Abstract
We have used homologous recombination to disrupt the mouse gene coding for the NaK2Cl cotransporter (NKCC2) expressed in kidney epithelial cells of the thick ascending limb and macula densa. This gene is one of several that when mutated causes Bartter's syndrome in humans, a syndrome characterized by severe polyuria and electrolyte imbalance. Homozygous NKCC2−/− pups were born in expected numbers and appeared normal. However, by day 1 they showed signs of extracellular volume depletion (hematocrit 51%; wild type 37%). They subsequently failed to thrive. By day 7, they were small and markedly dehydrated and exhibited renal insufficiency, high plasma potassium, metabolic acidosis, hydronephrosis of varying severity, and high plasma renin concentrations. None survived to weaning. Treatment of −/− pups with indomethacin from day 1 prevented growth retardation and 10% treated for 3 weeks survived, although as adults they exhibited severe polyuria (10 ml/day), extreme hydronephrosis, low plasma potassium, high blood pH, hypercalciuria, and proteinuria. Wild-type mice treated with furosemide, an inhibitor of NaK2Cl cotransporters, have a phenotype similar to the indomethacin-rescued −/− adults except that hydronephrosis was mild. The polyuria, hypercalciuria, and proteinuria of the −/− adults and furosemide-treated wild-type mice were unresponsive to inhibitors of the renin angiotensin system, vasopressin, and further indomethacin. Thus absence of NKCC2 in the mouse causes polyuria that is not compensated elsewhere in the nephron. The NKCC2 mutant animals should be valuable for uncovering new pathophysiologic and therapeutic aspects of genetic disturbances in water and electrolyte recovery by the kidney.
Keywords: gene targeting, NaK2Cl cotransporter, nonsteroidal anti-inflammatory drug, hydronephrosis
Bartter's syndrome, characterized by hypokalemic metabolic alkalosis, hyperreninemia, polyuria, and dehydration (1), includes several disorders of water and electrolyte handling by the kidney that share many physiologic derangements but differ in others (2, 3). Classic Bartter's syndrome is characterized by onset during infancy or early childhood, delayed growth, polyuria, hypokalemia, hyperreninemia, hypercalciuria, and high prostaglandin (PG) excretion in the urine (3). An antenatal variant exhibits a more severe phenotype, prematurity, hydramnios, massive polyuria, life-threatening episodes of dehydration, growth retardation, very high urinary PG, and calcium excretion and nephrocalcinosis (3–5). Gitelman's syndrome, although similar to Bartter's syndrome, is more frequent and differs in showing hypomagnesemia, hypocalciuria, normal urinary PG, and onset during childhood or adolescence (2, 6). Defects in genes coding for three proteins expressed in the thick ascending limb of Henle's loop of the kidney—the NKCC2 NaK2Cl cotransporter (7), the CLCNKB chloride channel (8), and the ROMK potassium channel (9)—have been implicated as causing antenatal Bartter's syndrome. Our present work is concerned with NKCC2.
The kidney-specific NaK2Cl cotransporter (NKCC2 or BSC1) (10, 11) is strongly expressed on the luminal membrane of renal tubular epithelial cells of the thick ascending limb of Henle's loop (12, 13), where it is essential for normal Na, K, Cl, Ca, and Mg reabsorption (14) and for the maintenance of the corticomedullary osmotic gradient, a key element in the urine concentrating mechanism (14). NKCC2 also is expressed in the specialized cells of the distal tubules that constitute the macula densa (15, 16). Macula densa cells sense the chloride concentration of the fluid in the adjacent lumen and, depending on this concentration, control the filtration rate of the connected upstream glomerulus (a mechanism known as tubuloglomerular feedback). In addition, the macula densa controls the secretion of renin from nearby juxtaglomerular cells (17). The signaling mechanism is not well understood.
To provide an animal model in which the complex pathophysiology of Bartter's syndrome can be related to the distribution and ionic functions of the NKCC2 cotransporter, we have used homologous recombination to inactivate the gene (Slc12a1) that codes for this protein in mice. The resulting mice have many similarities to humans with Bartter's syndrome.
Materials and Methods
Generation of Mutant Mice.
The NKCC2 gene was cloned from a strain 129/SvEvTac murine genomic DNA library. Gene targeting, as described (18), was used to disrupt the NKCC2 gene. The targeting plasmid, illustrated in Fig. 1a, was electroporated into the TC-1 embryonic stem cell line (19) derived from mouse strain 129/SvEvTac FBR. Targeting was confirmed by Southern blot analysis of cell DNA digested with BamHI and hybridized to probe a (Fig. 1a). Targeted clones have a 7.2-kb band in addition to an 18-kb endogenous band. Male chimeras carrying the disrupted allele were mated with 129/SvEv females to obtain animals with a genetically uniform background. Mice were handled by following the National Institutes of Health guidelines for the use and care of experimental animals.
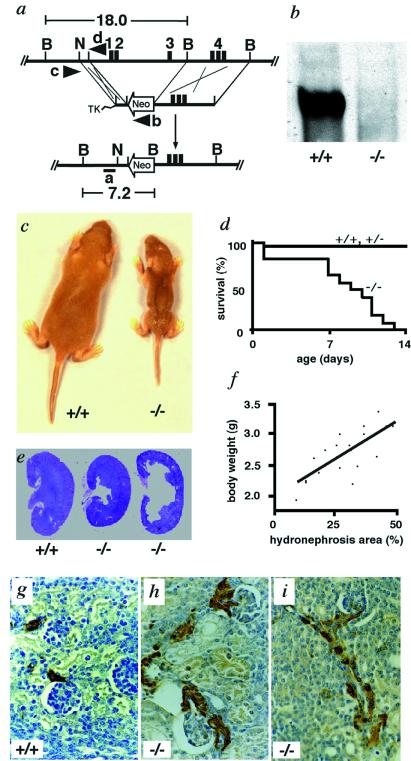
Figure 1.
Targeted inactivation of the NKCC2 gene and analysis of NKCC2−/− pups. (a) Gene targeting strategy. (Top) The region of the NKCC2 gene extending from the promoter to intron 4. (Exon 4 has three parts, 4B, 4A, and 4F.) (Middle) Targeting construct. (Bottom) Targeted allele in which the gene is disrupted and 12 kb from 2.5 kb upstream of transcription initiation site to part of intron 3 is replaced with the neomycin resistance gene, Neo. TK, Herpes simplex virus-thymidine kinase gene. 1–4 are exons 1–4. Restriction sites are B, BamHI; N, NheI. The positions of probe a and primers b–d are indicated. (b) Western blot analysis of kidney homogenate from +/+ and −/− mice. The blot was exposed to anti-NKCC2 antibody. (c) Growth retardation and poor turgor of −/− mice. Wild-type (+/+) (Left) and homozygous mutant mouse (−/−) (Right) from the same litter at 7 days after birth. (d) Mortality rate of −/− mice and control mice, +/+ and +/−. Dead animals were collected daily and genotyped. +/+, n = 15; +/−, n = 25; −/−, n = 13. (e) Kidneys from +/+ and −/− mice at 7 days of age stained with hematoxylin and eosin. (Original magnification: ×1.) (f) Correlation between severity of hydronephrosis and body weight of 7-day-old −/− pups. n = 19, correlation coefficient 0.7269, R2 = 0.528, P < 0.0001. (g–i) Renin immunohistochemistry with kidney sections from 7-day-old pups. (g) Renin staining (brown) is confined to the afferent arterioles in +/+. (h and i) Renin staining includes afferent arterioles and extends along them in the direction of the interlobular arteries. Efferent arterioles and glomeruli are also renin positive. (Original magnification: ×200.)
Genotyping.
Genotypes were determined by PCR with primers b, 5′-CTTCTATCGCCTTCTTGACG-3′; c, 5′-GCATCTTTACTCTTGGGAGC-3′; and d, 5′-CAATAGGCTGCTGAGATGAG-3′ (Fig. 1a). The presence of a 490-bp fragment, a 620-bp fragment, or both fragments identifies animals with the −/−, +/+, and +/− genotypes, respectively.
Indomethacin Protocol.
Subcutaneous injection of indomethacin (Sigma, 6 μg/g body weight, once a day) to all pups regardless of genotypes was started at 1 day after birth and continued for 3 weeks or until prior sacrifice.
Vasopressin Protocol.
Mice were given water containing 5% dextrose for 3 days before the experiment to ensure production of a relatively dilute urine. On the experimental day, urine was collected and 1 ng/g body weight of [desamino-Cys1, d-Arg8] vasopressin (DDAVP, desmopressin, Sigma) was injected i.p. Urine osmolality was measured at 1 and 2 h after the injection.
Analyses of Blood and Plasma Renin Concentration.
Blood samples were analyzed with a VT250 Chemical Analyzer (Johnson & Johnson) and Cobas Micro Hematology Analyzer (Roche Diagnostics). Plasma renin concentration was measured as described (20).
Analyses of Urine and Kidney Function.
Mice were maintained on a 12-h light/dark cycle and given water and food ad libitum. Body weight, amount of water and food intake, and urine excretion were measured every 24 h for 3 days. Osmolality of the urine and excretion of electrolyte and protein were measured (VT250 Chemical Analyzer) and normalized to a 20 g body weight. Urinary excretion of PGE2 was assayed with the PGE2 EIA system, RPN 222 (Amersham Pharmacia Biotech).
SDS/PAGE of Plasma and Urine Proteins.
Samples were preheated with β-mercaptoethanol for 5 min at 95°C, electrophoresed on a 4–15% gradient Tris⋅HCl SDS-polyacrylamide gel (Bio-Rad), and stained with Coomassie brilliant blue.
Western Blot Analyses.
Antibody against NKCC2 was a gift from Mark A. Knepper, (National Institutes of Health, Bethesda, MD), and was used as described (12).
Microscopy, Renin Immunohistochemistry, and Morphometric Analysis.
Immunohistochemistry for renin in kidney sections was performed as described (21, 22). Hydronephrosis was recorded for each kidney section as the percentage area of the pelvis relative to the total cross sectional area.
Statistics.
All values are expressed as mean ± SEM. Student's t test and tests of Pearson's correlation coefficient were used for statistical evaluations.
Results and Discussion
NKCC2 Mutant Mice.
Mice carrying a null allele of NKCC2 were generated from embryonic stem cells by deleting 12 kb of DNA that includes the promoter of the gene and exons 1–3 (Fig. 1a). Matings between +/− heterozygotes produced +/+, +/−, and −/− pups at the expected Mendelian ratios at birth (27 +/+, 49 +/−, and 24 −/−). Western blot analysis of kidney proteins showed that NKCC2 expression is absent in the −/− mice (Fig. 1b). Although hydramnios is common in human neonatal Bartter's syndrome (23), there was no obvious polyhydramnios during pregnancy in the −/− fetuses. Inspection of pups within 24 h after birth likewise revealed no obvious differences in appearance or body weight among the three genotypes. However, even by day 1, the −/− mice already showed signs of extracellular fluid volume depletion as judged by their hematocrits (50.8 ± 3.6% versus 37.0 ± 0.7% in wild type, n = 6, P < 0.01). By 7 days of age, the homozygotes were easily identified because of their poor turgor and wrinkled skin caused by fluid loss (Fig. 1c) and failure to thrive (body weight, on day 7, 3.2 ± 0.2 g versus 6.2 ± 0.4 g in wild type, n = 6, P < 0.0001). The −/− mice died within 2 weeks (Fig. 1d), and none of more than 100 −/− pups survived to weaning without treatment.
Blood Chemistry and Kidneys in 7-Day-Old NKCC2−/− Pups.
When mixed arterial and venous blood was collected at 7 days of age by decapitation, the NKCC2−/− mice proved significantly different from wild type in all parameters measured (Table 1). Of these, the higher hematocrit, Na, Cl, and total protein are indicative of a dehydration. In contrast, total plasma Ca was significantly less than normal, as was ionized Ca (2.9 ± 0.3 mg/dl versus 4.9 ± 0.1 mg/dl in wild type, n = 5, P < 0.0001). Plasma creatinine of the −/− pups was about three times wild type. However, because their body weights were about half wild type, creatinine normalized by muscle mass would be much higher, indicating severe renal insufficiency. The −/− pups had an impaired ability to concentrate their urine as judged by its osmolality even in the setting of dehydration (370 ± 7 mOsm vs. 420 ± 25 mOsm in wild type, n = 6, P < 0.01). Different from adult mice (see below), the concentration of urine protein in the −/− and +/+ pups was very low, often below a detectable level (<5 mg/dl). The −/− pups had higher plasma K and lower HCO3 than normal. They exhibited metabolic acidosis associated with an elevated anion gap, Na-(Cl + HCO3). Some human patients with Bartter's syndrome also exhibit metabolic acidosis (24, 25).
Table 1.
Blood data of 7-day-old pups
| NKCC2 genotype
|
||
|---|---|---|
| +/+ | −/− | |
| pH | 7.49 ± 0.01 | 7.29 ± 0.04* |
| HCO3, mmol/liter | 27.3 ± 1.0 | 13.3 ± 1.0* |
| Base excess, mmol/liter | 3.2 ± 1.0 | −11.8 ± 1.5* |
| Lactate, mmol/liter | 2.6 ± 0.1 | 4.1 ± 0.2* |
| pCO2, mmHg | 39.0 ± 1.5 | 27.3 ± 0.8* |
| Glucose, mg/dl | 168.6 ± 9.7 | 81.0 ± 8.5† |
| Hematocrit, % | 37.0 ± 0.5 | 46.6 ± 0.7† |
| Na, mmol/liter | 131.9 ± 0.5 | 163.7 ± 3.6* |
| K, mmol/liter | 6.1 ± 0.4 | 7.2 ± 0.1‡ |
| Cl, mmol/liter | 100.3 ± 1.1 | 117.0 ± 3.2* |
| Ca, mg/dl | 10.7 ± 0.2 | 9.1 ± 0.7† |
| Mg, mg/dl | 2.3 ± 0.04 | 3.1 ± 0.06* |
| Total protein, mg/dl | 3.2 ± 0.1 | 3.9 ± 0.5† |
| Creatinine, mg/dl | 0.35 ± 0.03 | 0.90 ± 0.17* |
| BUN, mg/dl | 28.2 ± 3.3 | 194.3 ± 60.7* |
| PRC, ng Ang I/ml per h | 127.6 ± 47.8 | 25,700 ± 1,840* |
Data are means ± SEM. n > 6. pCO2, CO2 pressure; BUN, blood urea nitrogen; PRC, plasma renin concentration.
*P vs. +/+ < 0.0001.
†P vs. +/+ < 0.005.
‡P vs. +/+ < 0.05.
The −/− pups showed bilateral hydronephrosis of varying severity, as judged by an abnormal dilatation of the renal collecting system (pelvis and calyx) with different degrees of atrophy of the renal parenchyma (Fig. 1e). This hydronephrosis was sometimes present as early as at term in utero. We observed a positive correlation between the severity of hydronephrosis and body weight (Fig. 1f). This correlation was still observed when kidney weight was subtracted from the body weight. The stomachs of the −/− pups were frequently empty, and their blood glucose concentration was low (Table 1), whereas the stomachs of the +/+ and +/− pups were always full of milk. It is possible that some of the −/− pups compete better for milk, grow better, and excrete more urine, causing more severe hydronephrosis. It is also possible that the hydronephrosis protects against fluid loss by decreasing glomerular filtration. The frequently empty stomachs of the −/− pups indicated inadequate nutrition compared with the wild type and heterozygous litter mates and probably led to their dehydration and early death.
Distribution of Renin.
Immunohistochemistry of the −/− kidneys showed a very marked increase in the expression of renin along the afferent arterioles and interlobular arteries, in the intraglomerular region, and along the efferent arterioles (Fig. 1 g–i); this is consistent with the 200× normal plasma renin concentration of the mutants (Table 1). This distribution of renin strongly resembles that seen in human Bartter's syndrome (1). The cause of the extremely high plasma renin concentration is an increase in the proportion of vascular smooth muscle cells, pericytes, and mesangial cells that produce renin (26). This increase most likely occurs because the macula densa cells transduce a signal falsely indicating a very low amount of chloride in the lumen (17, 27), and because dehydration and volume depletion (1, 28) diminish the stretch of the arterioles.
Rescue of NKCC2−/− Pups.
We first attempted to correct the dehydration and volume depletion of the −/− pups by s.c. injection of 0.45% saline (100 μl/g body weight, once a day). Survival was not improved. Human patients with prenatal Bartter's syndrome show an increased synthesis of PGE2 throughout the body (29, 30), and cyclooxygenase (COX) inhibitors have commonly been used for their treatment. In some patients this treatment reduces urine volume and urinary excretion of calcium and improves growth (31–33). Given these findings, we s.c. injected 6 μg/g per day of indomethacin, a potent nonselective COX inhibitor, into neonatal mice of all three genotypes starting at day 1 after birth and continuing until 3 weeks of age.
By day 7, the indomethacin treatment caused a 2-fold decrease in plasma renin concentration in the +/+ and the −/− mice, from 127.6 ± 47.8 ng angiotensin (Ang) I/ml per h to 77.8 ± 9.7 ng Ang I/ml per h and from 25,700 ± 1,840 ng Ang I/ml per h to 13,000 ± 745 ng Ang I/ml per h (P < 0.0001). We could not use higher doses of indomethacin because of frequent stomach perforation. Some of the indomethacin-treated −/− mice, when killed at 7 days of age, were indistinguishable from wild type in body weight, plasma Na, K, Cl, and Cr, but they all developed hydronephrosis. Indomethacin enabled survival of some −/− mice to weaning at 3 weeks, but most (74/83, 90%) died. The surviving weanlings showed a catch-up growth that continued after indomethacin was no longer given. The turgor of the surviving −/− mice was not distinguishable from wild type. Almost half of these surviving indomethacin-treated −/− weanlings have lived more than 10 months, but all developed extreme bilateral hydronephrosis, leading to a massively enlarged, paper-thin kidney with a greatly dilated calyx and pelvis and an almost completely destroyed medulla (Fig. 2a). The cortex was diminished to a very thin rim. The tubules were focally dilated (Fig. 2b). The severe hydronephrosis in the surviving −/− adults is most likely because the flow of dilute urine in these animals exceeds the maximum capability of the ureter and causes excessive pressure in the renal pelvis. In contrast, the kidneys from wild-type mice treated with indomethacin until 3 weeks after birth were indistinguishable from the kidneys of untreated wild-type mice.
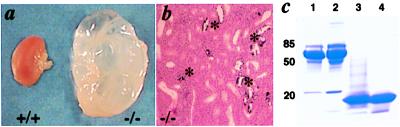
Figure 2.
Analysis of NKCC2−/− adult mice. (a) Kidneys from +/+ and −/− mice of the same litter at 5 months of age. Mice were treated with indomethacin to 3 weeks of age. (b) Kidney from −/− mouse 5 months of age stained with hematoxylin and eosin. *, Calcification in tubules. Note the focal dilatation of tubules. (Original magnification: ×100.) (c) Analysis of plasma and urine proteins from mice at 3 months of age. Lane 1, plasma from a wild-type male (1 μl); lane 2, plasma from a NKCC2−/− male (1 μl); lane 3, urine from a −/− female (20 μl of 5× concentrate); lane 4, urine from a −/− male (15 μl).
Blood/Urine Chemistry in Surviving NKCC2−/− Adult Mice.
Venous blood drawn from the retroorbital sinus of the −/− indomethacin-treated survivors at 3 months of age showed lower K, but pH and HCO3 were significantly above wild type (Table 2). Although the −/− mice that survived to weaning after 3 weeks of indomethacin had unlimited access to water and food and no longer had poor turgor, they had an extremely high hematocrit (70%) and a very high plasma renin concentration (Table 2), suggesting that they were still appreciably dehydrated. We do not know whether the high hematocrit is solely because of a decrease in plasma volume or whether excessive production of erythropoietin also is involved as is in the case of some human Bartter's syndrome patients (5). In the normal kidney, when a low Cl ion concentration is sensed by the macula densa, two major changes occur: glomerular filtration is increased, and renin is released from the adjacent juxtaglomerular cells (17, 27, 34). All our data are compatible with this Cl-sensing mechanism being disabled in the NKCC2−/− mice, so that the macula densa cells transmit the signal that normally results from a very low Cl ion concentration in the luminal fluid.
Table 2.
Blood data of adult mice
| NKCC2 genotype
|
||
|---|---|---|
| +/+ | −/− | |
| pH | 7.30 ± 0.01 | 7.42 ± 0.01* |
| HCO3, mmol/liter | 24.5 ± 1.4 | 30.6 ± 1.3† |
| Base excess, mmol/liter | −2.5 ± 0.9 | 4.9 ± 0.6* |
| Lactate, mmol/liter | 2.0 ± 0.4 | 2.2 ± 0.3 |
| pCO2, mmHg | 42.6 ± 3.6 | 48.2 ± 1.2 |
| Glucose, mg/dl | 199.6 ± 12.4 | 200.6 ± 11.6 |
| Hematocrit, % | 37.0 ± 3.2 | 70.0 ± 0.3* |
| Na, mmol/liter | 145.2 ± 0.6 | 154.8 ± 2.9† |
| K, mmol/liter | 5.8 ± 0.3 | 4.6 ± 0.1† |
| Ca, mg/dl | 9.8 ± 0.7 | 9.1 ± 1.4 |
| Creatinine, mg/dl | 0.15 ± 0.05 | 0.23 ± 0.09 |
| PRC, ng Ang I/ml per h | 32.4 ± 5.0 | 751.3 ± 76.8‡ |
Mice were 3 months old and treated with indomethacin to 3 weeks of age. pCO2, CO2 pressure; PRC, plasma renin concentration. Data are means ± SEM. n > 5.
*P vs. +/+ < 0.0005.
†P vs. +/+ < 0.05.
‡P vs. +/+ < 0.0001.
The most striking physiological abnormality in the surviving −/− mice at 3 months of age is the increased volume of their urine (equivalent to 30 liters of urine/day in a 60-kg human), which has an osmolality approaching that of plasma, indicating a virtually complete lack of ability to concentrate urine (Table 3). Deprivation of drinking water for 12 h induced a loss of more than 20% of body weight in the −/− mice. However, despite this volume depletion, the urine osmolality did not rise, and eventually the mice became anuric, suggesting that the mutants can no longer respond to antidiuretic signals. We tested this possibility by administration of an antidiuretic hormone analogue, [desamino-Cys1, d-Arg8] vasopressin (1 ng/g, i.p.). This treatment did not increase the urine osmolality of the −/− mice (405 ± 23 mOsm versus 395 ± 20 mOsm in untreated −/− control, n = 5), although it doubled the osmolality (1,390 ± 220 mOsm to 2,630 ± 130 mOsm, n = 4, P < 0.005) in wild-type mice. The simplest explanation of the failure of the −/− mice to respond to the vasopressin analogue or to water deprivation is their inability to set up a renal osmotic gradient combined with the damage to the kidney medulla caused by their hydronephrosis.
Table 3.
Water intake and urine excretion of adult mice
| NKCC2 genotype
|
||
|---|---|---|
| +/+ | −/− | |
| Water intake, ml/day | 3.3 ± 0.2 | 13.2 ± 1.0* |
| Urine volume, ml/day | 1.6 ± 0.2 | 10.0 ± 0.9* |
| Urine osmolality, mOsm | 2,314 ± 74 | 371 ± 20* |
| Na, μmol/day | 118.2 ± 19.8 | 119.4 ± 14.9 |
| K, μmol/day | 378.2 ± 14.4 | 415.3 ± 23.9 |
| Cl, μmol/day | 274.6 ± 23.4 | 301.4 ± 20.1 |
| Ca, mg/day | 0.12 ± 0.03 | 0.51 ± 0.08† |
| Mg, mg/day | 0.31 ± 0.04 | 0.52 ± 0.19 |
| Pi, mg/day | 1.95 ± 0.11 | 3.39 ± 0.20† |
| Glucose, mg/day | 0.55 ± 0.09 | 3.13 ± 0.83‡ |
| Protein (males), mg/day | 0.17 ± 0.03 | 10.10 ± 0.51* |
| Protein (females), mg/day | N.D. | 2.68 ± 0.15* |
| PGE2, ng/day | 4.0 ± 1.0 | 9.6 ± 1.2* |
Mice were 3 months old and treated with indomethacin to 3 weeks of age. N.D., not detected (below detectable level).
Data are means ± SEM. n > 5.
*P vs. +/+ < 0.0001.
†P vs. +/+ < 0.001.
‡P vs. +/+ < 0.005.
A gross deficit in the ability of the NKCC2−/− mice to concentrate their urine is not unexpected in view of the unique location of the NKCC2 on the luminal surface of the thick ascending limb cells (12, 13). Absence of transcellular NaCl transport via NKCC2 also is expected to abolish the lumen positive transepithelial voltage that enables paracellular reabsorption of Na and K across the wall of the tubule. The combined absence of transcellular and paracellular transport of salt across the thick ascending limb cells prevents the establishment of the normal osmotic gradient necessary for urine concentration.
Urinary calcium excretion in the −/− mice was four times wild type (Table 3) and was accompanied by calcification in renal tubules (Fig. 2b). However, the plasma Ca concentration and the food intake of the −/− mice were essentially the same as wild type. In contrast, the plasma concentration and daily excretion of magnesium of the −/− mice were not significantly different from wild type. PGE2 excretion was more than twice normal.
In contrast to the 7-day-old mice, which often had no detectable proteinuria, the surviving −/− adult mice excreted high amounts of a low molecular mass protein (≈20 kDa, Fig. 2c) in their urine with males excreting four times more protein than females (Table 3). The molecular size of the protein and the gender difference suggest that it is the mouse major urinary protein (MUP) (35). A Western blot with an antibody against MUP confirmed this identification (data not shown). MUP is produced mainly in the adult liver (36) and is excreted in the urine where it relates to sexual communication. MUP production is known to be increased by testosterone and growth hormone (37), but we are unaware of any published description of the relationship between MUP and diuretics or hydronephrosis. The mechanism leading to the increased proteinuria in the −/− mice is not clear. If it were because of glomerular damage, a higher amount of albumin excretion would be expected. Moreover, the −/− adult mice had at most only a trace amount of hematuria, and transmission electron microscopy of their glomeruli showed no noticeable morphological changes in the glomerular basement membranes, podocytes, and endothelial cells (data not shown). The proteinuria is therefore unlikely to be caused by glomerular damage. The fact that the rat MUP homologue, α2u-globulin, is a marker for low molecular weight protein reabsorption in the proximal tubules (38) suggests that the NKCC2−/− mice have an impaired ability of proximal tubule reabsorption.
Drug Treatments of the −/− Mice.
Because nonsteroidal anti-inflammatory drugs or the inhibition of renin Ang system ameliorate the symptoms of human patients with Bartter's syndrome, we tested the effects of several drugs known to alter renal function. Readministration of indomethacin (5 μg/g per day) to the surviving −/− mice did not ameliorate their polyuria or their daily urine volume, urinary Ca, and protein excretion. Similarly, oral administration of an Ang II type 1 receptor antagonist losartan (10 μg/g per day) or of an Ang-converting enzyme inhibitor enalapril (5 μg/g per day) in drinking water for 1 week did not significantly change the daily urine volume or urinary excretion of Ca or of protein by the surviving −/− mice, although the drugs at these doses both decrease systolic blood pressure of wild-type mice by 10–15 mmHg.
Phenotype of Furosemide-Treated Wild-Type Mice.
Because the phenotype of the −/− mice is likely to be affected by their severe hydronephrosis, we compared their phenotype with that of wild-type animals given a NaK2Cl transport inhibitor furosemide in their drinking water from 4 weeks of age (100 μg/g per day) for 4 weeks. This dose caused a urine output in +/+ mice comparable to that in the NKCC2−/− mice and induced a remarkably high plasma renin concentration of 4,600 ± 68 ng Ang I/ml per h; this renin concentration was more than six times that in the −/− adults, presumably because the kidneys of the furosemide-treated wild-type mice were minimally damaged compared with those of the −/− mice. Indomethacin decreased the plasma renin concentration of the furosemide-treated mice to 430 ± 31 ng Ang I/ml per h. The furosemide-treated mice developed mild hydronephrosis, but almost no tissue damage was observed. Therefore the gross effects of the hydronephrosis are virtually eliminated. Nevertheless the amount of daily urinary protein excretion in the furosemide-treated mice (9.5 ± 0.7 mg/day in males and 2.3 ± 0.3 mg/day in females, n = 6) was indistinguishable from that observed in the −/− mice. Again most of the protein was ≈20 kDa in size, as in the −/− surviving mice (data not shown). However, unlike the −/− mice, the furosemide-treated mice responded, although weakly, to the vasopressin analogue by changing their urine osmolality from 490 ± 40 mOsm to 670 ± 40 mOsm (n = 7, P < 0.01). The residual response in the furosemide-treated wild-type mice suggests that they can still set up an osmotic gradient, although much attenuated, which can be exploited by their relatively undamaged medulla. Losartan, enalapril, or indomethacin, as in the −/− mice, did not influence urine volume, Ca or protein excretion of the furosemide-treated mice. These observations suggest that the impaired ability of the NKCC2−/− mice and the furosemide-treated wild-type mice to concentrate urine is so overwhelming that correction of the concomitant disturbances in the renin-Ang system is insufficient to affect the phenotype.
Thus, most of the results seen in the −/− adults can be reproduced in an NKCC2-inhibited wild-type kidney that has minimal damage, as judged by the recovery of renal function when the furosemide treatment was stopped. Twenty four hours after stopping the furosemide treatment, the urine volume had fallen from 8.5 ± 0.8 ml/day (about 5 times normal), to 3.1 ± 0.3 ml/day (about 2 times normal), the osmolality had increased from 490 ± 40 mOsm, to 1430 ± 150 mOsm (about 0.6 times normal), and the urine protein had decreased from 4.0 ± 0.5 mg/day to 1.1 ± 0.1 mg/day, although this is still approximately 10 times higher than normal. Urine Ca excretion was reduced to an undetectable level. Four weeks after stopping furosemide, all urinary parameters including volume, osmolality, Ca, and protein had completely returned to normal, although a mild hydronephrosis remained.
Comparisons with Human Patients.
Much of the complex phenotype of the NKCC2−/− mice is similar to that of human patients with Bartter's syndrome. Like humans with Bartter's syndrome, surviving NKCC2−/− mice exhibit lower plasma potassium and higher bicarbonate, polyuria, hypercalciuria, nephrocalcinosis, hyperreninemia, and elevated urinary PG. As mentioned above, all of these alterations can be linked to the absence of NKCC2 in the thick ascending limb of Henle's loop, where under normal conditions approximately 30% of the filtered load of NaCl is reabsorbed, and to the absence of NKCC2 in macula densa cells, which regulate GFR and renin secretion.
Some features of the −/− mouse phenotype appear to represent a species difference. The marked proteinuria of the adult −/− mice falls into this category, because it involves the increased urinary excretion of a small molecular size protein, MUP, which is not present in human urine.
In contrast, most of the other differences between the two species appear to be quantitative rather than qualitative. Such differences include the following:
(i) Hydronephrosis is observed occasionally in human patients although it is not usually severe as in the NKCC2−/− mice. We presume that hydronephrosis arises in both species in response to the back pressure produced by the polyuria, and that the differences in severity of polyuria correlate with the severity of the hydronephrotic process. Human kidneys are more mature at birth than murine kidneys, although both continue to develop after birth. The immaturity of the neonatal kidney may contribute to the more severe hydronephrosis seen in mice compared with humans. In support of this, we note that furosemide started at birth in wild-type mice causes more severe hydronephrosis than when started at 4 weeks of age (unpublished observation).
(ii) The renal insufficiency and death of the NKCC2−/− pups involve not only their impaired renal conservation of water and electrolytes but also their failure to get sufficient milk to correct the dehydration and to allow normal growth. Human patients with Bartter's syndrome display similar problems in the absence of adequate fluid intake (23, 39).
(iii) Hypokalemia in the NKCC2−/− mice is unexpectedly mild, whereas human patients with Bartter's syndrome have profound hypokalemia. Mice could be less susceptible to hypokalemia because the amount of dietary potassium relative to sodium is several times higher in mice compared with humans. An impairment of distal tubular function to exchange Na and K because of hydronephrosis also might be involved.
Summary/Conclusions.
The NKCC2−/− pups exhibit renal insufficiency, metabolic acidosis, and bilateral hydronephrosis and die before weaning probably because they cannot get enough milk. Indomethacin treatment during the sucking period helps growth and rescues about 10% of the homozygotes. As adults, the surviving −/− mice show lower plasma K, higher plasma HCO3, and an above-normal pH, extreme polyuria and hydronephrosis, hypercalciuria, and proteinuria. Their kidney parameters, like those of furosemide-treated wild-type mice with only mild hydronephrosis, are unresponsive to inhibitors of the renin Ang system, vasopressin, and readministration of indomethacin. Thus absence of NKCC2 in the mouse causes a polyuria as a result of impaired reabsorption of NaCl across renal epithelial cells of the thick ascending limb and the macula densa that cannot be compensated elsewhere in the nephron.
Acknowledgments
We thank K. D. Kluckman, V. M. Stewart, and V. J. Madden for technical help, and Drs. W. J. Arendshorst, T. M. Coffman, S. K. Fellner, J. C. Jennette, H.-S. Kim, M. A. Knepper, T. Maack, N. Maeda, J. Schnermann, and G. Vanden Heuvel for discussions and critical reading of the manuscript. Our work was supported by the W. M. Keck Foundation and the National Institutes of Health (Grants HL49277, GM20069, DK42921, DK45179, and DK52612).
Abbreviations
- PG
prostaglandin
- MUP
major urinary protein
- Ang
angiotensin
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.090091297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.090091297
References
- 1.Bartter F, Pronove P, Gill J, MacCardle R. Am J Med. 1962;33:811–828. doi: 10.1016/0002-9343(62)90214-0. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz I. Kidney Int. 1998;54:1396–1410. doi: 10.1046/j.1523-1755.1998.00124.x. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz I, Alon U. J Nephrol. 1996;9:81–87. [Google Scholar]
- 4.Guay-Woodford L M. Am J Med. 1998;105:151–161. doi: 10.1016/s0002-9343(98)00196-x. [DOI] [PubMed] [Google Scholar]
- 5.Rodorigues-Soriano J. In: Pediatric Nephrology. Barratt T M, Avner E P, editors. Baltimore: Lippincott Williams & Wilkins; 1999. pp. 545–563. [Google Scholar]
- 6.Gitelman H J, Graham J B, Welt L G. Trans Assoc Am Physicians. 1966;79:221–235. [PubMed] [Google Scholar]
- 7.Simon D B, Karet F E, Hamdan J M, DiPietro A, Sanjad S A, Lifton R P. Nat Genet. 1996;13:183–188. doi: 10.1038/ng0696-183. [DOI] [PubMed] [Google Scholar]
- 8.Simon D B, Bindra R S, Mansfield T A, Nelson-Williams C, Mendonca E, Stone R, Schurman S, Nayir A, Alpay H, Bakkaloglu A, et al. Nat Genet. 1997;17:171–178. doi: 10.1038/ng1097-171. [DOI] [PubMed] [Google Scholar]
- 9.Simon D B, Karet F E, Rodriguez-Soriano J, Hamdan J H, DiPietro A, Trachtman H, Sanjad S A, Lifton R P. Nat Genet. 1996;14:152–156. doi: 10.1038/ng1096-152. [DOI] [PubMed] [Google Scholar]
- 10.Gamba G, Miyanoshita A, Lombardi M, Lytton J, Lee W S, Hediger M A, Hebert S C. J Biol Chem. 1994;269:17713–17722. [PubMed] [Google Scholar]
- 11.Igarashi P, Vanden Heuvel G B, Payne J A, Forbush B., 3rd Am J Physiol. 1995;269:F405–F418. doi: 10.1152/ajprenal.1995.269.3.F405. [DOI] [PubMed] [Google Scholar]
- 12.Ecelbarger C A, Terris J, Hoyer J R, Nielsen S, Wade J B, Knepper M A. Am J Physiol. 1996;271:F619–F628. doi: 10.1152/ajprenal.1996.271.3.F619. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan M R, Plotkin M D, Lee W S, Xu Z C, Lytton J, Hebert S C. Kidney Int. 1996;49:40–47. doi: 10.1038/ki.1996.6. [DOI] [PubMed] [Google Scholar]
- 14.Greger R. Physiol Rev. 1985;65:760–797. doi: 10.1152/physrev.1985.65.3.760. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen S, Maunsbach A B, Ecelbarger C A, Knepper M A. Am J Physiol. 1998;275:F885–F893. doi: 10.1152/ajprenal.1998.275.6.F885. [DOI] [PubMed] [Google Scholar]
- 16.Obermuller N, Kunchaparty S, Ellison D H, Bachmann S. J Clin Invest. 1996;98:635–640. doi: 10.1172/JCI118834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schnermann J, Ploth D W, Hermle M. Pflügers Arch. 1976;362:229–240. doi: 10.1007/BF00581175. [DOI] [PubMed] [Google Scholar]
- 18.Koller B H, Hagemann L J, Doetschman T, Hagaman J R, Huang S, Williams P J, First N L, Maeda N, Smithies O. Proc Natl Acad Sci USA. 1989;86:8927–8931. doi: 10.1073/pnas.86.22.8927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P. Cell. 1996;84:911–921. doi: 10.1016/s0092-8674(00)81069-7. [DOI] [PubMed] [Google Scholar]
- 20.Kim H S, Krege J H, Kluckman K D, Hagaman J R, Hodgin J B, Best C F, Jennette J C, Coffman T M, Maeda N, Smithies O. Proc Natl Acad Sci USA. 1995;92:2735–2739. doi: 10.1073/pnas.92.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez R A, Lynch K R, Chevalier R L, Everett A D, Johns D W, Wilfong N, Peach M J, Carey R M. Am J Physiol. 1988;254:F900–F906. doi: 10.1152/ajprenal.1988.254.6.F900. [DOI] [PubMed] [Google Scholar]
- 22.Romano L A, Ferder L, Inserra F, Ercole L, Gomez R A. Hypertension. 1994;23:889–893. doi: 10.1161/01.hyp.23.6.889. [DOI] [PubMed] [Google Scholar]
- 23.Proesmans W, Devlieger H, Van Assche A, Eggermont E, Vandenberghe K, Lemmens F, Sieprath P, Lijnen P. Int J Pediatr Nephrol. 1985;6:63–70. [PubMed] [Google Scholar]
- 24.Ammenti A, Montali S. Pediatr Nephrol. 1996;10:79–80. doi: 10.1007/s004670050072. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Soriano J, Vallo A, Oliveros R. Helv Paediatr Acta. 1978;33:141–151. [PubMed] [Google Scholar]
- 26.Kim H S, Maeda N, Oh G T, Fernandez L G, Gomez R A, Smithies O. J Biol Chem. 1999;274:14210–14217. doi: 10.1074/jbc.274.20.14210. [DOI] [PubMed] [Google Scholar]
- 27.Schnermann J, Briggs J. Fed Proc. 1986;45:1426–1430. [PubMed] [Google Scholar]
- 28.Fujita T, Sakaguchi H, Shibagaki M, Fukui T, Nomura M. Am J Med. 1977;63:467–474. doi: 10.1016/0002-9343(77)90287-x. [DOI] [PubMed] [Google Scholar]
- 29.Gans R, Hoorntje S. In: Oxford Textbook of Clinical Nephrology. Camerin S, Davison A, Grunfeld J, Kerr D, Ritz E, editors. New York: Oxford Univ. Press; 1992. pp. 782–789. [Google Scholar]
- 30.Bartter F C, Gill J R, Jr, Frolich J C, Bowden R E, Hollifield J W, Radfar N, Keiser H R, Oates J A, Seyberth H, Taylor A A. Trans Assoc Am Physicians. 1976;89:77–91. [PubMed] [Google Scholar]
- 31.Seyberth H W, Koniger S J, Rascher W, Kuhl P G, Schweer H. Pediatr Nephrol. 1987;1:491–497. doi: 10.1007/BF00849259. [DOI] [PubMed] [Google Scholar]
- 32.Mackie F E, Hodson E M, Roy L P, Knight J F. Pediatr Nephrol. 1996;10:756–758. doi: 10.1007/s004670050210. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto J, Han B K, Restrepo de Rovetto C, Welch T R. AJR Am J Roentgenol. 1989;152:1251–1253. doi: 10.2214/ajr.152.6.1251. [DOI] [PubMed] [Google Scholar]
- 34.Chou C L, Marsh D J. Am J Physiol. 1987;253:F366–F371. doi: 10.1152/ajprenal.1987.253.2.F366. [DOI] [PubMed] [Google Scholar]
- 35.Szoka P R, Gallagher J F, Held W A. J Biol Chem. 1980;255:1367–1373. [PubMed] [Google Scholar]
- 36.Hastie N D, Held W A, Toole J J. Cell. 1979;17:449–457. doi: 10.1016/0092-8674(79)90171-5. [DOI] [PubMed] [Google Scholar]
- 37.Kuhn N J, Woodworth-Gutai M, Gross K W, Held W A. Nucleic Acids Res. 1984;12:6073–6090. doi: 10.1093/nar/12.15.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neuhaus O W. Proc Soc Exp Biol Med. 1986;182:531–539. doi: 10.3181/00379727-182-42376. [DOI] [PubMed] [Google Scholar]
- 39.Seyberth H W, Rascher W, Schweer H, Kuhl P G, Mehls O, Scharer K. J Pediatr. 1985;107:694–701. doi: 10.1016/s0022-3476(85)80395-4. [DOI] [PubMed] [Google Scholar]