation of lordosis thought to primarily involve the classical genomic mechanism of action, mediated by PRs in the VMN and POA, was established by antisense approaches in rats and studies on PR knockout mice (6,7,8,9). This classical, genomic mechanism, characterized by the ligand-dependent gene regulation, has a delayed onset and is a protracted process (10,11,12). In addition to this delayed effect, rapid, short latency effects of P on facilitation of lordosis response in E2-primed female rats have also been reported (13,14,15). These nonclassical rapid effects of P are initiated at the plasma membrane and appear to be transmitted by the cytoplasmic second-messenger signaling cascades, independent of gene transcription (16,17,18,19,20). Interactions between the membrane-initiated P effects and intracellular PRs have also been observed in the facilitation of reproductive behavior in female hamsters (21,22), suggesting that both mechanisms act in concert rather than independently. Whereas the distinctions between the two mechanisms at the molecular level leading to the transcriptional activation of intracellular PRs are unclear, both mechanisms result in synthesis and/or down-regulation of new or existing proteins resulting in a successful mating behavior (23).
The nonclassical mechanisms involved in P-facilitated lordosis response in female rats have been demonstrated to involve stimulation of second messengers cAMP and cGMP, leading to the activation of signaling kinases (24,25,26,27). Chu and Etgen (28) have demonstrated the involvement of cGMP in the activation of protein kinase G, and also the requirement of PRs in this cGMP mediation of lordosis response. Several excellent studies also have demonstrated E2 regulation of protein kinase C (PKC) in the facilitation of lordosis in E2-primed female rats (29,30,31). A region-specific regulation of E2-stimulated PKC activity has been reported in the POA but not the hypothalamus (32). The involvement of both protein kinase A (PKA) and PKC in the facilitation of lordosis response by P and its metabolites, in E2-primed rats has also been demonstrated (33). However, none of these studies have examined the role of PKC in the nonclassical action of P in the facilitation of lordosis. Studies from our laboratory have demonstrated P-stimulated rapid elevations in hypothalamic cAMP and cAMP-dependent kinase (PKA) activity, the inhibition of which reduced P-facilitated lordosis response in E2-primed rats (34). Because P is likely to activate several intracellular signaling pathways, we questioned whether P, in the absence or presence of estradiol benzoate (EB) priming, can lead to the activation of PKC within the VMN and POA.
The PKC family of serine/threonine protein kinases is comprised of three distinct classes based on their cofactor requirement: conventional PKC (cPKC; α, βI, βII, and γ) requiring both calcium (Ca+2) and diacylglycerol (DAG) for activation, novel PKC (η and ε) requiring only DAG and atypical PKC (δ, ζ, and ι) requiring neither calcium nor DAG for activation (35,36,37). The ζ form of protein kinase M (PKM), uniquely found in the brain, is an alternately spliced form of PKCζ (38,39). All isoforms contain an ATP-binding domain that can be competitively inhibited by bisindolylmaleimide GF109203X (40). They are activated through a series of phosphorylation steps (except PKMζ) and share an allosteric mode of regulation in which a pseudosubstrate is displaced from the activation site of the kinase on autophosphorylation (41). The activated kinases are tethered to the plasma membrane by DAG and translocated to the cytosol on deactivation (41). The activated PKC species have been detected at pre- and postsynaptic termini, and it is now known that these molecules retain activity for a few hours past the initiation of the signaling event (35,42). Thus, the temporal and spatial regulation of PKC is imperative during signaling events in neuronal function (43,44,45). Such temporal and spatial correlations at the molecular level have not previously been examined in the P facilitation of reproductive behavior (2), thereby prompting our investigation of P on PKC activation in the VMN and POA.
Materials and Methods
Reagents and chemicals
All steroids, premixed protease and phosphatases inhibitor I cocktails (Sigma catalog no. P-8340 and P-2850, respectively), phosphatidyl serine, calmodulin and the calcium/calmodulin-dependent protein kinase II (CaMKII) inhibitor, KN-93, the PKA inhibitor R-diastereomer of adenosine-3′, 5′-cyclic monophosphothioate (Rp-cAMPS), PKC inhibitor GF109203X, 3-isobutyl-1-methylxanthine, 2-(N-morpholino) ethanesulfonic acid, and 8-bromoadenosine-cAMP were purchased from Sigma Chemicals, St. Louis, MO). Antibodies used in the study were from the following sources: pan-PKC from Upstate Biotechnology (Lake Placid, NY), pan-phosphorylated PKC (γThr514) from Cell Signaling Technology (Danvers, MA), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) and donkey antirabbit horseradish peroxidase-conjugated IgG from Amersham Pharmacia Biotechnology (Piscataway, NJ). DAG was obtained from Cayman Chemicals (Ann Arbor, MI) and the reagents for electrophoresis and Western blotting from Bio-Rad Laboratories (Hercules, CA). All other chemicals were of reagent grade and purchased from Sigma or Fisher Scientific (Pittsburgh, PA). All stereotaxic surgical supplies were obtained from Plastics One (Roanoke, VA).
Procedures
Ovariectomized Sprague Dawley rats (180–200 g) were obtained from the supplier (Charles River Laboratories, Wilmington, MA) a week after surgery. The animals were maintained on a 12-h light, 12-h dark reversed light cycle with lights off at 12:00 h and given food and water ad libitum. They were acclimatized to the facility and the light cycle for 4–6 wk after their arrival from the supplier before experimental manipulation. All animal studies were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine and were in compliance with the National Institutes of Health Guide for the care and use of Laboratory animals.
Stereotaxic surgeries and central administration of compounds
Stainless steel guide cannulae were stereotaxically implanted into the third cerebral ventricle of ovariectomized rats using a laboratory standard stereotaxic instrument (Stoelting, Wood Dale, IL) as previously described (8). Animals were allowed to recover from surgery for 1 wk before use in experiments. Experimental animals were primed sc with EB (2 μg per 100 μl in sesame oil). Forty-eight hours later, 1 μg P (1 μl in artificial cerebrospinal fluid) was administered intracerebroventricularly (icv) via the cannula. The dose of P was based on previous studies (8). In studies using the PKC inhibitor, GF109203X (100 pg per 1 μl artificial cerebrospinal fluid), the compound was administered icv 30 min before P administration. The inhibitor dose was chosen based on the published report on its ability to inhibit all classes of PKC isoforms in rat brain (46). Each treatment group consisted of six animals. Thirty minutes after P administration, the animals were killed under anesthesia (combination of ketamine 42.8 mg/ml, xylazine 8.6 mg/ml, and acepromazine 1.4 mg/ml), the brain isolated from the cranial cavity, placed in cold artificial cerebrospinal fluid, and the VMN of the hypothalamus, POA, and cerebral cortex (CTX) dissected.
Fresh brain dissection was carried out at 4 C. Using a McIlwain tissue chopper, the POA slab was cut beginning 2 mm rostral to the optic chiasm to 0.5 mm caudal to the chiasm. A second cut (the medial basal hypothalamus) was made 4 mm caudal to the caudal edge of the first slab. The slabs were placed on a cold microscope stage and brain areas of interest were viewed under a dissecting microscope with transillumination system (Stereo Discovery V8; Zeiss, Thornwood, NY). Bilateral punches were made using the Palkovits punch method from the slabs to dissect the VMN and POA using a 1-mm internal diameter stainless steel punch. The cerebral cortex included the frontal cortex without the white matter from the slabs. The punches were immediately frozen on dry ice/isopropanol and stored at −80 C until further analyses.
Tissue processing and sample preparation
The tissue samples were thawed and homogenized in buffer containing10 mm Tris-HCl (pH 7.4), 1 mm EGTA, 1 mm EDTA, 0.5 mm dithiothreitol (DTT), 1 mm thioglycerol, and a cocktail of protease and phosphatase inhibitors (1:100) in a handheld glass homogenizer on ice. The samples were centrifuged at 1000 × g for 10 min, the pellet consisting of nuclear, mitochondrial and cellular debris discarded, and the supernatant centrifuged at 20,000 × g for 20 min. The protein concentration was quantified using a BCA assay (Pierce Inc., Rockford, IL). The supernatant was stored at −80 C in aliquots for kinase assays and Western analyses.
Kinase assays
The PKC assays were performed using a modified procedure of Klann et al. (47), using a synthetic peptide (AAAKIQASFRGHMAR) derived from neurogranin (NG; amino acids 28–43), as the substrate. The reaction mixture (final volume, 25 μl) contained 50 mm HEPES (pH 7.4), 1 mm DTT, 2 mm sodium pyrophosphate, 100 μm ATP, 2 μCi [γ32P] ATP, and 10 μm NG. Assays for PKC activity were carried out with 1 mm EGTA and 1 mm EDTA in the reaction mixture for basal/calcium and cofactor-independent activity or in the presence of 4 mm CaCl2 and lipid cofactors for total activity [final concentrations: 320 μg/ml phosphatidyl serine (PS) and 30 μg/ml sn-1, DAG]. The reaction was initiated by the addition of 5 μl of the protein sample (∼1–5 μg) to the reaction mixture and incubated at 30 C for 2 min. In studies using the kinase-specific inhibitor GF109202X to inhibit PKC activity, the tissue homogenates were assayed for PKC in addition to CaMKII and PKA activities as described below.
CaMKII activity was measured using a synthetic peptide substrate, bearing the autophosphorylation sequence from the CaMKII sequence (autocamtide-3; KKALHRQETVDAL) (48). The assay was performed according to the method of Moore et al. (49) with minor modifications as described. Five milliliters of protein samples (∼1–5 μg) were added to the reaction mixture (final volume 25 μl) containing 50 mm HEPES (pH 7.4), 1 mm DTT, 20 μm substrate peptide, 20 μm ATP, 5 mm MgCl2, and 2 μCi [γ-32P]ATP (3000 Ci/mmol) and incubated at 30 C for 2 min. The assay was performed in the presence of 2 mm EGTA in the reaction mixture to determine the basal/calcium-independent activity and in the presence of 1 mm CaCl2 and 3 μm calmodulin to determine the total activity.
The PKA assays were performed as described in our earlier publication (34), using a synthetic peptide (kemptide-LRRASLG). The reaction buffer contained 37.5 mm of 2-(N-morpholino) ethanesulfonic acid (pH 6.0), 0.2 mm 3-isobutyl-1-methylxanthine, 15 mm MgOAc, 30 mm ATP, 2 μCi [γ32P] ATP, and 300 mm kemptide for the determination of the PKA basal activity. To determine total PKA activity, the reaction buffer contained 20 mm 8-bromoadenosine-cAMP in addition to the other reagents mentioned above. The reactions were carried out at 30 C for 5 min.
Control reactions for determination of background counts were run in parallel by omitting substrate peptide. After 2 min incubation, 17 μl of the reaction mixture were spotted on P-81 phosphocellulose filters and the reaction stopped by 75 mm phosphoric acid. The filters were washed three times, 10 min each, in the phosphoric acid solution, rinsed with methanol and air dried. The radioactivity on the phosphorylated peptide substrate was quantified by the Cerenkov method. Phosphorylation was determined to be linear with respect to time and protein concentration under these conditions (47). For each experimental determination, the values for control reactions lacking the substrate peptide were subtracted from those containing the substrate peptide. Counts were normalized using protein concentrations determined by the BCA assay. The assay was performed for the samples from each animal independently. Data are expressed as either specific activity (picomoles of phosphate transferred per minute per milligram of protein) for each of the treatment groups (mean ± sem; n = 6) or as a percent of vehicle control (taken as100%). The basal activities of all treatment groups are reported as a percent of basal activity of the vehicle (vehicle as 100%). The total activities of all treatment groups were compared with the total activity of the vehicle set at 100%. All activities were converted to a percent of their respective control so that the basal and the total activities could be presented in the same graph.
Western immunoblots for PKC
The tissue samples from VMN, POA, and CTX were subjected to Western immunoblotting. Equal amounts of protein (5 μg) from each sample were electrophoresed on SDS-PAGE (10% polyacrylamide) (50) followed by transfer to a nitrocellulose membrane for Western blotting (51). Magic Marker XP Western standards (Invitrogen, Carlsbad, CA) were included in the gels for molecular weight determination. The membranes were blocked and sequentially probed with commercially available antibody to pan-phosphorylated PKC (homologous to threonine 514 of γPKC), followed by a pan-antibody that recognized all the PKC isoforms at a 1:2000 dilution. The antibody to GAPDH (as a normalizing control) was used at 1:1000 dilutions in conjunction with both the PKC antibodies. The antibody binding was revealed by incubation with donkey antirabbit horseradish peroxidase-conjugated IgG (1:10,000) followed by detection with the enhanced chemiluminescence reagent ECL (Amersham Pharmacia Biotech). The PKC and GAPDH bands were quantified by densitometry using PhosphorImager:SF (Molecular Dynamics, Sunnyvale, CA). The immunoreactivity was normalized to the GAPDH. Linear range of signals for densitometry was obtained by exposing the chemiluminescent membranes to x-ray film for varying lengths of time. The protein extracts were also examined for presence of the neuronal membrane and cytosol components by probing for the 2′, 3′-cyclic nucleotide 3′-phosphodiesterase and the GAD67 protein (l-glutamic acid decarboxylase), respectively. These markers confirmed the presence of neuronal plasma membrane fraction in the protein extracts.
Statistical analysis
Statistical analyses were performed using activity values in counts per milligram per minute (in triplicates) from each animal. The mean ± sem of these counts (from six animals) was normalized to the mean ± sem of the counts from the vehicle control set as 100%. Statistical analyses were performed using Prism software (GraphPad, San Diego, CA). For each significant ANOVA, post hoc comparisons were made using Dunnett’s method for comparing all groups vs. the control group or the Newman-Keuls method for multiple comparisons.
Results
A synthetic peptide derived from NG was used to determine changes in PKC activity in physiological preparations (47). Both the Ca2+-independent (basal) PKC activity (in the presence of cation chelators, EGTA and EDTA), and the Ca+2-dependent (total) PKC activity, (in the presence of saturating levels of the cofactors, calcium and PS/DAG) were determined. The in vivo perturbation of the system by the steroid hormones is measured as the basal PKC activity, whereas a further stimulation by in vitro addition of cofactors measures the total activity.
A comparison of the two activities provided information on the activated fraction of PKC pool and the temporal nature of PKC regulation by the steroid hormones.
Progesterone increases basal activity of PKC in the VMN of the hypothalamus
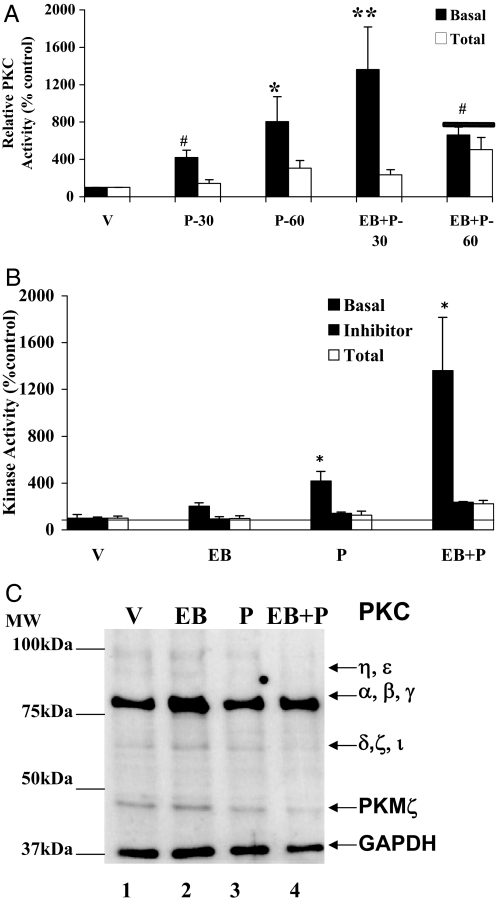
Earlier studies have established a role for E2-modulated PKC in the facilitation of lordosis in female rats (30,34). We demonstrated elevation in PKA activity within 30 min after icv administration of P in female rats (35). To determine whether P could stimulate PKC activity within the VMN of the hypothalamus, we measured PKC activity in tissue homogenates from in vivo-treated control and EB- and/or P-treated female rats. Figure 1A shows the results of a time-course experiment to determine the temporal change of basal PKC activity in response to icv P administration. There was a significant increase in P-stimulated basal PKC activity over vehicle treatment (shown as a threshold of 100%) at 30 min. This activity continued to increase at 60 min (P < 0.05). EB priming significantly augmented basal PKC activity at 30 min. By 60 min, this enhanced response had fallen to the levels seen with P alone. Because P treatment in the presence and absence of EB showed robust increases in basal PKC activity at 30 min, subsequent experimental analysis was carried out at this time point.
Figure 1.
Time course of P induction of PKC activity in the VMN. Time course (A), P modulation of PKC basal and total activities in the absence and presence of PKC inhibitor (B), and Western immunoblotting for PKC protein (C). Ovariectomized rats with chronic indwelling stainless steel cannulae in the third cerebral ventricle were either injected sc with EB or vehicle (V). Forty-eight hours later, P was administered icv, and the animals were killed and tissues isolated. A, The VMN homogenates were assayed for PKC basal and total activities at the indicated time points as described in Materials and Methods. The vehicle control (V) was set to 100% (mean ± sem). The basal and total PKC activities for each treatment group are presented and denoted by different pattern in the bar graph. ANOVA followed by Dunnett’s post hoc comparison demonstrated a statistically significant difference in the PKC basal activity between EB+P and vehicle treatments at 30 min (**, P < 0.001) and 60 min (#, P < 0.05). The P-induced PKC basal activity was statistically significant, compared with vehicle control, at 30 min (#, P < 0.05) and 60 min (*, P < 0.01) (n = 6 for each group). B, PKC inhibitor, GF109203X (GF), was infused (icv) 30 min before P or V treatment. The PKC activity was normalized to the vehicle in the presence of GF and expressed as percentage of the respective control (mean ± sem; 100%). The specific activity of V control in the presence of GF was 0.5 pmol/min·mg and in the absence of GF was 17.6 pmol/min·mg. Statistical analysis using ANOVA followed by Newman-Keuls post hoc multiple comparisons indicated statistically significant basal PKC activity for P and EB+P treatments (*, P < 0.05), compared with V or EB. C, Western immunoblotting for PKC protein. The experiment was performed six times to analyze all the samples from each group. A representative autoradiograph is shown. Each lane represents the sample from one animal per treatment group. The GAPDH band at 37 kDa was used as a normalizing control. Lane 1, Vehicle control (V); lane 2, EB; lane 3, P; lane 4, EB+P treatments. Magic marker molecular weight standards were run alongside the protein samples and the molecular weights are noted adjacent to lane 1.
As shown in Fig. 1B, the increase in basal PKC activity due to EB treatment alone was not significant (P > 0.05), compared with the basal PKC activity in vehicle-treated control. P treatment, in the absence of EB priming, stimulated statistically significant increase in basal PKC activity, compared with the vehicle control (P < 0.01). Administration of P, 48 h after EB priming, caused further elevation in basal PKC activity (P < 0.01). To determine the specificity of the basal PKC activity being measured, we examined the basal PKC activities in all treatment groups in the presence of PKC inhibitor bisindolylmaleimide GF109203X. The inhibitor significantly reduced the specific PKC basal activity measured in picomoles of phosphate transferred per minute per milligram protein (vehicle alone − GFX109203X = 15.7 ± 0.1; vehicle + GF109203 × = 0.7 ± 0.1). This inhibition was also significant in the EB- and/or P-stimulated basal PKC activities (Fig. 1B), demonstrating the specificity of the inhibitor to the measured PKC activity.
Progesterone alone has no further effect on Ca+2-inducible total PKC activity in the VMN of the hypothalamus
We proceeded to determine whether the P-induced PKC activity could be further stimulated in vitro by saturating levels of PS, DAG, and Ca+2. We refer to this stimulated PKC activity as the total PKC activity, which is a measure of the inducible fraction of PKC present in the cell over and above the basal PKC activity. This measurement provided additional information on the mechanism by which the fraction of PKC that can be activated by P differs from the effects of EB or P alone from that of EB+P. role of EB and P in the maintenance of activated PKC at the time point measured. Our initial time response data showed no significant differences in the Ca+2-dependent total PKC activity at 30 min vs. 60 min in response to P. This enabled us to use the 30 min time point as the early P-induced response for the remainder of the studies (Fig. 1A). Furthermore, it demonstrated that the persistently activated basal PKC was at its maximal and could not be further enhanced with Ca+2 and cofactors in vitro (Fig. 1, A and B). To estimate the proportion of the Ca+2 and PS/DAG-inducible total PKC activity that were potentially stimulatable by EB and/or P treatments, we determined the specific activity of total PKC in the vehicle controls and compared it with that of the basal PKC. The specific activity of Ca+2-inducible total PKC and basal PKC in the controls (vehicle) are 132.2 and 15.7 pmol of phosphate transferred per minute per milligram protein, respectively. This suggests that P activated only a fraction (12%) of the total pool of Ca+2-inducible total PKC molecules in the VMN at 30 min. In the presence of the inhibitor, this fraction was reduced to 0.5% when measured as picomoles of phosphate transferred per minute per milligram (vehicle − inhibitor = 132.2 ± 0.2; vehicle + inhibitor = 0.7 ± 0.1).
Progesterone does not alter PKC protein levels or phosphorylation
To determine whether the differences in basal and Ca+2-inducible total PKC activities were due to changes in PKC protein levels and/or changes in phosphorylation, the samples used in the kinase assays were subjected to Western blot analysis. Immunoblots probed with a pan-PKC antibody indicated multiple isoforms of the three classes of PKC in the range of 45–95 kDa (Fig. 1C). The cPKC class (α, βI, βII, and γ) was detected as an intense band at the 80 kDa molecular mass. The weak 90- to 95-kDa bands comprised the novel PKC (η and ε). The atypical PKC (δ, ζ and ι) corresponded to the 69- to 75-kDa band. The PKMζ isoform, abundantly expressed in the brain, was detectable as a 45-kDa band. Normalization of the protein content of the most abundant class of PKC, i.e. cPKC (α, β, and γ), using the GAPDH immunoreactive band, indicated no significant difference in the protein levels in EB- and/or P-treated samples (P > 0.05; Table 1). The phospho-cPKC (at 514Thr) values, normalized to the amount of cPKC present in the samples, showed no significant (P > 0.05) effects of EB and/or P treatments on phosphorylation levels of PKC (Table 2).
Table 1.
Total PKC
| Treatment | VMN | POA | CTX |
|---|---|---|---|
| V | 1 | 1 | 1 |
| EB | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.0 |
| P | 0.8 ± 0.1 | 0.8 ± 0.1 | 1.1 ± 0.0 |
| EB+P | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.1 |
Densitometric analyses of the cPKC protein (80 kDa) band in the Western immunoblots. The cPKC bands from VMN, POA, and CTX (Figs. 1C, 2B, and 3B) were quantified and normalized against the GAPDH (37 kDa) in each protein sample. The ratio of PKC to GAPDH in each lane was calculated. The ratio (PKC to GAPDH) of the steroid hormone treatments was compared with that of the vehicle control (V) set to 1. No significant differences were observed between the V and steroid hormone treatments in each of the regions of the brain examined (P > 0.05) (n = 6 per treatment group).
Table 2.
Ratio of phosphorylated PKC to total PKC
| Treatment | VMN | POA | CTX |
|---|---|---|---|
| V | 1 | 1 | 1 |
| EB | 1 ± 0.5 | 1.0 ± 0.1 | 3.6 ± 1.4 |
| P | 0.7 ± 0.2 | 0.7 ± 0.1 | 1.0 ± 0.2 |
| EB+P | 0.8 ± 0.2 | 0.8 ± 0.2 | 1.3 ± 0.7 |
Densitometric analysis of the phospho PKC to total PKC ratio from the Western immunoblots. The pan-phospho-PKC and total PKC bands were quantified from VMN, POA, and CTX (Figs. 1C, 2B, and 3B) and the ratios tabulated. No significant differences were observed in the ratios between the V and steroid hormone treatments in each of the regions of the brain examined (P > 0.05) (n = 6 per treatment group).
Progesterone alone has no effect on basal PKC activity in the POA
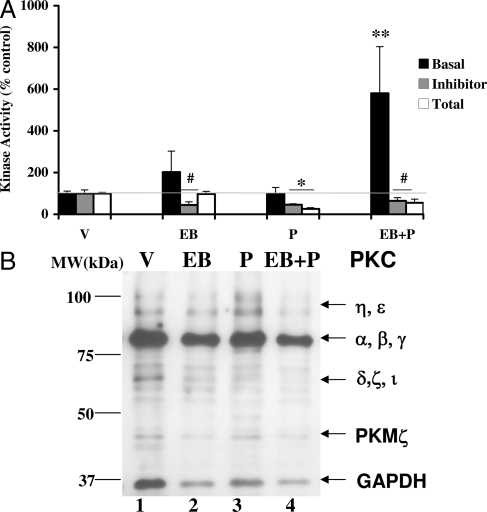
We also examined the effect of EB and/or P treatments on the basal PKC activity in the POA from the same animals (Fig. 2A). The increase in EB-stimulated basal activity was not significant, compared with the vehicle-treated controls (P > 0.05). In the absence of EB priming, P alone had no significant effect on the basal PKC activity. However, basal PKC activity was significantly increased as a result of P treatment in EB-primed animals (**, P < 0.001). In vivo administration of the PKC inhibitor, GF109203X, 30 min before P administration, attenuated the basal PKC activity in the POA, demonstrating the specificity of the inhibitor for PKC activity measured. This attenuation was significantly below the threshold level of the vehicle control (#, P < 0.05 in the case of EB and EB+P, and *, P < 0.01 for P; Fig. 2A). This inhibition is evident in the quantification of the specific activity (picomoles of phosphate transferred per minute per milligram protein) of basal PKC in the presence of the inhibitor, GF109203X (vehicle = 7.5 ± 0.1; GF109203X + vehicle = 1.3 ± 0.3).
Figure 2.
Progesterone modulation of PKC basal and total activities (A) and protein (B) in the POA. A, POA homogenates were assayed for PKC basal and total activities in the absence and the presence of PKC inhibitor, GF109203X as described in Materials and Methods. The PKC activity is expressed as percentage of vehicle control (mean ± sem; 100%). Statistical analysis using ANOVA followed by Dunnett’s post hoc multiple comparisons indicated statistically significant basal PKC activity for EB+P treatment (**, P < 0.001), compared with V (n = 6 for each treatment group). B, Western immunoblotting for PKC protein. Each lane represents the sample from one animal per treatment group. A representative autoradiograph is shown. The GAPDH band at 37 kDa was used as a normalizing control. Lane 1, Vehicle control (V); lane 2, EB; lane 3, P; lane 4, EB+P treatments. Magic marker molecular weight standards were run alongside the protein samples and the molecular weights are noted adjacent to lane 1.
Progesterone attenuates Ca+2-inducible total PKC activity in the POA
The total PKC activity quantified in vitro in the presence of saturating amounts of the cofactors, PS, DAG, and Ca+2 (Fig. 2A) was comparable in the EB and vehicle treatments. P alone, or in combination with EB priming, caused a significant reduction in the total PKC activity (*, P = P < 0.01; #, EB+P = P < 0.05). The specific activity of the vehicle control was calculated to be 145 ± 0.1 pmol phosphate transferred per minute per milligram protein. This value was comparable with the specific activity in the VMN (132.2 pmol phosphate transferred per minute per milligram protein).
Progesterone has no effect on PKC protein levels or phosphorylation in POA
To determine whether the changes in the PKC activities could be due to alterations in the protein levels or changes in phosphorylation of PKC, POA extracts were subjected to immunoblotting (Fig. 2B). Similar to that observed in the VMN samples, the 80-kDa band was the most intense in POA samples, suggesting that cPKC is the predominantly active species that has stronger affinity to the cPKC antibody. As in the VMN, the POA extracts showed no quantifiable changes in the cPKC isoform (80 kDa) protein levels, as a result of EB and/or P treatments when normalized to GAPDH immunoreactivity (Table 1). Additionally, the ratio of the phosphorylated PKC (p514Thr) to the total pool of PKC showed no significant changes between various treatments, suggesting that EB and/or P treatments did not modify this phosphorylation status of PKC (Table 2).
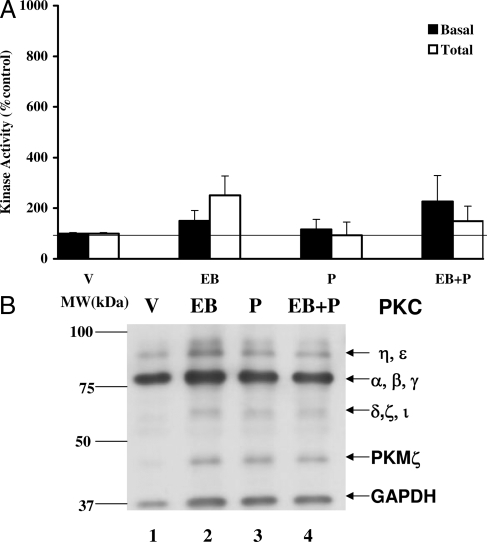
Progesterone has no effect on PKC activity in the cortex
We also analyzed the cerebral cortical tissues for both basal and Ca+2-inducible total PKC activities (Fig. 3A). EB and/or P had no significant effects on basal PKC activity, compared with the vehicle control. In these analyses, the specific activity of the basal PKC in vehicle control was calculated to be 11.8 ± 0.1 pmol phosphate transferred per minute per milligram. Ca+2-inducible total PKC activity was also not significantly affected by EB and/or P treatments in the cerebral cortex (P > 0.05). The total PKC activity in the cortex of the control group was 144.7 pmol phosphate transferred per minute per milligram protein. Furthermore, Western immunoblots for cPKC protein and phospho-PKC revealed no significant changes in either the protein levels or the phosphorylation due to the EB and/or P (Fig. 3B).
Figure 3.
P modulation of PKC basal and total activities (A) and protein (B) in the CTX. A, Cortical homogenates were assayed for basal and total PKC activities. The activity is expressed as percentage of vehicle control (mean ± sem; 100%). ANOVA followed by post hoc analyses using Dunnett’s or Newman-Keuls multiple comparison tests indicated no significant differences between the treatment groups and the vehicle (P > 0.05). B, The homogenates were subjected to Western immunoblot analysis. A representative autoradiograph is shown. The GAPDH was used as a normalizing control. Lane 1, Vehicle control (V); lane 2, EB; lane 3, P; lane 4, EB+P treatments. Magic marker molecular weight standards were run alongside the protein samples and the molecular weights are noted adjacent to lane 1.
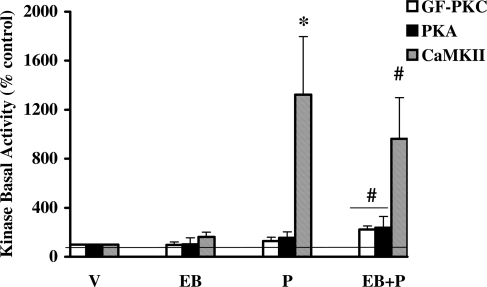
Inhibition of PKC activity by GF109203X did not block P-initiated basal activity of PKA and CaMKII in the VMN
Our finding that GF109203X blocked the increase in P-initiated basal PKC activity (Fig. 1B) prompted the following questions: 1) whether P stimulation of kinase activity was restricted to PKC alone and 2) whether the inhibition of PKC activity could influence the activities of the other P-activated kinases, PKA and CaMKII, known to be involved in P-facilitated reproductive behaviors. We therefore examined the basal activities of PKA (34) and CaMKII (another major calcium regulated Ser/Thr kinase abundant in the brain) (52) in the VMN homogenates from hormone-treated animals that received the PKC inhibitor in vivo (Fig. 4). The results shown in Fig. 4 indicate that the basal activities of PKA and CaMKII remain capable of being activated in the presence of GF109203X. The specific activities of basal and total PKA in the vehicle control in the presence of the PKC inhibitor were 1.8 ± 0.7 and 1.1 ± 0.4 pmol of phosphate transferred per minute per milligram, respectively. However, the specific activities of the basal and total PKA in the vehicle-treated animals were 123.5 ± 29.7 and 240.3 ± 44.6, in the absence of any inhibitor. Therefore, we find that the fraction of activated PKA was greater in the presence of GF109203X than in its absence (164 vs. 22%), albeit the overall individual activities were down.
Figure 4.
PKC inhibitor, GF109203X (GF), inhibited P-regulated PKC basal activity but not PKA and CaMKII in the VMN. GF-treated VMN homogenates were assayed for basal PKC, PKA, and CaMKII activities. The PKC activity was normalized to the vehicle in the presence of GF and expressed as percentage of the respective control (mean ± sem; 100%). ANOVA followed by post hoc Dunnett’s multiple comparisons demonstrated significant difference in CaMKII (*, P < 0.01) basal activity between P+GF and vehicle treatment. There was also a significant difference between EB+P and vehicle control in PKA (#, P < 0.05), PKC (#, P < 0.05), and CaMKII (#, P < 0.05) basal activities, respectively, in the GF treatment groups (n = 6 for each of treatment group).
The specific activity of the vehicle control was 0.9 ± 0.2 and 12.4 ± 0.1 pmol of phosphate transferred per minute per milligram in the presence and absence of the PKC inhibitor. The CaMKII total activity, calculated in the presence of calcium and calmodulin, were equal to 82.2 ± 12.1 and 151.2 ± 9.2 pmol of phosphate transferred per minute per milligram in the presence and absence of GF109203X, respectively. The ratio of CaMKII basal to total activities in the vehicle control in the presence of GF demonstrated that only a fraction of the CaMKII was activated in response to P administration (12%) within 30 min. This basal to total ratio for CaMKII activity was also greater in the absence of GF109203X (7%; CaMKII MS).
Discussion
The combinatorial action of PKA, CaMKII, and PKC signaling cascades in response to a number of plasticity-inducing stimuli are well documented (53,54,55,56,57,58,59,60). For example, the PKC and CaMKII cascades in the hippocampus have been shown to be both necessary and sufficient for the maintenance of long-term potentiation and hippocampal-dependent learning and memory (42). PKC activation, in conjunction with PKA, has been demonstrated in the consolidation of long-term memory (43,47). Studies from our laboratory have previously demonstrated that P initiates a rapid, nonclassical activation of cAMP-dependent PKA in the VMN of the female rat brain that is required for the P-facilitated lordosis response in female rats (34). Because P is likely to activate several intracellular signaling pathways, we questioned whether P, in the absence or presence of EB priming, can lead to the activation of other, nonclassical cascades within the VMN and/or POA. We determined both the Ca2+-independent basal PKC activity and the Ca+2-dependent total PKC activity in the VMN and POA in response to P. The former is a measure of the activated PKC in response to in vivo steroid hormone treatment. The latter represents the total pool of PKC in the milieu that can be activated further by saturating levels of cofactors and calcium in vitro. By measuring both the basal and total activities, the fraction of PKC activated by P at the specific time point could be determined. Furthermore, an increase in total activity in response to steroids suggests the possibility for sustained P action.
We report that icv administration of P initiated a rapid increase (30 min) in basal PKC activity within the VMN that could be significantly enhanced and sustained at 60 min, independent of EB priming. In contrast, EB priming amplified the P-dependent PKC activity, resulting in a robust increase within 30 min, which subsequently declined to the levels achieved by P at 60 min. This suggests that P activation of PKC signaling cascade is initiated in the VMN and is enhanced by EB priming. Consistent with this suggestion, Kow et al. (30) and Mobbs et al. (31) demonstrated that targeted infusion of the PKC activator 12-O-tetradecanoyl phorbol 13-acetate into the VMN of estrogen-primed rats could facilitate lordosis response. Additionally, the lack of change in total PKC activity at 30 and 60 min in P alone treatment demonstrated a maximal induction of PKC by P at these time points. EB+P-induced basal activity was maximal at 30 min as demonstrated by the lack of change in total PKC activity at the 30 min time point. By 60 min there was a residual total PKC activity that was significantly different from the vehicle or EB alone. In mechanistic terms, we infer that EB priming increases the fraction of PKC that can be further activated by P. Thus, these studies also distinguish the cellular effects of EB or P from that of EB+P. In summary, these observations suggest that the rapid activation of PKC within the VMN may be required for the P activation of intracellular signaling cascades that could provide alternate pathways to the subsequent PR-dependent lordosis response.
In contrast to that observed in the VMN, P treatment alone had no effect on basal PKC activity within the POA but required EB priming for a significant rapid but short-lived burst of activity. EB treatment, in the absence of P, showed no significant effects in basal PKC activity in both the POA and VMN. However, EB priming appears to enhance and sustain the P-stimulated basal PKC activity in both the VMN and POA, supporting the prevalent notion that EB priming is essential for physiological action. This finding is consistent with that reported by Ansonoff and Etgen (32), who demonstrated an increase in PKC catalytic activity in the POA, but not in the medial basal hypothalamus, in tissue slices taken from EB-primed female rats.
We also found that P caused a significant decrease in total PKC activity within the POA, compared with vehicle-treated controls. This attenuation was not observed in the VMN, suggesting a differential response of these two brain regions to P treatment. The attenuation of total PKC activity in the POA was observed as a result of the in vitro application of saturating levels of the cofactors phosphatidyl serine, DAG, and calcium and suggests that the amount of PKC available for activation within the milieu had changed in response to P treatment. Because this change could have resulted from the redistribution, degradation, or phosphorylation of PKC, we examined whether either a change in total protein levels or phosphorylation of Thr514, a key phosphorylation event in the activation of PKC (35,36,61), was associated with our observed decrease in total PKC activity. Although increases in PKCα mRNA in single neurons from the VMN of EB-treated ovariectomized rats (62) have been observed, our Western blot analysis did not reveal any significant differences in the levels of the α, β, and γ cPKC in the POA in response to either P alone, EB priming, or EB priming followed by P. Similarly, there was no observable change in the phosphorylation of Thr514. Whereas these results suggest that neither de novo protein turnover nor translocation is responsible for the P-mediated suppression of total PKC activity within the POA, we cannot rule out changes in one or more of the other phosphorylation events that are required for PKC activity (61).
Our results demonstrate that PKC, in addition to our previous report on PKA, is rapidly activated in the VMN and POA. Others and we have previously demonstrated that inhibition of PKA activity by Rp-diastereomer of adenosine-3′, 5′-cyclic monophosphothioate or PKC by bisindolymaleimide I hydrochloride (30,34) blocks the lordosis response in EB-primed female rats, demonstrating the necessity of these two cascades in sexual receptivity. Furthermore, the inhibitor studies suggest that these P-stimulated activities are independent yet interdependent, suggesting that multiple levels of cross talk can exist between these P-initiated kinase cascades. It remains to be established how the interplay of these pathways contribute to neuroendocrine function and female reproductive behavior. For example, PKA has been shown to phosphorylate, and alter the activity of dopamine- and cAMP-regulated phosphoprotein 32 kDa, a protein critical for lordosis response in EB-primed female rats (34). It is also possible that these rapid signaling events potentially converge in the nucleus to integrate into PR-mediated gene-regulated pathways (5,63). Future research will help identify the targets of these kinase cascades and provide further insight into the role of these nonclassical signaling cascades that act in concert with the genomic mechanisms to regulate the P-facilitated reproductive behavior.
Recent studies have revealed several mechanisms mediating the rapid P effects both in vivo and in vitro (64,65,66). Some of these effects are thought to be mediated via G protein-coupled membrane PRs (64,65) and/or mediated by a subpopulation of the intracellular PRs that associate with Src signaling kinases in the cytoplasm (66). Whereas the membrane receptors have been localized to the medial basal hypothalamus in the mouse brain (67), the presence of these in the VMN and POA in the female rat brain remains to be established. In addition, the association of the Src signaling cascade with intracellular PRs requires further investigation. Whereas the current studies highlight the importance of P-mediated kinase cascades in the nonclassical mechanism of P action, the integration of these mechanisms with the PR-mediated genomic pathway modulating the lordosis response is under investigation. The integration of nonclassical and classical actions could provide a mechanism through which the neuronal responses to diverse stimuli such as steroid hormones, neurotransmitters and environmental cues could alter physiology and reproductive behavior.
Acknowledgments
Antibodies to GAD67 and 2′, 3′-cyclic nucleotide 3′-phosphodiesterase were kindly donated by Dr. Cecilia Ljungberg (Children’s Nutrition Research Center, Houston, TX). We thank Dr. Dinakar Iyer for providing the PKC antibodies to pan-PKC (Baylor College of Medicine, Houston, TX). The kinase peptide substrates were synthesized by Dr. Flora (Biomedical Instrumentation Center, Uniformed Services University of Health Sciences, Bethesda, MD).
Footnotes
This work was supported by the National Institutes of Health Grants MH-63954 (to S.K.M.) and NS053588, NS049160, and MH072933 (to P.K.D.).
Disclosure Statement: The authors have nothing to declare.
First Published Online July 10, 2008
Abbreviations: CaMKII, Calmodulin-dependent protein kinase II; cPKC, conventional PKC; CTX, cerebral cortex; DAG, diacylglycerol; DTT, dithiothreitol; E2, estradiol; EB, estradiol benzoate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; icv, intracerebroventricular; NG, neurogranin; P, progesterone; PKA, protein kinase A; PKC, protein kinase C; PKM, protein kinase M; POA, preoptic area; PR, progestin receptor; PS, phosphatidyl serine; VMN, ventromedial nucleus of the hypothalamus.
References
- Pfaff DW, Schwartz-Giblin S, McCarthy MM, Kow LM 1994 Cellular and molecular mechanisms of female reproductive behavior In: Knobil E, Neill JD, eds. Physiology of reproduction. New York: Raven Press; 107–220 [Google Scholar]
- Pfaff DW 2005 Hormone-driven mechanisms in the central nervous system facilitate the analysis mammalian behavior. J Endocrinol 184:447–453 [DOI] [PubMed] [Google Scholar]
- Ronnnekleiv OK, Kelly MJ 2005 Diversity of ovarian steroid hormones in the hypothalamus. Front Neuroendocrinol 26:65–84 [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Erskine MS 2002 Sexual behavior: cellular integration of hormonal and afferent information In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, eds. Hormones, brain and behavior. Vol 1. San Diego: Academic Press; 139–214 [Google Scholar]
- Blaustein JD, Mani SK 2007 Feminine sexual behavior from the neuroendocrine and molecular neurobiological perspectives In: Blaustein JD, ed. Lajtha A, series ed. Handbook of neurochemistry and molecular neurobiology: behavioral neurochemistry and neuroendocrinology. Vol 21. Berlin, Heidelberg: Springer-Verlag; 95–150 [Google Scholar]
- Ogawa S, Olazábal UE, Parhar IS, Pfaff DW 1994 Effects of intrahypothalamic administration of antisense DNA for progesterone receptor mRNA on reproductive behavior and progesterone receptor immunoreactivity in female rat. J Neurosci 14:1766–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollio G, Xue P, Zainisi A, Nicolin A, Maggi A 1993 Antisense oligonucleotide blocks progesterone-induced lordosis behavior in ovariectomized rats. Mol Brain Res 19:135–139 [DOI] [PubMed] [Google Scholar]
- Mani SK, Blaustein JD, Allen JM, Law SW, O'Malley BW, Clark JH 1994 Inhibition of rat sexual behavior by antisense oligonucleotides to the progesterone receptor. Endocrinology 135:1409–1414 [DOI] [PubMed] [Google Scholar]
- Mani SK, Allen JM, Lydon JP, Mulac-Jericevic B, Blaustein JD, DeMayo FJ, Conneely OM, O'Malley BW 1996 Dopamine requires the unoccupied progesterone receptor to induce sexual behavior in mice. Mol Endocrinol 10:1728–1737 [DOI] [PubMed] [Google Scholar]
- Parsons B, MacLusky NJ, Krey L, Pfaff DW, McEwen BS 1980 The temporal relationship between estrogen-inducible PRs in the female rat brain and the time course of estrogen activation of mating behavior. Endocrinology 107:774–779 [DOI] [PubMed] [Google Scholar]
- Etgen A 1984 Progestin receptors and the activation of female reproductive behavior: a critical review. Horm Behav 18:411–430 [DOI] [PubMed] [Google Scholar]
- Mani SK, Blaustein JD, O'Malley BW 1997 Progesterone receptor function from a behavioral perspective. Horm Behav 31:244–255 [DOI] [PubMed] [Google Scholar]
- Lisk RD 1960 A comparison of the effectiveness of intravenous as opposed to subcutaneous injection of progesterone for the induction of estrous behavior in the rat. Can J Biochem Physiol 38:1381–1383 [PubMed] [Google Scholar]
- Kubli-Garfias C, Whalen RE 1977 Induction of lordosis behavior in female rats by intravenous administration of progestins. Horm Behav 9:380–386 [DOI] [PubMed] [Google Scholar]
- Meyerson B 1972 Latency between intravenous injection of progestins and the appearance of oestrous behavior in estrogen-treated ovariectomised rats. Horm Behav 3:1–10 [DOI] [PubMed] [Google Scholar]
- Schumacher M, Coirini H, Pfaff DW, McEwen BS 1990 Behavioral effects of progesterone associated with rapid modulation of oxytocin receptors. Science 250:691–694 [DOI] [PubMed] [Google Scholar]
- Schumacher M, Coirini H, Robert E, Guennoun R, El-Etr M 1999 Genomic and membrane actions of progesterone: implications for reproductive physiology and behavior. Behav Brain Res 105:37–52 [DOI] [PubMed] [Google Scholar]
- Beyer C, Gonzalez-Flores O, Garcia-Juarez M, Gonzalez-Mariscal G 2003 Non-ligand activation of estrous behavior in rodents: cross-talk at the progesterone receptor. Scand J Psychol 44:221–229 [DOI] [PubMed] [Google Scholar]
- Leonhardt SA, Boonyaratanakornkit V, Edwards DP 2003 Progesterone receptor transcription and non-transcription signaling mechanisms. Steroids 68:761–770 [DOI] [PubMed] [Google Scholar]
- Mani SK, O'Malley BW 2002 Molecular mechanisms of Progesterone receptor action. In: Pfaff DW, Etgen AM, Fahrbach SE, Rubin RT, eds. Hormones, brain and behavior. Vol 3. San Diego: Academic Press; 643–682 [Google Scholar]
- DeBold JF, Frye CA 1994 Genomic and non-genomic actions of progesterone in the control of female hamster sexual behavior. Horm Behav 28:445–453 [DOI] [PubMed] [Google Scholar]
- DeBold JF, Frye CA 1994 Progesterone and the neural mechanisms of hamster sexual behavior. Psychoneuroendocrinology 19:563–579 [DOI] [PubMed] [Google Scholar]
- Jones KJ, Pfaff, DW, McEwen BS 1987 Hormonal control of sexual behavior in the female rat: molecular, cellular and neurochemical studies. Biol Reprod 36:37–45 [DOI] [PubMed] [Google Scholar]
- Limbird LE 1981 Activation and attenuation of adenylate cyclase: the role of GTP binding proteins as macromolecular messengers in receptor-cyclase coupling. Biochem J 195:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen RE, Lauber AH 1986 Progesterone substitutes: cGMP mediation. Neurosci Biobehav Rev 10:47–53 [DOI] [PubMed] [Google Scholar]
- Beyer C, Canchola E, Larsson K 1981 Facilitation of progesterone induced lordosis behavior by phosphodiesterase inhibitors in estrogen primed rats. Physiol Behav 27:731–733 [DOI] [PubMed] [Google Scholar]
- Beyer C, González-Mariscal G 1986 Elevation in hypothalamic cyclic AMP as a common factor in the facilitation of lordosis in rodents: a working hypothesis. Ann NY Acad Sci 474:270–281 [DOI] [PubMed] [Google Scholar]
- Chu HP, Etgen AM 1998 A potential role of cyclic GMP in the regulation of lordosis behavior of female rats. Horm Behav 32:125–132 [DOI] [PubMed] [Google Scholar]
- Kow LM, Brown HE, Pfaff DW 1994 Activation of protein kinase C in the hypothalamic ventromedial nucleus or the midbrain central gray facilitates lordosis. Brain Res 660:241–248 [DOI] [PubMed] [Google Scholar]
- Kow LM, Mobbs CV, Pfaff DW 1994 Roles of second messenger systems and neuronal activity in the regulation of lordosis by neurotransmitters, neuropeptides and estrogen: a review. Neurosci Biobehav Rev 18:251–268 [DOI] [PubMed] [Google Scholar]
- Mobbs CV, Rothfeld JM, Saluja R, Pfaff DW 1989 Phorbol esters and forskolin infused into midbrain central gray facilitate lordosis. Pharmacol Biochem Behav 34:665–667 [DOI] [PubMed] [Google Scholar]
- Ansonoff MA, Etgen MA 1998 Estradiol elevates protein kinase C catalytic activity in the preoptic area of female rats. Endocrinology 139:3050–3056 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Flores O, Ramirez-Orduna JM, Lima-Hernandez FJ, Garcia-Juarez M, Beyer C 2006 Differential effect of kinase A and C blockers on lordosis facilitation by progesterone and its metabolites in ovariectomized estrogen-primed rats. Horm Behav 49:398–404 [DOI] [PubMed] [Google Scholar]
- Mani SK, Fienberg AA, O'Callaghan JP, Snyder GL, Allen PB, Dash PK, Moore AN, Mitchell AJ, Bibb J, Greengard P, O'Malley BW 2000 Requirement for DARPP-32 in progesterone-facilitated sexual receptivity in female rats and mice. Science 287:1053–1056 [DOI] [PubMed] [Google Scholar]
- Shirai Y, Sakai N, Saito N 1998 Subspecies-specific targeting mechanism of protein kinase C. Jap J Pharmacol 78:411–4177 [DOI] [PubMed] [Google Scholar]
- Mellor H, Parker PJ 1998 The extended protein kinase C superfamily. Biochem J 332:281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton A 2003 Regulation of the ABC kinases by phosphorylation: protein kinase C as paradigm. Biochem J 370:361–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez AI, Blace N, Crary JF, Serrano PA, Leitges M, Libien JM, Weinstein G, Tcherapanov A, Sacktor TC 2003 Protein kinase Mζ synthesis from a brain mRNA encoding an independent protein kinase Cζ catalytic domain: implications for the molecular mechanism of memory. J Biol Chem 278:40305–40316 [DOI] [PubMed] [Google Scholar]
- Oster H, Eichele G, Leitges M 2004 Differential expression of atypical PKCs in the adult mouse brain. Mol Brain Res 127:79–88 [DOI] [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H 1991 The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem 266:15771–15781 [PubMed] [Google Scholar]
- Seki T, Matsubayashi H, Amano T, Shirai Y, Saito N, Sakai N 2005 Phosphorylation of PKC activation loop plays an important role in receptor-mediated translocation of PKC. Genes Cells 10:225–239 [DOI] [PubMed] [Google Scholar]
- Sweatt JD 2003. Biochemical mechanisms for short-term information storage at the cellular level. In: Sweatt JD, ed. The mechanisms of memory. San Diego: Academic Press; 194–232 [Google Scholar]
- Wang JH, Kelly PT 1995 Postsynaptic injection of CA2+/CaM induces synaptic potentiation requiring CaMKII and PKC activity. Neuron 15:443–452 [DOI] [PubMed] [Google Scholar]
- Meyer T, Shen S 2000 In and out of the postsynaptic region: signalling proteins on the move. Trends Cell Biol 10:238–244 [DOI] [PubMed] [Google Scholar]
- Saito N, Shirai Y 2002 Protein kinase Cγ (PKCγ): function of neuron specific isotype. J Biochem (Tokyo) 132:683–687 [DOI] [PubMed] [Google Scholar]
- Wilkinson SE, Parker PJ, Nixon JS 1993 Isoenzyme specificity of bisindolylmaleimides selective inhibitors of protein kinase C. Biochem J 294:335–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klann E, Chen SJ, Sweatt JD 1993 Mechanism of protein kinase C activation during the induction and maintenance of long-term potentiation probed using a selective peptide substrate. Proc Natl Acad Sci USA 90:8337–8341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RR, Kwon YG, Taing M, Lawrence DS, Edelman AM 1998 Definition of optimal substrate recognition motifs of Ca2+-calmodulin-dependent protein kinases IV and II reveals shared and distinctive features. J Biol Chem 273:3166–3172 [DOI] [PubMed] [Google Scholar]
- Moore AM, Waxham MN, Dash PK 1996 Neuronal activity increases the phosphorylation of the transcription factor cAMP response element-binding protein (CREB) in rat hippocampus and cortex. J Biol Chem 14214–14220 [DOI] [PubMed] [Google Scholar]
- Laemmli UK 1970 Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J 1979 Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith LC, Liu CS, Sun XX 2003 CamK—an enzyme on the move: regulation of temporospatial localization. Mol Interventions 3:365–403 [DOI] [PubMed] [Google Scholar]
- Matsumoto S, Shamloo M, Matsumoto E, Isshiki A, Wieloch T 2004 Protein kinase C-γ and calcium/calmodulin-dependent protein kinase II-α are persistently translocated to cell membranes of the rat brain during and after middle cerebral artery occlusion. J Cereb Blood Flow Metab 24:54–61 [DOI] [PubMed] [Google Scholar]
- Selcher JC, Weeber EJ, Varga AW, Sweatt JD, Swank M 2002 Protein kinase signal transduction cascades in mammalian associative conditioning. Neuroscientist 8:122–131 [DOI] [PubMed] [Google Scholar]
- Micheau J, Riedel G 1999 Protein kinases: which one is the memory molecule? Cell Mol Life Sci 55:534–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleegal MA, Sumners C 2003 Drinking behavior elicited by central injection of angiotensin II: roles for protein kinase C and Ca2+/calmodulin-dependent protein kinase II. Am J Physiol Regul Integr Comp Physiol 285:R632–R640 [DOI] [PubMed] [Google Scholar]
- Mani S 2006 Signalling mechanisms in progesterone-neurotransmitter interactions. Neuroscience 138:773–774 [DOI] [PubMed] [Google Scholar]
- Mani S 2003 G protein-coupled receptor signalling in neuroendocrine systems: signalling mechanisms in progesterone-neurotransmitter interactions. J Mol Endocrinol 30:127–137 [DOI] [PubMed] [Google Scholar]
- Naor Z, Harris D, Shacham S 1998 Mechanism of GnRH receptor signaling: combinatorial cross talk of Ca2+ and PKC. Front Neuroendocrinol 19:1–19 [DOI] [PubMed] [Google Scholar]
- MacNicol M, Schulman H 1992 Cross talk between protein kinase C and multifunctional Ca2+/calmodulin-dependent protein kinase. J Biol Chem 267:12197–12201 [PubMed] [Google Scholar]
- Cameron AJ, De Rycker M, Calleja V, Alcor D, Kjaer S, Kostelecky B, Saurin A, Faisal A, Laguerre M, Hemmings BA, McDonald N, Larijani B, Parker PJ 2007 Protein kinases from B to C. Biochem Soc Trans 35:1013–1017 [DOI] [PubMed] [Google Scholar]
- Devidze N, Mong JA, Jasnow AM, Kow LM, Pfaff DW 2005 Sex and estrogenic effects on coexpression of mRNAs in single ventromedial hypothalamic neurons. Proc Natl Acad Sci USA 102:14446–14451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Kow LM, Pfaff DW 2005 Integration of steroid hormone initiated membrane action to genomic function in the brain. Steroids 70:388–396 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Rice CD, Pang Y, Pace M, Thomas P 2003 Cloning, expression and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA 100:2231–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Bond J, Thomas P 2003 Identification, classification and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci USA 100:2237–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonyaratanakornkit V, Bi Y, Rudd M, Edwards DP 2008 The role and mechanism of progesterone receptor activation of extra-nuclear signaling pathways in the regulating gene transcription ad cell cycle progression. Steroids 73:922–928 [DOI] [PubMed] [Google Scholar]
- Ashley RL, Clay CM, Farmerie GD, Niswender G, Nett TM 2006 Cloning and characterization of an ovine intracellular seven transmembrane receptor for progesterone that mediates calcium mobilization. Endocrinology 147:4151–4159 [DOI] [PubMed] [Google Scholar]