Abstract
In addition to the activation of classical progestin receptor-dependent genomic pathway, progesterone (P) can activate nonclassical, membrane-initiated signaling pathways in the brain. We recently demonstrated rapid P activation of second-messenger kinases, protein kinase A, and protein kinase C in the ventromedial nucleus (VMN) and preoptic area (POA) of rat brain. To determine whether P can activate yet another Ca+2dependent kinase, we examined the rapid P modulation of calcium and calmodulin-dependent protein kinase II (CaMKII) in the VMN and POA in female rats. A rapid P-initiated activation of CaMKII basal activity was observed in the VMN but not the POA at 30 min. Estradiol benzoate (EB) priming enhanced this CaMKII basal activity in both the VMN and POA. CaMKII protein levels and phosphorylation of Thr-286 moiety on CaMKII, however, remained unchanged with EB and/or P treatments, suggesting that the changes in the CaMKII kinase activity are due to rapid P modulation of the kinase activity and not its synthesis or autoactivation. Furthermore, intracerebroventricular (icv) administration of a CaMKII-specific inhibitor, KN-93, 30 min prior to the P infusion, in EB-primed, ovariectomized female rats inhibited CaMKII activation but not protein kinase A and protein kinase C activities. Interestingly, icv administration of KN-93 30 min prior to P infusion (icv) resulted in a reduction but not total inhibition of P-facilitated lordosis response in EB-primed female rats. These observations suggest a redundancy or, alternately, a hierarchy in the P-regulated activation of kinase signaling cascades in female reproductive behavior.
THE GONADAL HORMONES estradiol and progesterone (P) play a pivotal role in the neuroendocrine regulation of brain and reproductive behavior in mammals. Whereas genomic effects of P have traditionally been assumed to be the primary pathway for its action in the brain, numerous reports of P’s short-latency effects suggest the involvement of nonclassical effects mediated by putative cell surface receptors, ion channels, and mechanisms coupled to second messenger signaling cascades (1). We and others have reported the involvement of at least two second-messenger systems, protein kinase A (PKA) and protein kinase C (PKC), mediating the rapid effects of steroid hormones in the hypothalamus and preoptic areas (POA) of the female rat (2,3,4,5,6,7). We now extend our studies on the nonclassical effects of P on the ventromedial nucleus (VMN) of the hypothalamus and the POA (6,7) of the female rat to another family of calcium-mediated serine/threonine kinases, the calcium (Ca2+)/calmodulin-dependent protein kinases (CaMKs).
Ca+2-dependent CaMKII is the most abundant protein (2% of total protein) in the mammalian brain (8). The dodecameric holoenzyme is formed from α- and β-polypeptides. Similar to PKC kinases, CaMKII is also allosterically regulated by cofactors and multiple phosphorylation steps (8,9). The autophosphorylation of Ca+2-dependent CaMKII at Thr 286/287 increases its affinity for cofactors and transforms it into an autoactive kinase, whose activity then becomes independent of Ca2+ and calmodulin. The latter form, albeit less active, is retained in the neurons for longer durations, as revealed by immunohistochemical staining of presynaptic sites (10). Whereas the involvement of Ca+2 in the estradiol regulation in the brain has been extensively studied (11,12,13,14,15), the individual role of Ca+2-dependent and -independent CaMKII in P regulation has not been examined.
In this study, we present evidence for the involvement of both Ca+2-dependent- and -independent CaMKII basal activity in the nonclassical effects of P. Intracerebroventricular administration of P into the third cerebral ventricle of ovariectomized rats resulted in stimulation of basal CaMKII activity in the VMN and the POA of the hypothalamus within 30 min. Estradiol benzoate (EB) priming significantly enhanced this activity in both the regions. This increase in activity was not due to the de novo synthesis or changes in the phosphorylation state of CaMKII (Thr286/7). CaMKII-specific inhibitor, KN-93, inhibited both the P- and EB+P-regulated basal CaMKII activity to control levels in the VMN. However, KN-93 administered 30 min [intracerebroventricular (icv)] before P infusion (icv) was unable to completely inhibit P-facilitated lordosis response.
Materials and Methods
Reagents and chemicals
All steroids, calmodulin, the CaMKII inhibitor KN-93, PKA inhibitor R-diastereoisomer of adenosine-3′,5′-cyclic monophosphothioate (Rp-cAMPS), PKC inhibitor GF109203X, 3-isobutyl-1-methylxanthine, 2-(N-morpholino) ethanesulfonic acid, 8-bromoadenosine-cAMP, premixed protease and phosphatase inhibitor I cocktails (Sigma catalog no. P-8340 and P-2850, respectively), diacylglycerol, and phosphatidyl serine were purchased from Sigma Chemicals (St. Louis, MO). Rabbit polyclonal antibodies to CaMKII and phosphorylated-CaMKII (Thr286/7) were purchased from Cell Signaling Biotechnology (Danvers, MA). Rabbit polyclonal antibody to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA) and donkey antirabbit horseradish peroxidase-conjugated-IgG from Amersham Pharmacia Biotechnology (Piscataway, NJ). The reagents for electrophoresis and Western blotting were obtained from Bio-Rad Laboratories (Hercules, CA). All other chemicals were of reagent grade and purchased from Sigma or Fisher Scientific (Pittsburgh, PA). All stereotaxic surgical supplies were obtained from Plastics one (Roanoke, VA).
Procedures
Ovariectomized Sprague Dawley rats (180–200 g) and male proven breeders (280–300 g) were purchased from the supplier (Harlan Sprague Dawley Inc., Indianapolis, IN). The animals were maintained on a 12-h light, 12-h dark reversed light cycle with lights off at 12:00 h and provided food and water ad libitum. The animals arrived in the facility a week after surgery and were allowed to acclimatize to the facility and the reverse light cycle for 4–6 wk before experimental manipulation. The female rats were individually housed in hanging wire cages. The male rats were also housed individually in polystyrene cages (50 × 45 × 24 cm) on sanichip bedding. Animal studies were conducted in accordance with protocols approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine and were in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Stereotaxic surgeries and central administration of compounds
Stainless steel guide cannulae were stereotaxically implanted into the third cerebral ventricle of ovariectomized rats under combination anesthesia (ketamine 42.8 mg/ml, xylazine 8.6 mg/ml, and acepromazine 1.4 mg/ml) using a Lab Standard stereotaxic instrument (Stoelting, Wood Dale, IL) as previously described (16). Animals were allowed to recover from surgery for 1 wk before their use in the experiments. Two micrograms of EB (100 μl in sesame oil) was administered sc followed by icv infusion of P [1 μg/1 μl of artificial cerebrospinal fluid (aCSF)] 48 h later. In studies using the CaMKII inhibitor KN-93, the inhibitor (100ng/μl in aCSF) was administered icv 30 min before P treatment. The dose of KN-93 was chosen based on its ability to specifically inhibit α- and β-subunits of CaMKII in the rat brain extracts, as demonstrated previously (17). Thirty minutes after P administration, the animals were killed under combination anesthesia (ketamine 42.8 mg/ml, xylazine 8.6 mg/ml, and acepromazine 1.4 mg/ml); the brains isolated and placed in cold aCSF; and the VMN of the hypothalamus, POA, and cerebral cortex (CTX) dissected for the kinase assays and Western analyses.
Fresh brain dissection was carried out at 4 C. Using a McIlwain tissue chopper, the POA slab was cut beginning 2 mm rostral to the optic chiasm to 0.5 mm caudal to the chiasm. A second cut (the medial basal hypothalamus) was made 4 mm caudal to the caudal edge of the first slab. The slabs were placed on a cold microscope stage and brain areas of interest were viewed under a dissecting microscope with transillumination system (Stereo Discovery V8; Zeiss, Thornwood, NY). Bilateral punches were made using the Palkovits punch method from the slabs to dissect the VMN and POA using a 1-mm internal diameter stainless steel punch. The cerebral cortex included the frontal cortex without the white matter from the slabs. The punches were immediately frozen on dry ice/isopropanol and stored at −80 C until further analyses.
Tissue isolation and processing
Tissue samples were homogenized in buffer containing10 mm Tris-HCl (pH 7.4), 1 mm EGTA, 1 mm EDTA, 0.5 mm dithiothreitol (DTT), 1 mm thioglycerol, and a cocktail of protease inhibitors and phosphatase inhibitors (1:100) in a handheld glass homogenizer on ice. The samples were centrifuged at 1000 × g for 10 min; the pellet consisting of nuclear, mitochondrial, and cellular debris discarded; and the supernatant solution centrifuged at 20,000 × g for 20 min. The protein was quantified using a BCA assay (Pierce Inc., Rockford, IL). The supernatant was stored at −80 C in aliquots for the kinase assays and Western analysis.
Kinase assays
CaMKII activity was measured using a synthetic peptide substrate, bearing the autophosphorylation sequence from the CaMKII sequence (autocamtide-3; KKALHRQETVDAL) (18). The assay was performed according to the method of Moore et al. (19) with minor modifications as described. Five microliters of protein samples (∼1–5 mg) were added to the reaction mixture (final volume 25 μl) containing 50 mm HEPES (pH 7.4), 1 mm DTT, 20 μm substrate peptide, 20 μm ATP, 5 mm MgCl2, 2μCi [γ-32P]ATP (3000 Ci/mmol) and incubated at 30 C for 2 min. The assay was performed in the presence of 2 mm EGTA in the reaction mixture to determine the basal activity and in the presence of 1 mm CaCl2 and 3 μm calmodulin to determine the total activity. In studies using the CaMKII-specific inhibitor KN-93 to inhibit CaMKII activity, the tissue homogenates were assayed for CaMKII in addition to PKC and PKA activities as described below.
The PKC assays were performed using a modified procedure of Klann et al. (20), using a synthetic peptide (AAAKIQASFRGHMAR) derived from neurogranin (amino acids 28–43) as the substrate. The reaction mixture (final volume 25 μl) contained 50 mm HEPES (pH 7.4), 1 mm DTT, 2 mm sodium pyrophosphate, 100 μm ATP, 2μCi of [γ32P] ATP, and 10 μm neurogranin. Assays for PKC activity were carried out with 1 mm EGTA and 1 mm EDTA in the reaction mixture for basal activity or in the presence of 4 mm CaCl2 and lipid cofactors for total activity (final concentrations: 320 μg/ml phosphatidyl serine and 30 μg/ml sn-1, diacylglycerol). The reaction was started by the addition of 5 μl of protein extract (∼1–5 μg) to aliquots (20 μl) of the reaction buffer either containing NG or not and incubated at 30 C for 2 min. The PKA assays were performed as described in the earlier publication (7), using a synthetic peptide (kemptide-LRRASLG). The reaction buffer contained 37.5 mm of [2-(N-morpholino) ethanesulfonic acid (pH 6.0)], 0.2 mm 3-isobutyl-1-methylxanthine, 15 mm MgOAc, 30 mm ATP, 2 μCi [γ32P] ATP, and 300 μm kemptide for the determination of PKA basal activity. PKA total activity was determined by adding 20 μm 8-bromoadenosine-cAMP to the reaction mixture containing the other reagents mentioned above. The reactions were carried out at 30 C for 5 min. After the incubation, all the reactions from the three assays were similarly processed. The control reactions for background determination were run in parallel by omitting substrate peptide. Seventeen microliters of the reaction mixture were spotted on P-81 phosphocellulose filters and the reaction stopped by 75 mm phosphoric acid. The filters were washed twice (10 min each) in fresh phosphoric acid solution, rinsed in methanol, and air dried. The radioactivity on the phosphorylated peptide substrate was quantified by the Cerenkov method.
Autocamtide phosphorylation was determined to be linear with respect to time and protein concentration under these conditions. For each experimental determination, the values for control reactions lacking the substrate peptide were subtracted from those containing the substrate peptide. The counts were normalized using protein concentrations determined by the BCA assay. The assay was performed for the samples from each animal independently. Data are expressed as either specific activity (picomoles of phosphate transferred per minute per milligram of protein) for each of the treatment groups (average of six per group) or as a percent of vehicle control group (set to 100%). The basal activities of all treatment groups are reported as a percent of basal activity of the vehicle (vehicle as 100%). The total activities of all treatment groups are compared with the total activity of the vehicle set at 100%. All specific activities (mean ± sem) activities were converted to a percent of their respective control so that the basal and the total activities could be presented in the same graph.
Western blot analysis
The tissue samples from VMN, POA, and CTX used in the kinase assays were subjected to Western immunoblotting. Equal amounts of protein (5 μg) from each sample were electrophoresed on SDS-PAGE (10% polyacrylamide) (21) followed by transfer to a nitrocellulose membrane for Western blotting (22). Magic Marker XP Western standards (Invitrogen, Carlsbad, CA) were included in the gels for molecular weight determination. The membranes were blocked and sequentially probed with commercially available antibodies to phosphorylated-CaMKII (phospho-Thr286/7) and total CaMKII at 1:2000 dilution. The antibody to GAPDH (as a normalizing control) was used at 1:1000 dilutions in conjunction with both the CaMKII antibodies. The antibody binding was revealed by incubation with donkey antirabbit horseradish peroxidase-conjugated IgG (1:10,000) followed by chemiluminescence detection with the enhanced chemiluminescence reagent ECL (Amersham Pharmacia Biotech). The intensities of the total and phosphorylated CaMKII (α-subunit = 50 kDa and β-subunit = 60 kDa) and the GAPDH bands in each of the treatment groups were quantified by densitometry using a PhosphorImager-SF (Molecular Dynamics, Sunnyvale, CA). The CaMKII protein levels were normalized to GAPDH for each sample. The ratio of pCaMKII to CaMKII was also calculated. Experiments were carried out using at least six animals for each treatment group.
Behavioral studies
Lordosis response in female rats was determined as described in our published protocols (16,23). Briefly, ovariectomized female rats with indwelling stainless steel cannulae in the third cerebral ventricle were subcutaneously primed with vehicle or EB (2 μg per 100 μl in sesame oil). Forty-eight hours later, the animals were given icv infusion of CaMKII inhibitor KN-93 (100 ng/μl in aCSF) or vehicle (1 μl in aCSF). Vehicle or P (1 μg per 1 μl aCSF) was administered icv 30 min after KN-93 treatment. Thirty minutes after P (or vehicle) infusion, the females were tested for lordosis response in the home cages of sexually active males. Lordosis response of the female rats to the mounting by male rats was scored for 10 mounts or 30 min (whichever came first). Lordosis response was quantified and represented as lordosis quotient (LQ; number of lordosis/number of mounts × 100). Steroid and drug treatments and behavioral observations were performed under red light illumination during the dark phase of the reversed light-dark cycle.
Statistical analysis
For kinase assays, statistical analyses were performed using activity values in counts per milligram per minute (in triplicates) from each animal. The mean ± sem of these counts (from six animals) was normalized to the mean ± sem of the counts from the vehicle control set as 100%. The data were analyzed by either of two methods, as appropriate. Analyses were performed using ANOVA followed by post hoc comparisons with Dunnett’s method for comparison of all groups vs. the control group or the Newman-Keuls method for multiple comparisons (Prism; GraphPad, San Diego, CA).
Results
Among the three identified forms of CaMK, CaMKII is localized to the cytosol and CaMKIV is nuclear (8,24), whereas CaMKI is restricted to skeletal cells. Because our studies focused on nonclassical P-initiated cytosolic second-messenger signaling cascades, we examined the changes in cytosolic CaMKII activity in the P-sensitive regions of the brain known to be associated with female reproductive behavior. Basal CaMKII activity in the presence of calcium chelator (EGTA) and the total CaMKII activity in the presence of exogenous cofactors, calcium and calmodulin, provided information on the temporal nature of CaMKII regulation by P. The modulation of the kinase activity, in vivo, by progesterone is referred to as CaMKII basal activity. A further in vitro stimulation of CaMKII activity on addition of the specific cofactors described above represents the pool of CaMKII that can be stimulated beyond the basal activity within the cell and is referred to as the total activity.
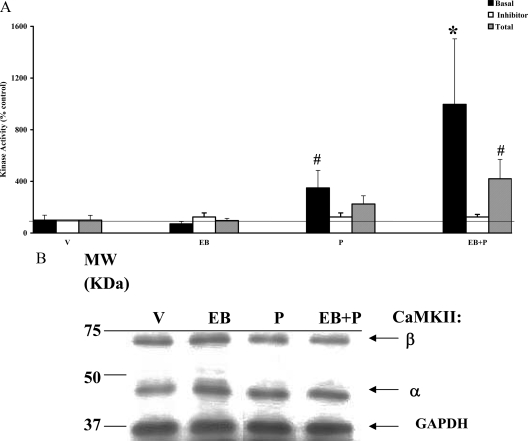
P infusion results in rapid increase in basal CaMKII activity in the VMN of the hypothalamus
Our accompanying studies demonstrated the involvement of calcium as a second messenger in the nonclassical activation by P in the VMN via PKC signaling. We further explored the role of calcium in the intracellular signaling initiated by P by examining the involvement of CaMKII in response to steroid hormones, EB and/or P. As shown in Fig. 1A, P treatment alone caused a significant increase in basal CaMKII activity over the vehicle control within 30 min (#, P < 0.05). EB priming alone showed no significant changes in the basal CaMKII activity. However, EB priming significantly augmented the P-induced basal activity (*, P < 0.01) over control. KN-93, a CaMKII-specific inhibitor, administered icv 30 min before P treatment, blocked this increase, returning CaMKII activity to that seen in the control (vehicle) group, demonstrating the specificity of the inhibitor for CaMKII activity. The inhibitor was effective in reducing the specific activity (in picomoles phosphate transferred per minute per milligram protein) of CaMKII basal activity (vehicle = 12.4 ± 0.6; KN-93 + vehicle = 0.2 ± 0.01).
Figure 1.
P modulation of CaMKII basal and total activities in the presence and absence of CaMKII inhibitor KN-93 (A) and Western immunoblotting for CaMKII protein (B) in the VMN. Ovariectomized rats with chronic in-dwelling stainless steel cannulae in the third cerebral ventricle were primed with EB or vehicle (V) sc. Forty-eight hours later, P was administered icv and killed 30 min later. A, The VMN tissue homogenates were assayed for CaMKII basal and total activities in the absence and presence of CaMKII inhibitor KN-93 (inhibitor) infused (icv) 30 min before P or V treatment. The CaMKII basal and total activities are expressed as percentage of vehicle control (mean ± sem; 100%). The CaMKII activity was normalized to the vehicle in the presence of KN-93 and expressed as percentage of the respective control (mean ± sem; 100%). The specific activity of V control in the presence of KN-93 was 0.2 pmol/min·mg and in the absence of KN-93 was 12.4 pmol/min·mg. Statistical analysis using ANOVA followed by Dunnett’s or Newman-Keuls post hoc multiple comparisons indicated statistically significant differences between V and P treatment (#, P < 0.05) and between V and EB+P (*, P < 0.01). The CaMKII total activity due to EB+P, compared with vehicle, was significant (#, P < 0.05) (n = 6 for each group). B, The homogenates were subjected to Western immunoblot analysis as described. Each lane represents the sample from one animal per treatment group. A representative autoradiograph is shown. The GAPDH band at 37 kDa was used as a normalizing control. Lane 1 corresponds to vehicle control (V); lane 2, EB; lane 3, P; and lane 4, EB+P treatments. Magic marker molecular weight (MW) standards were run alongside the protein samples and the MWs are noted adjacent to lane 1.
P activates a fraction of total CaMKII in the VMN on EB priming
To examine whether CaMKII can be further stimulated, in vitro, the tissue homogenates from the various treatment groups were also assayed for total CaMKII activity in the presence of saturating levels of exogenous calcium and calmodulin (Fig. 1A). Treatment with P or EB alone had no significant effect on the total CaMKII activity, suggesting that the activated CaMKII was functioning at its peak at 30 min (measured as basal CaMKII activity in vivo) and could not be further enhanced with the addition of exogenous cofactors, Ca+2 and calmodulin, in vitro. In contrast, EB+P treatment caused a significant (#, P < 0.05) increase in total CaMKII, suggesting that CaMKII activity had not reached its maximal activity level and could be further enhanced by cofactor addition in vitro. A comparison of the specific activities of the total (151.2 pmol phosphate transferred per minute per milligram protein) and basal (12.2 pmol phosphate transferred per minute per milligram protein) CaMKII concurred with this observation and additionally indicated that only a fraction (8%) of the total pool of CaMKII was active in vivo at the 30-min time point in the VMN.
Changes in CaMKII activity in the VMN are independent of de novo protein synthesis or phosphorylation status
To examine the contribution of de novo protein synthesis or changes in phosphorylation status to the increase in CaMKII activity we observed, we performed Western immunoblot analyses on the samples used in the kinase assays. As shown in Fig. 1B, two major CaMKII bands were evident at 59 kDa (β) and 50 kDa (α). Normalization of the protein content of the individual bands, quantified by densitometry, indicated no significant difference in the protein levels due to EB and/or P treatments (P > 0.05; Table 1). The phospho-CaMKII (phosho-Thr286/287 CaMKII) values normalized to the amount of total CaMKII also showed no significant (P > 0.05) effects of EB and/or P treatments on phosphorylation levels of CaMKII (Table 2).
Table 1.
Total CaMKII (α = 50 kDa) protein levels
| Treatment | VMN | POA | CTX |
|---|---|---|---|
| V | 1.0 | 1.0 | 1.0 |
| EB | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 |
| P | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.2 |
| EB+P | 1.0 ± 0.3 | 1.0 ± 0.2 | 1.2 ± 0.2 |
Analysis of the Western blots from the extracts used in the kinase assays are shown. The intensities of the total CaMKIIα (50-kDa band only) and the GAPDH (37 kDa) bands in each of the treatment groups were quantified using a densitometer. The ratio of CaMKIIα and -β to GAPDH in each lane was calculated. Because the values were similar, only the values for the 50-kDa subunit are presented. This ratio for the vehicle (V) was equated to 1 and compared with those of the steroid treatments. Column 1 indicates the various treatments (V, EB, P, and EB+P). Columns 2, 3, and 4 represent the average (n = 6) values of total CaMKII in the VMN, POA, and CTX for each treatment group. ANOVA showed no significant differences in the mean values (P < 0.05) for VMN, POA, and CTX samples. Dunnett’s post hoc multiple comparisons showed no significant differences between the groups in each region of the brain (P > 0.05; n = 6).
Table 2.
Ratio of phosphorylated CaMKII to total CaMKII (α = 50 kDa) protein levels
| Treatment | VMN | POA | CTX |
|---|---|---|---|
| V | 1.0 | 1.0 | 1.0 |
| EB | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.9 ± 0.5 |
| P | 1.0 ± 0.3 | 1.0 ± 0.2 | 2.7 ± 1.3 |
| EB+P | 1.2 ± 0.2 | 1.0 ± 0.2 | 1.9 ± 0.6 |
Analysis of the fraction of phosphorylated CaMKII in each treatment group are shown. The Western blots were probed with an antibody specific for Thr286/287 phosphorylated CaMKII. The intensities of the phosphorylated and total CaMKIIα band (50 kDa only) were quantified using a densitometer. The ratio of pCaMKIIα to total CaMKIIα for each treatment group was quantified. Column 1 represents the treatment groups [(vehicle (V), EB, P, and EB+P], and columns 2, 3, and 4 are the average of the calculated ratio (n = 6). ANOVA showed no significant differences between means (P > 0.05) for VMN, POA, and CTX. Newman-Keuls’ post hoc multiple comparison tests showed no significant differences between the vehicle control and the treatment groups (P > 0.05; n = 6).
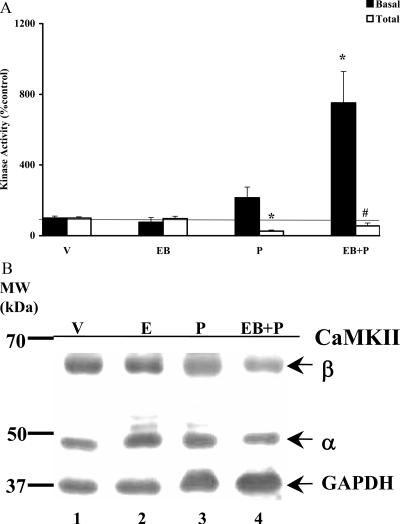
P alone had no significant effect on basal CaMKII activity in the POA
We were also interested in determining the P-mediated effects on CaMKII activity in POA, the other major region of the brain known to be involved in female reproductive behavior. The results are shown in Fig. 2A. Neither P nor EB treatments by themselves had any significant effects on CaMKII basal activity. Unlike the VMN, EB priming was absolutely necessary for P activation of CaMKII basal activity in the POA, which was significantly enhanced (*, P < 0.01). The specific activity of the vehicle control was 2.8 pmol ATP transferred per minute per milligram protein.
Figure 2.
P modulation of CaMKII basal and total activities (A) and Western analysis of CaMKII protein (B) in the POA. A, POA homogenates from the experimental animals were analyzed for their CaMKII basal and total activities as described above. The CaMKII activity is expressed as percentage of vehicle control (mean ± sem; 100%). Statistical analysis using ANOVA followed by Dunnett’s post hoc multiple comparisons indicated statistically significant increase in CaMKII basal activity for EB+P treatment (*, P < 0.01), compared with vehicle control (V). The CaMKII total activities for P and EB+P treatments were also significantly different from V (*, P < 0.01 and #, P < 0.05, respectively) (n = 6 for each treatment group). B, The POA homogenates were subjected to Western immunoblot analysis. Each lane represents the sample from one animal per treatment group. A representative autoradiograph is shown. The GAPDH band at 37 kDa was used as a normalizing control. Lane 1 corresponds to vehicle control (V); lane 2, EB; lane 3, P; and lane 4, EB+P treatments. Magic marker molecular weight (MW) standards were run alongside the protein samples and the MWs are noted adjacent to lane 1.
POA demonstrated attenuation in the CaMKII total activity in response to P
CaMKII total activity measured in the presence of exogenous cofactors in vitro demonstrated no additional activity in EB-treated group compared with the vehicle, suggesting that this signaling system could not be further stimulated at 30 min in vitro (Fig. 2A). P significantly reduced the total CaMKII activity in comparison with vehicle control in the presence (#, P < 0.05) and absence of EB priming (*, P < 0.01). The specific activity of the vehicle control was equal to 76.7 pmol ATP transferred per minute per milligram protein.
P-initiated changes in CaMKII activity is independent of kinase protein levels in the POA
The lack of inducible CaMKII total activities in the steroid hormone-treated samples could be due to a reduction in the proteins levels in the POA. Therefore, we performed a Western immunoblot analyses to quantify the protein levels of CaMKII in these samples. Because the changes in the 59 kDa (β) and 50 kDa (α) were identical, we present the data of the quantified 50-kDa (α) band, which is the more abundant species in the brain, as a representative of the CaMKII protein. The results are shown in Fig. 2B and tabulated in Table 1. There were no differences (P > 0.05) in the protein levels as a consequence of steroid(s) treatments. We also determined that the ratio of phospho-CaMKII (phospho-Thr286) to total CaMKII in the POA (Table 2) was not significantly different between the groups (P > 0.05).
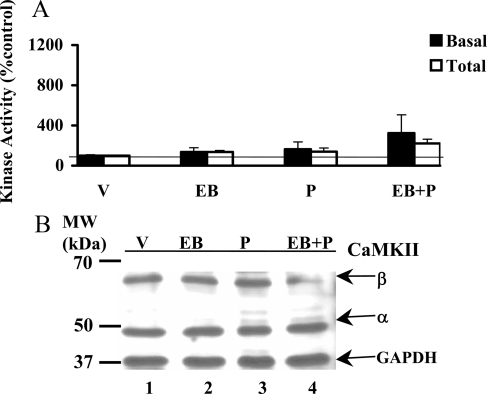
P has no effect on CaMKII basal and total activities or protein levels in the cortex
We analyzed CaMKII basal and total activities in the extracts of the frontal cortex from the animals treated with P and/or EB. The results shown in Fig. 3A did not reveal statistically significant differences in both CaMKII basal and total activities between the treatment groups and vehicle control. The specific activities (picomoles of phosphate transferred per minute per milligram protein) of basal and total CaMKII of vehicle control were equal to 5.96 and 10.31 pmol, respectively. Interestingly the ratio of total to basal activity was 12-fold in the VMN and 25-fold in the POA, attesting to the selective response to the steroids EB and P, in these two regions and its lack thereof in the cortex (2-fold). Western immunoblot analyses revealed no significant differences in the protein levels (Fig. 3B and Table 1) or phosphorylation status (Table 2) of CaMKII in response to steroid treatments.
Figure 3.
P modulation of CaMKII basal and total activities (A) and protein (B) in the CTX. A, Cortical tissue homogenates were analyzed for basal and total CaMKII. The CaMKII activity is expressed as percentage of vehicle control (V; mean ± sem; 100%). ANOVA followed by post hoc analyses using Dunnett’s or Newman-Keuls multiple comparison tests indicated no significant differences between the treatment groups and the vehicle (P > 0.05). B, The homogenates were subjected to Western immunoblot analysis a representative autoradiograph is shown. The GAPDH band at 37 kDa was used as a normalizing control. Lane 1 corresponds to vehicle control (V); lane 2, EB; lane 3, P; lane 4, EB+P treatments. Magic marker molecular weight (MW) standards were run alongside the protein samples and the MWs are noted adjacent to lane 1.
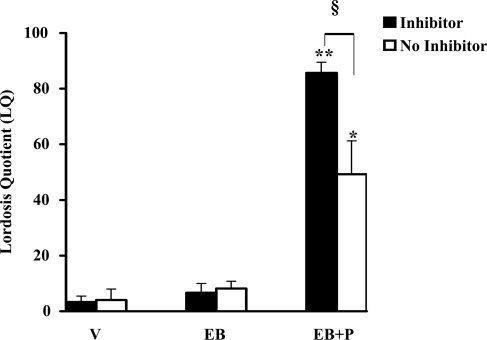
CaMKII activity inhibition does not completely abolish female receptive behavior
We next examined whether CaMKII signaling pathway is involved in the P-facilitated lordosis response in female rats (Fig. 4). The icv infusion of KN-93, an inhibitor of CaMKII, 30 min before P infusion (icv), reduced P-facilitated lordosis response in EB-primed female rats measured at 30 min after P. This reduction was statistically significant (§, P < 0.001), compared with the EB+P treatment group, in the absence of inhibitor. KN-93 had no effect on lordosis response in vehicle, EB-primed, or P-treated rats. The, EB+P treatment group exhibited high levels of lordosis in the absence of the inhibitor (**, P < 0.001).
Figure 4.
KN-93 was capable of inhibiting CaMKII basal activity in the VMN but not the lordosis response in female rats. Ovariectomized rats with stereotaxically implanted stainless steel cannula into the third cerebral ventricle were primed with 2 μg EB or vehicle (V) (sc). Forty-eight hours later, the rats were infused with vehicle (aCSF) or CaMKII inhibitor KN-93 in aCSF, followed by icv P or vehicle 30 min later. The animals were examined for their lordosis response in the presence of a male (proven breeder) 30 min after P administration (icv). The results were expressed as LQ. ANOVA followed by Newman-Keul’s multiple comparison tests indicated significant differences in the lordosis response between EB+P and V in the absence of KN-93 (**, P < 0.001) and in its presence (*, P < 0.01). Statistically significant differences were observed in the LQ in the absence and presence of KN-93 (§, P < 0.001) (n = 6 for each treatment group).
CaMKII activity inhibition does not affect PKC basal activities in the VMN
Whereas KN-93 completely inhibited CaMKII basal activity (Fig. 1A), it was able to significantly reduce but not completely inhibit the P-facilitated lordosis response in EB-primed female rats. We therefore examined whether EB+P maintains its ability to activate PKA and PKC in the presence of KN-93. The results shown in Fig. 5 indicate that the basal activities of PKA and PKC remain capable of being activated in the presence of KN-93 (#, P < 0.05).
Figure 5.
P regulation of PKA and PKC basal activities remained unaltered by CaMKII inhibitor KN-93 in the VMN. VMN tissue sample homogenates from the treatments groups administered KN-93 in Fig. 4 were processed for kinase assays as described in Materials and Methods. The homogenates were assayed for basal PKA, PKC, and CaMKII activities. The CaMKII activity was normalized to the vehicle (V) in the presence of KN-93 and expressed as percentage of the respective control (mean ± sem; 100%). ANOVA followed by Dunnett’s post hoc multiple comparisons demonstrated significant differences between P-stimulated PKA (#, P < 0.05) and PKC (#, P < 0.05) basal activities, compared with the vehicle control, in the presence of KN-93. Significant difference in the PKC basal activity was also observed between EB+P and vehicle control (#, P < 0.05) in the presence of KN-93 (n = 6 in each treatment group).
Discussion
CaMKII, with its absolute dependence on calcium, is uniquely able to serve as a Ca+2 sensor and mediates signaling events associated with Ca+2 fluctuations in vivo (25,26). Calcium involvement in nonclassical action of P has been evidenced in several physiological processes such as the oocyte maturation in the ovary (27,28) and sperm activity during fertilization (29,30). In the neuroendocrine system, steroid hormone mobilization of intracellular Ca+2 in the rat pituitary gonadotropes and hypothalamic astrocytes (11,13,15) has been well established. Calcium channel blockers have also been shown to inhibit P-facilitated lordosis response in estradiol-primed female rats, suggesting the involvement of Ca+2/calmodulin system in this P-facilitated response (12). We reasoned that intracellular signaling processes mediating the P-modulated changes in Ca+2 levels could involve CaMKII, the most abundant CaMK protein in the brain. The current observations extend and add to our previous studies demonstrating a rapid nonclassical activation of signaling kinases by P in the VMN and POA.
P alone caused a significant activation of basal CaMKII in the VMN but not the POA. Interestingly, EB priming significantly enhanced P-initiated rapid activation of basal CaMKII activity in both the VMN and POA. This P-initiated activation was not accompanied by de novo protein synthesis or changes in a crucial phosphorylation event necessary for CaMKII autoactivity. The CaMKII-specific inhibitor KN-93 inhibited CaMKII basal activity when infused (icv) before P treatment, demonstrating the specificity of the activities being measured. However, P-facilitated lordosis response in the EB-primed female rats could only be partially inhibited by KN-93. Furthermore, our studies also demonstrate sustained PKA and PKC activities in the absence of CaMKII. These differences in CaMKII regulation by EB and P were specific to the two known steroid-sensitive regions involved in the regulation of female reproductive behavior, the VMN and the POA, and were not observed in the cortex.
Our data suggest that the VMN is programmed for a rapid and robust response to P, whereas the POA response, albeit rapid, was constrained in comparison. Whereas there was a P-dependent increase in the specific activity of basal CaMKII in the VMN, it was not the case in the POA. Thus, there is a differential, rapid P response in these two regions of the brain that regulate reproductive behavior. This concurs with and extends previous behavioral and biochemical studies demonstrating a difference in the P-regulated response in the VMN and the POA (31). Lesions in the VMN abolished lordosis, whereas those in the POA promoted the receptive component but not the proceptivity of the lordosis response (32,33).
Our studies dealing with the P regulation of second messengers in the cytosol of the VMN and POA reveal the induction of multiple signaling cascades within 30 min in response to the gonadal steroids EB and P and are in agreement with published reports both in vivo and in vitro (3,4,5). Kow et al. (34) demonstrated the involvement of PKC in lordosis using both activators and inhibitors. We previously demonstrated that Rp-cAMPS pretreatment 30 min before P infusion inhibits P-facilitated lordosis response (7), suggesting that nonclassical signaling pathways initiated at the membrane are essential for downstream genomic response involving progestin receptors (PRs). In contrast to the PKA results, we observed only a partial inhibition of P-facilitated lordosis on administration of CaMKII-specific inhibitor before P infusion. Collectively, these findings demonstrate: 1) the requirement of intracellular kinase cascades for the P facilitation of lordosis, 2) the possible existence of a hierarchy in the activation of signaling cascades, and 3) the differences in the P activation of signaling cascade in the presence and absence of EB priming.
In terms of lordosis response, in the VMN and POA, EB priming results in multifaceted actions that include both the nonclassical signaling cascades and the classical receptor-ligand activation of ERE-regulated genes, including the PRs. The former is illustrated by our observations that whereas P is sufficient to activate both CaMKII and PKC in the VMN, EB priming was essential for these kinase activities in both the VMN and POA. Thus, it appears that EB priming enables the activation of a bigger fraction of the kinase pool that can be rapidly modulated by the early actions of P in vivo. EB priming modulates signaling cascades and gene transcription and sets the threshold for the synthesis of proteins necessary for the subsequent P action through the PRs.
Rapid effects of progesterone on facilitation of lordosis response in estrogen-primed female rats have been reported (35,36,37). Our studies suggest that these rapid effects involve activation of signaling kinases. Intracellular kinase cascades could potentially modulate gene expression via multiple transcription factors and/or transcription coactivators, providing an alternative pathway to the genome (38,39,40,41). For example, signaling mechanisms that elevate the levels of cAMP or calcium can increase the phosphorylation of Ca2+/cAMP response element binding protein and other transcription factors (e.g. activating transcription factor-1), enabling them to become transcriptional regulators (38,39,40,41). Interactions between membrane-initiated P effects and intracellular progestin receptors have also been observed in vivo in the facilitation of lordosis response in female hamsters (42,43), suggesting that the classical and nonclassical mechanisms are not mutually exclusive and that signals generated at the membrane can enhance gene expression regulated by classical genomic pathways.
Nonclassical steroid hormone effects are thought to be mediated by binding to putative cell surface membrane receptors (44) that gate ion channels (45,46) and are coupled to certain second-messenger systems (47,48). A novel membrane PR (mPR) that is a transmembrane G protein-coupled receptor and unrelated to the classical PR was identified in the teleost and sheep (49,50,51). The α- and β-subtypes of this mPR have been localized to the preoptic anterior hypothalamic area in the mouse brain (52). The involvement of these mPRs in the P induction of kinase cascades and the molecular mechanisms leading to the activation of PR per se are under investigation.
Acknowledgments
The kinase peptide substrates were synthesized by Dr. Flora (Biomedical Instrumentation Center, Uniformed Services University of Health Sciences, Bethesda, MD).
Footnotes
This work was supported by National Institutes of Health Grants MH 63954 (to S.K.M.) and NS035457, NS049160, and MH72933 (to P.K.D.).
Disclosure Statement: The authors have nothing to declare.
First Published Online July 10, 2008
Abbreviations: aCSF, Artificial cerebrospinal fluid; CaMK, calmodulin-dependent protein kinase; CTX, cerebral cortex; DTT, dithiothreitol; EB, estradiol benzoate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; icv, intracerebroventricular; LQ, lordosis quotient; mPR, membrane PR; P, progesterone; PKA, protein kinase A; PKC, protein kinase C; POA, preoptic area; PR, progestin receptor; VMN, ventromedial nucleus.
References
- Schumacher M, Coirini H, Pfaff DW, McEwen BS 1990 Behavioral effects of progesterone associated with rapid modulation of oxytocin receptors. Science 250:691–694 [DOI] [PubMed] [Google Scholar]
- Beyer C, Gonzalez-Mariscal G 1986 Elevation in hypothalamic cAMP as a common factor in the facilitation of lordosis in rodents: a working hypothesis. Ann NY Acad Sci 474:270–281 [DOI] [PubMed] [Google Scholar]
- Kow LM, Mobbs CV, Pfaff DW 1994 Role of second-messenger systems and neuronal activity in the regulation of lordosis by neurotransmitters neuropeptides and estrogen: a review. Neurosci Bio Behav Rev 18:251–268 [DOI] [PubMed] [Google Scholar]
- Pettiti N, Etgen AM 1989 Progesterone depression of norepinephrine-stimulated cAMP accumulation in hypothalamic slices. Brain Res Mol Brain Res 5:109–119 [DOI] [PubMed] [Google Scholar]
- Pettiti N, Etgen AM 1990 β1-Adrenoreceptor augmentation of β-stimulated cAMP formation is enhanced by estrogen and reduced by progesterone in rat hypothalamic slices. J Neurosci 10:2842–2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian B, Portillo W, Reyna A, Chen JZ, Moore AN, Dash PK, Mani SK 2008 Nonclassical mechanisms of progesterone action in the brain: I. Protein kinase C activation in the hypothalamus of female rats. Endocrinology 149:5509–5517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SK, Fienberg AA, O'Callaghan JP, Snyder GL, Allen PB, Dash PK, Moore AN, Mitchell AJ, Bibb J, Greengard P, O'Malley BW 2000 Requirement for DARPP-32 in progesterone-facilitated sexual receptivity in female rats and mice. Science 287:1053–1056 [DOI] [PubMed] [Google Scholar]
- Griffith LC, Liu CS, Sun XX 2003 CaMKII—an enzyme on the move: regulation of temporospatial localization. Mol Interv 3:385–403 [DOI] [PubMed] [Google Scholar]
- Cameron AJ, De Rycker M, Calleja V, Alcor D, Kjaer S, Kostelecky B, Saurin A, Faisal A, Laguerre M, Hemmings BA, McDonald N, Larijani B, Parker PJ 2007 Protein kinases, from B to C. Biochem Soc Trans 35:1013–1017 [DOI] [PubMed] [Google Scholar]
- Irvine EE, von Hertzen LS, Plattner F, Giese KP 2006 αCaMKII autophosphorylation: a fast track to memory. Trends Neurosci 29:459–465 [DOI] [PubMed] [Google Scholar]
- Micevych P, Sinchak K 2008 Synthesis and function of hypothalamic neuroprogesterone in reproduction. Endocrinology 149:2739–2742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canchola E, Rodriguez-Medine N, Duenas-Tentori H, Mercado E, Rosado A 1996 Ca2+/calmodulin system: participation in the progesterone-induced facilitation of lordosis behavior in the ovariectomized estrogen primed rat. Pharmacol Biochem Behav 54:403–407 [DOI] [PubMed] [Google Scholar]
- Turgeon DW, Waring JL 2006 Estradiol inhibition of voltage-activated and gonadotropin-releasing hormone-induced currents in mouse gonadotrophs. Endocrinology 147:5798–5805 [DOI] [PubMed] [Google Scholar]
- Lee SJ, Campomanes CR, Sikat PT, Greenfield AT, Allen PB, McEwen BS 2004 Estrogen induces phosphorylation of cyclic AMP response element binding (pCREB) in primary hippocampal cells in a time-dependent manner. Neuroscience 124:549–560 [DOI] [PubMed] [Google Scholar]
- Chaban VV, Lakhter AJ, Micevych P 2004 A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology 145:3788–3795 [DOI] [PubMed] [Google Scholar]
- Mani SK, Blaustein JD, Allen JM, Law SW, O'Malley BW, Clark JH 1994 Inhibition of rat sexual behavior by antisense oligonucleotides to the progesterone receptor. Endocrinology 135:1409–1414 [DOI] [PubMed] [Google Scholar]
- Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, Hidaka H 1991 The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem Biophy Res Commun 181:968–975 [DOI] [PubMed] [Google Scholar]
- White RR, Kwon YG, Taing M, Lawrence DS, Edelman AM 1998 Definition of optimal substrate recognition motifs of Ca2+-calmodulin-dependent protein kinases IV and II reveals shared and distinctive features. J Biol Chem 273:3166–3172 [DOI] [PubMed] [Google Scholar]
- Moore AN, Waxham MN, Dash PK 1996 Neuronal activity increases the phosphorylation of the transcription factor cAMP response element-binding protein (CREB) in rat hippocampus and cortex. J Biol Chem 271:14214–14220 [DOI] [PubMed] [Google Scholar]
- Klann E, Chen SJ, Sweatt JD 1993 Mechanism of protein kinase C activation during the induction and maintenance of long-term potentiation probed using a selective peptide substrate. Proc Natl Acad Sci USA 90:8337–8341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK 1970 Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J 1979 Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SK, Allen JM, Clark JH, Blaustein JD, O'Malley BW 1994 Convergent pathways for steroid hormone- and neurotransmitter-induced rat sexual behavior. Science 265:1246–1249 [DOI] [PubMed] [Google Scholar]
- Schulman H 2004 Activity-dependent regulation of calcium/calmodulin-dependent protein kinase II localization. J Neuroscience 24:8399–8403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shobe J 2002 The role of PKA, CaMKII, and PKC in avoidance conditioning: permissive or instructive? Neurobiol Learn Mem 77:291–312 [DOI] [PubMed] [Google Scholar]
- Soderling TR 1993 Calcium/calmodulin-dependent protein kinase II: role in learning and memory. Mol Cell Biochem 28:93–101 [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Fernandez G, Pappalardo A, White BA 2002 Membrane-initiated events account for progesterone’s ability to regulate intracellular free calcium levels and inhibit rat granulosa cell mitosis. Biol Reprod 67:379–385 [DOI] [PubMed] [Google Scholar]
- Ferrell Jr JE 1999 Xenopus oocyte maturation: new lessons from a good egg. Bioessays 833–842 [DOI] [PubMed] [Google Scholar]
- Blackmore PF 1999 Extra genomic actions of progesterone in human sperm and progesterone metabolites in human platelets. Steroids 64:149–156 [DOI] [PubMed] [Google Scholar]
- Luconi M, Bonaccorsi L, Maggi M, Pecchioli P, Krausz C, Forti G, Baldi E 1998 Identification and characterization of functional nongenomic progesterone receptors on human sperm membrane. J Clin Endocrinol Metab 83:877–885 [DOI] [PubMed] [Google Scholar]
- McEwen B, Jones KJ, Pfaff DW 1987 Hormonal control of sexual behavior in the female rat: molecular, cellular and neurochemical studies. Biol Reprod 36:37–45 [DOI] [PubMed] [Google Scholar]
- Powers B, Valenstein ES 1972 Sexual receptivity: facilitation by medial preoptic lesions in female rats. Science 175:1003–1005 [DOI] [PubMed] [Google Scholar]
- Hoshina Y, Takeo T, Nakano K, Sato T, Sakuma Y 1994 Axon-sparing lesion of the preoptic area enhances receptivity and diminishes proceptivity among components of female rat sexual behavior. Behav Brain Res 61:197–204 [DOI] [PubMed] [Google Scholar]
- Kow LM, Brown HE, Pfaff DW 1994 Activation of protein kinase C in the hypothalamic ventromedial nucleus or the midbrain central gray facilitates lordosis. Brain Res 660:241–248 [DOI] [PubMed] [Google Scholar]
- Lisk RD 1960 A comparison of the effectiveness of intravenous as opposed to subcutaneous injection of progesterone for the induction of estrous behavior in the rat. Can J Biochem Physiol 38:1381–1383 [PubMed] [Google Scholar]
- Kubli-Garfias C, Whalen RE 1977 Induction of lordosis behavior in female rats by intravenous administration of progestins. Horm Behav 9:380–386 [DOI] [PubMed] [Google Scholar]
- Meyerson B 1972 Latency between intravenous injection of progestins and the appearance of oestrous behavior in estrogen-treated ovariectomised rats. Horm Behav 3:1–10 [DOI] [PubMed] [Google Scholar]
- Watters JJ, Campbell JS, Cunnigham MJ, Krebs EG, Dorsa DM 1997 Rapid membrane effects of steroids in neuroblastoma cells: effects of estrogen on mitogen activated protein signaling cascade and c-fos immediate early gene transcription. Endocrinology 138:4030–4033 [DOI] [PubMed] [Google Scholar]
- Watters JJ, Dorsa DM 1998 Transcriptional effects on neuronal neurotensin gene expression involve cAMP/protein kinase A-dependent signaling mechanisms. J Neurosci 18:6672–6680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RC, Montiminy MR 1993 Transsynaptic control of gene expression. Annu Rev Neurosci 16:17–29 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Watters JJ, Dorsa DM 1996 Estrogen rapidly induces the phosphorylation of the cAMP response element binding protein in rat brain. Endocrinology 137:2163–2166 [DOI] [PubMed] [Google Scholar]
- DeBold JF, Frye CA 1994 Genomic and non-genomic actions of progesterone in the control of female hamster sexual behavior. Horm Behav 28:445–453 [DOI] [PubMed] [Google Scholar]
- DeBold JF, Frye CA 1994 Progesterone and neural mechanisms of hamster sexual behavior. Psychoneuroendocrinology 19:563–579 [DOI] [PubMed] [Google Scholar]
- Ramirez VD, Zheng J, Siddique KM 1996 Membrane progesterone receptors for estrogen, progesterone and testosterone in the rat brain: fantasy or reality. Cell Mol Neurobiol 16:175–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee KW, Chang WC, Briton RE, McEwen BS 1987 GABA-dependent modulation of the Cl-ionophore by steroids in rat brain. Eur J Pharmacol 136:419–423 [DOI] [PubMed] [Google Scholar]
- McEwen BS 1991 Non-genomic and genomic effects of steroids on neural activity. Trends Pharmacol Sci 12:141–146 [DOI] [PubMed] [Google Scholar]
- McEwen BS 1994 Steroid hormone actions in the brain: when is genome involved? Horm Behav 28:396–405 [DOI] [PubMed] [Google Scholar]
- Ronnekleiv O, Malyala A, Kelly MJ 2007 Membrane-initiated signaling of estrogen in the brain. Sem Reprod Med 25:165–177 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Rice CD, Pang Y, Pace M, Thomas P 2003 Cloning, expression and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA 100:2231–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Bond J, Thomas P 2003 Identification, classification and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci USA 100:2237–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley RL, Clay CM, Farmerie GD, Niswender G, Nett TM 2006 Cloning and characterization of an ovine intracellular seven transmembrane receptor for progesterone that mediates calcium mobilization. Endocrinology 147:4151–4159 [DOI] [PubMed] [Google Scholar]
- Thomas P 2008 Characteristics of membrane progestin receptor α (mPRα) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front Neuroendocrinol 29:292–312 [DOI] [PMC free article] [PubMed] [Google Scholar]