Abstract
Purpose
Sonic hedgehog (Shh) signaling is essential for photoreceptor differentiation and retinal cell survival in embryonic zebrafish. The study was conducted to determine whether adult heterozygous carriers of mutant alleles for the shh gene display retinal abnormalities.
Methods
Retinal cryosections from young, middle-aged, and senescent wild-type and sonic-you+/− (syu+/−) zebrafish were probed with retinal cell type–specific markers. Contralateral retinal flatmounts from these fish, and from adult albino zebrafish subjected to light-induced photoreceptor damage followed by regeneration, were hybridized with blue cone opsin cRNA for quantitative analysis of the blue cone pattern. Retinal expression of shh mRNA was measured by quantitative RT-PCR.
Results
Regions of cone loss and abnormal cone morphology were observed in the oldest syu+/− zebrafish, although no other retinal cell type was affected. This phenotype was age-related and genotype-specific. Cone distribution in the oldest syu+/− zebrafish was predominantly random, as assessed by measuring the short-range pattern, whereas that of wild-type fish and the younger syu+/− zebrafish was statistically regular. A measure of long-range pattern revealed atypical cone aggregation in the oldest syu+/− zebrafish. The light-treated albino zebrafish displayed random cone patterns immediately after light toxicity, but showed cone aggregation on regeneration. Retinas from the syu+/− fish showed reduced expression of shh mRNA compared with those of wild-type siblings.
Conclusions
The syu+/− zebrafish presents a model for the study of hereditary age-related cone abnormalities. The syu+/− retinas most likely experience progressive cone photoreceptor loss, accompanied by cone regeneration. Shh signaling may be required to maintain cone viability throughout life.
The extracellular signaling molecule, Sonic hedgehog (Shh),plays multiple roles in the morphogenesis of the vertebrate eye, and regulates proliferation, differentiation, and survival of retinal cells.1,2 During retinal development, Shh is synthesized by retinal ganglion cells3-5 and amacrine cells.6 The retinal pigmented epithelium (RPE) is also a source of Hedgehog signals: shh and tiggy-winkle hedgehog (twhh) in the zebrafish7; banded hedgehog (bhh) and cephalic hedgehog (chh) in Xenopus8; and Indian hedgehog (Ihh) in the rat.9 Hedgehog proteins emanating from these sources influence the development of rod photoreceptors. For example, treatment of cultured rat embryonic retinal cells with recombinant Shh promotes the expression of rod photoreceptor–specific genes.9 In addition, Shh stimulates proliferation of rat Müller glia and the generation of rhodopsin-expressing cells in vitro, and in vivo subsequent to retinal damage.10 However, experiments using mouse retinal explants demonstrated that Shh inhibits rhodopsin synthesis,11 suggesting the role of Shh in rod differentiation is complex and may be species-specific.
The use of anamniote vertebrate models has uncovered important roles for Hedgehog signaling in the development of rod and cone photoreceptors and the RPE. In zebrafish embryos, reduction in Shh signaling through molecular, pharmacologic, or genetic techniques results in multiple retinal abnormalities including reduced rod and cone photoreceptor differentiation.6,7,12,13 Homozygous sonic-you (syu−/−) mutant zebrafish,14 display extensive retinal cell death before embryonic mortality.13 In Xenopus embryos, Hedgehog signaling is necessary for RPE differentiation and maintenance.8 Thus, in addition to regulating progenitor cell proliferation and photoreceptor differentiation, Hedgehog signaling may function in the survival and maintenance of retinal and RPE cells.
Many inherited retinal degenerative diseases, including those related to age, are characterized by rod and/or cone photoreceptor death.15 The underlying genetic causes of many of these disorders are known and mostly involve photoreceptor-specific genes.16,17 Murine models of inherited retinal degenerations have been valuable both in uncovering mechanisms driving photoreceptor loss and in searching for therapeutic interventions.18 As a complement to the mouse models, zebrafish offer several advantages for studying retinal disorders. For example, zebrafish have a duplex retina with a substantial number of cone photoreceptors arranged in a precise mosaic that can be quantitatively assessed.19,20 In addition, zebrafish are amenable to large-scale genetic manipulation.21,22 As an example of their experimental power, the recent characterization of a zebrafish model for choroideremia, an X-linked retinal degeneration, revealed that the loss of photoreceptors in larval fish is a secondary consequence of defects in the RPE.23
Zebrafish are also a model for aging and age-related disease.24,25 Their lifespan and generation time are relatively short, and potential disease treatments can be administered systemically through the water, which allows for convenient pharmacologic screening. In addition, zebrafish exhibit extraordinary neuronal regeneration, including the retinal photoreceptors, making them an excellent adult model for the study of regenerative therapies for acute disease or genetic conditions.22,26,27 In addition, new zebrafish models of adult-onset retinal degenerations recently have been identified and characterized.28,29 Zebrafish, however, have not yet been used to study retinal disorders associated with senescence, defined herein as the postreproductive portion of the lifespan.
In the present study, we tested the hypothesis that Hedgehog signaling is important for the maintenance and survival of photoreceptor cells throughout life. We report a novel zebrafish cone photoreceptor phenotype related to organismal senescence in heterozygous animals carrying a single mutant allele of the shh gene (syu+/−), and with correspondingly lower levels of shh expression in the retina. The retinas of the aging syu+/− zebrafish display cone loss, abnormalities of cone morphology, and significant disruptions in cone organization. These disruptions in the cone mosaic are quantitatively similar to those of the light-treated and regenerated zebrafish retina, suggesting that syu+/− retinas exhibit a compensatory cone replacement along with cone loss.
Methods
Animals, BrdU Cell Labeling, and Light Treatments
All experiments involving animals conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research. Heterozygous carriers of two different sonic you (syu) alleles were obtained from Anand Chandrasekhar (University of Missouri, Columbia, MO), the Zebrafish International Resource Center (syut4; Eugene, OR), or Rolf Karlstrom (syutbx; University of Massachusetts, Amherst, MA). The syut4 allele is a deletion spanning the entire shh coding region as well as 4 and 2.2 kb of 5′ and 3′ flanking sequence, respectively.14 The syutbx allele is null and is caused by a point mutation in the shh gene.14 Genotypically wild-type adult zebrafish were either the genotypically wild-type siblings of the syut4+/− fish (Tuebingen [Tu] background strain) or of our outbred strain originally obtained from Scientific Hatcheries (SciH; Huntington Beach, CA). Fish were bred and raised at the University of Idaho according to Westerfield.30 Fish were kept in monitored aquatic housing units (14 hours–10 hours light– dark cycle) employing filtered, recirculated water (28.5°C).
BrdU treatments were performed as published.31,32 The fish were incubated in 5 mM BrdU in system water for 5 days with solutions changed on day 3. The fish were fed before the solution change. In these experiments, the fish were killed immediately after the 5-day BrdU treatment.
For the light-lesion experiments, 1.5-year postfertilization (pf) albino (alb) zebrafish were dark treated for 14 days and exposed to constant, intense light for 3 days, to cause rod and cone photoreceptor death in the dorsal retina.26,33 To verify the light-induced photoreceptor damage and the initiation of retinal cell proliferation that characterizes the teleost retinal regeneration response, some fish were killed at the conclusion of the light treatment, and the eyes were processed for frozen section immunolocalization of rod opsin, blue cone opsin, and proliferating cell nuclear antigen (PCNA). 34 As expected, the immunolabeling demonstrated severe reductions in the rod and blue cone cells and a large number of PCNA+ cells within the inner and outer nuclear layers of the dorsal retina (data not shown). Both eyes from the remaining light-treated fish were used to examine the blue opsin gene expression pattern by wholemount retina in situ hybridizations. Thus, retinas from six fish were harvested after 3 days of light treatment, to assess the pattern of blue cone cell loss. In addition, six light-treated fish were returned to normal facility light conditions for 33 days and the retinas collected to assess the pattern of regenerated blue cone cells. Non–light-treated controls for this experiment included alb fish that were maintained in normal facility light conditions (three fish) and 14-day dark-treated alb fish that were returned to facility lighting (three fish).
Tissue Processing and Immunohistochemistry
Fish were anesthetized in buffered MS-22230 and were killed by spinal cord transection. Subsequent to enucleations, lenses were removed through corneal perforations. The left eyes of each fish were processed for frozen section cell labeling, whereas the retinas were dissected from the right eyes of the fish for wholemount in situ RNA hybridizations. Dissected whole retinas were obtained from both eyes of fish from the light treatment experiment. Eyes processed for frozen section analysis were fixed in phosphate-buffered 4% paraformaldehyde-5% sucrose for 1 hour, washed in buffered 5% sucrose, and cryoprotected overnight in buffered 20% sucrose. The eyes were frozen embedded in a 2:1 solution of buffered 20% sucrose-OCT (Sakura Finetek, Torrance, CA) and cryosectioned at 3 to 5 μm.35 For wholemount retinal analysis, dissected, fixed retinas were washed in buffered 5% sucrose and stored in 100% methanol at 4°C.
The following antibodies (and their dilutions) were used: rat monoclonal anti-BrdU (1:50; Accurate Chemical, Westbury, NY); mouse monoclonals zpr-1, zpr-3, and anti-HuC/D (all at 1:200; Zebrafish International Resource Center, University of Oregon); anti-glutamine synthetase (GS; 1:5000; BD Biosciences, San Diego, CA); anti-protein kinase C (PKC; 1:200; Santa Cruz Biotechnology, Santa Cruz, CA); and anti-PCNA (1:50; Biomeda Corp., Foster City, CA). Before primary antibody application, the sections for BrdU or PCNA detection were incubated in a 1:1 solution of 4 N HCl-PBS-0.05% Triton X-100 (PBST) for 30 minutes, followed by washes in PBST and PBS. In all cases, the sections were blocked in 20% normal goat serum and the primary antibodies were incubated overnight at 4°C. The sections were washed with PBST and incubated in the appropriate secondary antibody (Jackson ImmunoResearch, West Grove, PA) for 2 hours at room temperature. The immunolabeled sections were washed in PBST, and coverslips were mounted (Vectashield; Vector Laboratories, Burlingame, CA).
Tissue In Situ RNA Hybridization
Hybridization was performed as previously described.36 Digoxigenin-labeled (DIG) cRNA probes were prepared from full-length cDNAs corresponding to blue cone and rod opsin using components of the Genius Kit (Roche, Indianapolis, IN). Cryosections were hydrated through an ethanol series before incubation with 1 mg/mL proteinase K at 37°C for 2 minutes (sections) or 30 minutes (whole retinas). Tissues were treated with acetic anhydride in triethanolamine buffer for 10 minutes, dehydrated, and hybridized with 4 mg/mL DIG probe in a hybridization buffer containing 50% formamide overnight at 65°C. Hybridization was detected with an anti-DIG antibody conjugated to alkaline phosphatase and the color substrates 4-nitroblue tetrazolium chloride and 5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP).
Cell Death Detection
Detection of cell death was performed using an in situ cell death detection kit based on the terminal dUTP nick-end labeling (TUNEL) method (Roche) according to the manufacturer’s instructions. Incorporated fluorescein-tagged dUTPs were amplified using peroxidase-conjugated anti-fluorescein followed by a diaminobenzidine color reaction.13 Endogenous peroxidases were quenched before the TUNEL procedure by incubating slides in 0.3% H2O2.
Microscopy, Imaging, and Pattern Analysis
Sections were viewed on an epifluorescence microscope (DMR; Leica, Deerfield, IL) and/or bright-field or Nomarski optics. Images were collected with a digital camera (Spot; Diagnostic Instruments, Sterling Heights, MI) and associated software. Each section was imaged in its entirety to enable visual inspection of the entire retina for any photoreceptor abnormalities. In the BrdU-exposed animals, the total number of BrdU+ cells in each retinal layer was counted for each section, and the individual section counts were averaged for each fish.
Images of wholemounted retinas processed for blue cone opsin in situ hybridization were used for spatial pattern analysis. In the light-treated alb retinas, we evaluated the dorsal retina, but did not include the ventral retina in our analysis because minimal light-induced in this retinal region fails to stimulate a significant regenerative response.33 This lack of response was evident in the light-treated eyes because the ventral retinas retained the precise blue cone mosaic pattern. We randomly selected 3 to 10 optically coplanar, 110 × 110 μm regions that were free of folds, wrinkles, or tears, for quantitative pattern analysis. These images were imported into ImageJ (available by ftp at zippy.nimh.nih.gov/ or at http://rsb.info.nih.gov/nih-image; developed by Wayne Rasband, National Institutes of Health, Bethesda, MD) for assignment of unique x,y coordinates corresponding to the labeled blue cones. The x,y coordinates were subjected to two methods of spatial pattern analysis using custom-made software36,37: nearest neighbor distance analysis (NND)32; and quadrat analysis.36,38 After NND analysis, conformity ratios (CRs) were calculated as the mean NND/SD; CRs were used to determine whether a particular pattern was significantly different from a random pattern.39 Quadrat analysis involved the calculation of a statistical measure (var/mean)(N – 1), which allows for the assignment of pattern attributes as significantly more regular, or more aggregated than those expected for a Poisson distribution.36 Statistical comparisons of proportions of retinal regions having specific assigned pattern attributes were performed using the nonparametric Kolmogorov-Smirnov test. Statistical comparisons of planimetric densities were performed by using one-way ANOVA followed by a Fisher’s post hoc least significant differences test.
RT-PCR and Quantitative RT-PCR
Wild-type (n = 3) and syu+/− (n = 3) zebrafish were anesthetized and killed by spinal cord transection, and the retinas were harvested for RNA extraction. Tissue was homogenized, and RNA extraction was performed (RNeasy Mini Kit; Qiagen, Valencia, CA) followed by oncolumn DNase treatment. The quantity of RNA was determined spectrophotometrically by the A260/A280 ratio (Nanodrop 1000; Thermo-Scientific, Wilmington, DE).
RNA from adult wild-type and syu+/− zebrafish retinas was amplified by semiquantitative RT-PCR (One-Step kit; Qiagen). Samples were amplified using the following conditions: 5 minutes at 94°C, 40 cycles of 45 seconds at 94°C, 45 seconds at 58°C, 2 minutes at 72°C. The following primers were used: β-actin, 5′-TGGTATTGTGATGGACTCTGG (forward) and 5′-AGCACTGTGTTGGCATACAGG (reverse) resulting in a 450-bp product40; ptc-2, 5′-CTACAGCCCTCCACCCTCCTAC (forward) and 5′-CGTATACCAGAATCCCCAAACTGAG (reverse) resulting in a 333-bp product7; and shh 5′-CCACTACGAGGGAAGAGCTG (forward) and 5′-GCTGACCGCTATCATCAACA (reverse) resulting in a 500-bp product, designed using the Web-based application, Primer 3.41
Expression levels of shh mRNA in wild-type and syu+/− zebrafish retinas were quantified by quantitative real-time RT-PCR (qRT-PCR). For each sample, first-strand cDNA was synthesized with 100 ng of total RNA, 100 ng random primers (Invitrogen, Carlsbad, CA), and 50 U reverse transcriptase (Superscript II; Invitrogen), according to the manufacturer’s protocol. DNase I (Fermentas, Glen Burnie, MD) was used to remove possible DNA contamination. qRT-PCR was performed (model 7900HT Fast Real-Time PCR System using SYBR-Green PCR Master Mix; Applied Biosystems, Inc. [ABI], Foster City, CA) according to the manufacturer’s protocol. cDNAs were diluted 1:5 with TE (pH 8.0) and used as a template in the reactions. Specificity of the reactions was verified with melting-curve analysis. Three biological replicates and two technical replicates were performed for each tissue type. 18S rRNA was used as the endogenous reference gene.34 Before statistical analysis, CT values for both the target genes and the reference gene were averaged across technical replicates. The target gene was then normalized to the endogenous control, and an unpaired t-test was used to test for differences in shh expression between wild-type and syu+/− tissues.
Results
Regional Cone Loss and Morphologic Abnormalities in the Retinas of Senescent syut4+/− Zebrafish
The organization and morphology of double cone photoreceptors in wild-type and syu heterozygotes (syu+/−) were analyzed by indirect immunofluorescence of retinal frozen sections using the zpr-1 antibody, which labels a surface epitope on both the red-and green-sensitive cones of the zebrafish retina.42 In young (3–10 months pf) wild-type fish, the zpr-1 immunofluorescence revealed the double cone synaptic terminals, axons, cell bodies, and inner and outer segments (Fig. 1; Table 1). The young syu+/− fish also displayed a normal double-cone–labeling pattern with the double cone cell morphology displaying laminar positioning that was identical with that in the wild-type. Similarly, the double cones of older (1.5 years pf), though not reproductively senescent, syu+/− fish also appeared similar to those in the wild-type (Fig. 1; Table 1). The retinas from reproductively senescent (2 years and older) wild-type fish also displayed no detectable abnormalities in the double-cone arrangement or morphology based on the frozen section immunolabeling (Fig. 1; Table 1). However, half of the retinas (n = 12) derived from 2 years pf syut4+/− fish contained regions of double-cone loss and abnormalities including a lack of outer segments. In some cases these regions of cone abnormality were adjacent to retinal regions that were characterized by increased cone density (Fig. 1F). Retinas derived from syutbx+/− fish did not display these overt abnormalities, although some retinas possessed regions of reduced zpr-1+ cone density (Table 1, and data not shown). This cone loss phenotype was therefore both age- and genotype-specific.
Figure 1.
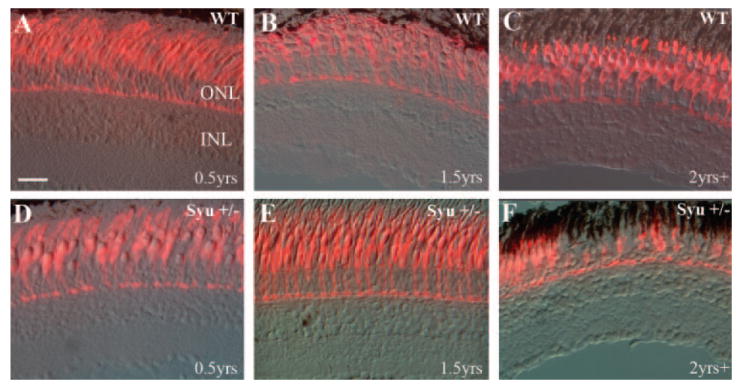
Double-cone photoreceptor abnormalities in aging syu+/− zebrafish. Retinal cryosections from young (3–10 months pf; A, D), middle-aged (1.5 years pf; B, E), and reproductively senescent (2 years plus; C, F) wild-type (A–C) and syut4+/− (D–F) zebrafish were immunolabeled with the monoclonal antibody zpr-1 to visualize the double-cone cells. Zpr-1+ cones were abundant and regularly spaced in all wild-type retinas (A–C) and the youngest and middle-aged syut4+/− retinas (D, E). Sections from the oldest syut4+/− fish, however, demonstrated sporadic loss and dysmorphism of the double-cone photoreceptors (F). ONL, outer nuclear layer; INL, inner nuclear layer. Scale bar, 25 μm.
Table 1.
Cone Pattern Abnormalities Are Both Genotype-and Age-Specific
| Genotype | Age | Fish (n) | Average of Sections Analyzed/Fish* (n) | Fish with Cone Loss/Abnormality* (%) | Fish with Cone Pattern Abnormality† (%) |
|---|---|---|---|---|---|
| Wild-type | 3–10 mo | 1 | 10 | 0 | 0 |
| (Tu and SciH) | 1.5 y | 5 | 45 | 0 | 0 |
| >2 y | 5 | 45 | 0 | 0 | |
| Syut4+/− | 3–10 mo | 2 | 39 | 0 | 0 |
| 1.5 y | 10 | 39 | 0 | 75 | |
| >2 y | 12 | 42 | 50 | 100 | |
| Syutbx+/− | >2 y | 4 | 30 | 0 | 50 |
Sections: zpr-1 (red/green cone) labeling.
Wholemounts: blue cone opsin labeling.
To confirm the regional cone loss and cone morphologic abnormalities exhibited by the 2-year-old syu+/− fish were not restricted to the double-cone cell population, and to evaluate cone loss across entire retinas, we examined the long single-cone (blue cones) organization by wholemount in situ RNA hybridization. Retinas were obtained from young, middle-aged, and old (<10 months pf, 1.5 years pf, and >2 years pf, respectively) wild-type and syut4+/− zebrafish, processed for in situ hybridization with a blue opsin cRNA probe and flat-mounted for imaging the long single-cone population. Retinas of wild-type fish of all ages and the youngest syut4+/− fish displayed a geometrically precise blue cone mosaic (Figs. 2A-D; Table 1). Likewise, the middle-aged syut4+/− fish displayed a precise cone mosaic, although in a few cases a small number of cones were missing from the mosaic (Fig. 2E; Table 1) or appeared structurally irregular (not shown). In marked contrast, all retinas obtained from the oldest syut4+/− fish exhibited a highly irregular blue cone pattern with regions of low cone density alternating with regions of higher density (Fig. 2F; Table 1). Unusual patterns similar to those of middle-aged syut4+/− fish were also regularly observed in retinas obtained from old syutbx+/− zebrafish (Fig. 2G; Table 1).
Figure 2.
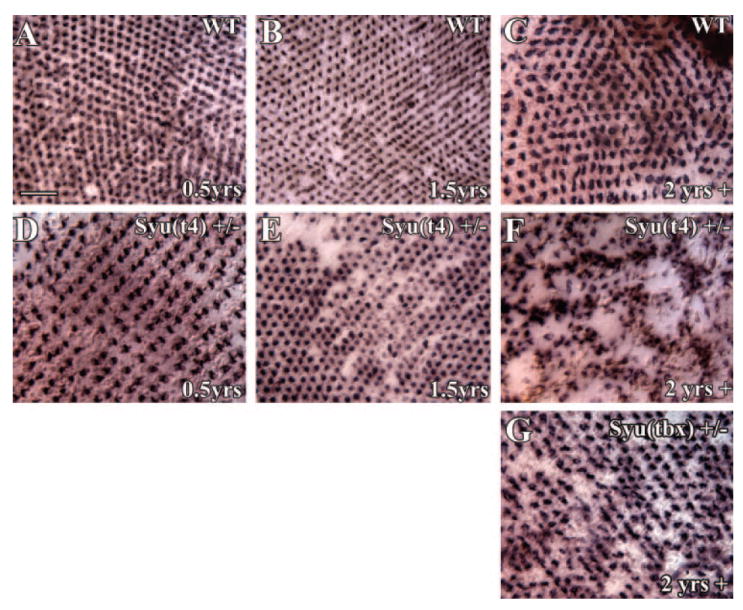
Age-dependent loss and maldistribution of blue cones in aging syu+/− zebrafish. Retinal whole-mounts from young (3–10 months pf; A, D), middle-aged (1.5 years pf; B, E), and old (2 years plus; C, F) wild-type (A–C) and syut4+/− (D–F) zebrafish were hybridized in situ with a blue opsin cRNA probe to identify the blue cones specifically. A precise blue cone mosaic was present in the wild-type retinas (A–C) and the youngest syut4+/− retinas (D). A few retinas from middle-aged syut4+/− fish were missing blue cones (E), whereas at least half of the oldest syut4+/− retinas were characterized by large regions lacking this cone photoreceptor type (F). Notice the blue cone-deficient regions are surrounding clusters of very densely spaced blue opsin-positive cells. (G) A disrupted blue cone mosaic, reminiscent of that observed in middle-aged syut4+/− fish, is evident in old syutbx+/− (see also Fig. 6). Scale bar, 25 μm.
To determine whether the retinal abnormalities in aging syut4+/− zebrafish were cone-specific, several retinal cell type-specific markers were examined by immunolabeling retinal cryosections from wild-type and syut4+/− zebrafish at 1.5 and 2+ years pf. Zpr-3, a monoclonal antibody selective for rod opsin, labeled rod outer segments in all fish examined including the oldest syu+/− fish (Figs. 3A-D; n = 3 for each genotype/age, average of 18 sections examined per eye). In the zpr-3-labeled sections, there were no indications of rod outer segment loss or damage, even in regions exhibiting cone abnormalities. Tissue in situ hybridizations of retinal sections using a rod opsin cRNA probe were also performed to assess further the potential abnormalities in the rod photoreceptors. This test failed to reveal any unusual rod cell labeling patterns or morphologies (data not shown). Sections were also immunolabeled by using antibodies to detect protein kinase C (PKC, rod bipolar cells; Figs. 3E-H), HuC/D (ganglion and amacrine cells; Figs. 3I-L), and glutamine synthetase (GS, Müller glia; Figs. 3M-P). In all cases, the labeling patterns in the syu+/− retinas appeared equivalent to wild-type (n = 3–7 for each genotype/age, average of 18, 28, and 40 sections examined per eye for PKC, HuC/D, and GS, respectively). Thus, the rod photoreceptors, amacrine, and ganglion cells, as well as the rod bipolar and Müller glia in the aged syu+/− retinas appeared normal in terms of organization and cellular morphology. These data indicate the syu+/− retinal phenotype is cone-specific.
Figure 3.
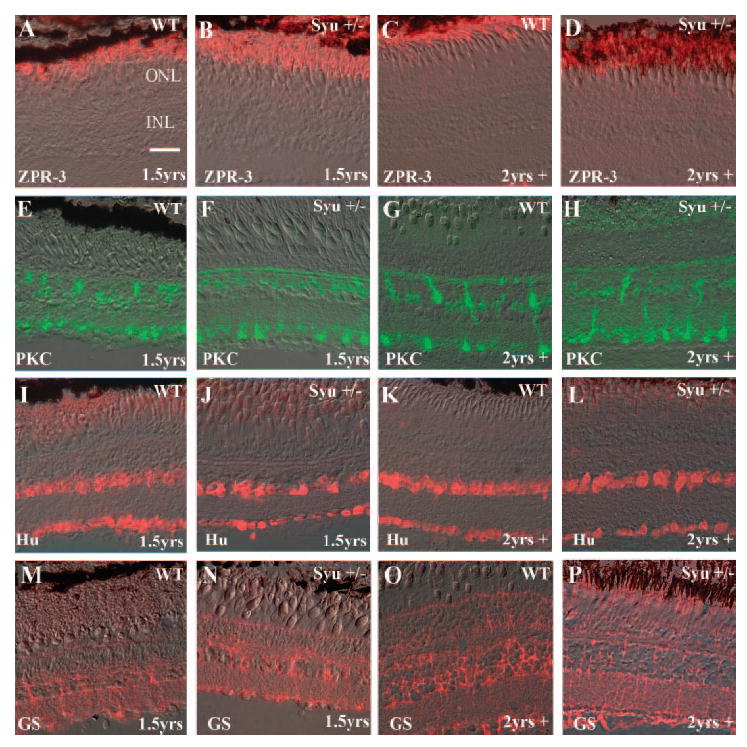
Retinal cell-type analysis demonstrates that the cellular defects in the syut4+/− retinas were cone-specific. Retina cell-type-specific markers were immunolocalized in eye frozen sections from aging wild-type and syu+/− fish. (A–D) The monoclonal antibody zpr-3 labeled rhodopsin in the rod outer segments. The rod photoreceptor number and morphology in the middle-aged (1.5 years; B) and older (2 years plus; D) syut4+/− retinas were equivalent to wild-type (A, C). Anti- PKC labeled the cell bodies and processes of the rod bipolar cells. Anti-PKC immunolabeling demonstrated rod bipolar cells in the retinas of the middle-aged (F) and older syut4+/− (H) fish were equivalent to the wild-type control (E, G). Amacrine and ganglion cells were identified by HuC/D protein expression. Retinal cryosections from middle-aged (I, J) and older (K, L) wild-type (I, K) and syut4+/− (J, L) zebrafish possessed an equivalent number of amacrine and ganglion cells with wild-type morphology and organization. The glutamine synthetase (GS) protein expression pattern, which characterizes the Müller glia was also equivalent in the middle-aged (M, N) and reproductively senescent (O, P) wild-type (M, O) and syut4+/− (N, P) zebrafish. ONL, outer nuclear layer; INL, inner nuclear layer. Scale bar, 25 μm.
Cone Patterns in Aging syut4+/− Retinas and in Retinas Subjected to Acute Photoreceptor Damage
The geometrically precise cone cell pattern of teleost retinas becomes disrupted after light-induced photoreceptor damage26 and is statistically distinct from the undamaged, wild-type condition after retinal regeneration.36 To gain additional insights into the cone loss phenotype in aging syut4+/− retinas, we compared the syu heterozygote cone pattern with the cone pattern in a model of acute cone cell loss and regeneration caused by light-induced.26 Whole retinas were obtained from alb zebrafish subjected to 3 days of constant, intense light or from light-treated alb fish after a 33-day period of regeneration. These retinas were processed for wholemount in situ hybridizations using a blue cone opsin cRNA probe and flatmounted for imaging and analysis. Qualitatively, the blue cone patterns of all non–light-treated control alb fish displayed the geometrically precise cone mosaic (Fig. 4A). In contrast, the blue cone pattern in the dorsal retinal regions of the light-treated alb zebrafish was sparse and irregular (Fig. 4B). The alb regenerated retinas also displayed an irregular blue cone pattern, but the cells were more densely arranged than in the retinas at the conclusion of the light treatment (Fig. 4C).
Figure 4.
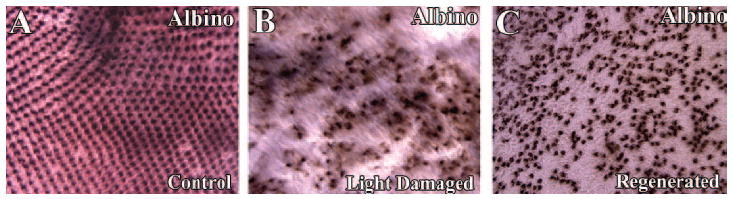
Blue cone distribution in light-damaged and regenerated alb zebrafish retinas. Wholemount in situ hybridizations using a blue opsin cRNA probe of retinas from the following experimental groups are shown: (A) non–light-treated control, (B) 3 days intense exposure to light, and (C) 33 days after treatment with light. A precise blue cone mosaic was present in the control retina (A), although the light-damaged retina exhibited a severely disrupted blue cone mosaic, with many missing cone cells (B). In comparison, the regenerated retina possessed more blue cones than did the light-damaged retina, but displayed a very irregular cone cell distribution (C). Scale bar, 25 μm.
Images such as those in Figures 2 and 4 were used to identify unique x,y coordinates of labeled cells, and the resultant cone distributions were quantitatively evaluated with NND and quadrat analyses.36,39 The application of NND analysis, which emphasizes local patterning attributes, to the blue cone pattern revealed predominantly statistically regular patterns in all wild-type retinas and in all untreated control alb retinas, with a normal distribution of NND values and a correspondingly high conformity ratio (Fig. 5A, top histograms). In many regions of the 1.5 years pf syut4+/− retinas, and in most regions of the oldest syut4+/− retinas, blue cones showed a progressively less normal distribution, and conformity ratios indicating a pattern not significantly different from random (Fig. 5A, middle histogram). Similarly, the blue cone patterns of the light-damaged alb dorsal retina showed statistically random patterns of blue cones (Fig. 5A, bottom right histogram). In the regenerated dorsal retinas of the light-treated alb zebrafish, NND analysis again showed statistically random patterns of blue cones (Fig. 5A, bottom left). These analyses suggest that local blue cone pattern abnormalities associated with syut4+/− and light-induced damage were statistically similar.
Figure 5.
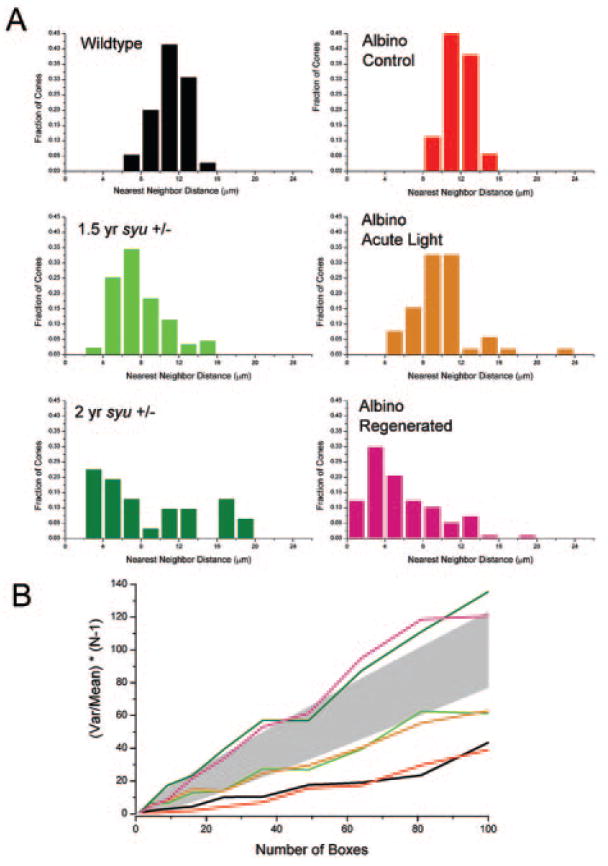
Cone distributions in aged syut4+/− and light-damaged and regenerated alb zebrafish retinas are quantitatively similar. (A) NND histograms reveal a normal distribution of blue cones in wild-type (black) and control alb retinas (red) retinas. NND distributions are less “normal” in retinas from aging syut4+/− (light and dark green), light-damaged (orange), and the light-damaged regenerated retinas (pink). (B) Quadrat analysis of blue cone pattern in wild-type and control alb retinas (black and red lines, respectively) resulted in values of (var/mean)(N – 1) that are significantly lower (P = 0.05 criterion) than expected for a Poisson distribution, which is consistent with a highly regular pattern. Pattern analysis of the blue cones in middle-aged syut4+/− retinas (light green line) and alb retinas immediately after light-induced damage (orange line) showed values of (var/mean)(N – 1) that are not significantly different from those expected for a Poisson distribution (represented by shaded area), which are consistent with random patterns. Analysis of the blue cone pattern in an old syut4+/− retina and a light-damaged regenerated alb retina (dark green and pink lines, respectively) resulted in values of (var/mean)(N – 1) that are significantly higher than expected for a Poisson distribution, consistent with a clumped pattern.
The application of quadrat analysis to the blue cone organization allowed quantitative evaluation of global pattern attributes, including regularity and aggregation (“clumping”36,38). All the patterns categorized as “regular” by NND analysis were also found by quadrat analysis to be regular (Fig. 5B). Blue cone patterns in middle-aged (1.5 years) syut4+/− retinas were characterized by quadrat analysis as not significantly different from random. Similarly, blue cone patterns of alb retinas obtained immediately after light-induced damage were also random (Fig. 5B). However, in some regions of the syut4+/− retinas and in some regions of the alb retinas allowed to regenerate after light treatment, quadrat analysis indicated statistically clumped patterns (Fig. 5B). Collectively, these data suggest quantitative similarities in the blue cone pattern of syut4+/− retinas and the retinas subjected to photoreceptor-specific damage. The pattern changes, however, were not uniform across all regions within a retina, nor between individual fish.
Figure 6 summarizes the pattern trends for the analyzed ages, genotypes, and treatments. NND analysis revealed consistently regular patterns in wild-type animals, an increasing tendency toward random patterns with age in syut4+/− and syutbx+/− retinas, and predominantly random patterns in the dorsal retinas of alb zebrafish subjected to light toxicity, both before and after a period of regeneration (Fig. 6A). The shift from predominantly regular to random patterns was statistically significant (P < 0.05) between young and old syu+/− retinas of both genotypes and between any two of the three conditions for the alb retinas. Quadrat analysis revealed consistently regular patterns in wild-type animals, tendencies toward both randomness and clumping in syut4+/− and the regenerated dorsal alb retinas, and tendencies toward randomness in the dorsal alb retinas immediately after acute light toxicity (Fig. 6B). The reduction in proportions of the analyzed regions showing long-range regularity was statistically significant (P < 0.05) between any two of the four conditions for the syu+/− retinas, and between control and light-treated alb retinas. The increase in proportions of analyzed regions showing blue cone clumping was statistically significant (P < 0.05) between young and old syut4+/− retinas and between regenerated and nonregenerated alb retinas. Planimetric density of blue cones did not change significantly with age in either wild-type or syut4+/− retinas (Fig. 6C). The density of blue cones decreased significantly however, compared with the control after light-induced damage (P < 0.05) and then increased significantly after regeneration (P < 0.05; Fig. 6C). These results are consistent with a regenerative response that has not fully resolved the native local or global pattern of blue cones.
Figure 6.
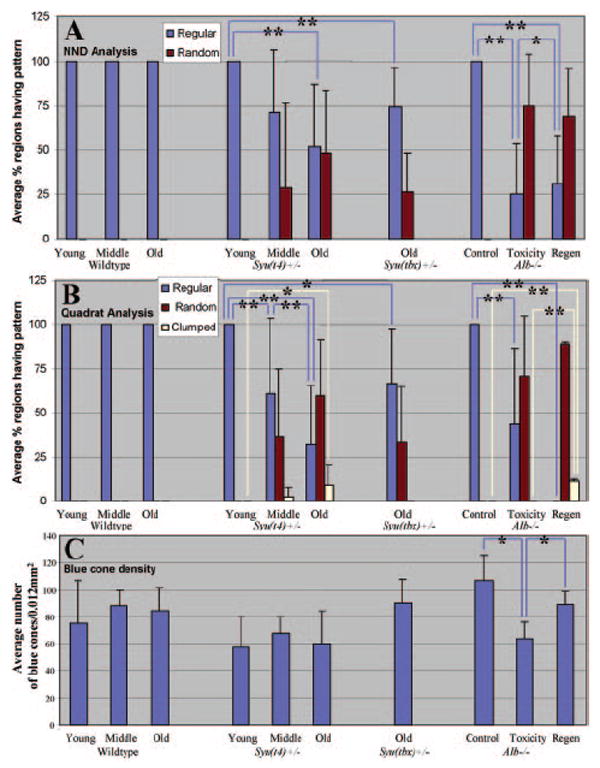
Summary of blue cone pattern analyses. (A) Average percentage of retinal regions having regular (blue columns) or random (red columns) patterns, as determined by NND analysis. Regions were scored as significantly regular or random based on the value of the conformity ratio.32 Proportions of regular versus random patterns were averaged over the number of retinas analyzed for each age, treatment, and genotype (two to five retinas in each case). Significant reductions in the percentage of regions showing locally regular patterns are indicated by blue brackets (*0.005 < P < 0.05 and **P < 0.005; Kolmogorov-Smirnov test). (B) Average percentage of retinal regions having regular (blue), random (red), or clumped (yellow) patterns as determined by quadrat analysis. Regions were scored as regular, random, or clumped based on the values of (var/mean)(N – 1).33,35 The different proportions of each pattern type (regular, random, or clumped) were averaged over the number of retinas analyzed for each age, treatment, and genotype (two to five retinas in each case). Significant reductions in the percentage of regions showing globally regular patterns are indicated by blue brackets (*0.005 < P < 0.05 and **P < 0.005; Kolmogorov-Smirnov test). Significant increases in the percentage of regions showing clumped patterns are indicated by yellow brackets (*0.005 < P < 0.05 and **P < 0.005; Kolmogorov-Smirnov test). (C) Mean (±SD) planimetric density of labeled blue cones. Significant differences are indicated by blue brackets (*P < 0.05; ANOVA and post hoc analysis).
Retinal Cell Death and Proliferation
To determine whether and when cone photoreceptors die, we processed retinal sections from wild-type and syut4+/− zebrafish at 1.5 and 2+ years pf for TUNEL. We used sectioned syut4−/− embryos as positive controls (not shown), as their retinal tissue invariably displays widespread cell death.13 TUNEL+ cells were rarely observed in any of the adult retinal sections (data not shown), and the number of TUNEL+ cells in syut4+/− retinas was not significantly different from that in the wild-type retinas (Table 2).
Table 2.
Cell Death and Cell Proliferation in Wild-Type and Syut4+/− Retinas
| TUNEL Labeling
|
BrdU Labeling
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Average TUNEL+ Cells/Section (n)
|
Average BrdU+ Cells/Section (n)
|
||||||||
| Genotype | Age (y) | Fish (n) | Average Sections Analyzed/Fish (n) | ONL | INL | Fish (n) | Average Sections Analyzed/Fish (n) | ONL | INL |
| Wildtype (Tu and SciH) | 1.5 | 2 | 5 | 0 | 0 | 5 | 27 | 0.5 | 0.3 |
| >2 | 2 | 5 | 0 | 0.1 | 5 | 12 | 0.3 | 0.3 | |
| Syu(t4)+/− | 1.5 | 6 | 5 | 0.1 | 0.1 | 6 | 45 | 0.4 | 0.3 |
| >2 | 6 | 5 | 0.1 | 0.2 | 7 | 24 | 0.1 | 0.6 | |
The zebrafish retina is capable of robust retinal cell regeneration after both widespread and cell-selective injuries.22,27 The regenerated cells arise from a proliferative population of inner nuclear layer cells, at least some of which correspond to Müller glia.34,43-45 To determine whether the syut4+/− retinal cone cell loss was stimulating a cone regeneration response as indicated by increased retinal cell divisions, we treated age-matched wild-type and syu+/− zebrafish systemically with 5 mM BrdU for 5 days and obtained retinal sections to assess BrdU incorporation. In rare cases, we observed BrdU+ cells within the ONL and the outer half of the INL (Fig. 7; Table 2). However, the number of BrdU+ cells in syut4+/− retinas was not significantly different from that of wild-type retinas (Table 2). Experiments using an antibody to detect PCNA, an independent indicator of cell cycle progression32 similarly revealed no significant proliferative activity in the syut4+/− retinas (data not shown). Taken together, the TUNEL, BrdU incorporation, and PCNA immunohistochemistry suggest that the cell death and cell proliferation responses in the syu+/− retinas must occur at very low levels over a prolonged time course.
Figure 7.
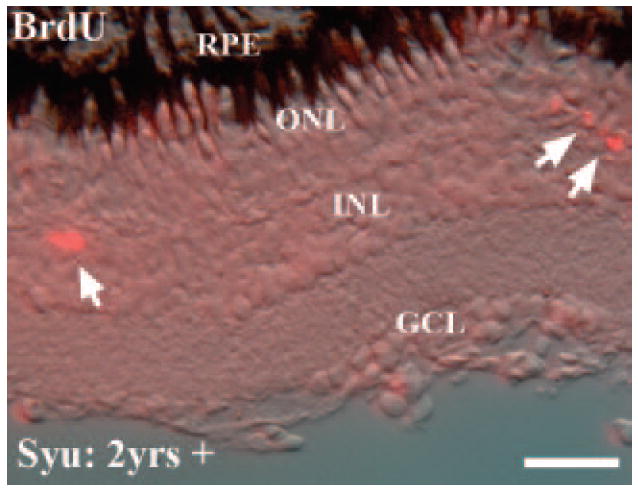
Proliferating cells in the inner and outer nuclear layers of aging syut4+/− zebrafish. Fish were systemically exposed to 5 mM BrdU for 5 days to identify dividing cells by indirect immunofluorescence with an anti-BrdU antibody. The cryosection displays the typical locations of the very few BrdU+ cells (arrows) that were observed in the oldest wild-type and syut4+/− retinas (2 years plus). RPE, retinal pigmented epithelium; ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bar, 50 μm.
Expression of Sonic Hedgehog and Patched2 mRNA
To determine whether shh expression and signaling were reduced in the syut4+/− fish compared with wild-type, we performed semiquantitative RT-PCR using specific primers for shh and patched-2 (ptc2) on RNA obtained from isolated retinas. Expression of Patched genes is positively regulated by Shh signaling,46 and ptc2 expression has been demonstrated in the embryonic zebrafish retina.7 PCR products of the appropriate size were consistently amplified from 10, 5, and 2.5 ng of total RNA. Isolated retinas from syu+/− fish contained lower quantities of both shh and ptc2 mRNA (Fig. 8A). qRT-PCR analysis confirmed these findings for shh expression, with significant difference between shh expression in the retina of wild-type versus syu+/− (P = 0.0265; Fig. 8B). Mean normalized ΔCT values for wild-type and syu+/− retina were 19.68187 and 20.73061, respectively.
Figure 8.
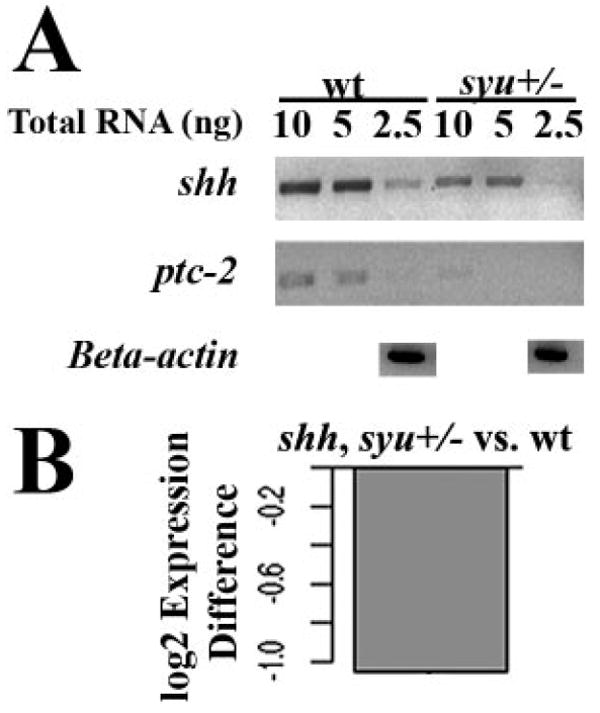
Expression of shh and ptc-2 mRNA in adult wild-type and syut4+/− zebrafish retinas. (A) Semiquantitative RT-PCR of shh and ptc-2; PCR products amplified from retinas of adult syu+/− zebrafish and their wild-type (wt) siblings. (B) qRT-PCR analysis showing that expression of shh mRNA (normalized to that of 18s rRNA) is reduced in syu+/− retinas compared with wild-type.
Discussion
Age-Related, Cone-Restricted Phenotype in the syu+/− Zebrafish Retina
This study revealed an age-related detrimental effect of heterozygosity at the syu locus that specifically affects cone photoreceptors. In young adult syu+/− zebrafish and in all ages of wild-type fish examined, cone photoreceptors displayed normal morphology and a regular mosaic distribution. In contrast, the oldest syu+/− zebrafish displayed retinal regions characterized by missing cones, clumped cones, or cones with abnormal morphology. This phenotype was clearly associated with the syu+/− genotype and was more pronounced in carriers of the shh deletion (syut4) than in those of the point mutation (syutbx). Rod photoreceptors and other retinal cell types were unaffected, based on immunolocalizations and in situ hybridizations to detect various retinal cell-type markers. Although the syut4+/− blue cone pattern phenotype exhibits incomplete penetrance in middle-aged fish (1.5 years), it is fully penetrant in old, reproductively senescent individuals. At this stage of the zebrafish lifespan, retinal growth is negligible,47 indicating the cone degeneration occurred within extant retina. This constitutes the first report in zebrafish of retinal abnormalities associated with postreproductive senescence.
Statistical comparisons of the cone patterns in aged syu+/− retinas and light-damaged retinas demonstrated the perturbations in syu+/− cone pattern are consistent with cone loss followed by, or accompanied by, a cone regeneration response (see also Refs. 26,36). However, the changes in the syu+/− cone pattern were not accompanied by detectable alterations in cell death or cell proliferation. This result may be expected for several reasons. The cone abnormalities in the syut4+/− retinas did not become evident until late adulthood. This phenotype indicates the cone disease develops over an protracted time course. Our sampling of single time points during this extended period would be expected to miss relatively infrequent cone cell death events. These low levels of photoreceptor cell death may not be adequate to stimulate the high levels of cell proliferation previously observed in zebrafish models of retinal cell regeneration, in which widespread cell death was induced by treatment with intense light or acute trauma.26,32,36,44,45 Regenerative cell proliferation may also be influenced by the mitogenic function of Shh5,10,13,48; reduction of Shh in the syu+/− retinas may limit the extent of the retinal cell proliferation response. The requirement for supplemental Shh in the regenerating embryonic chick retina supports this hypothesis.49
Sonic Hedgehog for Adult Cone Maintenance and Survival
Our results suggest Shh activity is necessary for the maintenance and survival of the cone photoreceptor cells in the adult retina. During retinal development, shh is expressed in ganglion cells, amacrine cells,3,6 and the RPE.7-9 Experimental reductions in Shh signaling resulted in reduced retinal progenitor proliferation,13 a reduction in differentiated ganglion cells4 and photoreceptors,7,12,13 and RPE defects.8 We report here that expression of shh persists in the adult, wild-type zebrafish retina, but shh mRNA is significantly reduced in the retinas of the syut4+/− fish. Our semiquantitative RT-PCR results suggest further that Shh signaling activity is also reduced within the retina so that expression of the target gene ptc2 is affected. The age-related cone abnormalities are therefore associated with reduced shh expression and signaling in the retina. Cone photoreceptors may require some threshold amount of Shh throughout the zebrafish lifespan, and this becomes limiting in the oldest syu+/− fish. An analysis of Shh-related gene expression—including that of twhh— in isolated retina and RPE tissue will address the question of whether hedgehog gene expression and signaling is also affected in the RPE of adult, aging syu+/− fish.7
Mutations in Shh or in the molecular components of its signal transduction pathway have not been directly associated with any human retinal disorder or any other animal model of retinal disease. However, major risk factors for human age-related macular degeneration (AMD) include metabolic errors in cholesterol homeostasis.50 In addition, cholesterol deficiency or elevated sterol precursors cause profound congenital abnormalities, including ocular defects51 and retinal degeneration.52 The Shh signal transduction pathway requires cholesterol, and Shh signaling can be altered by sterols.53 Thus, proper regulation of cholesterol homeostasis and Shh signaling may be necessary to maintain photoreceptor health in the human retina.
The age-related cone abnormality phenotype was less pronounced in carriers of the tbx (point mutation) allele than in those of the t4 (deletion) allele. Both alleles are considered null, and homozygous mutants exhibit nearly identical embryonic phenotypes.14 The retinal phenotype associated with heterozygosity may have uncovered incomplete penetrance of the tbx allele. Alternatively, the more severe syut4+/− retinal phenotype may be due to the synergistic effect of the deletion on a nearby gene or gene regulatory elements. The deleted region of the syut4 allele encompasses the entire shh coding region, along with 4.0 kb of 5′ sequence and 2.2 kb of 3′ sequence.14 However, the nearest predicted open reading frame is located approximately 15.0 kb upstream of shh, based on a single expressed sequence tag (EST) identified from this genomic region. The expression of this gene may be affected by the syut4 deletion through the loss of putative cis-regulatory elements. Characterization of carriers of other Hh pathway mutations is under way, to explore these possibilities. The syu+/− zebrafish nevertheless represents a genetically tractable model for investigating molecular determinants of human retinal dysgenesis and disease.
The syu+/− cone photoreceptors are disrupted in the absence of any overt impact on the rod photoreceptors, suggesting that the cone population is more sensitive to limiting levels of Shh signaling. Alternatively, the rod population may also be sensitive, but mechanisms for their continual addition may be compensating.22,27 This cone-specific effect in the zebrafish syu+/− retinas is distinct from most human photoreceptor degenerations, including AMD, where rod photoreceptor death precedes cone cell death.54-56 Mammalian models that mimic this sequence of photoreceptor loss were used to identify cone survival factors generated by rods that become limiting during rod degeneration.57 However, other human retinal diseases are characterized by either the exclusive loss of cones, or the concurrent loss of both the cone and rod cells.58,59 Similar to the aged syu+/− fish, larval-age gnn zebrafish mutants lose cones with little loss of rods.60 In contrast, the Xops:mCFP transgene causes rod photoreceptor loss in zebrafish without detectable effects on the cone cell population.61 Collectively, these zebrafish models confirm the variable degree of cone–rod interdependence and emphasize that non–cell-autonomous factors function to maintain cone viability and should be considered for their therapeutic potential.
Cone Patterning in Environmental and Genetic Models of Photoreceptor Damage
In zebrafish, retinal cells are regenerated in response to acute trauma or cellular insult that results in some threshold level of cell death.22,27 The present study suggests that retinal regeneration is also stimulated in response to a genetic defect that results in cone photoreceptor degeneration. The cone regeneration response after photoreceptor-selective damage due to intense light treatment resulted in a disrupted cone mosaic that qualitatively and quantitatively resembled the disrupted cone mosaic after more widespread forms of retinal damage, such as whole-retina destruction with a toxin,36 or surgical removal of a portion of retina.20 This quantitatively predictable disruption in cone cell pattern was also observed in the aged syu+/− retinas. The fish retina may therefore incur a conserved cellular response to photoreceptor damage, resulting in densely clustered cones whose aberrant organization contributes to the surrounding region’s cone loss phenotype. These findings also indicate that, after photoreceptor damage, cone positional information contributed by the other retinal layers or remaining photoreceptors is not sufficient to restore the native cone mosaic pattern.
Acknowledgments
The authors thank Anand Chandrasekhar (University of Missouri); and Rolf Karlstrom (University of Massachusetts) for providing the syu+/− fish; Bhavani Kashyap, Savjit Singh, Jessica Thompson, and Rob Drew (University of Idaho) for technical assistance; and the University of Notre Dame Center for the use of the Zebrafish Research and the Freimann Life Sciences Center.
Supported by the American Health Assistance Foundation’s Macular Degeneration Program (DLS), National Eye Institute Grant EY012146 (DLS), and National Science Foundation Grant 0351250 (DAC).
Footnotes
Disclosure: D.L. Stenkamp, None; R. Satterfield, None; K. Muhunthan, None; T. Sherpa, None; T.S. Vihtelic, None; D.A. Cameron, None
References
- 1.Stenkamp DL. Multiple roles for hedgehog signaling in zebrafish eye development. In: Fisher CE, Howie SEM, editors. Shh and Gli Signaling and Development. Landes Bioscience; Georgetown, TX: 2006. pp. 58–68. [Google Scholar]
- 2.Wallace VA. Proliferative and cell fate effects of Hedgehog signaling in the vertebrate retina. Brain Res. 2008;1192:61–75. doi: 10.1016/j.brainres.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Wallace VA, Raff MC. A role for Sonic hedgehog in axon-to-astrocyte signalling in the rodent optic nerve. Development. 1999;126(13):2901–2909. doi: 10.1242/dev.126.13.2901. [DOI] [PubMed] [Google Scholar]
- 4.Neumann CJ, Nuesslein-Volhard C. Patterning of the zebrafish retina by a wave of sonic hedgehog activity. Science. 2000;289(5487):2137–2139. doi: 10.1126/science.289.5487.2137. [DOI] [PubMed] [Google Scholar]
- 5.Zhang XM, Yang XJ. Regulation of retinal ganglion cell production by Sonic hedgehog. Development. 2001;128(6):943–957. doi: 10.1242/dev.128.6.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shkumatava A, Fischer S, Müller F, Strahle U, Neumann CJ. Sonic hedgehog, secreted by amacrine cells, acts as a short-range signal to direct differentiation and lamination in the zebrafish retina. Development. 2004;131(16):3849–3858. doi: 10.1242/dev.01247. [DOI] [PubMed] [Google Scholar]
- 7.Stenkamp DL, Frey RA, Prabhudesai SN, Raymond PA. Function for Hedgehog genes in zebrafish retinal development. Dev Biol. 2000;220(2):238–252. doi: 10.1006/dbio.2000.9629. [DOI] [PubMed] [Google Scholar]
- 8.Perron M, Boy S, Amato MA, et al. A novel function for Hedgehog signalling in retinal pigment epithelium differentiation. Development. 2003;130(8):1565–1577. doi: 10.1242/dev.00391. [DOI] [PubMed] [Google Scholar]
- 9.Levine EM, Roelink H, Turner J, Reh TA. Sonic hedgehog promotes rod photoreceptor differentiation in mammalian retinal cells in vitro. J Neurosci. 1997;17(16):6277–6288. doi: 10.1523/JNEUROSCI.17-16-06277.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan J, Zheng H, Xiao HL, She ZJ, Zhou GM. Sonic hedgehog promotes stem-cell potential of Muller glia in the mammalian retina. Biochem Biophys Res Commun. 2007;363(2):347–354. doi: 10.1016/j.bbrc.2007.08.178. [DOI] [PubMed] [Google Scholar]
- 11.Yu C, Mazerolle CJ, Thurig S, et al. Direct and indirect effects of hedgehog pathway activation in the mammalian retina. Mol Cell Neurosci. 2006;32(3):274–282. doi: 10.1016/j.mcn.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Stenkamp DL, Frey RA. Extraretinal and retinal hedgehog signaling sequentially regulate retinal differentiation in zebrafish. Dev Biol. 2003;258(2):349–363. doi: 10.1016/s0012-1606(03)00121-0. [DOI] [PubMed] [Google Scholar]
- 13.Stenkamp DL, Frey RA, Mallory DE, Shupe EE. Embryonic retinal gene expression in sonic-you mutant zebrafish. Dev Dyn. 2002;225(3):344–350. doi: 10.1002/dvdy.10165. [DOI] [PubMed] [Google Scholar]
- 14.Schauerte HE, van Eeden FJ, Fricke C, Odenthal J, Strähle U, Haffter P. Sonic hedgehog is not required for the induction of medial floor plate cells in the zebrafish. Development. 1998;125(15):2983–2993. doi: 10.1242/dev.125.15.2983. [DOI] [PubMed] [Google Scholar]
- 15.Szamier RB, Berson EL. Retinal ultrastructure in advanced retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1977;16(10):947–962. [PubMed] [Google Scholar]
- 16.Michaelides M, Hardcastle AJ, Hunt DM, Moore AT. Progressive cone and cone-rod dystrophies: phenotypes and underlying molecular genetic basis. Surv Ophthalmol. 2006;51(3):232–258. doi: 10.1016/j.survophthal.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Wang DY, Chan WM, Tam PO, et al. Gene mutations in retinitis pigmentosa and their clinical implications. Clin Chim Acta. 2005;351(12):5–16. doi: 10.1016/j.cccn.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Dalke C, Graw J. Mouse mutants as models for congenital retinal disorders. Exp Eye Res. 2005;81(5):503–512. doi: 10.1016/j.exer.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Raymond PA, Barthel LK, Rounsifer ME, Sullivan SA, Knight JK. Expression of rod and cone visual pigments in goldfish and zebrafish: a rhodopsin-like gene is expressed in cones. Neuron. 1993;10(6):1161–1174. doi: 10.1016/0896-6273(93)90064-x. [DOI] [PubMed] [Google Scholar]
- 20.Stenkamp DL, Cameron DA. Cellular pattern formation in the retina: retinal regeneration as a model system. Mol Vis. 2002;8:280–293. [PubMed] [Google Scholar]
- 21.Easter SS, Jr, Malicki JJ. The zebrafish eye: developmental and genetic analysis. Results Probl Cell Differ. 2002;40:346–370. doi: 10.1007/978-3-540-46041-1_17. [DOI] [PubMed] [Google Scholar]
- 22.Stenkamp DL. Neurogenesis in the fish retina. Int Rev Cytol. 2007;259:173–224. doi: 10.1016/S0074-7696(06)59005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krock BL, Bilotta J, Perkins BD. Noncell-autonomous photoreceptor degeneration in a zebrafish model of choroideremia. Proc Natl Acad Sci U S A. 2007;104(11):4600–4605. doi: 10.1073/pnas.0605818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keller ET, Murtha JM. The use of mature zebrafish (Danio rerio) as a model for human aging and disease. Comp Biochem Physiol C Toxicol Pharmacol. 2004;138(3):335–341. doi: 10.1016/j.cca.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 25.Kishi S, Uchiyama J, Baughman AM, Goto T, Lin MC, Tsai SB. The zebrafish as a vertebrate model of functional aging and very gradual senescence. Exp Gerontol. 2003;38(7):777–786. doi: 10.1016/s0531-5565(03)00108-6. [DOI] [PubMed] [Google Scholar]
- 26.Vihtelic TS, Hyde DR. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J Neurobiol. 2000;44(3):289–307. doi: 10.1002/1097-4695(20000905)44:3<289::aid-neu1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 27.Hitchcock P, Ochocinska M, Sieh A, Otteson D. Persistent and injury-induced neurogenesis in the vertebrate retina. Prog Retin Eye Res. 2004;23(2):183–194. doi: 10.1016/j.preteyeres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Maaswinkel H, Riesbeck LE, Riley ME, et al. Behavioral screening for nightblindness mutants in zebrafish reveals three new loci that cause dominant photoreceptor cell degeneration. Mech Ageing Dev. 2005;126(10):1079–1089. doi: 10.1016/j.mad.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 29.Maaswinkel H, Mason B, Li L. ENU-induced late-onset night blindness associated with rod photoreceptor cell degeneration in zebrafish. Mech Ageing Dev. 2003;124(1012):1065–1071. doi: 10.1016/j.mad.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) 4. Eugene, OR: University of Oregon Press; 2000. [Google Scholar]
- 31.Otteson DC, D’Costa AR, Hitchcock PF. Putative stem cells and the lineage of rod photoreceptors in the mature retina of the goldfish. Dev Biol. 2001;232(1):62–76. doi: 10.1006/dbio.2001.0163. [DOI] [PubMed] [Google Scholar]
- 32.Sherpa T, Fimbel SM, Mallory DE, et al. Ganglion cell regeneration following whole-retina destruction in zebrafish. Dev Neurobiol. 2008;68:166–181. doi: 10.1002/dneu.20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vihtelic TS, Soverly JE, Kassen SC, Hyde DR. Retinal regional differences in photoreceptor cell death and regeneration in light-lesioned albino zebrafish. Exp Eye Res. 2006;82(4):558–575. doi: 10.1016/j.exer.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 34.Kassen SC, Ramanan V, Montgomery JE, et al. Time course analysis of gene expression during light-induced photoreceptor cell death and regeneration in albino zebrafish. Dev Neurobiol. 2007;67(8):1009–1031. doi: 10.1002/dneu.20362. [DOI] [PubMed] [Google Scholar]
- 35.Barthel LK, Raymond PA. Improved method for obtaining 3-microns cryosections for immunocytochemistry. J Histochem Cytochem. 1990;38(9):1383–1388. doi: 10.1177/38.9.2201738. [DOI] [PubMed] [Google Scholar]
- 36.Stenkamp DL, Powers MK, Carney LH, Cameron DA. Evidence for two distinct mechanisms of neurogenesis and cellular pattern formation in regenerated goldfish retinas. J Comp Neurol. 2001;431(4):363–381. doi: 10.1002/1096-9861(20010319)431:4<363::aid-cne1076>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Cameron DA, Carney LH. Cell mosaic patterns in the native and regenerated inner retina of zebrafish: implications for retinal assembly. J Comp Neurol. 2000;416(3):356–367. [PubMed] [Google Scholar]
- 38.Grieg-Smith P. Quantitative Plant Ecology. 2. London: Butterworth; 1964. [Google Scholar]
- 39.Cook JE. Spatial properties of retinal mosaics: an empirical evaluation of some existing measures. Vis Neurosci. 1996;13(1):15–30. doi: 10.1017/s0952523800007094. [DOI] [PubMed] [Google Scholar]
- 40.DeCarvalho AC, Cappendijk SL, Fadool JM. Developmental expression of the POU domain transcription factor Brn-3b (Pou4f2) in the lateral line and visual system of zebrafish. Dev Dyn. 2004;229(4):869–876. doi: 10.1002/dvdy.10475. [DOI] [PubMed] [Google Scholar]
- 41.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 42.Larison KD, Bremiller R. Early onset of phenotype and cell patterning in the embryonic zebrafish retina. Development. 1990;109(3):567–576. doi: 10.1242/dev.109.3.567. [DOI] [PubMed] [Google Scholar]
- 43.Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci. 2007;27(7):1712–24. doi: 10.1523/JNEUROSCI.5317-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006;26(23):6303–6313. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yurco P, Cameron DA. Responses of Muller glia to retinal injury in adult zebrafish. Vision Res. 2005;45(8):991–1002. doi: 10.1016/j.visres.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 46.Concordet JP, Lewis KE, Moore JW, et al. Spatial regulation of a zebrafish patched homologue reflects the roles of sonic hedgehog and protein kinase A in neural tube and somite patterning. Development. 1996;122(9):2835–2846. doi: 10.1242/dev.122.9.2835. [DOI] [PubMed] [Google Scholar]
- 47.Marcus RC, Delaney CL, Easter SS., Jr Neurogenesis in the visual system of embryonic and adult zebrafish (Danio rerio) Vis Neurosci. 1999;16(3):417–424. doi: 10.1017/s095252389916303x. [DOI] [PubMed] [Google Scholar]
- 48.Moshiri A, McGuire CR, Reh TA. Sonic hedgehog regulates proliferation of the retinal ciliary marginal zone in posthatch chicks. Dev Dyn. 2005;233(1):66–75. doi: 10.1002/dvdy.20299. [DOI] [PubMed] [Google Scholar]
- 49.Spence JR, Madhavan M, Ewing JD, et al. The hedgehog pathway is a modulator of retina regeneration. Development. 2004;131(18):4607–4621. doi: 10.1242/dev.01298. [DOI] [PubMed] [Google Scholar]
- 50.Chamberlain M, Baird P, Dirani M, Guymer R. Unraveling a complex genetic disease: age-related macular degeneration. Surv Ophthalmol. 2006;51(6):576–586. doi: 10.1016/j.survophthal.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Javitt NB. Oxysterols: functional significance in fetal development and the maintenance of normal retinal function. Curr Opin Lipidol. 2007;18(3):283–288. doi: 10.1097/MOL.0b013e328133851e. [DOI] [PubMed] [Google Scholar]
- 52.Fliesler SJ, Peachey NS, Richards MJ, Nagel BA, Vaughan DK. Retinal degeneration in a rodent model of Smith-Lemli-Opitz syndrome: electrophysiologic, biochemical, and morphologic features. Arch Ophthalmol. 2004;122(8):1190–1200. doi: 10.1001/archopht.122.8.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen MM., Jr The hedgehog signaling network. Am J Med Genet A. 2003;123(1):5–28. doi: 10.1002/ajmg.a.20495. [DOI] [PubMed] [Google Scholar]
- 54.Curcio CA. Photoreceptor topography in ageing and age-related maculopathy. Eye. 2001;15:376–383. doi: 10.1038/eye.2001.140. [DOI] [PubMed] [Google Scholar]
- 55.Reme CE, Grimm C, Hafezi F, Iseli HP, Wenzel A. Why study rod cell death in retinal degenerations and how? Doc Ophthalmol. 2003;106(1):25–29. doi: 10.1023/a:1022423724376. [DOI] [PubMed] [Google Scholar]
- 56.Chader GJ. Animal models in research on retinal degenerations: past progress and future hope. Vision Res. 2002;42(4):393–399. doi: 10.1016/s0042-6989(01)00212-7. [DOI] [PubMed] [Google Scholar]
- 57.Leveillard T, Mohand-Saïd S, Lorentz O, et al. Identification and characterization of rod-derived cone viability factor. Nat Genet. 2004;36(7):755–759. doi: 10.1038/ng1386. [DOI] [PubMed] [Google Scholar]
- 58.Michaelides M, Hunt DM, Moore AT. The cone dysfunction syndromes. Br J Ophthalmol. 2004;88(2):291–297. doi: 10.1136/bjo.2003.027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Simunovic MP, Moore AT. The cone dystrophies. Eye. 1998;12:553–565. doi: 10.1038/eye.1998.145. [DOI] [PubMed] [Google Scholar]
- 60.Biehlmaier O, Neuhauss SC, Kohler K. Double cone dystrophy and RPE degeneration in the retina of the zebrafish gnn mutant. Invest Ophthalmol Vis Sci. 2003;44(3):1287–1298. doi: 10.1167/iovs.02-0363. [DOI] [PubMed] [Google Scholar]
- 61.Morris AC, Schroeter EH, Bilotta J, Wong RO, Fadool JM. Cone survival despite rod degeneration in XOPS-mCFP transgenic zebrafish. Invest Ophthalmol Vis Sci. 2005;46(12):4762–4771. doi: 10.1167/iovs.05-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]


