Abstract
The periosteum is now widely recognized as a homeostatic and therapeutic target for actions of sex steroids and intermittent PTH administration. The mechanisms by which estrogens suppress but PTH promotes periosteal expansion are not known. In this report, we show that intermittent PTH(1–34) promotes differentiation of periosteal osteoblast precursors as evidenced by the stimulation of the expression or activity of alkaline phosphatase as well as of targets of the bone morphogenetic protein 2 (BMP-2) and Wnt pathways. In contrast, 17β-estradiol (E2) had no effect by itself. However, it attenuated PTH- or BMP-2-induced differentiation of primary periosteal osteoblast progenitors. Administration of intermittent PTH to ovariectomized mice induced rapid phosphorylation of the BMP-2 target Smad1/5/8 in the periosteum. A replacement dose of E2 had no effect by itself but suppressed PTH-induced phosphorylation of Smad1/5/8. In contrast to its effects to stimulate periosteal osteoblast differentiation, PTH promoted and subsequently suppressed proliferation of periosteal osteoblast progenitors in vitro and in vivo. E2 promoted proliferation and attenuated the antiproliferative effect of PTH. Both hormones protected periosteal osteoblasts from apoptosis induced by various proapoptotic agents. These observations suggest that the different effects of PTH and estrogens on the periosteum result from opposing actions on the recruitment of early periosteal osteoblast progenitors. Intermittent PTH promotes osteoblast differentiation from periosteum-derived mesenchymal progenitors through ERK-, BMP-, and Wnt-dependent signaling pathways. Estrogens promote proliferation of early osteoblast progenitors but inhibit their differentiation by osteogenic agents such as PTH or BMP-2.
BONE IS SURROUNDED by a layer of dense connective tissue, the periosteum, which contains mesenchymal progenitors, osteoblasts (bone-forming cells), and fibroblasts as well as microvessels and sympathetic nerves. The periosteum possesses osteogenic potency because, under appropriate stimulation, mesenchymal progenitors adjacent to the bone surface can differentiate into osteoblasts. The periosteal envelope has now been identified as a target for therapeutic intervention because 1) changes in periosteal bone mass have a higher impact on bone strength than changes in any other bone compartment, 2) the added strength afforded by periosteal expansion occurs independent of increases in bone mineral density, and 3) periosteal expansion compensates, in part, for reductions in bone strength caused by postmenopausal bone loss (1,2,3,4).
Estrogens (and androgens) protect the adult skeleton against loss of cancellous bone by suppressing the rate of bone turnover and maintaining a balance between bone formation and resorption (5,6). The latter results from opposite effects on the life span of osteoblasts/osteocytes and osteoclasts: an antiapoptotic effect on the former and a proapoptotic effect on the latter cell type (7,8,9). Both the antiapoptotic and proapoptotic effects are conferred by kinase-mediated actions of the estrogen receptor (ER) or androgen receptor (AR) that are distinct from classical genotropic actions (7,8). Similar to their anti-osteoblastogenic actions on cancellous bone, estrogens suppress (whereas androgens promote) periosteal expansion in the growing, adult, and aging skeleton of rodents, primates, and humans (10,11,12,13,14). It is believed that this effect of androgen on the periosteal surface gives men a biomechanical advantage and, thus, helps to protect them from fragility or fractures (15,16).
PTH, administered intermittently and in low dosage, is anabolic for the human and rodent skeleton (17,18,19,20,21). One of the mechanisms by which PTH is anabolic relates to its effects to increase periosteal bone formation (22,23). The biomechanical basis for the salutary improvement in bone strength is due to the actions of PTH to increase cross-sectional diameter of bone by stimulating periosteal bone apposition (24,25,26). The molecular mechanisms by which PTH is protective in this way of bone strength may relate to its effects to prevent osteoblast apoptosis via activation of cAMP/protein kinase A-dependent inactivation of the proapoptotic protein Bad and transcription of survival genes such as Bcl-2 and runt-related transcription factor 2 (Runx2) (27,28,29). These are important mechanisms of the anabolic actions of intermittent PTH in cancellous bone. Stimulation of osteogenic cell proliferation and differentiation in bone marrow-derived cells also contribute to the anabolic actions of intermittent PTH administration (17,30,31).
The cellular targets and the molecular and cellular mechanisms of the suppressive actions of estrogens and the beneficial effects of PTH on periosteal expansion in the adult and aging skeleton are unknown. Moreover, the extent to which and the mechanisms by which estrogens may contribute to or antagonize the anabolic actions of PTH on the periosteum are also not known. The demonstration that estrogen (ERα and ERβ) and PTH (PTH1R) receptors are expressed in periosteal cells (32,33) suggests that estrogens and PTH can have direct effects on periosteal osteoblasts. In this investigation, we studied potential cellular targets of intermittent PTH and sex steroids actions on the periosteum and investigated in vitro and in vivo the molecular mechanisms and signaling events that could help account for their differential effects. The results provide new insights into how these hormonal regulators of the periosteum act and interact with each other.
Materials and Methods
Chemicals
PTH(1–34) was kindly provided by Drs. John T. Potts, Jr., and Thomas J. Gardella (Massachusetts General Hospital and Harvard Medical School, Boston, MA). 17β-Estradiol (E2), dihydrotestosterone (DHT), and epidermal growth factor (EGF) were purchased from Sigma Chemical Co. (St. Louis, MO). Recombinant human bone morphogenetic protein 2 (BMP-2), recombinant mouse noggin, and recombinant mouse Wnt3a were purchased from R&D Systems (Minneapolism MN). Dickopff 1 (DKK-1) was kindly provided by Dr. Georges Rawadi (Proskelia a Galapagos Co., Romainville, France). PD98059 was obtained from Promega (Madison, WI).
Animal experiments
Sprague Dawley rats were obtained from Harlan (Indianapolis, IN), and C57BL/6 mice were obtained from Jackson Laboratories (Bar Harbor, ME). For the experiments with ovariectomized animals, mice were anesthetized by inhalation of 2–5% isoflurane. The back was shaved and cleansed with betadine, and an incision was made to expose the ovaries, which were then removed. The incision was then closed with a wound clip. All animals were housed in a temperature-controlled room (22 ± 2 C) with a daily 12-h light, 12-h dark schedule. During the study, animals had free access to water and were fed a phytoestrogen-free diet (2014 Teklad Global 14% protein rodent maintenance diet; Harlan Teklad, Madison, WI). Animals were euthanized by inhalation of 5% isoflurane followed by cervical dislocation. All procedures were approved by the Institutional Animal Care and Use Committee.
Isolation and culture of periosteal osteoblast precursors
Periosteal tissue was dissected from the proximal tibias and femurs of 3-wk-old C57BL/6 mice or Sprague Dawley rats with a scalpel and placed in Hanks’ balanced salt solution containing 10% fetal bovine serum (FBS). Periosteal flaps were washed in 4 mm EDTA to remove the serum and were incubated in 10 ml Hanks’ balanced salt solution containing 0.3% (or 400 U) collagenase type II twice for 40 min at 37 C with shaking. The digestion was stopped by addition of an equal volume of FBS, and the cells were collected, centrifuged, and resuspended in growth medium containing 10% FBS in DMEM. Cells were plated in a T75 flask and expanded for approximately 15 d until they reached 90% confluency. Half of the medium was replaced every 5 d. For experiments, cells were trypsinized with 0.25% trypsin in PBS and replated at appropriate density. In all experiments described, FBS was charcoal treated to assure that the medium was not contaminated with fetal steroids.
Characterization of periosteal osteoblasts
The isolation of osteoblastic cells from periosteal tissue was achieved by measuring the expression of periostin by immunocytochemistry. Periostin is a secreted cell adhesion protein that is highly and preferentially expressed in the embryonic and adult periosteum and periodontal ligament as well as in cardiac valves (34). Inactivation of the protein results in defects in these tissues. Periostin supports the recruitment, attachment, and spreading of osteoblast precursors in the periosteum and of osteoblastic cell lines. We selected periostin as a periosteal osteoblast marker because, unlike osteocalcin, it is expressed in multipotential osteoblast progenitors, and, unlike alkaline phosphatase, which can be strongly expressed in fibroblasts, periostin is expressed at low levels in these cells (34).
Measurement of alkaline phosphatase (ALP) activity
Primary cultures of periosteal osteoblast precursors were seeded at a density of 1.2 × 104/cm2 in 10% FBS. The following day, cells were placed in 5% serum-containing medium and were pretreated with vehicle, noggin, human recombinant DKK-1 or PD98580 for 1 h. Vehicle, BMP-2, PTH (1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34), and steroids were added to the cells, and cultures were continued for 16 d. Cells were lysed in 100 mm glycine, 1 mm MgCl2, and 1% Triton X-100 (pH 10). ALP activity in the cell lysate was determined using a buffer containing 2-amino-2-methylpropanol and p-nitrophenylphosphate (Sigma-Aldrich). ALP activity was normalized for total protein concentration, determined using a Bio-Rad (Hercules, CA) DC protein assay kit.
Cell proliferation in vitro
Periosteal cells were cultured in 96-well plates at 1 × 104 cells per well. Twenty four hours before treatments, cells were switched to medium containing 2% FBS. Proliferation of periosteal osteoblast precursors was assessed during the last 4 h of treatments using a commercially available assay (CellTiter 96* AQueous One; Promega), which measures bioreduction of a tetrazolium compound by dehydrogenase enzymes found in metabolically active cells to a formazan product. The quantity of formazan product as measured by the amount of absorbance is directly proportional to the number of living cells in culture.
Cell proliferation in vivo
Mice were injected ip with 50 mg/kg of the thymidine analog bromodeoxyuridine (BrdU). BrdU is incorporated into proliferating cells (S-phase) and is then detected by a monoclonal anti-BrdU antibody. Animals were killed 4 h after BrdU injection. Tibia were removed, dissected longitudinally at the midshaft, and fixed in 10% formalin/PBS at 4 C for 24 h. Bones were demineralized in 25% EDTA at 37 C for 3 d. EDTA was replenished every day. Demineralized bones were washed in H2O to remove EDTA, dehydrated, and embedded in paraffin. BrdU labeling was measured in 4μm sections along the tibial or femoral midshaft using commercially available kit (Zymed Laboratories, San Francisco, CA). Sections were counterstained with hematoxylin. Osteoblasts (cuboidal cells lining the bone surface) with dark brown nuclear staining were counted as BrdU positive.
Western blot analysis
The phosphorylation status of Smad1/5/8 was analyzed by immunoblotting using antibodies recognizing phosphorylated Smad1/Smad5(Ser463/465)/Smad8(Ser426/428) (Cell Signaling, Beverly, MA) and Smad4 (Santa Cruz Biotechnology, Santa Cruz, CA). Primary antibodies were detected with a horseradish peroxidase-conjugated secondary antibody (Santa Cruz) and SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL).
Real-time PCR
Total RNA was extracted and real-time PCR performed as previously described (8,35). The primers and probes for alkaline phosphatase, Axin2, Smad6, and 18S rRNA (used as the housekeeping gene) were manufactured by Assays-by-Demand Service (Applied Biosystems, Foster City, CA). The probe and primers for BMP-2 and osteocalcin were also obtained from Applied Biosystems and were as follows: BMP-2 forward 5′-CCCGCGTGCTTCTTAGAC-3′, reverse 5′-GAAGCAGCAACGCTAGAAGACA-3′, and probe 5′-CATGGTCGACCTTTAG-3′ and osteocalcin forward 5′-GCTGCGCTCTGTCTCTCTGA-3′, reverse 5′-TGCTTGGACATGAAGGCTTTG-3′, and probe 5′-AAGCCCAGCGGCC-3′.
Apoptosis
Apoptosis in cultures of primary periosteal cells was determined by measuring caspase 3 activity in cell lysates as described before (36).
Statistical analysis
The data were analyzed by ANOVA, and the Student-Newman-Keuls method was used to estimate the level of significance of differences between means. Results shown were reproduced in at least three experiments. Dunn’s test on ANOVA by ranks was used for comparisons in which variances were not equivalent.
Results
Characterization of periosteal osteoblasts
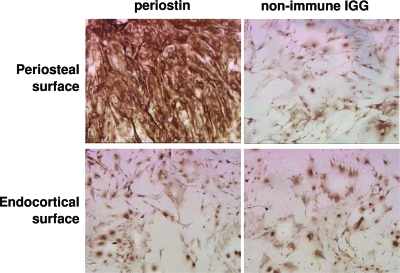
We first established that osteoblastic cells can be efficiently isolated from the periosteal surface of long bones. We measured expression of periostin, an osteoblastic marker, in rat periosteal cells isolated and cultured as described in Materials and Methods. Cells derived from the periosteum of long bones from 3-wk-old rats were immunostained with a periostin-specific antibody (Fig. 1). Periostin was highly expressed in periosteal cells (top left) but not in cells isolated from the remaining area of cortical bone (bottom left). The intensity of the staining in approximately 90% of the cells in culture indicates high levels of osteoblastic lineage homogeneity in the isolated periosteal cell population. The specificity of immunostaining in these experiments was demonstrated using as negative control periosteal or periosteal cell-free cortical bone cultures stained with an antibody recognizing nonimmune IgG (right panels).
Figure 1.
Expression of periostin in periosteal cells. Periosteal cells (top) or cells from the remaining area of cortical bone were isolated from tibias and femurs of 3-wk-old Sprague Dawley rats and were immunostained with a periostin-specific antibody or a nonimmune IgG control. Cells were photographed at magnification ×20.
PTH and sex steroids differentially regulate differentiation of periosteal osteoblast progenitors in vitro and in vivo: involvement of BMP-2 and Wnt signaling
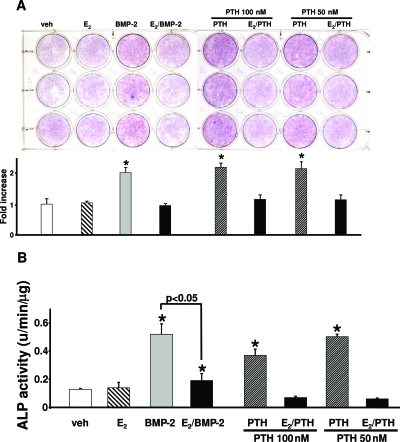
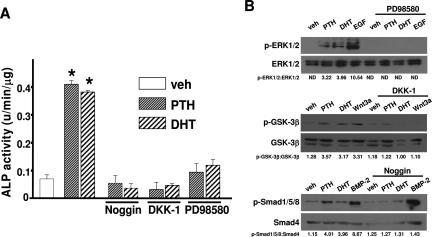
Based on the evidence that PTH has anabolic properties on periosteal bone, but estrogens limit periosteal bone mass in vivo, we next investigated potential biological mechanisms by which PTH could exert an advantageous effect over estradiol. The three mechanisms to consider are effects on periosteal osteoblast differentiation, osteoblast proliferation, or apoptosis. We first examined whether PTH could induce osteoblastic lineage differentiation in periosteal cells. We employed intermittent PTH administration, a setting in which its anabolic properties are most evident (31,37). PTH administration under these conditions has been shown to simulate an intermittent mode of administration of the hormone and stimulate cAMP/protein kinase A- and protein kinase C-dependent ALP activity and mineralization in bone marrow cells. Accordingly, periosteal osteoblast precursors were exposed to the biologically active but foreshortened amino-terminal fragment of PTH [PTH(1–34)] for the first 6 h of each 48-h period, after which the medium was removed, the cell layer was rinsed with warm medium, and fresh medium without PTH(1–34) was added for the remaining 42 h. PTH up-regulated the expression and activity of ALP, a gene responsible for elaboration of mineralized matrix, within 16 d of treatment (Fig. 2, A and B). Continuous treatment of periosteal osteoblast cultures with PTH suppressed basal levels of ALP activity (data not shown), probably reflecting, at least in part, the bone catabolic actions of chronic exposure to the hormone. BMP-2, used as a positive control due to its established prodifferentiating/osteogenic potential, had similar actions. In contrast, E2 had no effect by itself but it attenuated PTH- or BMP-2-induced stimulation of ALP expression or activity.
Figure 2.
PTH promotes but E2 inhibits PTH- or BMP-2- induced ALP expression or activity in primary cultures of periosteal cells. A, Top, Primary rat periosteal cells (0.2 × 105 per 12-well plate) were cultured in 10% FBS. Vehicle, BMP-2 (100 ng/ml), E2 (10−8 m), human PTH(1–34) (50 or 100 nm), or a combination of E2 and PTH or E2 and BMP-2 were added to the cells, and cultures were continued for 16 d. Cells were immunostained with an ALP-specific antibody. Bottom, Resulting stained cultures were quantitated by image analysis. Fold increase in the y-axis indicates increase over vehicle-treated cells (1 is the staining intensity of vehicle-treated cells). B, Primary rat periosteal cells were cultured and treated as described in A for 16 d. ALP activity was determined in cell lysates using a commercially available kit (Sigma). ALP activity was normalized for total protein concentration. Bars indicate means ± sd of triplicate determinations. *, P < 0.05 vs. vehicle by ANOVA.
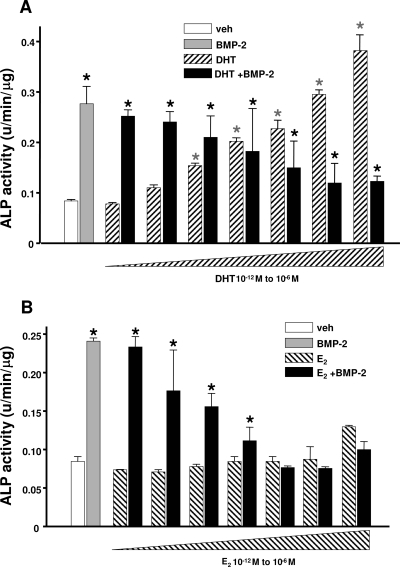
Similar to PTH, and consistent with its ability to promote periosteal expansion in rodents and humans, DHT dose-dependently up-regulated ALP activity in rat periosteal osteoblast precursors (Fig. 3A). In contrast, E2 dose-dependently antagonized BMP-2-induced ALP activity in the same cells (Fig. 3B). Next, we examined whether PTH, DHT, and E2 affect the differentiation of periosteal osteoblast progenitors by interacting with components of the BMP-2 or Wnt signaling cascades. The rational for these studies was based on evidence that Wnts promote osteoblast differentiation, proliferation, or survival via inactivation of glycogen synthase kinase-3β (GSK-3β), stabilization of β-catenin, and β-catenin-mediated activation of transcription factors such as the T cell factor (TCF) (35,38). BMPs, acting via their receptors, induce osteoblast differentiation by phosphorylation of Smad1, Smad5, and Smad8 and subsequent recruitment of Smad4 to the phosphorylated Smad complex. Eventually, the complex translocates to the nucleus and induces expression of ALP and Runx2, a transcription factor required for expression of the osteoblast phenotype (39).
Figure 3.
DHT promotes ALP activity, but E2 inhibits BMP-2-induced ALP activity in rat periosteal cells. Primary rat periosteal cells were treated as described in Fig. 2A. Bars indicate means ± sd of triplicate determinations. *, P < 0.05 vs. vehicle (veh) by ANOVA.
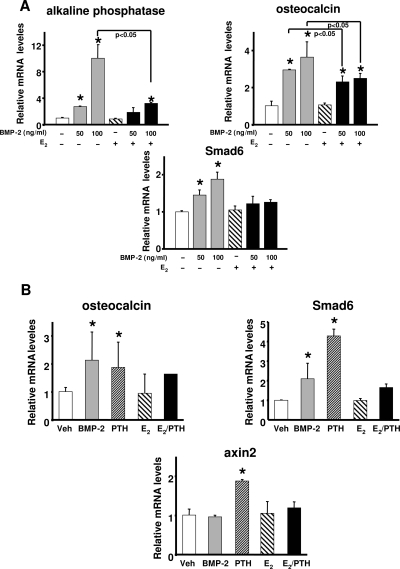
E2, similar to its suppressive effects on ALP expression and activity, also attenuated the stimulatory effect of BMP-2 on the up-regulation of ALP, osteocalcin, and Smad6 (a transcriptional target of BMP-2) expression in murine periosteal cells (Fig. 4A). In contrast to E2, and consistent with its pro-differentiating actions, PTH up-regulated the expression of axin2 (a transcriptional target of Wnt/β-catenin), osteocalcin, and Smad6 in rat periosteal cells (Fig. 4B). E2 attenuated PTH-induced up-regulation of axin2, osteocalcin, and Smad6 in the same cells. Confirming the involvement of BMP and Wnt signaling in the pro-differentiating actions of PTH and DHT, induction of ALP activity by either hormone was abrogated by the BMP inhibitor noggin or the Wnt inhibitor DKK-1 (Fig. 5A).
Figure 4.
PTH promotes whereas E2 inhibits BMP-2-induced expression of β-catenin and BMP targets in primary cultures of murine and rat periosteal cells. Primary murine (A) or rat (B) periosteal cells were treated as described in Fig. 2A. Gene expression was measured by real-time PCR. Bars indicate means ± sd of triplicate determinations. *, P < 0.05 vs. vehicle (Veh) by ANOVA.
Figure 5.
BMP, Wnt, and ERK signaling are required for the pro-differentiating actions of PTH or DHT. A, Primary rat periosteal cells were pretreated with noggin (50 ng/ml), human recombinant DKK-1 (25 ng/ml), or PD98580 (25 μm) for 1 h. Vehicle (veh), PTH (100 nm), or DHT (10−8 m) were added to the cells, and cultures were continued for 16 d. Bars indicate means ± sd of triplicate determinations. *, P < 0.05 vs. vehicle by ANOVA. B, Primary rat periosteal cells were pretreated with PD98580 (25 μm), DKK-1 (25 ng/ml), or noggin (50 ng/ml) for 1 h. Vehicle (veh), PTH (100 nm), DHT (10−8 m), EGF (5 ng/ml), BMP-2 (100 ng/ml), or recombinant Wnt3a (50 ng/ml) were added to the cells as indicated. Cultures were continued for 5 min for the determination of ERK phosphorylation, 1 h for the determination of GSK-3β phosphorylation, and 30 min for the determination of Smad1/5/8 phosphorylation. Total, ERK, GSK-3β, and Smad4 were used for protein normalization.
Possible regulators of the positive actions of PTH and DHT on differentiation of periosteal osteoblasts are kinases such as ERK1/2. It has been shown previously that kinase-mediated actions of the ER not only control bone cell survival but, in the absence of classical genotropic actions, also promote differentiation of bone marrow-derived osteoblast progenitors by concomitantly activating the BMP and Wnt signaling cascades (7,8,40). Thus, we examined whether activation of ERK1/2 is required for the pro-differentiating actions of DHT and PTH on periosteal osteoblast progenitors. Pretreatment of primary periosteal cultures with the ERK inhibitor PD98580 suppressed the induction of ALP activity by PTH or DHT (Fig. 5A).
We examined the ability of PTH and DHT to induce BMP, Wnt, and ERK signaling in our experimental setting. Activation of BMP signaling was assessed by measuring the phosphorylation status of Smad1/5/8. Induction of Wnt signaling was evaluated by analyzing the phosphorylation and subsequent inactivation of GSK-3β. Phosphorylation of ERK1/2 was also measured. EGF, recombinant Wnt3a, and BMP-2 were used as positive controls for the induction of ERK, Wnt, and BMP signaling. PTH and DHT induced the phosphorylation of ERK1/2, GSK-3β, and Smad1/5/8 in periosteal cells (Fig. 5B). Pretreatment of cultures with recombinant noggin or DKK1 or PD98580 abolished the effects of either hormone on the activation of the respective pathways.
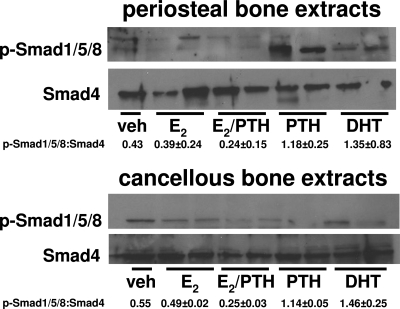
These in vitro studies were followed by an in vivo protocol to further confirm the actions of sex steroids and PTH to differentially regulate BMP signaling. We examined 3-month-old C57BL/6 mice. In agreement with the in vitro observations, administration of an anabolic dose of intermittent PTH (40 ng/g) (28) or a bone-protective dose of DHT (300 ng/g) (41,42) to these ovariectomized C57BL/6 mice induced, within 1 h, phosphorylation of Smad1/5/8 in the periosteum as assessed by Western blot analysis of femoral periosteal lysates. A replacement dose of E2 (30 ng/g) had no effect by itself but suppressed PTH-induced phosphorylation of Smad1/5/8. None of the three hormones affected phosphorylation of Smad1, -5, or -8 in trabecular bone from the femurs of the same experimental animals (Fig. 6).
Figure 6.
PTH and DHT stimulate but E2 inhibits Smad1/5/8 phosphorylation in periosteal bone in vivo. Four-month-old C57BL/6 mice were ovariectomized. Ovariectomized animals were left untreated for 5 d and then received a single ip injection of human PTH(1–34) (40 ng/g) or single sc injections of E2 (30 ng/g) or DHT (300 ng/g) or combination of E2 and PTH. One hour later, femurs were obtained and lysates were made for periosteal and cancellous bone separately. Smad1/5/8 phosphorylation was determined in extracts by Western blot analysis. Smad4 was used for protein normalization. Each lane represents one mouse.
PTH and sex steroids regulate proliferation of periosteal osteoblast progenitors in vitro and in vivo
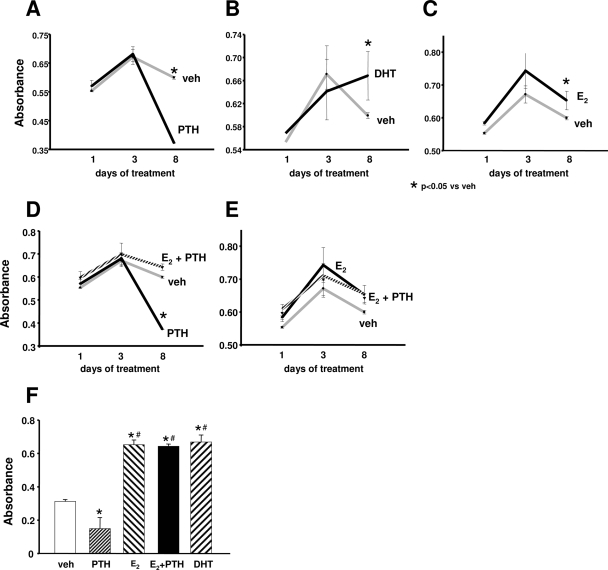
We next studied the action of PTH and sex steroids on another important aspect of periosteal cell function, namely proliferation. In contrast to its pro-differentiating actions, PTH had no effect up to 3 d of treatment (Fig. 7A). However, in two independent experiments, PTH suppressed proliferation of primary periosteal osteoblast progenitors after 8 d of treatment (Fig. 7, D and F). In contrast, E2 promoted proliferation in both experiments (Fig. 7, C and F). It also attenuated the late, antiproliferative effect of PTH (Fig. 7, D and E). Similar to E2, DHT increased the proliferative activity of periosteal osteoblasts (Fig. 7B).
Figure 7.
Proliferative actions of sex steroids and PTH in primary periosteal osteoblast progenitors. In two separate experiments, primary periosteal cells were treated with vehicle (veh), E2, DHT (10−8 m), or PTH(1–34) for 1, 3, or 8 d (A–E) or 8 d (F). Proliferation was assessed during the last 4 h of treatments using a commercially available assay (CellTiter 96* AQueous One; Promega) that measures bioreduction of a tetrazolium compound by dehydrogenase enzymes found in metabolically active cells to a formazan product. The quantity of formazan product as measured by the amount of absorbance is directly proportional to the number of living cells in culture. Bars indicate means ± sd of triplicate determinations. *, P < 0.05 vs. vehicle; #, P < 0.05 vs. PTH by ANOVA. In A–E, results obtained from the same experiment are depicted in five different graphs to facilitate comparisons between different treatment groups.
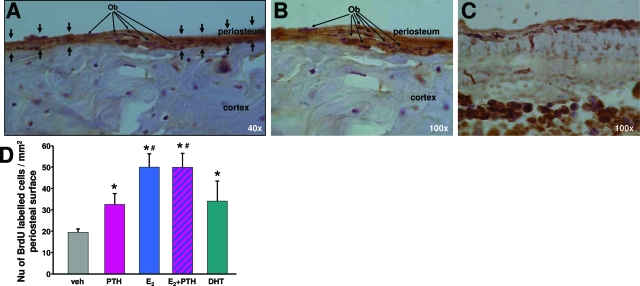
These studies in vitro on the action of PTH and sex steroids were followed by an in vivo protocol of the ovariectomized mice as noted above. Three-month-old ovariectomized mice were administered the anabolic dose of PTH and the bone-protective doses of E2 or DHT. After 24 h, BrdU was administered for an additional 4 h to quantify the extent of cellular proliferation in the tibia. In agreement with the in vitro observations, both E2 and DHT within 24 h stimulated the proliferation of periosteal osteoblasts in the tibia of adult ovariectomized C57BL/6 mice (Fig. 8). Intermittent PTH also promoted the proliferation of periosteal osteoblasts at this early time point. Combination treatment with E2 and PTH increased periosteal cell proliferation to levels higher than PTH alone.
Figure 8.
Proliferative actions of sex steroids and PTH in vivo. Four-month-old C57BL/6 mice were ovariectomized. Ovariectomized animals were left untreated for 5 d and then received a single ip injection of human PTH(1–34) (40 ng/g) or single sc injections of E2 (30 ng/g) or DHT (300 ng/g) or combination of E2 and PTH. Twenty-four hours later, mice were injected ip with 50 mg/kg BrdU and killed 4 h later. Tibias were removed and BrdU labeling was measured as described in Materials and Methods. A and B, Magnifications of tibial cross-sections from BrdU-infused animals (×40 and ×100); C, magnification of tibia from non-BrdU-treated animals (×100). D, BrdU-positive cells were counted using the Osteomeasure Software (Osteometrix Inc., Atlanta, GA). Each bar indicates means ± sd of all the animals in each group. (n = 4). *, P < 0.05 vs. untreated ovariectomized mice; #, P < 0.05 vs. PTH-treated mice by ANOVA. Ob indicates representative periosteal osteoblast progenitors.
PTH and sex steroids regulate apoptosis of periosteal osteoblasts
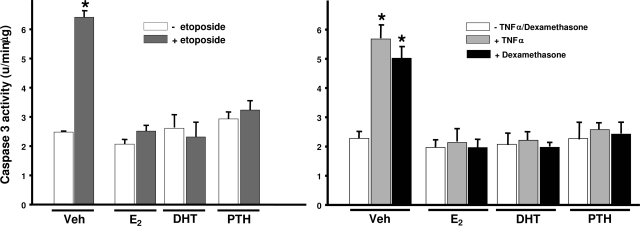
Intermittent PTH as well as estrogens and androgens promote the survival of osteoblasts in the bone marrow (7,8,27,43). Thus, we examined whether the three hormones influence periosteal bone expansion by actions on osteoblast apoptosis. Consistent with their actions on bone marrow-derived osteoblastic cells, E2, DHT, or PTH each protected periosteal osteoblasts from apoptosis induced by three different proapoptotic stimuli: the topoisomerase II inhibitor of DNA synthesis etoposide, TNFα, and dexamethasone (Fig. 9).
Figure 9.
E2, DHT, and PTH protect rat periosteal cells from apoptosis. Primary rat periosteal cells (0.2 × 105 per 12-well plate) were cultured in 10% FBS. Cells were treated with vehicle, E2, DHT (10−8 m) or bovine PTH(1–34) (50 nm) for 1 h before the addition of etoposide (50 μm), TNFα (1 nm), or dexamethasone (10−7 m) for an additional 6 h. Apoptosis was assessed by measuring caspase 3 activity in cell lysates. Caspase 3 activity was normalized for total protein concentration. Bars indicate means ± sd of triplicate determinations. *, P < 0.05 vs. vehicle by ANOVA.
Discussion
In this report, we have shown that intermittent PTH administration promotes, whereas estrogens suppress, osteoblast differentiation from periosteum-derived mesenchymal progenitors in vitro and in vivo. In contrast to its pro-differentiating actions, intermittent PTH exerts a biphasic effect on the proliferation of periosteal osteoblast progenitors; initially it promotes but subsequently suppresses their proliferation. Estrogens promote proliferation of periosteal osteoblast progenitors. Moreover, estrogens inhibit the antiproliferative and pro-differentiating actions of intermittent PTH. These observations suggest that the different effects of estrogens and PTH on the periosteum result from opposing actions on the recruitment of early periosteal osteoblast progenitors. Estrogens promote the expansion of early osteoblast progenitors but inhibit their differentiation by osteogenic agents such as PTH or BMP-2.
The property of estrogens to increase periosteal osteoblast precursor number yet maintain them in an undifferentiated state may reflect actions of the hormone on a cell population distinct from that affected by PTH. Indeed, the existence of an early osteoblast progenitor in the periosteum has been confirmed by several studies (44,45,46,47). However, it is likely that periosteal cell cultures will contain a heterogeneous cell population including mesenchymal osteoblast progenitors and committed preosteoblasts as well as mature osteoblasts. It is possible that estrogens, androgens, and PTH may act on uncommitted osteoblast precursors at a different stage during their differentiation to the osteoblastic lineage. Further studies that will identify the stage-specific cell target of each one of the three hormones are needed.
Similar to PTH, DHT had a pro-differentiating action on periosteal osteoblasts as evidenced by the dose-dependent induction of ALP activity in periosteal cultures and Smad1/5/8 phosphorylation in bone in vivo. In contrast to DHT, but similar to estrogens, DHT promoted proliferation of periosteal osteoblasts in vitro and in vivo.
The molecular mechanisms that underlie the pro-differentiating effects of intermittent PTH and androgens as well as the anti-osteoblastogenic effects of estrogens on periosteal osteoblast precursors involve modulation of BMP and Wnt signaling. This was shown by the induction, by PTH and DHT, or suppression, by E2, of Smad1/5/8 activity and Wnt/β-catenin-mediated transcription in periosteal cells in vitro and in vivo. Inhibitors of Wnt or BMP signaling abrogated the pro-differentiating actions of PTH or DHT. This finding indicates that interactions between each hormone and components of BMP or Wnt signaling are a prerequisite for the development and maturation of periosteal osteoblasts from their precursors. In support of our findings, components of Wnt and BMP signaling are known to interact with the ER or the AR. The formation of a complex between β-catenin or Smad1/5/8 or Smad4 proteins and the ER or AR up-regulated the transcriptional activity of the two receptors at the expense of β-catenin- or Smad1/5/8-mediated transcription (for a review see Ref. 48). We have previously shown that selective activation of kinase-mediated actions of the ER or AR can potentiate β-catenin-mediated transcription, increase BMP-2 expression and Smad1/5/8 phosphorylation, and promote differentiation of pluripotent mesenchymal progenitors to the osteoblastic lineage in vitro (40). It is therefore possible that the balance between stimulation of Smad1/5/8 and/or β-catenin-mediated transcription and classical transcriptional activity of the ER or AR may determine the final outcome of estrogens compared with androgens on periosteal osteoblast differentiation.
Consistent with our observations that intermittent PTH promotes differentiation of periosteal osteoblast progenitors, in vitro studies under conditions that simulate administration of PTH in an intermittent fashion show stimulation of osteoblastic lineage commitment in bone marrow-derived cells or a bipotential cell line (17,30,31). PTH also rapidly and transiently stimulates the expression and activity of the osteoblast-specific transcription factor Runx2 as well as the expression of ALP and type I procollagen in cultured bone marrow or calvaria cells (31,37). The pro-differentiating actions of PTH in periosteal osteoblast progenitors required BMP and Wnt signaling and involved stimulation of Smad1/5/8 activity and β-catenin-mediated transcription. Similar to our findings in periosteal cells, intermittent PTH regulates components of BMP and Wnt signaling and requires the Wnt receptor secreted Frizzled-related protein 1 (SFRP1) for its bone anabolic actions (49,50,51,52,53).
The discrepancy between the in vitro and in vivo observations regarding the proliferative actions of PTH may have resulted from differences in the duration of treatment with the hormone. A biphasic effect of PTH on the proliferation of periosteal osteoblast precursors in combination with its ability to promote the differentiation of early periosteal cells to the osteoblastic lineage are characteristics of potent differentiating agents. Indeed, an early phase of rapid proliferation of progenitor cells is followed by exit from the cell cycle, which allows the differentiation process to specific lineages to initiate and proceed. More to the point, PTH targets cells in the G1/S phase of the cell cycle, increases expression of the cell cycle inhibitors p27Kip1 and p21Cip1, and attenuates the expression of the cell cycle marker H4 and cyclin D1 in osteoblastic cell lines or cultures of primary cells (54,55,56). Alternatively, the discrepancy between the antiproliferative effect of intermittent PTH in periosteal cultures and its proliferative actions in the periosteum in vivo may be specific to the differentiation/developmental stage.
In cancellous bone, prevention of osteoblast apoptosis is an important mechanism of the actions of estrogens and intermittent PTH. We show that estrogens and PTH protect periosteal osteoblasts from apoptosis induced by various proapoptotic stimuli. However, it has been suggested that the effect of PTH on periosteal expansion cannot be solely caused by abrogation of osteoblast apoptosis because the normal periosteal bone formation rate is very low (57). It is possible that whereas antiapoptosis is a crucial mechanism by which estrogens and intermittent PTH exert their beneficial effects on cancellous bone, and may contribute to their actions on the periosteum, it is their proliferative and pro-differentiating effects on early osteoblast progenitors that determine periosteal bone formation. The extent to which antiapoptosis contributes to the affects of the hormones will need to be further explored.
PTH is believed to be efficacious as a treatment for osteoporosis by increasing periosteal bone apposition (in addition to its major effects on cancellous bone mass). This outer envelope of bone is thus identified as a therapeutic target. Our studies provide the first mechanistic evidence of how PTH and sex steroids serve to have beneficial actions at this important skeletal site.
Acknowledgments
We thank Gerard Karsenty for critically reviewing this paper before its submission. We are grateful to John T. Potts, Jr. and Thomas J. Gardella for providing PTH(1–34) and to Georges Rawadi for providing DKK-1. We also thank Maria Almeida and Li Han (University of Arkansas for Medical Sciences) for preliminary experiments pertinent to this study.
Footnotes
This work was supported by the National Institutes of Health (AR051187 to S.K.).
Disclosure Statement: S.K., M.O., M.T.R., and E.D. have nothing to disclose. J.P.B. consults for and received lecture fees from Amgen, Procter & Gamble, Novartis, Sanofi-Aventis, and Merck.
First Published Online July 10, 2008
Abbreviations: ALP, Alkaline phosphatase; AR, androgen receptor; BMP-2, bone morphogenetic protein 2; BrdU, bromodeoxyuridine; DHT, dihydrotestosterone; DKK-1, Dickopff 1; E2, 17β-estradiol; EGF, epidermal growth factor; ER, estrogen receptor; FBS, fetal bovine serum; GSK-3β, glycogen synthase kinase-3β; Runx2, runt-related transcription factor 2.
References
- Seeman E 2003 The structural and biomechanical basis of the gain and loss of bone strength in women and men. Endocrinol Metab Clin North Am 32:25–38 [DOI] [PubMed] [Google Scholar]
- Seeman E 2001 Sexual dimorphism in skeletal size, density, and strength. J Clin Endocrinol Metab 86:4576–4584 [DOI] [PubMed] [Google Scholar]
- Orwoll ES 2003 Toward an expanded understanding of the role of the periosteum in skeletal health. J Bone Miner Res 18:949–954 [DOI] [PubMed] [Google Scholar]
- Carter DR, Beaupré GS 2001 Skeletal function and form: mechanobiology of skeletal development. Cambridge, UK: Cambridge University Press [Google Scholar]
- Manolagas SC 2000 Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21:115–137 [DOI] [PubMed] [Google Scholar]
- Manolagas SC, Kousteni S, Jilka RL 2002 Sex steroids and bone. Recent Prog Horm Res 57:385–409 [DOI] [PubMed] [Google Scholar]
- Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han K, DiGregorio G, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC 2001 Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell 104:719–730 [PubMed] [Google Scholar]
- Kousteni S, Han L, Chen J-R, Almeida M, Plotkin LI, Bellido T, Manolagas SC 2003 Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J Clin Invest 111:1651–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JR, Plotkin LI, Aguirre JI, Han L, Jilka RL, Kousteni S, Bellido T, Manolagas SC 2005 Transient versus sustained phosphorylation and nuclear accumulation of ERKs underlie anti-versus pro-apoptotic effects of estrogens. J Biol Chem 280:4632–4638 [DOI] [PubMed] [Google Scholar]
- Turner RT, Hannon KS, Demers LM, Buchanan J, Bell NH 1989 Differential effects of gonadal function on bone histomorphometry in male and female rats. J Bone Miner Res 4:557–563 [DOI] [PubMed] [Google Scholar]
- Coxam V, Bowman BM, Mecham M, Roth CM, Miller MA, Miller SC 1996 Effects of dihydrotestosterone alone and combined with estrogen on bone mineral density, bone growth, and formation rates in ovariectomized rats. Bone 19:107–114 [DOI] [PubMed] [Google Scholar]
- Gunness M, Orwoll E 1995 Early induction of alterations in cancellous and cortical bone histology after orchiectomy in mature rats. J Bone Miner Res 10:1735–1744 [DOI] [PubMed] [Google Scholar]
- Lea C, Kendall N, Flanagan AM 1996 Casodex (a nonsteroidal antiandrogen) reduces cancellous, endosteal, and periosteal bone formation in estrogen-replete female rats. Calcif Tissue Int 58:268–272 [DOI] [PubMed] [Google Scholar]
- Kim BT, Mosekilde L, Duan Y, Zhang XZ, Tornvig L, Thomsen JS, Seeman E 2003 The structural and hormonal basis of sex differences in peak appendicular bone strength in rats. J Bone Miner Res 18:150–155 [DOI] [PubMed] [Google Scholar]
- Seeman E 2002 Pathogenesis of bone fragility in women and men. Lancet 359:1841–1850 [DOI] [PubMed] [Google Scholar]
- Beck TJ, Ruff CB, Scott Jr WW, Plato CC, Tobin JD, Quan CA 1992 Sex differences in geometry of the femoral neck with aging: a structural analysis of bone mineral data. Calcif Tissue Int 50:24–29 [DOI] [PubMed] [Google Scholar]
- Dempster DW, Cosman F, Parisien M, Shen V 1993 Anabolic actions of parathyroid hormone on bone. Endocr Rev 14:690–709 [DOI] [PubMed] [Google Scholar]
- Hodsman AB, Fraher LJ, Watson PH 1998 Parathyroid hormone: the clinical experience and prospects. In: Whitfield JF, Morley P, eds. Anabolic treatments for osteoporosis. 1st ed. Boca Raton, FL: CRC Press; 83–108 [Google Scholar]
- Bilezikian JP, Rubin MR, Finkelstein JS 2005 Parathyroid hormone as an anabolic therapy for women and men. J Endocrinol Invest 28:41–49 [PubMed] [Google Scholar]
- Rubin MR, Cosman F, Lindsay R, Bilezikian JP 2002 The anabolic effects of parathyroid hormone. Osteoporos Int 13:267–277 [DOI] [PubMed] [Google Scholar]
- Jilka RL 2007 Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone 40:1434–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, Shane E, Plavetic K, Muller R, Bilezikian J, Lindsay R 2001 Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res 16:1846–1853 [DOI] [PubMed] [Google Scholar]
- Rehman Q, Lang TF, Arnaud CD, Modin GW, Lane NE 2003 Daily treatment with parathyroid hormone is associated with an increase in vertebral cross-sectional area in postmenopausal women with glucocorticoid-induced osteoporosis. Osteoporos Int 14:77–81 [DOI] [PubMed] [Google Scholar]
- Hirano T, Burr DB, Cain RL, Hock JM 2000 Changes in geometry and cortical porosity in adult, ovary-intact rabbits after 5 months treatment with LY333334 (hPTH 1–34). Calcif Tissue Int 66:456–460 [DOI] [PubMed] [Google Scholar]
- Zhou H, Shen V, Dempster DW, Lindsay R 2001 Continuous parathyroid hormone and estrogen administration increases vertebral cancellous bone volume and cortical width in the estrogen-deficient rat. J Bone Miner Res 16:1300–1307 [DOI] [PubMed] [Google Scholar]
- Parfitt AM 2002 Parathyroid hormone and periosteal bone expansion. J Bone Miner Res 17:1741–1743 [DOI] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC 1999 Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J Clin Invest 104:439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellido T, Ali AA, Plotkin LI, Fu Q, Gubrij I, Roberson PK, Weinstein RS, O'Brien CA, Manolagas SC, Jilka RL 2003 Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J Biol Chem 278:50259–50272 [DOI] [PubMed] [Google Scholar]
- Krishnan V, Moore TL, Ma YL, Helvering LM, Frolik CA, Valasek KM, Ducy P, Geiser AG 2003 Parathyroid hormone bone anabolic action requires Cbfa1/Runx2-dependent signaling. Mol Endocrinol 17:423–435 [DOI] [PubMed] [Google Scholar]
- Miao D, Tong XK, Chan GK, Panda D, McPherson PS, Goltzman D 2001 Parathyroid hormone-related peptide stimulates osteogenic cell proliferation through protein kinase C activation of the Ras/mitogen-activated protein kinase signaling pathway. J Biol Chem 276:32204–32213 [DOI] [PubMed] [Google Scholar]
- Locklin RM, Khosla S, Turner RT, Riggs BL 2003 Mediators of the biphasic responses of bone to intermittent and continuously administered parathyroid hormone. J Cell Biochem 89:180–190 [DOI] [PubMed] [Google Scholar]
- Bord S, Horner A, Beavan S, Compston J 2001 Estrogen receptors α and β are differentially expressed in developing human bone. J Clin Endocrinol Metab 86:2309–2314 [DOI] [PubMed] [Google Scholar]
- Kasperk C, Helmboldt A, Borcsok I, Heuthe S, Cloos O, Niethard F, Ziegler R 1997 Skeletal site-dependent expression of the androgen receptor in human osteoblastic cell populations. Calcif Tissue Int 61:464–473 [DOI] [PubMed] [Google Scholar]
- Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A 1999 Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor β. J Bone Miner Res 14:1239–1249 [DOI] [PubMed] [Google Scholar]
- Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S 2005 Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by β-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem 280:41342–41351 [DOI] [PubMed] [Google Scholar]
- Kousteni S, Chen J-R, Bellido T, Han L, Ali AA, O'Brien C, Plotkin LI, Fu Q, Mancino AT, Wen Y, Vertino AM, Powers CC, Stewart SA, Ebert R, Parfit AM, Weinstein RS, Jilka RL, Manolagas SC 2002 Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science 298:843–846 [DOI] [PubMed] [Google Scholar]
- Ishizuya T, Yokose S, Hori M, Noda T, Suda T, Yoshiki S, Yamaguchi A 1997 Parathyroid hormone exerts disparate effects on osteoblast differentiation depending on exposure time in rat osteoblastic cells. J Clin Invest 99:2961–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf JJ, Kahler RA, Schroeder TM 2004 Wnt signaling in osteoblasts and bone diseases. Gene 341:19–39 [DOI] [PubMed] [Google Scholar]
- Chen D, Zhao M, Mundy GR 2004 Bone morphogenetic proteins. Growth Factors 22:233–241 [DOI] [PubMed] [Google Scholar]
- Kousteni S, Almeida M, Han L, Bellido T, Jilka RL, Manolagas SC 2007 Induction of osteoblast differentiation by selective activation of kinase-mediated actions of the estrogen receptor. Mol Cell Biol 27:1516–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher AC, Chambers TJ, Tobias JH 1996 Androgens contribute to the stimulation of cancellous bone formation by ovarian hormones in female rats. Am J Physiol Endocrinol Metab 270:E407–E412 [DOI] [PubMed] [Google Scholar]
- Tobias JH, Gallagher A, Chambers TJ 1994 5α-Dihydrotestosterone partially restores cancellous bone volume in osteopenic ovariectomized rats. Am J Physiol Endocrinol Metab 267:E853–E859 [DOI] [PubMed] [Google Scholar]
- Bellido T, Plotkin LI, O'Brien CA, Manolagas SC, Jilka RL 2002 PTH-mediated control of proteasome-mediated degradation of Runx2/Cbfa1: a pivotal determinant of the longevity of PTH-initiated anti-apoptosis signaling in osteoblastic cells. J Bone Miner Res 17:S128 [Google Scholar]
- Wlodarski KH 1989 Normal and heterotopic periosteum. Clin Orthop Relat Res 241:265–277 [PubMed] [Google Scholar]
- Uchida A, Kikuchi T, Shimomura Y 1988 Osteogenic capacity of cultured human periosteal cells. Acta Orthop Scand 59:29–33 [DOI] [PubMed] [Google Scholar]
- Breitbart AS, Grande DA, Kessler R, Ryaby JT, Fitzsimmons RJ, Grant RT 1998 Tissue engineered bone repair of calvarial defects using cultured periosteal cells. Plast Reconstr Surg 101:567–574 [DOI] [PubMed] [Google Scholar]
- Zhang X, Xie C, Lin AS, Ito H, Awad H, Lieberman JR, Rubery PT, Schwarz EM, O'Keefe RJ, Guldberg RE 2005 Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. J Bone Miner Res 20:2124–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland DJ, Dedhar S, Coetzee GA, Nelson CC 2005 Interaction of nuclear receptors with Wnt/β-catenin/Tcf signaling axis: Wnt you like to know? Endocr Rev 26:898–915 [DOI] [PubMed] [Google Scholar]
- Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O'Brien CA, Manolagas SC, Jilka RL 2005 Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 146:4577–4583 [DOI] [PubMed] [Google Scholar]
- Keller H, Kneissel M 2005 SOST is a target gene for PTH in bone. Bone 37:148–158 [DOI] [PubMed] [Google Scholar]
- Bodine PVN, Kharode YP, Seestaller-Wehr L, Green. P, Milligan C, Bex FJ 2007 The bone anabolic effects of parathyroid hormone (PTH) are blunted by deletion of the Wnt antagonist secreted frizzled-related protein (sFRP)-1. J Cell Physiol 210:352–357 [DOI] [PubMed] [Google Scholar]
- Guo J, Schipani E, Liu M, Bringhurst FR, Kronenberg HM 2005 Suppression of Dkk1 is not essential for PTH-mediated activation of canonical Wnt signaling in bone. J Bone Miner Res 20(Suppl 1):p S2 (Abstract) [Google Scholar]
- Li X, Liu H, Qin L, Tamasi J, Bergenstock M, Shapses S, Feyen JH, Notterman DA, Partridge NC 2007 Determination of dual effects of parathyroid hormone on skeletal gene expression in vivo by microarray and network analysis. J Biol Chem 282:33086–33097 [DOI] [PubMed] [Google Scholar]
- Onyia JE, Bidwell J, Herring J, Hulman J, Hock JM 1995 In vivo, human parathyroid hormone fragment (hPTH 1–34) transiently stimulates immediate early response gene expression, but not proliferation, in trabecular bone cells of young rats. Bone 17:479–484 [DOI] [PubMed] [Google Scholar]
- Qin L, Li X, Ko JK, Partridge NC 2005 Parathyroid hormone uses multiple mechanisms to arrest the cell cycle progression of osteoblastic cells from G1 to S phase. J Biol Chem 280:3104–3111 [DOI] [PubMed] [Google Scholar]
- Wang YH, Liu Y, Rowe DW 2007 Effects of transient PTH on early proliferation, apoptosis, and subsequent differentiation of osteoblast in primary osteoblast cultures. Am J Physiol Endocrinol Metab 292:E594–E603 [DOI] [PubMed] [Google Scholar]
- Balena R, Shih M-S, Parfitt AM 1992 Bone resorption and formation on the periosteal envelope of the ilium: a histomorphometric study in healthy women. J Bone Miner Res 7:1475–1482 [DOI] [PubMed] [Google Scholar]