Abstract
Carbohydrate metabolism in pregnancy reflects the balance between counterregulatory hormones, which induce insulin resistance, and lactogenic hormones, which stimulate β-cell proliferation and insulin production. Here we explored the interactions of prolactin (PRL) and glucocorticoids in the regulation of β-cell gene expression, fatty acid oxidation, and glucose-stimulated insulin secretion (GSIS). In rat insulinoma cells, rat PRL caused 30–50% (P < 0.001) reductions in Forkhead box O (FoxO)-1, peroxisome proliferator activator receptor (PPAR)-γ coactivator-1α (PGC-1α), PPARα, and carnitine palmitoyltransferase 1 (CPT-1) mRNAs and increased Glut-2 mRNA and GSIS; conversely, dexamethasone (DEX) up-regulated FoxO1, PGC1α, PPARα, CPT-1, and uncoupling protein 2 (UCP-2) mRNAs in insulinoma cells and inhibited GSIS. Hydrocortisone had similar effects. The effects of DEX were attenuated by coincubation of cells with PRL. In primary rat islets, PRL reduced FoxO1, PPARα, and CPT-1 mRNAs, whereas DEX increased FoxO1, PGC1α, and UCP-2 mRNAs. The effects of PRL on gene expression were mimicked by constitutive overexpression of signal transducer and activator of transcription-5b. PRL induced signal transducer and activator of transcription-5 binding to a consensus sequence in the rat FoxO1 promoter, reduced nuclear FoxO1 protein levels, and induced its phosphorylation and cytoplasmic redistribution. DEX increased β-cell fatty acid oxidation and reduced fatty acid esterification; these effects were attenuated by PRL. Thus, lactogens and glucocorticoids have opposing effects on a number of β-cell genes including FoxO1, PGC1α, PPARα, CPT-1, and UCP-2 and differentially regulate β-cell Glut-2 expression, fatty acid oxidation, and GSIS. These observations suggest new mechanisms by which lactogens may preserve β-cell mass and function and maternal glucose tolerance despite the doubling of maternal cortisol concentrations in late gestation.
THE PREGNANT MOTHER in late gestation develops severe resistance to insulin action, with postprandial free fatty acidemia and a 50% reduction in insulin-mediated glucose disposal (1,2,3). Maternal insulin resistance spares glucose, amino acids, essential fatty acids, and ketones for placental-fetal transport and is therefore obligatory for normal fetal development and growth. In uncomplicated pregnancies, the fall in insulin sensitivity in maternal tissues triggers a striking rise in pancreatic insulin production (1,3), which derives from increases in maternal β-cell mass, insulin synthesis, and glucose-stimulated insulin secretion (GSIS). Preexisting β-cell dysfunction and/or failure of the β-cell adaptive response lead to maternal glucose intolerance and, in more severe cases, gestational diabetes (3,4,5).
The insulin resistance and free fatty acidemia of pregnancy result from progressive increases in counterregulatory hormones and inflammatory cytokines (2,6,7,8,9,10,11,12,13,14) including placental GH, cortisol, progesterone, and TNFα. Placental lactogen (PL) and prolactin (PRL) may also play contributory roles. Interestingly, several of the factors that reduce insulin sensitivity in the mother have detrimental effects on β-cell mass and/or function; for example, glucocorticoids inhibit β-cell proliferation and reduce β-cell viability, insulin production, and GSIS (15,16,17,18,19). Likewise, TNFα and free fatty acids in chronic excess inhibit GSIS and may induce β-cell death (20,21). Yet insulin production is sustained through late gestation in the majority of women, who thereby maintain plasma glucose concentrations within the normal range.
How is maternal insulin production sustained in late gestation in the face of a doubling of plasma cortisol concentrations (2) and a rise in free fatty acids and inflammatory cytokines? The inhibitory effects of glucocorticoids on β-cell function are countered by progressive increases in the lactogenic hormones PRL and PL. The lactogens and glucocorticoids have opposing effects on β-cell proliferation, insulin production, glucokinase activity, and GSIS in insulinoma cells (2,6,18,19,22), and PRL blunts (but does not abolish) the apoptotic effect of dexamethasone (DEX) in rat islets (18).
The molecular mechanisms that govern the interactions of the glucocorticoids and lactogens are thus far unknown. The metabolic effects of the glucocorticoids in liver are mediated by induction of Forkhead box O (FoxO)-1, peroxisome proliferator activator receptor (PPAR)-γ coactivator (PGC)-1α, and PPARα, which promote hepatic gluconeogenesis, glycogenolysis, and fatty acid oxidation under conditions of fasting and stress (23,24,25,26). Although their regulation by glucocorticoids in pancreatic islets has not been studied previously, the transcriptional regulators FoxO1, PGC1α, and PPARα play important roles in β-cell growth and function. Constitutive expression of FoxO1 in pancreatic islets suppresses β-cell proliferation, increases β-cell apoptosis, and reduces insulin production (27,28,29,30). Conversely, haploinsufficiency or phosphorylation and inactivation of FoxO1 by insulin/IGF signaling increases nuclear expression of PDX-1 and the D cyclins, thereby promoting β-cell proliferation (27,28,29,31). Induction of islet PGC1α in β-cells in vitro is accompanied by increases in expression of PPARα, uncoupling protein 2 (UCP-2), and carnitine palmitoyltransferase (CPT)-1, the rate limiting enzyme in fatty acid oxidation (32,33,34,35,36,37,38,39). Activation of PPARα in vivo antagonizes the effects of high-fat feeding and insulin resistance on islet insulin secretion (40,41); in vitro, PPARα induces UCP-2 expression and in some (but not all) studies suppresses GSIS (35). Conversely, deletion of PPARα and reductions in β-cell fatty acid oxidation increase GSIS in fasted mice (34), whereas overexpression of CPT-1 in islet β-cells inhibits GSIS (36,37,38,39,42).
The effects of fasting and glucocorticoids, which inhibit β-cell growth and GSIS, contrast with those of the lactogenic hormones, which promote β-cell proliferation and GSIS. We therefore hypothesized that PRL would counteract the effects of nutrient deprivation and glucocorticoids on β-cell FoxO1, PGC1α, PPARα, UCP-2, and CPT-1 expression and thereby attenuate their effects on β-cell fatty acid oxidation and insulin secretion. To test that hypothesis, we examined the interactions of PRL and DEX in the control of β-cell gene expression and function in rat insulinoma (INS-1) cells and primary rat islets.
Materials and Methods
Materials
RPMI 1640, DMEM, l-glutamine, antibiotic/antimycotic solution, fetal bovine serum (FBS), and Trizol reagent were purchased from Life Technologies (Rockville, MD). DEX, hydrocortisone, and ovine PRL were from Sigma Corp. (St. Louis, MO). Rat PRL (lot AFP7545E) was purchased from Dr. Albert Parlow (Hormone Distribution Program, National Institute of Diabetes and Digestive and Kidney Diseases, Torrance, CA). The Bradford protein reagents were from Sigma. Rat Insulin ELISA kits were purchased from Linco Research (St. Charles, MO). Insulin RIA DPC Coat-A-Count kits were purchased from Diagnostic Products Corp. (Los Angeles, CA). The high-capacity cDNA archive kits and SYBR Green PCR master mixes were purchased from Applied Biosystems Inc. (Foster City, CA). The NE-PER nuclear and cytoplasmic extraction reagents kits and the Halt protease inhibitor cocktail kit were purchased from Pierce Biotechnology (Rockford, IL). Rabbit polyclonal antibodies to FoxO1 and phosphorylated FoxO1 were purchased from Cell Signaling (Beverly, MA). A mouse monoclonal antibody to γ-tubulin was from Sigma. Rabbit polyclonal antibodies to signal transducer and activator of transcription (STAT)-5 and to STAT5a were from Upstate Biotechnologies (Bedford, MA) and Santa Cruz Biotechnologies (Santa Cruz, CA), respectively.
Cell culture
Parental rat INS-1 cells (originally supplied by Dr. Claes Wollheim, Geneva, Switzerland) and an INS-1 cell line with high-glucose responsivity (832/13 cells, from Dr. Christopher Newgard, Duke University, Durham, NC) were used for these experiments. The two cell lines gave similar results in parallel experiments; however, we elected to use the 832/13 cells specifically for studies of GSIS. The INS-1 cells were grown in RPMI 1640 (11.1 mm glucose) supplemented with 10% FBS, 50 μm 2-mercaptoethanol, 1 mm sodium pyruvate, 10 mm HEPES, and 1% antibiotic/antimycotic solution in 5% CO2 at 37 C. At approximately 80% confluence, the cells were washed and incubated with hormones or diluents for 20 h (h) in basal medium containing DMEM with 5.5 mm glucose, 0.1% human serum albumin, 10 μg/ml human transferrin, 50 μm ethanolamine, 0.1 nm T3, 50 μm phosphoethanolamine, and 1% antibiotic/antimycotic solution. To study the effects of serum repletion on hormone action, we performed additional experiments in INS-1 cells incubated in medium containing 1 or 10% calf serum.
Primary rat islets were isolated from about 250 g male Wistar rats by a previously described procedure (43). Use of the rats for this purpose was approved by the Duke University Institutional Animal Care and Use Committee. The preincubation medium (used during the first 24 h after isolation) was RPMI 1640 containing 6.8 mm glucose, 10% FBS, 10 mm HEPES, and 1% antibiotic/antimycotic solution. After washing, the islets were incubated for 20 h with hormones or diluents in RPMI basal medium containing 6.8 mm glucose, 0.1% human serum albumin, 10 mm HEPES, and 1% antibiotic/antimycotic solution.
To assess cell viability in serum-free basal medium, we grew INS-1 cells to confluence in RPMI containing 10% FBS. The cells were then washed and incubated for 20 h in serum-free basal medium or basal medium containing 1 or 10% calf serum. After trypsinization (0.05% trypsin/EDTA), the cells were washed and incubated with trypan blue (0.4%), counted by hemocytometry, and replated in RPMI/10% FBS. Under these conditions, there were no differences in cell viability among the three experimental groups [serum-free: cell counts 966,000 ± 45,299 (mean ± se, n = 4), trypan blue positive < 0.5%, replating efficiency > 99%; 1% calf serum: cell counts 885,000 ± 16,523 (n = 4), trypan blue positive < 0.5%, replating efficiency > 99%; 10% calf serum: cell counts 873,000 ± 21,564 (n = 4), trypan blue positive < 0.5%, replating efficiency > 99%].
Overexpression of Stat5 b in INS-1 cells
A constitutively active, murine STAT5b adenovirus was a gift from Dr. Nils Billestrup (Novo, Copenhagen, Denmark) and has been described previously (44). A recombinant adenovirus containing green fluorescent protein served as a control. INS-1 cells were transduced with adenovirus for 24 h and cultured for an additional 24 h. The transfected cells were subsequently incubated in serum-free basal medium for an additional 24 h with hormones or diluents.
Basal and GSIS
Basal insulin secretion and GSIS were assessed using INS-1 832/13 cells. To assess the effects of serum deprivation on basal and GSIS, the cells were grown to 80% confluence and washed with basal media. Half the cells were then incubated for 16 h in basal media containing 5.5 mm glucose; the remaining cells were reincubated for 16 h in growth medium containing 10% FBS. All the cells were then washed and incubated for 2 h in basal medium containing 2.8 mm glucose. After collection of the conditioned media, the cells were incubated for an additional 2 h in basal medium containing 15 mm glucose. The conditioned media samples were then analyzed for insulin content.
To assess the effects of PRL and DEX on basal and GSIS the cells were grown to 80% confluence, washed, and incubated in basal medium (5.5 mm glucose) containing rat PRL (20 nm), DEX (0.1 or 1 μm, which gave similar results), a combination of the two, or diluent(s) for 16 h. The cells were then washed with a secretion buffer (SAB) [114 mm NaCl, 4.7 mm KCl, 1.2 mm KH2PO4, 1.16 mm MgSO4, 2.5 mm CaCl2, 25 mm NaHCO3, 20 mm HEPES, and 1% fatty acid-free BSA (pH 7.4)] and incubated for 2 h in SAB containing 2.8 mm glucose in the presence or absence of the various hormones. The cells were then washed and incubated for 2 h in fresh SAB containing 2.8 or 15 mm glucose; the media were then analyzed for basal and glucose stimulated insulin secretion. Insulin was measured with an insulin ELISA kit or with the Coat-A-Count kit (Diagnostic Products Corp.) and expressed per milligram of cell protein.
Quantification of mRNA expression
INS-1 cells and rat islets were incubated with hormones or diluents for 16–20 h as described above. Total RNA was extracted using Trizol reagent according to the manufacturer’s protocol. cDNA was synthesized from 2.0 μg RNA using the high capacity cDNA Archive kit (Applied Biosystems) according to the manufacturer’s protocol. Quantitative real-time PCR was performed using an ABI 7300 real-time PCR system. Oligonucleotide primers were designed using the Primer Express program (Applied Biosystems). Amplicon lengths ranged from 90 to 150 bp; all primer pairs spanned introns. Negative controls were processed without reverse transcriptase. All samples from a single experiment were run using a single PCR mixture. Expression levels were normalized against the levels of acidic ribosomal phoshoprotein PO (riboprotein), a housekeeping gene that shows little change during cellular growth or differentiation (45).
The levels of mRNA were quantified using the comparative threshold cycle (CT) method. Table 1 shows the oligonucleotide primer pairs, all of which encode rat genes, and CT values obtained in cells at 80% confluence (before the change to basal medium).
Table 1.
Analysis of gene expression in INS-1 cells by quantitative real-time PCR
| Gene | Accession no. | Primer sequence (top forward, bottom reverse, 5′–3′) | CT |
|---|---|---|---|
| Rat PGC1α | NM_031347 | GCT GGA TGG CTT GGG ACA T | 25 |
| CAA CCA GGG CAG CAC ACT CT | |||
| Rat PPARα | NM_013196 | CCG TCG GGA TGT CAC ACA | 24 |
| TTG CTT TCT CAG ATC TTG GCA TT | |||
| Rat PPARγ | AF246458 | GGA TGT CTC ACA ATG CCA TCA G | 29 |
| CGC CAA CAG CTT CTC CTT CT | |||
| Rat FoxO1 | XM_001056726 | TGT GCC CTA CTT CAA GGA TAA GG | 22 |
| GTG GCG AAT TGA ATT CTT CCA | |||
| Rat UCP2 | NM_019354 | AAG ACC ATT GCA CGA GAG GAA | 17 |
| TGG CAT TTC GGG CAA CAT | |||
| Rat CPT1 | NM_031559 | AAT TGC AGT GGT ATT TGA AGC TAA AA | 22 |
| GAT ATA TTC TTC CCA CCA GTC ACT CA | |||
| Rat ADD1 | AF286470 | GCT ACC GTT CCT CTA TCA ATG ACA | 20 |
| GCA AGA CAG CAG ATT TAT TCA GCT T | |||
| Rat Glut2 | NM_012879 | GCT TCC AGT ACA TTG CGG ACT T | 17 |
| AGG ACC ACC CCA GCA AAA A | |||
| Rat riboprotein | X15096 | CCC AGA GGT GCT GGA CAT CA | 16 |
| GCG GAC ACC CTC TAG GAA GC |
Values were obtained in cells grown in RPMI 1640 (11.1 mm glucose) supplemented with 10% FBS, 50 μm 2-mercaptoethanol, 1 mm sodium pyruvate, 10 mm HEPES, and 1% antibiotic/antimycotic solution.
FoxO1 protein content and cellular distribution
INS-1 832/13 cells at 80% confluence were washed and preincubated for 4 h in serum-free basal medium. The cells were then incubated with hormones or diluent for 15 min or for 16 h to assess changes in FoxO1 phosphorylation and the cellular distribution of FoxO1 protein. The cells were collected and centrifuged at 200 × g for 5 min. The resulting cell pellets were then either frozen at −80 C or used immediately for cytoplasmic and nuclear extraction in preparation for Western blotting. Cytoplasmic and nuclear extracts were prepared on ice using the NE-PER nuclear and cytoplasmic extraction reagents kit (Pierce) with the addition of protease inhibitors (Halt protease inhibitor cocktail kit; Pierce).
The distribution and levels of FoxO1 protein were estimated by Western blot. 35 μg of each extract protein were separated on a 4–12% Bis-Tris SDS-PAGE gel (Invitrogen, Carlsbad, CA) and transferred by electrophoresis to a polyvinylidine floride membrane. The membrane was washed in Tris-buffered saline and then blocked in 3% milk buffer (Chemicon, Temecula, CA) for 15 min followed by quick washes in Tris-buffered saline. Membranes were probed with primary antibodies (rabbit polyclonal anti-FoxO1 or anti-phospho-FoxO1) according to manufacturer’s guidelines. The membranes were exposed to chemiluminescent substrate (ECL Advance Western blotting detection kit; GE Health Care, Piscataway, NJ) and imaged using the VersaDoc imaging system (Bio-Rad, Los Angeles, CA). Mouse monoclonal antitubulin antibody was used as internal control. Proteins were quantified by densitometric analysis of the blots using Image J software (rsb.info.nih.gov/ij).
Gel EMSAs
To determine whether PRL induces binding of STAT5 to consensus sequences in the rat FoxO1 promoter we incubated INS-1 cells in RPMI containing 10% FBS until 60–70% confluence. The cells were then washed, incubated for 4 h in serum-free basal medium, and treated with PRL (20 nm) or diluent for 30 min. Nuclear extracts, prepared as described previously (46,47), were incubated for 30 min at room temperature with radiolabeled double-stranded oligonucleotide probes encoding putative STAT5 consensus sequences in the rat FoxO1 promoter (5′-ccggttTTCTTGGAAgcctca-3′, bp −1241 to −1221) or the rat β-casein promoter (5′-agatTTCTAGGAAttcaatcc-3′). Parallel incubations included a rabbit polyclonal antibody to STAT5a or a 200-fold excess of nonradiolabeled (cold competitor) double-stranded oligonucleotide primers encoding the STAT5 consensus sequence in the rat β-casein promoter. The complexes were resolved by PAGE as described previously (46,47).
1–14C-palmitate oxidation
Palmitate oxidation was assessed by measuring the production of 1–14C-labeled acid-soluble metabolites, tricarboxylic acid intermediates, and acetyl esters (incomplete oxidation), and [14C] CO2 (complete oxidation), by use of a modified 48-well microtiter plate described by Kim et al. (48,49). 1–14C-palmitate was dried under nitrogen and incubated in media for 15 min at 37 C; under these conditions the 1–14C-palmitate complexes with media protein. INS-1 cells were grown and treated with PRL (20 nm), DEX (1 μm), or a combination of the two as described previously. After 20 h, the media were aspirated; the cells were then incubated for 2 h at 37 C in media containing 1–14C-palmitate (1 μCi/ml). Aliquots of conditioned media were added to odd-numbered rows of the modified microtiter plate (Costar, Cambridge MA). Two hundred microliters of 1 n NaOH were added to adjoining even-numbered rows. The plate was sealed with a rubber gasket and 100 μl of 70% perchloric acid was added to the conditioned media in the odd-numbered rows; this produces [14C]CO2, which then diffuses into the adjoining even-numbered well containing NaOH. This reacts with the CO2 to form radiolabeled NaHCO3 during incubation at room temperature for 75 min on a shaker set at 125 rpm. At the end of the incubation, 150 μl of the labeled NaHCO3 solution was transferred to scintillation vials and counted using high salt scintillation fluid (complete oxidation).
To quantify acid soluble metabolites (incomplete oxidation), the acidified media samples were collected from the plate, incubated at 4 C overnight and then centrifuged at 14,000 × g for 10 min at 4 C. The supernatants were transferred and centrifuged at 14,000 × g for 10 min at 4 C; 300 μl of the final supernatant was assayed for radioactivity by liquid scintillation counting.
The total fatty acid oxidation is derived from the sum of radioactivity obtained from CO2 absorption (complete oxidation) and acid-soluble metabolites (incomplete oxidation). The results were expressed as nanomoles per milligram protein per hour. Protein was determined by the BCA assay (50) using BSA as standard.
1–14C-Palmitate esterification
The incorporation of 1–14C-palmitate into cellular lipid was assessed using procedures described previously (48,49,51). INS-1 cells were grown and treated with PRL, DEX, or a combination of the two as described previously. After 24 h, the media were aspirated; the cells were then incubated for 2 h at 37 C with 1–14C-palmitate (1 μCi/ml). The resulting conditioned media were collected for measurements of fatty acid oxidation (see above); the remaining media were aspirated and the cells were washed with PBS, extracted with methanol-PBS (2:3), collected, and centrifuged at 700 × g for 5 min. The supernatant was aspirated and the pellet was washed with 1 ml of PBS and recentrifuged at 700 × g for 5 min. The resulting supernatant was removed and 200 μl of 0.2 m NaCl were added to each pellet. The cell pellet was frozen in a dry ice/ethanol bath, thawed at room temperature, and extracted by vortexing in 750 μl CHCl3-MeOH (2:1) and 50 μl 0.1 n KOH. Each sample was then centrifuged at 2000 × g for 20 min; the top aqueous layer and the white protein precipitate were removed. Two hundred microliters of MeOH-H2O-CHCl3 (48:47:3) were added to the bottom layer; after vortexing, the tubes were centrifuged at 2000 × g for 10 min. Two hundred microliters of top aqueous phase was assayed for analysis of residual glycerol products, and 200 μl of the bottom phase containing lipid were counted by liquid scintillation. The results were expressed as nanomoles per milligram protein per hour, calculated using the following formula: [(disintegrations per minutesample − disintegrations per minuteblank)/volume]/(disintegrations per minute/nanomole)]/milligram protein]/2.
Statistical analysis
All assays were performed in triplicate or quadruplicate unless otherwise noted. Data are expressed as mean ± sem of all values obtained in two to five independent experiments. Differences among sample means were tested by ANOVA, followed by the Neuman-Keuls or Bonferroni tests of multiple comparisons. P < 0.05 was considered statistically significant.
Results
Effects of serum deprivation on β-cell gene expression and insulin secretion
β-Cell growth and function are regulated by glucose availability and nutritional status. To assess the effects of nutrient deprivation on β-cell gene expression and insulin production, we compared the levels of FoxO1, PGC1α, PPARα, UCP-2, and CPT-1 mRNAs and the rates of basal and GSIS in INS-1 cells subjected to serum starvation for 20 h with those in cells growing in serum-replete medium. The concentration of glucose (5.5 mm) in serum-free basal medium was lower than the concentration of glucose in serum-containing growth medium (11.1 mm). As noted previously (Materials and Methods), the viability of cells incubated for 20 h in serum-free basal medium was comparable with that of cells incubated for 20 h in basal medium containing 1 or 10% calf serum.
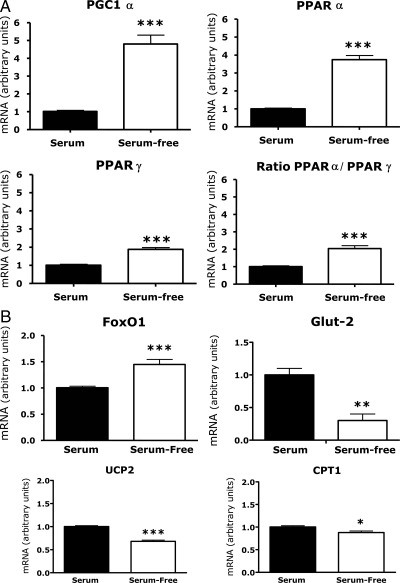
As shown in Fig. 1, A and B, deprivation of serum and reduction in ambient glucose concentrations (from 11.1 to 5.5 mm glucose) stimulated a 50% increase in FoxO1 mRNA, 4- to 5-fold increases in PGC1α and PPARα mRNAs, a smaller increase in PPARγ mRNA, and a 2-fold increase in the ratio of PPARα to PPARγ (all P < 0.01). In contrast, glucose transporter (Glut)-2 mRNA declined 70% (P < 0.01), and there were small but statistically significant reductions (P < 0.05) in UCP-2 and CPT-1 mRNAs.
Figure 1.
Effects of serum deprivation and reduction in ambient glucose concentrations on β-cell gene expression. INS-1 cells were grown in RPMI 1640 containing 10% FCS and 11.1 mm glucose (growth medium). At 80% confluence the cells were washed and incubated for 24 h in growth medium or in serum-free DMEM (5.5 mm glucose, 0.1% human serum albumin). RNA levels were measured by quantitative PCR. Values for cells incubated in growth medium (serum) were adjusted so that the mean equaled 1.0; values for cells in serum-free medium were calculated as a function of the mean of serum-replete values. The figures show the means ± sem of all data from three independent experiments, each of which contained four flasks per treatment group. Statistically significant differences between the experimental groups are indicated by asterisks. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
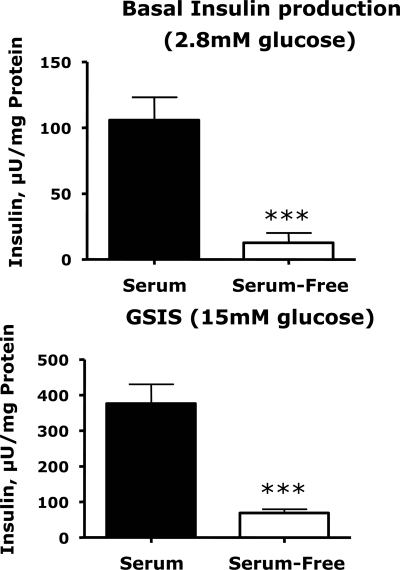
These changes in gene expression were accompanied by sharp reductions in basal and glucose-stimulated insulin secretion (Fig. 2). Serum deprivation did not abolish the insulin secretory response to glucose: under serum-free conditions, media insulin concentrations were 4.5-fold higher in cells treated with 15 mm glucose than with 2.8 mm glucose. However, the absolute basal and glucose-stimulated insulin concentrations were 80% lower (P < 0.01) in cells preincubated in serum-free medium than in cells preincubated in serum-replete medium. Indeed, glucose (15 mm)-stimulated insulin concentrations in cells preincubated in serum-free medium were 40% lower than insulin concentrations in cells preincubated in serum and then treated with 2.8 mm glucose.
Figure 2.
Effects of serum deprivation on basal and glucose-stimulated insulin secretion. INS-1 832/13 cells were grown in RPMI 1640 (11.1 mm glucose) supplemented with 10% FBS (growth medium); at 80% confluence the cells were washed and incubated in growth medium or basal serum-free medium for 16 h. Insulin secretion was measured as described in Materials and Methods. Values represent the means ± sem of six samples in a representative experiment. The figures show the means ± sem of all data from two independent experiments, each of which contained six wells per treatment group. Statistically significant differences between the experimental groups are indicated by asterisks. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Effects of PRL and glucocorticoids on β-cell gene expression
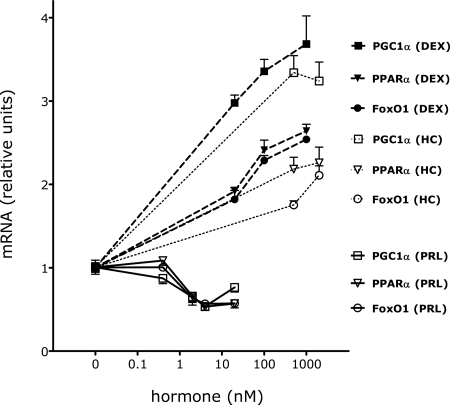
The effects of PRL on β-cell gene expression were studied in INS-1 cells and primary islets incubated in serum-free basal medium. Preliminary experiments (Fig. 3) showed that rat PRL (10–500 ng/ml, 0.4–20 nm) caused dose-dependent reductions in the levels of FoxO1, PGC1α, and PPARα mRNAs in INS-1 cells; the half-maximal concentration of PRL approximated 25 ng/ml. Ovine PRL had similar effects (data not shown). Subsequent experiments used PRL at a concentration of 20 nm.
Figure 3.
Dose-dependent effects of PRL and glucocorticoids on gene expression in INS-1 cells. INS-1 cells were incubated for 20 h in serum-free basal medium in the presence or absence of rat PRL (10–500 ng/ml, 0.4–20 nm), DEX (0.02–1 μm, 20–1000 nm), hydrocortisone (HC; 15–60 μg/dl, 410–1640 nm), or diluent. mRNA levels were measured by quantitative PCR. Values in diluent-treated control cells were adjusted so that the mean equaled 1.0; values for hormone-treated cells were calculated as a function of the mean of control values. The figure shows the mean ± sem of four flasks per treatment group. PRL had statistically significant effects (P < 0.01) on gene expression at concentrations exceeding 10 ng/ml (0.4 nm). DEX and HC had statistically significant effects (P < 0.001) on gene expression at all concentrations tested.
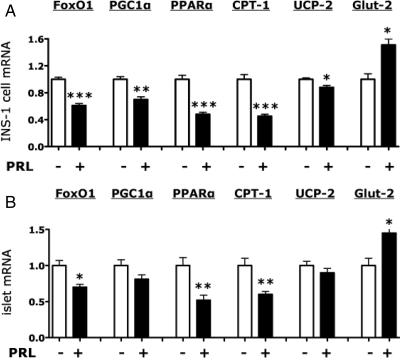
In INS-1 cells (Figs. 4A and 5A), rat PRL reduced by 30–50% the expression of FoxO1, PGC1α, PPARα, and CPT-1 (all P < 0.001); PRL also reduced by 12–25% the levels of UCP-2 mRNA (P < 0.05). In contrast, PRL stimulated a 65% increase in Glut-2 mRNA (P < 0.01). In primary rat islets, (Fig. 4B), PRL caused a 30% reduction in FoxO1 mRNA (P < 0.05), a 48% reduction in PPARα mRNA (P < 0.01), and a 40% reduction in CPT-1 mRNA (P < 0.01). In contrast, PRL stimulated a 35% increase in Glut-2 mRNA (P < 0.05) but had no significant effect on PGC1α (-18%, P = 0.1) or UCP-2 mRNAs.
Figure 4.
Effects of PRL on β-cell gene expression in INS-1 cells and primary rat islets. INS-1 cells (A) or primary rat islets (B) were incubated for 20 h in serum-free DMEM (5.5 mm glucose, 0.1% human serum albumin for INS-1 cells) or serum-free RPMI (6.8 mm glucose, 0.1% human serum albumin for rat islets) in the presence or absence of rat PRL (20 nm). mRNA levels were measured by quantitative PCR. Values in diluent-treated control cells (−) were adjusted so that the mean equaled 1.0; values for hormone-treated cells were calculated as a function of the mean of control values. The figures show the means ± sem of all data from two (islets) to five (INS-1 cells) independent experiments, each of which contained four flasks per treatment group. Statistically significant differences between the PRL and control (−) groups are indicated by asterisks. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Figure 5.
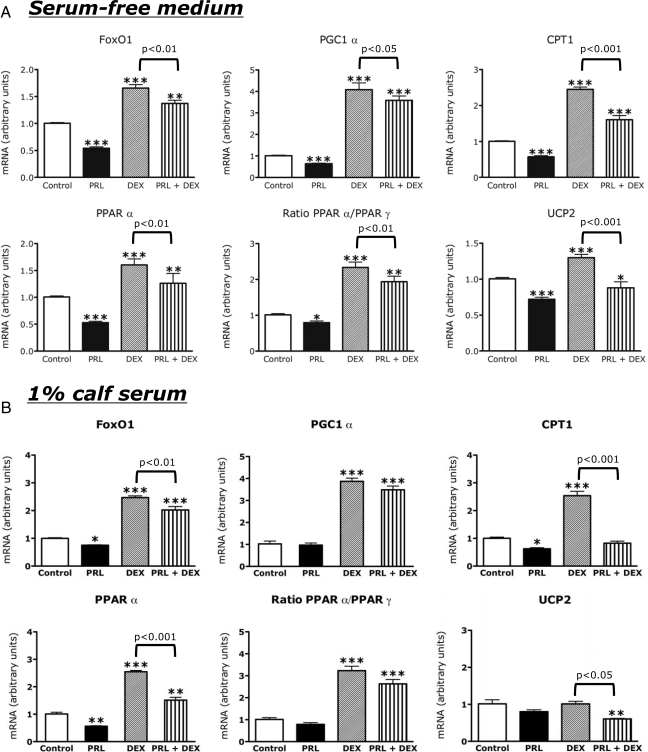
Effects of PRL and DEX on gene expression in INS-1 cells. INS-1 cells were incubated for 20 h in serum-free basal medium (A) or basal medium containing 1% (B) or 10% (C) calf serum in the presence or absence of rat PRL (20 nm), DEX (0.1 μm), a combination of the two, or diluent. mRNA levels were measured by quantitative PCR. Values in diluent-treated control cells were adjusted so that the mean equaled 1.0; values for hormone-treated cells were calculated as a function of the mean of control values. The figures show the means ± sem of four flasks per treatment group. Statistically significant differences between the experimental (hormone treated) and control groups are indicated by asterisks. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Statistical differences between the DEX group and the PRL + DEX group are indicated by the P values above the horizontal connecting lines.
To assess the effects of glucocorticoids on β-cell gene expression, we conducted preliminary studies using DEX and hydrocortisone. As shown in Fig. 3, DEX (0.02–1 μm, 20–1000 nm) stimulated dose-dependent increases in FoxO1, PGC1α, and PPARα mRNAs; the effect of DEX was mimicked by hydrocortisone at concentrations (15 μg/dl, 410 nm) within the range of maternal cortisol concentrations during pregnancy (10–55 μg/dl, 280-1520 nm) (52). Subsequent experiments used DEX at concentrations of 0.1 or 1 μm, which gave similar results.
In INS-1 cells, DEX stimulated 2- to 5-fold increases in the expression of FoxO1, PGC1α, PPARα, and CPT-1 mRNAs (all P < 0.001) and increased by 18% (P < 0.05) the levels of UCP-2 mRNA (Fig. 5A). The effects of DEX were attenuated by coincubation of cells with PRL; in all cases, the effects of PRL plus DEX on gene expression differed significantly from those of DEX alone. Neither PRL nor DEX had significant effects on levels of mRNA encoding rat adipose differentiation and development 1 (also called srEBPc, data not shown). In primary islets DEX stimulated a 73% increase in FoxO1 mRNA (P < 0.01), a 79% increase in PGC1α mRNA (P < 0.01), and a 40% increase in UCP-2 mRNA (P < 0.05, figure not shown) but had no significant effect on Glut-2 mRNA (−15%, P = 0.21), PPARα, or CPT-1 mRNAs. The combination of PRL and DEX yielded a 38% increase in FoxO1 mRNA (P = 0.07 vs. DEX alone) and a 26% increase in UCP-2 mRNA (P = 0.18 vs. DEX alone).
Effects of serum repletion on hormone action
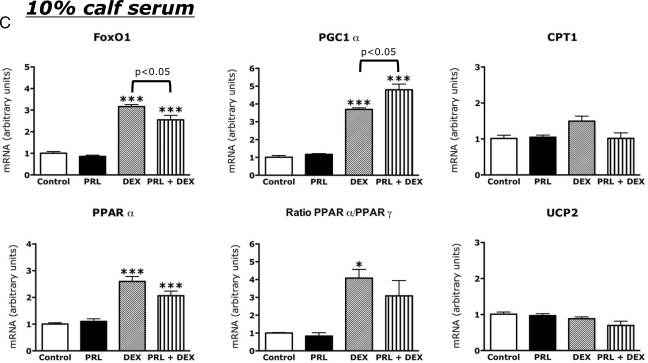
To assess the effects of serum repletion on hormone action, we compared the effects of PRL and DEX on gene expression in cells incubated in 1% (Fig. 5B) or 10% (Fig. 5C) calf serum to their effects on gene expression in cells incubated in serum-free medium (Fig. 5A). The effects of hormones in 1% serum were similar to those in serum-free medium. However in 1% serum, PRL had no significant effect on PGC1α mRNA and had lesser (and statistically insignificant) effects on UCP-2 mRNA and the ratio of PPARα to PPARγ. In cells incubated in 10% serum, the induction of FoxO1, PGC1α, and PPARα by DEX was maintained. However, the effect of DEX on CPT-1 was blunted and its induction of UCP-2 was abolished. PRL alone had no significant effects on gene expression in the presence of 10% calf serum but attenuated the effect of DEX on FoxO1 expression.
Effects of PRL and DEX on FoxO1 protein content, phosphorylation, and cellular distribution
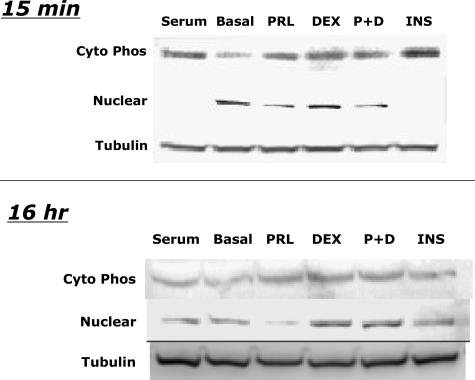
FoxO1 activity can be regulated by changes in total cellular FoxO1 content or in the phosphorylation and cellular distribution of the protein (27,28,29,31). Dephosphorylation and nuclear localization of FoxO1 increases FoxO1 activity, whereas phosphorylation and cytoplasmic redistribution of FoxO1 suppresses FoxO1 action. To examine the effects of PRL and DEX on FoxO1 content, phosphorylation, and cellular distribution, we incubated INS-1 cells in RPMI 1640 containing 10% FBS until they were 70–80% confluent. The cells were then washed and incubated in serum-replete or serum-free basal medium with PRL (40 nm), DEX (1 μm), PRL+DEX (P+D), Insulin (INS, 1 μm), or diluent for 15 min or 16 h to assess effects on levels of phosphorylated (cyto phos) and total FoxO1 in cytoplasmic and nuclear compartments. Cell extracts were analyzed by Western blot (Fig. 6).
Figure 6.
Effects of PRL and DEX on FoxO1 content, phosphorylation, and cellular distribution. INS-1 832/13 cells were grown in serum-containing medium and then incubated in serum free in the presence of rat PRL (40 nm), DEX (1 μm), a combination of the two, or diluent in basal medium for 15 min or 16 h. Phosphorylated FoxO1 (cyto phos), nuclear FoxO1, and tubulin were detected by Western blot, as described in Materials and Methods. Similar results were obtained in three experiments.
Serum depletion acutely increased nuclear FoxO1 and decreased cytoplasmic, phosphorylated FoxO1, whereas insulin reduced nuclear FoxO1 and increased cytoplasmic, phosphorylated FoxO1 (Fig. 6). PRL reduced the effect of serum deprivation on appearance of FoxO1 in the nucleus by 35–40% (P < 0.05). At 15 min and 16 h of incubation, PRL increased cytoplasmic phosphorylated FoxO1 levels relative to basal controls and thereby increased the ratio of cytoplasmic phosphorylated FoxO1 to nuclear FoxO1 by 52% (P < 0.05).
Induction of STAT5 binding to a consensus sequence in the rat FoxO1 promoter
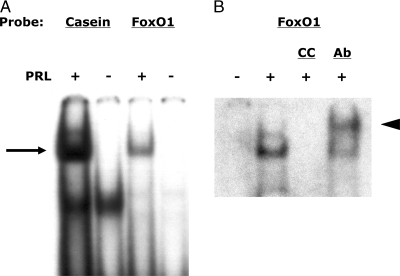
Many of the biological actions of PRL in target tissues are mediated through activation and induction of binding of STAT5 to consensus sequences in the promoters of target genes. An analysis of the promoters of the various genes studied here identified a potential STAT5 consensus sequence 5′-TTCTTGGAA-3′ in the promoter (−1235 to −1227 bp) of the rat FoxO1 gene. To determine whether that sequence could bind activated STAT5 we performed an electrophoretic mobility shift assay using double-stranded oligonucleotide primers encoding −1241 to −1221 bp of the rat FoxO1 promoter. A consensus STAT5 sequence in the rat β-casein promoter served as a positive control. As shown in Fig. 7A, PRL stimulated the binding of STAT5 to its consensus sequence in the rat FoxO1 promoter; the radiolabeled complex was abolished by the addition of nonradiolabeled primers encoding the consensus STAT5 sequence in the β-casein promoter and supershifted by addition of anti-STAT5a antisera to the preelectrophoresis incubation mix (Fig. 7B). These findings suggest that PRL might regulate the expression of FoxO1 through induction of STAT5.
Figure 7.
PRL induces STAT5 binding to a consensus sequence on the rat FoxO1 promoter. Nuclear proteins were prepared from INS-1 cells treated with PRL (20 nm) or diluent for 30 min. The proteins were incubated with radiolabeled double stranded oligonucleotides encoding STAT5 consensus sequences in the rat β-casein promoter or the rat FoxO1 promoter (Fig. 6A). Parallel incubations contained a 200-fold excess of cold competitor (CC) double-stranded oligonucleotides encoding the STAT5 sequence in the rat β-casein gene or 2 μg of polyclonal anti-STAT5a antibody (Ab, Fig. 6B). The protein-DNA complexes were separated by PAGE. The shifted bands (arrow) represent binding of STAT5 to DNA. The arrowhead (Fig. 6B) represents the supershifted complex.
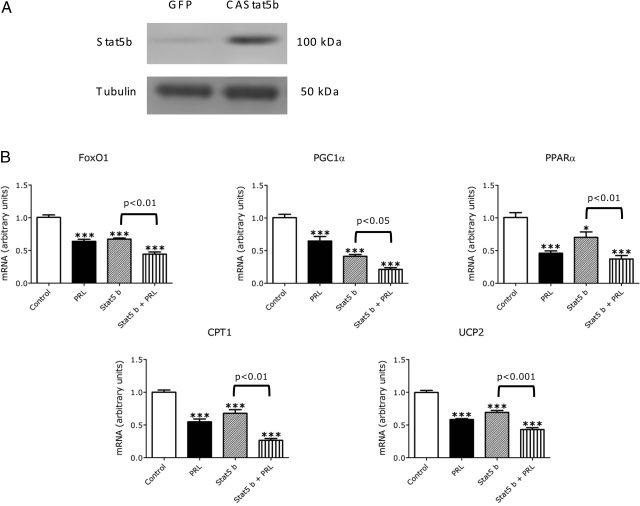
Effects of overexpression of STAT5b on β-cell gene expression
To assess further the role of STAT5 activation in PRL action, we examined the effects of overexpression of STAT5 on β-cell gene expression. INS-1 cells were transfected transiently with an adenoviral vector encoding a constitutively activated STAT5b or a control vector expressing green fluorescent protein. Transfection of INS-1 cells with activated STAT5b increases STAT5b mRNA and protein levels about 5-fold (Fig. 8A). The cells were analyzed after incubation for 20 h in the presence or absence of PRL or diluent.
Figure 8.
Overexpression of STAT5b mimics and potentiates the effects of PRL on β-cell gene expression. INS-1 832/13 cells were transfected with recombinant adenoviruses expressing a constitutively active, murine STAT5b adenovirus, or green fluorescent protein (GFP; control). After 24 h of transduction, the conditioned media was analyzed (A) for STAT5 protein using a polyclonal antibody to STAT5. The cells were washed with basal media and then incubated for an additional 24 h with PRL (20 nm) or diluent. mRNA levels were measured by quantitative PCR. Values in diluent-treated control cells expressing green fluorescent protein (control) were adjusted so that the mean equaled 1.0; values for STAT5b-expressing and hormone-treated cells were calculated as a function of the mean of control values. B, Means ± sem of all data from two independent experiments, each of which contained four flasks per group. Statistically significant differences between the experimental (hormone treated) and control groups are indicated by asterisks. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Statistical differences between the DEX group and the PRL + DEX group are indicated by the P values above the horizontal connecting lines.
As shown in Fig. 8B, STAT5b overexpression alone reduced expression of FoxO1, PGC1α, PPARα, UCP-2, and CPT-1, mimicking the effects of PRL. The effects of STAT5b were potentiated by concurrent treatment with PRL; the effects of PRL plus STAT5b on gene expression exceeded (P < 0.05) the effects of STAT5b or PRL alone. These findings suggest that the effects of PRL on β-cell gene expression might be mediated, at least in part, by activation of STAT5.
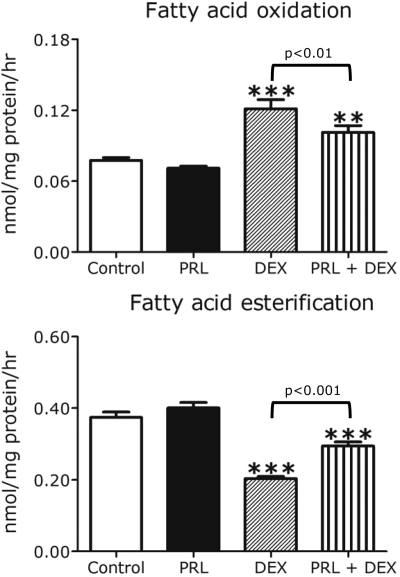
Effects of PRL and DEX on fatty acid oxidation and esterification
CPT-1 is the rate-limiting step in β-cell fatty acid oxidation. To assess the effects of PRL and DEX on fatty acid oxidation and esterification, we incubated INS-1 cells in basal medium (5.5 mm glucose) with hormones or diluent for 20 h and then exposed the cells for 2 h to 1–14C-palmitate. As shown in Fig. 9, PRL alone had no significant effect on cellular fatty acid oxidation but attenuated (P < 0.01) the effect of DEX, which stimulated a 56% increase (P < 0.001) in [14C]CO2 production. DEX also reduced fatty acid esterification (the incorporation of palmitate into total cellular lipid) by 50% (P < 0.01); the effect of DEX was attenuated (P < 0.001) by coincubation of cells with PRL.
Figure 9.
Effects of PRL and DEX on INS-1 cell fatty acid oxidation and esterification. INS-1 cells were incubated for 24 h in serum-free RPMI (11.1 mm glucose, 0.1% human serum albumin) in the presence or absence of rat PRL (20 nm), DEX (1 μm), a combination of the two, or diluent (control). Rates of 14C-palmitate oxidation and esterification were measured as described in Materials and Methods. Values, expressed as nanomoles per milligram protein per hour, represent the means ± se of all data from two independent experiments, each of which contained six wells per group. Statistically significant differences between the experimental (hormone treated) and control groups are indicated by asterisks. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
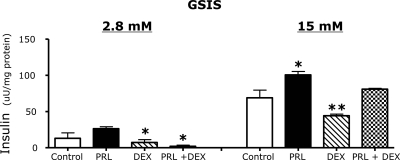
Effects of PRL and DEX on GSIS
In parallel experiments we examined the effects of PRL and DEX on GSIS in INS-1 832/13 cells. As shown in Fig. 10, PRL stimulated a 48% increase in GSIS (P < 0.05) during a 24-h incubation in serum-free medium. In contrast, DEX reduced GSIS by 50% (P < 0.01). The effects of DEX were reversed by PRL (P < 0.01 PRL+DEX vs. DEX alone).
Figure 10.
Effects of PRL and DEX on glucose-stimulated insulin secretion in INS-1 832/13 cells. INS-1 832/13 cells were grown in RPMI 1640 (11.1 mm glucose) supplemented with 10% FBS; at 80% confluence the cells were washed and incubated for 16 h in basal medium (DMEM with 5.5 mm glucose) containing rat PRL (20 nm), DEX (0.1 μm), a combination of the two, or diluent (control), and insulin secretion was measured as described in Materials and Methods. Values represent the means ± se of all data from two independent experiments, each of which contained five samples per group. Statistically significant differences between the experimental (hormone treated) and control groups are indicated by asterisks. *, P < 0.05; **, P < 0.01.
Discussion
Maintenance of glucose tolerance in mid- to late gestation requires a 2- to 2.5-fold increase in maternal insulin production (1,3,4,5,53) to counter the insulin resistance induced by counterregulatory hormones, cortisol, and TNFα (2,6,7,8,9,10,11,12,13,14). That the lactogenic hormones subserve a critical role in the β-cell adaptive response is suggested by several lines of evidence. First, the increase in maternal insulin secretion during mid- to late gestation parallels the rise in maternal PL and PRL concentrations (2) and the increased expression of islet PRL receptors (54), which bind PL as well as PRL. Second, the lactogens stimulate β-cell proliferation, insulin gene expression, and GSIS in human and rodent islets and insulinoma cells and inhibit β-cell apoptosis (6,18,19,22,55,56,57,58,59,60,61). Third, exogenous administration or constitutive expression of PRL or PL protects against the development of diabetes in streptozotocin-treated mice (57,60,62,63,64). Finally, insulin production is increased in men and women with hyperprolactinemia (65,66), whereas targeted deletion of the PRL receptor in mice causes 30–40% reductions in β-cell mass, pancreatic insulin mRNA, and GSIS (67).
In this study we explored the interactions of lactogens and glucocorticoids in the regulation of β-cell gene expression and function. Our initial studies were conducted using rat insulinoma (INS-1) cells. We demonstrated that serum deprivation and reduction in ambient glucose concentrations stimulated increases in β-cell expression of key transcriptional determinants of energy metabolism including FoxO1, PGC1α, and PPARα and increased the ratio of PPARα to PPARγ. These changes in gene expression were accompanied by striking reductions in β-cell Glut-2 expression and insulin secretion. We then showed that PRL inhibits expression of FoxO1, PGC1α, PPARα, CPT-1, and UCP-2 in serum-free medium and blunts or reverses their induction by DEX. The effects of PRL on β-cell gene expression were observed at concentrations (half-maximal dose ∼25 ng/ml) comparable with or less than (2) those observed in nonpregnant adults and children (3–20 ng/ml), pregnant women (50–180 ng/ml), and late fetal and newborn boys and girls (50–400 ng/ml). Repletion of serum, which contains a panoply of β-cell growth factors and insulinotropic hormones, obscured most of the effects of PRL on gene expression. In accordance with its suppression of CPT-1 and induction of Glut-2 mRNAs, PRL blunted the effects of DEX on β-cell fatty acid oxidation and increased GSIS. It should be noted that the effect of PRL on cellular fatty acid oxidation was less striking in magnitude than the effect of the hormone on CPT-1 expression or insulin production, possibly because the absolute level of fatty acid oxidation in β-cells is relatively low.
The effects of PRL and DEX on gene expression in primary rat islets differed in certain respects from the effects of the hormones in INS-1 cells. In primary islets as in INS-1 cells, PRL reduced expression of FoxO1, PPARα, and CPT-1 mRNAs and stimulated an increase in Glut-2 mRNA. However, PRL had no effect on PGC1α or UCP-2 mRNAs in primary islets. DEX stimulated increases in FoxO1, PGC1α, and UCP-2 mRNAs in primary islets as well as INS-1 cells but had no effect on islet Glut-2, PPARα, or CPT-1 expression. Differential effects of the hormones in INS-1 cells and islets may reflect differences in cell composition (clonal β-cells vs. mixed islet cells), glucose sensitivity (higher in islets than in parental INS-1 cells), proliferation rates (high in INS-1 cells, low in primary adult islets), and sensitivity to apoptosis/necrosis (higher in INS-1 cells, low in islets).
The effects of PRL on FoxO1, PPARα, and CPT-1 mRNAs and β-cell fatty acid oxidation resemble in some ways those of glucose (38,42,68). Glucose uptake and use likely play roles in PRL action because the hormone increases Glut-2 mRNA and glucokinase activity in pancreatic β-cells (this study and Refs. 19,56,69). However unlike glucose, PRL is not an acute insulin secretagogue (18,19,22). Moreover, PRL and glucose have synergistic effects on β-cell replication (70), rat insulin gene expression (47,56), and GSIS (18,19). These observations suggest that PRL and glucose exert insulinotropic effects through overlapping mechanisms of action.
At least two mechanisms appear to mediate the effect of PRL on FoxO1 expression. PRL reduced FoxO1 mRNA during a 16- to 20-h incubation and increased the ratio of cytoplasmic, phosphorylated (inactivated) FoxO1 to nuclear (active) FoxO1. The effects of PRL on FoxO1 mRNA might be exerted at the level of transcription because the hormone-induced binding of STAT5 to a consensus sequence in the rat FoxO1 promoter, and overexpression of STAT5 reduced FoxO1 mRNA and potentiated the (suppressive) effect of PRL. However, additional experiments will be required to define the role of Stat5 in this action of PRL. The mechanism by which PRL induces cytoplasmic redistribution of phosphorylated (inactive) FoxO1 is currently unknown, but similar effects are achieved through induction of Akt by other hormones that induce β-cell proliferation including insulin, IGF-II, glucagon-like peptide-1, and glucose-dependent insulinotropic polypeptide (29,30,68,71). Glucose also induces FoxO1 phosphorylation; this effect appears to be mediated by induction of insulin secretion (68,71). A role for glucose and/or insulin action in PRL induction of FoxO1 phosphorylation is possible, given that PRL increases β-cell Glut-2 expression and GSIS.
Activation and/or overexpression of nuclear FoxO1 inhibits β-cell proliferation and induces β-cell apoptosis (27,28,29,31). In contrast, haploinsufficiency of FoxO1 promotes an increase in β-cell mass (27). In our studies PRL, which induces β-cell replication and inhibits β-cell apoptosis (60,61,70), reduced FoxO1 expression. Conversely DEX, which reduces β-cell replication and induces β-cell apoptosis (16,18), increased FoxO1 mRNA levels. Thus, the PRL-dependent reduction of FoxO1 expression provides a new mechanism by which the lactogens may promote β-cell growth and increase β-cell mass.
FoxO1 suppression might also explain in part the effects of PRL on other β-cell genes. Overexpression of FoxO1 increases PGC1α mRNA in skeletal muscle and liver (72,73), whereas deletion of FoxO1 reduces hepatic PGC1α mRNA levels (26). In β-cells, induction of PGC1α induces expression of PPARα and CPT-1 and up-regulates expression of UCP-2 (34,35,36,37,38,39,42,73). Thus, inhibition of FoxO1 by PRL might initiate a cascade that reduces expression of PGC1α and PPARα and thereby inhibits β-cell expression of UCP-2 and CPT-1.
Together with induction of glucose uptake and utilization, these changes in gene expression may facilitate the effects of PRL on β-cell insulin production. This is because overexpression of FoxO1, PGC1α, UCP-2, or CPT-1 inhibits GSIS (30,32,74,75,76,77,78). UCP-2 reduces insulin secretion by limiting cellular ATP production from glucose use, whereas up-regulation of CPT-1 reduces insulin production by increasing rates of fatty acid oxidation. Induction of β-cell fatty acid oxidation does not alter glucose oxidation or ATP production and plays no role in the ATP-sensitive potassium channel-dependent insulin secretory response to glucose (38,42). Rather, induction of fatty acid oxidation may reduce the levels of critical lipid mediators that regulate protein kinases and thereby amplify insulin secretion through a ATP-sensitive potassium channel-independent pathway (38,42). In our studies, PRL suppressed CPT-1 expression and blunted the induction of β-cell fatty acid oxidation by DEX; concurrently, PRL stimulated an increase in GSIS and blocked the inhibitory effect of DEX. Although the magnitude of the effect of PRL on insulin production exceeded the magnitude of its effect on fatty acid oxidation, our findings are consistent with previous investigations that showed an inverse relationship between fatty acid oxidation and GSIS (38,74,75,76,77,78,79,80). Other studies, however, report that activation of PPARα and induction of fatty acid oxidation may increase β-cell insulin secretion (74); moreover, attenuation of the effect of glucose on fatty acid oxidation (81) and inhibition of fatty acid synthase in INS-1 832/13 cells or primary rat islets are reported to have no effect on GSIS (82,83). Thus, the roles of fatty acid oxidation in β-cell insulin secretion and PRL action remain unclear.
In summary, the lactogens and glucocorticoids have opposing effects on a number of β-cell genes including FoxO1, PGC1α, PPARα, CPT-1, and UCP2 and differentially regulate β-cell Glut-2 expression, insulin production, and fatty acid oxidation. These observations suggest new mechanisms by which lactogens may preserve β-cell mass and function and maternal glucose tolerance despite the doubling of maternal cortisol concentrations in late gestation.
It should be noted that PRL only partially antagonized the effect of DEX on gene expression and fatty acid metabolism in INS-1 cells and primary rat islets. There are a number of possible explanations for this finding; most important, the in vitro cell culture model clearly does not mimic perfectly the metabolic milieu of pregnancy. For example, the timing, duration, and level of exposure to DEX or hydrocortisone in vitro do not replicate perfectly the timing, duration and level of exposure to hydrocortisone in vivo; and PRL is only one of a number of pregnancy serum factors (e.g. placental GH, progesterone, menin, insulin, IGF, incretins, etc.) that may regulate maternal β-cell mass and GSIS.
It is also clear that the insulinotropic effects of PRL are not mediated solely by suppression of FoxO1, PPARα or CPT-1 or changes in β-cell lipid metabolism. In addition to up-regulating glucose uptake and use, PRL stimulates β-cell proliferation and inhibits β-cell apoptosis through induction of cyclin D expression (44) and Bcl-XL (61) and suppression of menin (84). PRL also promotes insulin gene transcription (47,56) and enhances the expression of soluble N-ethylmaliemide-sensitive fusion factor attachment protein receptor proteins involved in insulin secretion (85). Thus, PRL is a multifunctional β-cell tropin with complex effects on growth and function.
Our studies of the interactions between lactogens and glucocorticoids may have implications for the physiology of insulin production in states other than pregnancy. For example, β-cell mass and insulin production increase markedly in the late gestational fetus and newborn infant despite a surge in cortisol secretion (86); the high levels of lactogens in fetal (PL and PRL) and neonatal (PRL) blood (55,67,87) may promote or sustain β-cell proliferation and insulin production at this stage of development. The ability of PRL to maintain insulin secretion during nutrient deprivation or in the presence of DEX (this study) may also implicate a role for lactogens in the preservation of β-cell function during fasting, stress, or states of glucocorticoid excess. Finally, because FoxO1, PGC1α, and UCP-2 are overexpressed in islets of diabetic humans and rodents (29,32,33,88,89), our findings may suggest novel pathways by which lactogens might prevent or reverse β-cell failure in types 1 and 2 diabetes.
Footnotes
This work was supported by Grant HD024192 from the National Institute of Child Health and Human Development and Pfizer Corp. (to M.F.) and the Duke Children’s Miracle Network (to R.A.).
Disclosure Statement: The authors have nothing to declare.
First Published Online July 3, 2008
Abbreviations: CPT, Carnitine palmitoyltransferase; CT, threshold cycle; DEX, dexamethasone; FBS, fetal bovine serum; Glut, glucose transporter; GSIS, glucose-stimulated insulin secretion; FoxO, Forkhead box O; INS-1, insulinoma; PGC, PPARγ coactivator; PL, placental lactogen; PPAR, peroxisome proliferator activator receptor; PRL, prolactin; SAB, secretion buffer; STAT, signal transducer and activator of transcription; UCP, uncoupling protein.
References
- Catalano PM, Huston L, Amini SB, Kalhan SC 1999 Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol 180:903–916 [DOI] [PubMed] [Google Scholar]
- Freemark M 2006 Regulation of maternal metabolism by pituitary and placental hormones: roles in fetal development and metabolic programming. Horm Res 65(Suppl 3):41–49 [DOI] [PubMed] [Google Scholar]
- Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE 2007 Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care 30(Suppl 2):S112–S119 [DOI] [PubMed] [Google Scholar]
- Di Cianni G, Seghieri G, Lencioni C, Cuccuru I, Anichini R, De Bellis A, Ghio A, Tesi F, Volpe L, Del Prato S 2007 Normal glucose tolerance and gestational diabetes mellitus: what is in between? Diabetes Care 30:1783–1788 [DOI] [PubMed] [Google Scholar]
- Buchanan TA, Xiang AH 2005 Gestational diabetes mellitus. J Clin Invest 115:485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson RL, Brelje TC, Roth C 1993 Effects of steroid and lactogenic hormones on islets of Langerhans: a new hypothesis for the role of pregnancy steroids in the adaptation of islets to pregnancy. Endocrinology 133:2227–2234 [DOI] [PubMed] [Google Scholar]
- Ryan EA, Enns L 1988 Role of gestational hormones in the induction of insulin resistance. J Clin Endocrinol Metab 67:341–347 [DOI] [PubMed] [Google Scholar]
- Fleenor D, Oden J, Kelly PA, Mohan S, Alliouachene S, Pende M, Wentz S, Kerr J, Freemark M 2005 Roles of the lactogens and somatogens in perinatal and postnatal metabolism and growth: studies of a novel mouse model combining lactogen resistance and growth hormone deficiency. Endocrinology 146:103–112 [DOI] [PubMed] [Google Scholar]
- Lacroix MC, Guibourdenche J, Frendo JL, Muller F, Evain-Brion D 2002 Human placental growth hormone—a review. Placenta 23(Suppl A):S87–S94 [DOI] [PubMed] [Google Scholar]
- Barbour LA, Shao J, Qiao L, Leitner W, Anderson M, Friedman JE, Draznin B 2004 Human placental growth hormone increases expression of the p85 regulatory unit of phosphatidylinositol 3-kinase and triggers severe insulin resistance in skeletal muscle. Endocrinology 145:1144–1150 [DOI] [PubMed] [Google Scholar]
- Brăniçsteanu DD, Mathieu C 2003 Progesterone in gestational diabetes mellitus: guilty or not guilty? Trends Endocrinol Metab 14:54–56 [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Ilias I 2003 Maternal and fetal hypothalamic-pituitary-adrenal axes during pregnancy and postpartum. Ann NY Acad Sci 997:136–149 [DOI] [PubMed] [Google Scholar]
- Kirwan JP, Hauguel-De Mouzon S, Lepercq J, Challier JC, Hutson-Presley L, Friedman JE, Kalhan SC, Catalano PM 2002 TNF-α is a predictor of insulin resistance in human pregnancy. Diabetes 51:2207–2213 [DOI] [PubMed] [Google Scholar]
- Altinova AE, Toruner F, Bozkurt N, Bukan, N, Karakoc A, Yetkin I, Ayvaz G, Cakir N, Arslan M 2007 Circulating concentrations of adiponectin and tumor necrosis factor-α in gestational diabetes mellitus. Gynecol Endocrinol 23:161–165 [DOI] [PubMed] [Google Scholar]
- Lambillotte C, Gilon P, Henquin JC 1997 Direct glucocorticoid inhibition of insulin secretion. An in vitro study of dexamethasone effects in mouse islets. J Clin Invest 99:414–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranta F, Avram D, Berchtold S, Dufer M, Drews G, Lang F, Ullrich S 2006 Dexamethasone induces cell death in insulin-secreting cells, an effect reversed by exendin-4. Diabetes 55:1380–1390 [DOI] [PubMed] [Google Scholar]
- Ullrich S, Berchtold S, Ranta F, Seebohm G, Henke G, Lupescu A, Mack AF, Chao CM, Su J, Nitschke R, Alexander D, Friedrich B, Wuff P 2005 Serum- and glucocorticoid-inducible kinase 1 (SGK1) mediates glucocorticoid-induced inhibition of insulin secretion. Diabetes 54:1090–1099 [DOI] [PubMed] [Google Scholar]
- Weinhaus AJ, Bhagroo NV, Brelje TC, Sorenson RL 2000 Dexamethasone counteracts the effect of prolactin on islet function: implications for islet regulation in late pregnancy. Endocrinology 141:1384–1393 [DOI] [PubMed] [Google Scholar]
- Shao J, Qiao L, Friedman JE 2004 Prolactin, progesterone, and dexamethasone coordinately and adversely regulate glucokinase and cAMP/PDE cascades in MIN6 β-cells. Am J Physiol Endocrinol Metab 286:E304–E310 [DOI] [PubMed] [Google Scholar]
- Saldeen J 2000 Cytokines induce both necrosis and apoptosis via a common Bcl-2-inhibitable pathway in rat insulin-producing cells. Endocrinology 141:2003–2010 [DOI] [PubMed] [Google Scholar]
- Collier JJ, Fueger PT, Hohmeier HE, Newgard CB 2006 Pro- and antiapoptotic proteins regulate apoptosis but do not protect against cytokine-mediated cytotoxicity in rat islets and β-cell lines. Diabetes 55:1398–1406 [DOI] [PubMed] [Google Scholar]
- Brelje TC, Scharp DW, Lacy PE, Ogren L, Talamantes F, Robertson M, Friesen HG, Sorenson RL 1993 Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology 132:879–887 [DOI] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM 2006 Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 27:728–735 [DOI] [PubMed] [Google Scholar]
- Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W 1999 Peroxisome proliferator-activated receptor α mediates the adaptive response to fasting. J Clin Invest 103:1489–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnich A, Tian N, Byan L, Bilder G 2001 A potent PPARα agonist stimulates mitochondrial fatty acid β-oxidation in liver and skeletal muscle. Am J Physiol Endocrinol Metab 280:E270–E279 [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D 2007 Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor foxo1 in liver. Cell Metab 6:208–216 [DOI] [PubMed] [Google Scholar]
- Kawamori D, Kaneto H, Nakatani Y, Matsuoka TA, Matsuhisa M, Hori M, Yamasaki Y 2006 The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. J Biol Chem 281:1091–1098 [DOI] [PubMed] [Google Scholar]
- Nakae J, Biggs 3rd WH, Kitamura T, Cavenee WK, Wright CV, Arden KC, Accili D 2002 Regulation of insulin action and pancreatic β-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet 32:245–253 [DOI] [PubMed] [Google Scholar]
- Okamoto H, Hribal ML, Lin HV, Bennett WR, Ward A, Accili D 2006 Role of the forkhead protein FoxO1 in β cell compensation to insulin resistance. J Clin Invest 116:775–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buteau J, Shlien A, Foisy S, Accili D 2007 Metabolic diapause in pancreatic β-cells expressing a gain-of-function mutant of the forkhead protein Foxo1. J Biol Chem 282:287–293 [DOI] [PubMed] [Google Scholar]
- Kitamura T, Nakae J, Kitamura Y, Kido Y, Biggs 3rd WH, Wright CV, White MF, Arden KC, Accili D 2002 The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic β cell growth. J Clin Invest 110:1839–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JC, Xu G, Deeney JT, Yang SN, Rhee J, Puigserver P, Levens AR, Yang R, Zhang CY, Lowell BB, Berggren PO, Newgard CB, Bonnie-Weir S, Weir G, Spiegelman BM 2003 Suppression of β cell energy metabolism and insulin release by PGC-1α. Dev Cell 5:73–83 [DOI] [PubMed] [Google Scholar]
- Soyal S, Krempler F, Oberkofler H, Patsch W 2006 PGC-1α: a potent transcriptional cofactor involved in the pathogenesis of type 2 diabetes. Diabetologia 49:1477–1488 [DOI] [PubMed] [Google Scholar]
- Gremlich S, Nolan C, Roduit R, Burcelin R, Peyot ML, Delghingaro-Augusto V, Desvergne B, Michalik L, Prentki M, Wahli W 2005 Pancreatic islet adaptation to fasting is dependent on peroxisome proliferator-activated receptor α transcriptional up-regulation of fatty acid oxidation. Endocrinology 146:375–382 [DOI] [PubMed] [Google Scholar]
- Tordjman K, Standley KN, Bernal-Mizrachi C, Leone TC, Coleman T, Kell PA, Semenkovich CF 2002 PPARα suppresses insulin secretion and induces UCP2 in insulinoma cells. J Lipid Res 43:936–943 [PubMed] [Google Scholar]
- Oberkofler H, Klein K, Felder TK, Krempler F, Patsch W 2006 Role of peroxisome proliferator-activated receptor-γ coactivator-1α in the transcriptional regulation of the human uncoupling protein 2 gene in INS-1E cells. Endocrinology 147:966–976 [DOI] [PubMed] [Google Scholar]
- Li LX, Skorpen F, Egeberg K, Jorgensen IH, Grill V 2002 Induction of uncoupling protein 2 mRNA in β-cells is stimulated by oxidation of fatty acids but not by nutrient oversupply. Endocrinology 143:1371–1377 [DOI] [PubMed] [Google Scholar]
- Herrero L, Rubi B, Sebastian D, Serra D, Asins G, Maechler P, Prentki M, Hegardt FG 2005 Alteration of the malonyl-CoA/carnitine palmitoyltransferase I interaction in the β-cell impairs glucose-induced insulin secretion. Diabetes 54:462–471 [DOI] [PubMed] [Google Scholar]
- Sadana P, Zhang Y, Song S, Cook GA, Elam MB, Park EA 2007 Regulation of carnitine palmitoyltransferase I (CPT-Iα) gene expression by the peroxisome proliferator activated receptor γ coactivator (PGC-1) isoforms. Mol Cell Endocrinol 267:6–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holness MJ, Smith ND, Greenwood GK, Sugden MC 2005 Interactive influences of peroxisome proliferator-activated receptor α activation and glucocorticoids on pancreatic β cell compensation in insulin resistance induced by dietary saturated fat in the rat. Diabetologia 48:2062–2068 [DOI] [PubMed] [Google Scholar]
- Holness MJ, Greenwood GK, Smith ND, Sugden MC 2006 Peroxisome proliferator-activated receptor-α and glucocorticoids interactively regulate insulin secretion during pregnancy. Diabetes 55:3501–3508 [DOI] [PubMed] [Google Scholar]
- Rubi B, Antinozzi PA, Herrero L, Ishihara H, Asins G, Serra D, Wollheim, CB, Maechler P, Hegardt FG 2002 Adenovirus-mediated overexpression of liver carnitine palmitoyltransferase I in INS1E cells: effects on cell metabolism and insulin secretion. Biochem J 364:219–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milburn Jr JL, Hirose H, Lee YH, Nagasawa Y, Ogawa A, Ohneda M, BeltrandelRio H, Newgard CB, Johnson JH, Unger RH 1995 Pancreatic β-cells in obesity. Evidence for induction of functional, morphologic, and metabolic abnormalities by increased long chain fatty acids. J Biol Chem 270:1295–1299 [DOI] [PubMed] [Google Scholar]
- Friedrichsen BN, Richter HE, Hansen JA, Rhodes CJ, Nielsen JH, Billestrup N, Moldrup A 2003 Signal transducer and activator of transcription 5 activation is sufficient to drive transcriptional induction of cyclin D2 gene and proliferation of rat pancreatic β-cells. Mol Endocrinol 17:945–958 [DOI] [PubMed] [Google Scholar]
- Dheda K, Huggett J, Bustin SA, Johnson MA, Rook G, Zumla A 2004 Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques 37:112–119 [DOI] [PubMed] [Google Scholar]
- Fleenor D, Arumugam R, Freemar M 2006 Growth hormone and prolactin receptors in adipogenesis: STAT-5 activation, suppressors of cytokine signaling, and regulation of insulin-like growth factor I. Horm Res 66:101–110 [DOI] [PubMed] [Google Scholar]
- Fleenor DE, Freemark M 2001 Prolactin induction of insulin gene transcription: roles of glucose and signal transducer and activator of transcription 5. Endocrinology 142:2805–2810 [DOI] [PubMed] [Google Scholar]
- Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA 2000 Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab 279:E1039–E1044 [DOI] [PubMed] [Google Scholar]
- Kim JY, Koves TR, Yu GS, Gulick T, Cortright RN, Dohm GL, Muoio DM 2002 Evidence of a malonyl-CoA-insensitive carnitine palmitoyltransferase I activity in red skeletal muscle. Am J Physiol Endocrinol Metab 282:E1014–E1022 [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto AK, Goeke NM, Olson BJ, Klenk DC 1985 Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85 [DOI] [PubMed] [Google Scholar]
- Antinozzi PA, Segall L, Prentki M, McGarry JD, Newgard CB 1998 Molecular or pharmacologic perturbation of the link between glucose and lipid metabolism is without effect on glucose-stimulated insulin secretion. A re-evaluation of the long-chain acyl-CoA hypothesis. J Biol Chem 273:16146–16154 [DOI] [PubMed] [Google Scholar]
- Lindsay JR, Nieman LK 2005 The hypothalamic-pituitary-adrenal axis in pregnancy: challenges in disease detection and treatment. Endocr Rev 26:775–799 [DOI] [PubMed] [Google Scholar]
- Kuhl C 1998 Etiology and pathogenesis of gestational diabetes. Diabetes Care 21(Suppl 2):B19–B26 [PubMed] [Google Scholar]
- Moldrup A, Petersen ED, Nielsen JH 1993 Effects of sex and pregnancy hormones on growth hormone and prolactin receptor gene expression in insulin-producing cells. Endocrinology 133:1165–1172 [DOI] [PubMed] [Google Scholar]
- Fleenor D, Petryk A, Driscoll P, Freemark M 2000 Constitutive expression of placental lactogen in pancreatic β cells: effects on cell morphology, growth, and gene expression. Pediatr Res 47:136–142 [DOI] [PubMed] [Google Scholar]
- Petryk A, Fleenor D, Driscoll P, Freemark M 2000 Prolactin induction of insulin gene expression: the roles of glucose and glucose transporter-2. J Endocrinol 164:277–286 [DOI] [PubMed] [Google Scholar]
- Vasavada RC, Garcia-Ocana A, Zawalich WS, Sorenson RL, Dann P, Syed M, Ogren L, Talamantes F, Stewart AF 2000 Targeted expression of placental lactogen in the β cells of transgenic mice results in β cell proliferation, islet mass augmentation, and hypoglycemia. J Biol Chem 275:15399–15406 [DOI] [PubMed] [Google Scholar]
- Parsons JA, Bartke A, Sorenson RL 1995 Number and size of islets of Langerhans in pregnant, human growth hormone-expressing transgenic, and pituitary dwarf mice: effect of lactogenic hormones. Endocrinology 136:2013–2021 [DOI] [PubMed] [Google Scholar]
- Labriola L, Montor WR, Krogh K, Lojudice FH, Genzini T, Goldberg AC, Eliaschewitz FG, Sogayar MC 2007 Beneficial effects of prolactin and laminin on human pancreatic islet-cell cultures. Mol Cell Endocrinol 263:120–133 [DOI] [PubMed] [Google Scholar]
- Fujinaka Y, Sipula D, Garcia-Ocana A, Vasavada RC 2004 Characterization of mice doubly transgenic for parathyroid hormone-related protein and murine placental lactogen: a novel role for placental lactogen in pancreatic β-cell survival. Diabetes 53:3120–3130 [DOI] [PubMed] [Google Scholar]
- Fujinaka Y, Takane K, Yamashita H, Vasavada RC 2007 Lactogens promote β cell survival through JAK2/STAT5 activation and Bcl-XL upregulation. J Biol Chem 282:30707–30717 [DOI] [PubMed] [Google Scholar]
- Holstad M, Sandler S 1999 Prolactin protects against diabetes induced by multiple low doses of streptozotocin in mice. J Endocrinol 163:229–234 [DOI] [PubMed] [Google Scholar]
- Atwater I, Gondos B, DiBartolomeo R, Bazaes R, Jovanovic L 2002 Pregnancy hormones prevent diabetes and reduce lymphocytic infiltration of islets in the NOD mouse. Ann Clin Lab Sci 32:87–92 [PubMed] [Google Scholar]
- Jackerott M, Moldrup A, Thams P, Galsgaard ED, Knudsen J, Lee YC, Nielsen JH 2006 STAT5 activity in pancreatic β-cells influences the severity of diabetes in animal models of type 1 and 2 diabetes. Diabetes 55:2705–2712 [DOI] [PubMed] [Google Scholar]
- Maccario M, Grottoli S, Razzore P, Procopio M, Oleandri SE, Ciccarelli E, Camanni F, Ghigo E 1996 Effects of glucose load and/or arginine on insulin and growth hormone secretion in hyperprolactinemia and obesity. Eur J Endocrinol 135:205–210 [DOI] [PubMed] [Google Scholar]
- Foss MC, Paula FJ, Paccola GM, Piccinato CE 1995 Peripheral glucose metabolism in human hyperprolactinaemia. Clin Endocrinol (Oxf) 43:721–726 [DOI] [PubMed] [Google Scholar]
- Freemark M, Avril I, Fleenor D, Driscoll P, Opara E, Kendall W, Oden J, Bridges S, Binart N, Breant B, Kelly PA 2002 Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology 143:1378–1385 [DOI] [PubMed] [Google Scholar]
- Martinez SC, Cras-Meneur C, Bernal-Mizrachi E, Permutt MA 2006 Glucose regulates Foxo1 through insulin receptor signaling in the pancreatic islet β-cell. Diabetes 55:1581–1591 [DOI] [PubMed] [Google Scholar]
- Weinhaus AJ, Stout LE, Bhagroo NV, Brelje TC, Sorenson RL 2007 Regulation of glucokinase in pancreatic islets by prolactin: a mechanism for increasing glucose-stimulated insulin secretion during pregnancy. J Endocrinol 193:367–381 [DOI] [PubMed] [Google Scholar]
- Hugl SR, Merger M 2007 Prolactin stimulates proliferation of the glucose-dependent β-cell line INS-1 via different IRS-proteins. JOP 8:739–752 [PubMed] [Google Scholar]
- Kim SJ, Winter K, Nian C, Tsuneoka M, Koda Y, McIntosh CH 2005 Glucose-dependent insulinotropic polypeptide (GIP) stimulation of pancreatic β-cell survival is dependent upon phosphatidylinositol 3-kinase (PI3K)/protein kinase B (PKB) signaling, inactivation of the forkhead transcription factor Foxo1, and down-regulation of bax expression. J Biol Chem 280:22297–22307 [DOI] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM 2003 Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1α interaction. Nature 423:550–555 [DOI] [PubMed] [Google Scholar]
- Southgate RJ, Bruce CR, Carey AL, Steinberg GR, Walder K, Monks R, Watt MJ, Hawley JA, Birnbaum MJ, Febbraio MA 2005 PGC-1α gene expression is down-regulated by Akt-mediated phosphorylation and nuclear exclusion of FoxO1 in insulin-stimulated skeletal muscle. FASEB J 19:2072–2074 [DOI] [PubMed] [Google Scholar]
- Ravnskjaer K, Boergesen M, Dalgaard LT, Mandrup S 2006 Glucose-induced repression of PPARα gene expression in pancreatic β-cells involves PP2A activation and AMPK inactivation. J Mol Endocrinol 36:289–299 [DOI] [PubMed] [Google Scholar]
- Nolan CJ, Madiraju MS, Delghingaro-Augusto V, Peyot ML, Prentki M 2006 Fatty acid signaling in the β-cell and insulin secretion. Diabetes 55(Suppl 2):S16–S23 [DOI] [PubMed] [Google Scholar]
- Newsholme P, Keane D, Welters HJ, Morgan NG 2007 Life and death decisions of the pancreatic β-cell: the role of fatty acids. Clin Sci (Lond) 112:27–42 [DOI] [PubMed] [Google Scholar]
- Wang X, Zhou L, Li G, Luo T, Gu Y, Qian L, Fu X, Li F, Li J, Luo M 2007 Palmitate activates AMP-activated protein kinase and regulates insulin secretion from β cells. Biochem Biophys Res Commun 352:463–468 [DOI] [PubMed] [Google Scholar]
- Chan CB, Saleh MC, Koshkin V, Wheeler MB 2004 Uncoupling protein 2 and islet function. Diabetes 53(Suppl 1):S136–S142 [DOI] [PubMed] [Google Scholar]
- Roduit R, Morin J, Masse F, Segall L, Roche E, Newgard CB, Assimacopoulos-Jeannet F, Prentki M 2000 Glucose down-regulates the expression of the peroxisome proliferator-activated receptor-α gene in the pancreatic β-cell. J Biol Chem 275:35799–35806 [DOI] [PubMed] [Google Scholar]
- Guay C, Madiraju SR, Aumais A, Joly E, Prentki M 2007 A role for ATP-citrate lyase, malic enzyme, and pyruvate/citrate cycling in glucose-induced insulin secretion. J Biol Chem 282:35657–35665 [DOI] [PubMed] [Google Scholar]
- Mulder H, Lu D, Finley J 4th, An J, Cohen J, Antinozzi PA, McGarry JD, Newgard CB 2001 Overexpression of a modified human malonyl-CoA decarboxylase blocks the glucose-induced increase in malonyl-CoA level but has no impact on insulin secretion in INS-1-derived (832/13) β-cells. J Biol Chem 276:6479–6484 [DOI] [PubMed] [Google Scholar]
- Joseph JW, Odegaard ML, Ronnebaum SM, Burgess SC, Muehlbauer J, Sherry AD, Newgard CB 2007 Normal flux through ATP-citrate lyase or fatty acid synthase is not required for glucose-stimulated insulin secretion. J Biol Chem 282:31592–31600 [DOI] [PubMed] [Google Scholar]
- Chakravarthy MV, Zhu Y, Lopez M, Yin L, Wozniak DF, Coleman T, Hu Z, Wolfgang M, Vidal-Puig A, Lane MD, Semenkovich CF 2007 Brain fatty acid synthase activates PPARα to maintain energy homeostasis. J Clin Invest 117:2539–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnik SK, Chen H, McLean GW, Heit JJ, Gu X, Zhang AY, Fontaine M, Yen MH, Kim SK 2007 Menin controls growth of pancreatic β-cells in pregnant mice and promotes gestational diabetes mellitus. Science 318:806–809 [DOI] [PubMed] [Google Scholar]
- Cunha DA, Amaral ME, Carvalho CP, Collares-Buzato CB, Carneiro EM, Boschero AC 2006 Increased expression of SNARE proteins and synaptotagmin IV in islets from pregnant rats and in vitro prolactin-treated neonatal islets. Biol Res 39:555–566 [DOI] [PubMed] [Google Scholar]
- Freemark M 1999 The fetal adrenal and the maturation of the growth hormone and prolactin axes. Endocrinology 140:1963–1965 [DOI] [PubMed] [Google Scholar]
- Freemark M 2001 Ontogenesis of prolactin receptors in the human fetus: roles in fetal development. Biochem Soc Trans 29:38–41 [DOI] [PubMed] [Google Scholar]
- Del Guerra S, Lupi R, Marselli L, Masini M, Bugliani M, Sbrana S, Torri S, Pollera M, Boggi U, Mosca F, Del Prato S, Marchetti P 2005 Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes 54:727–735 [DOI] [PubMed] [Google Scholar]
- Anello M, Lupi R, Spampinato D, Piro S, Masini M, Boggi U, Del Prato S, Abuazzo AM, Purrello F, Marchetti P 2005 Functional and morphological alterations of mitochondria in pancreatic β cells from type 2 diabetic patients. Diabetologia 48:282–289 [DOI] [PubMed] [Google Scholar]