Abstract
In support of a new clinical trial designed to compare the effects of crushed fresh garlic and two types of garlic supplement tablets (enteric-coated dried fresh garlic and dried aged garlic extract) on serum lipids, the three garlic products have been characterized for (a) composition (14 sulfur and 2 non-sulfur compounds), (b) stability of suspected active compounds, and (c) availability of allyl thiosulfinates (mainly allicin) under both simulated gastrointestinal (tablet dissolution) conditions and in vivo. The allyl thiosulfinates of blended fresh garlic were stable for at least two years when stored at −80 °C. The dissolution release of thiosulfinates from the enteric-coated garlic tablets was found to be >95%. The bioavailability of allyl thiosulfinates from these tablets, measured as breath allyl methyl sulfide, was found to be complete and equivalent to that of crushed fresh garlic. S-allylcysteine was stable for 12 months at ambient temperature. The stability of the suspected active compounds under the conditions of the study and the bioavailability of allyl thiosulfinates from the dried garlic supplement have validated the use of these preparations for comparison in a clinical trial.
Keywords: garlic, allicin, bioavailability, stability, dissolution, enteric coated, aged garlic extract, allyl methyl thiosulfinate, S-allylcysteine, γ-glutamyl-S-allylcysteine, γ-glutamyl-S-trans-1-propenylcysteine, γ-glutamylphenylalanine, arginine
INTRODUCTION
Since 1981, 52 randomized controlled trials of at least four weeks duration have been published on the ability of a variety of garlic supplements to affect serum lipids.(1) However, the results have been inconsistent. Although most of the earlier trials reported positive effects, 10 of the 13 trials published since 1995 have found no effect on serum cholesterol or serum triglyceride.(2) Systematic reviews (meta-analyses) of these trials have concluded that most of the positive studies had significant design problems.(1,3–5) The authors of most of the meta-analyses and of the trials that showed no effect have concluded that “garlic,” rather than the particular supplement used in the trials, has little, if any, effect on serum lipids. However, this conclusion assumes that garlic supplements contain similar amounts of compounds as garlic itself and that they are as effective as crushed fresh garlic in delivering active compounds to the body, assumptions that have long been suspected as doubtful,(6–10) but which has been given little attention in prior trials.
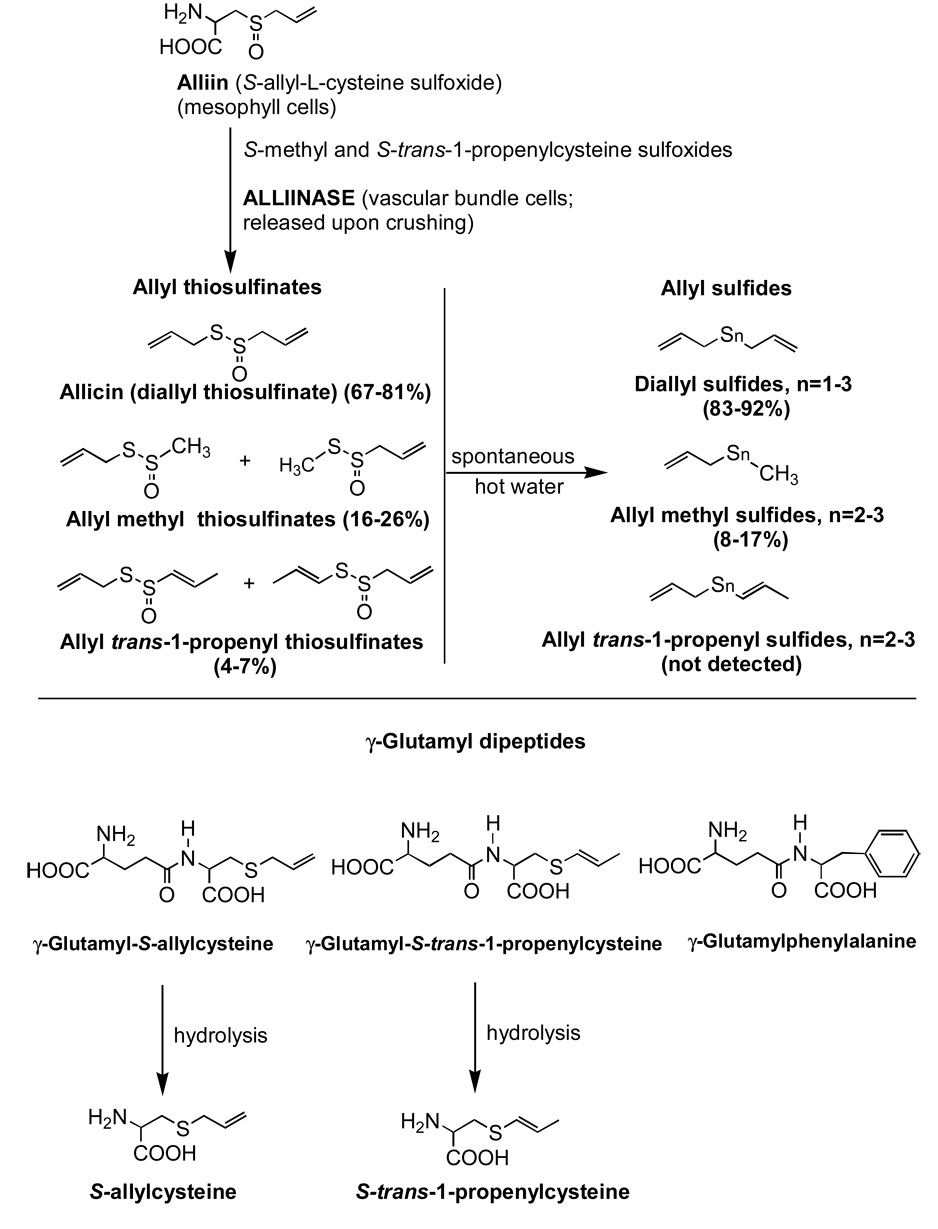
Although several forms of garlic supplements have been used in clinical trials, the most common form has been garlic powder (dried garlic) supplements.(1,10) In fact, most garlic powder supplements claim to lower serum cholesterol and to be standardized on allicin (diallyl thiosulfinate) yield, including those used in clinical trials. Considerable evidence has indicated that allicin is responsible for most of the effects of garlic on serum lipids.(10) However, it has been known since 1944 that allicin is absent from garlic and garlic powders until the enzyme, alliinase, has been activated, by the crushing of cloves or the wetting of powder, allowing the transformation of the amino acid, alliin, to allicin and other allyl thiosulfinates (Figure 1).(6,11,12) When fresh garlic is crushed or blended, allicin formation is complete in approximately six seconds, well before consumption.(74) With supplements the opportunity for allicin formation does not occur until after consumption, when the tablets dissolve. However, because the activity of alliinase is dramatically affected by the gastrointestinal environment (gastric acid, intestinal proteases) and by supplement processing procedures,(7,9,13) the amount of allicin produced in the body from supplements, and, hence, confidence in extrapolating clinical trial results to fresh garlic, can be highly questionable.
Figure 1.
Structures and formation of compounds analyzed
We have determined that upon subjecting garlic powder tablets to the simulated gastrointestinal dissolution conditions defined in the U.S. Pharmacopeia/National 4 Formulary,(14,15) that the allicin released from the tablet brand used in most of the clinical trials from 1994–2000 varied from 14% to 18% (compared to 100% upon adding crushed tablets to water).(2) The same evaluation of 24 brands of acid-protected (enteric-coated) tablets revealed an allicin release of 3–94%, with an average of 13%, clearly demonstrating the difficulty of allicin formation from supplements under gastrointestinal conditions and the need to establish allicin release in vivo.(13) Indeed, allicin bioavailability, measured as exhaled allyl methyl sulfide, has been demonstrated to be complete (>95%) for the brand that gave a dissolution allicin release of 94%, while tablets of the same alliin content, but lower alliinase activity, gave allicin bioavailability values as low as 5%.(13,16) Hence, confidence in the ability of a garlic powder supplement to represent crushed fresh garlic in a clinical trial can only be established based on bioavailability studies, although some confidence can be obtained by subjecting the tablets to simulated conditions. To date, no clinical trial has been conducted with a garlic supplement of known allicin bioavailability, and only one trial has reported the dissolution allicin release.(17) Therefore, the results of past clinical trials on serum lipids can only be applied to the particular product used in the trial and cannot be considered valid for crushed fresh garlic.
The authors have undertaken a large-scale clinical trial to address this issue (June 2002–June 2005). This trial will test the effects of blended raw garlic, an enteric-coated powdered garlic supplement of known high allicin bioavailability, and a dried aged garlic extract supplement on plasma LDL-cholesterol concentrations among approximately 200 moderately hypercholesterolemic adults. A major focus of the current clinical trial is not only that of high methodological design quality but also to more thoroughly define the quality of the garlic products being consumed than has been done in the past, particularly with respect to (1) composition of the unique sulfur compounds of garlic, (2) stability of suspected active compounds under the lengthy storage and particular usage conditions of the clinical trial, and (3) bioavailability of suspected active compounds when bioavailability is in question. The intent of the results to be presented here is to address issues of composition, stability, and bioavailability, separate from and preceding the trial results.
MATERIALS AND METHODS
Blended fresh garlic
In September 2002, 68 kg of recently harvested (July/August) California Early garlic bulbs, colossal size, were purchased at Christopher Ranch in Gilroy, California, USA. After verifying that the yield of allicin and other allyl thiosulfinates of several random bulbs from this batch fell within the typical range, the bulbs were broken into cloves and the clove skins manually removed from the larger cloves with the aid of rubber tube garlic peelers (Selandia, Spokane, WA 99224, USA). The peeled cloves (41 kg) were then placed as 700-gram portions into a 2-L Vita-Mix (Cleveland, OH 44138, USA) blender along with 280 mL water (0.40 mL/g) and blended (homogenized) at high speed (24,000 rpm) until homogeneous, 30–45 sec. The blendates were poured into four 19-L pails, thoroughly stirred, poured into 1-L containers and all but the first container to be used were frozen (−20°C). Four samples from each pail were analyzed within two days for allyl thiosulfinates to determine the amount of blendate needed to contain an equimolar amount of S-allyl groups as when four Garlicin tablets are pulverized and placed in water. After this parcel value was determined for each pail (5.68 ± 0.08 g blendate, n=4, containing 4.06 g fresh garlic), the blendates were thawed (one day’s work load at a time), accurately weighed (±0.1g) into approximately 10,000 plastic weighing dishes (4 × 4 × 0.8 cm), covered with aluminum foil, placed into small plastic bags, sealed, and placed in a cryofreezer (−80°C) until consumption. The parceling procedure was labor intensive, requiring four workers for seven days.
The blended garlic was prepared for consumption by the Stanford University Hospital General Clinical Research Kitchen by thawing the parcel, removing it with the aid of a rubber spatula, and mixing it with about 15 mL of a condiment. The condiments mixtures were placed on the sandwiches by the participants themselves, immediately prior to consumption. Three sandwiches were prepared at one time, placed in insulated containers containing ice packs, picked up by study participants on the day of preparation, stored in the participant’s home refrigerator, and consumed over three days (one sandwich per day). Only those condiments were used in which allicin had been shown to be stable at refrigeration temperature for three days.
Garlic supplements
In August 2002, 38,000 tablets of a single lot (916243) of Garlicin®, Nature’s Way Products, Inc., Springville, Utah 84663, USA, and 57,000 tablets of a single lot (2E06A) of Kyolic® Hi-Po Formula 100 (Kyolic), Wakunaga of America Co., Ltd., Mission Viejo, California 92691, USA were purchased from WebVitamins.com, an independent vendor. The bottles were emptied and repackaged into unlabelled bottles, each containing a 2-week supply: 48 Garlicin tablets (4 tablets/day, 6 days/week) or 72 Kyolic tablets (6 tablets/day, 6 days/week). Garlicin tablets (room temperature expiration date 1-2004) were stored at 4°C. Kyolic tablets (room temperature expiration date 7-2006) were stored at room temperature until October 2004 and thereafter, at 4°C.
The label on Garlicin claims that each tablet is enteric-coated, contains 350 mg of garlic powder and is standardized to release 3200 mcg of allicin under USP method 724A dissolution conditions.(14,15) The label on Kyolic claims that each tablet contains 300 mg of aged garlic extract powder. Kyolic is standardized on unspecified amounts of S-allylcysteine;(18) however, no standardization claim is on the package label. The tablet weights were determined to be 684±2 mg and 500.5±0.8 mg for Garlicin and Kyolic, respectively.
Placebo
The placebo tablets were made by Nature’s Way Products, Inc. to match the Garlicin tablets. The tablets had the same appearance and contained the same coating and other contents as Garlicin, except that the garlic powder was replaced by cellulose, the main excipient in Garlicin.
Standards
Alliin, L(+)-S-allylcysteine sulfoxide, was purchased from Extrasynthese (Lyon-Nord, France). S-allylcysteine, diallyl disulfide, and diallyl trisulfide were purchased from LKT Laboratories, Inc. (St. Paul, MN). Allyl methyl sulfide and diallyl sulfide were purchased from Aldrich (Milwaukee, WI). Arginine and γ-glutamylphenylalanine were purchased from Sigma (St. Louis, MO). γ-Glutamyl-S-allylcysteine was purchased from U.S. Pharmacopeia (Rockville, MD). γ-Glutamyl-S-trans-1-propenylcysteine and S-trans-1-propenylcysteine were identified as previously described.(19) Allicin was prepared by oxidation of diallyl disulfide with hydrogen peroxide, as previously described.(13).
Allyl thiosulfinates
Blended fresh garlic (thiosulfinates already maximally present) was extracted for 15 s with water at 20 mL/g blendate (20.8 mL total volume/g blendate). Garlicin and Kyolic tablets were pulverized in an electric coffee bean grinder and extracted for 5 min with water at 50 mL/g and 10 mL/g, respectively. Prior to HPLC analysis, protein was precipitated by addition of one volume of acetonitrile, followed by high speed centrifugation (microfuge), giving final dilutions of 41.6 mL/g blendate and 100 mL/g tablet. Thiosulfinates were analyzed by C18 HPLC at 240 nm, upon elution with acetonitrile/water (45/55) at 1 mL/min, using a cooled (4°C) autosampler, similar to that which was previously described.(20) Pure allicin (0.100 mg/mL water) was used as the standard for quantitation of all thiosulfinates. Quantitation of the other allyl thiosulfinates was based on dividing by the relative response factors, which were calculated from published extinction coefficients and relative abundance of the regioisomers.(20) For the allyl methyl thiosulfinates, the response factor was found to be essentially the same as for allicin. Trans-1-propenyl allyl thiosulfinate has a substantially higher extinction coefficient (4040 M−1 in HPLC eluant) than allicin (2395 M−1), while that of the less abundant allyl trans-1-propenyl isomer is assumed to be the same as allicin.(20) Their relative abundance was determined by HPLC upon elution with methanol/water (50/50) because of better isomer separation than with acetonitrile-water. The trans-1-propenylallyl/allyl trans-1-propenyl ratio was found to be >50 for blended garlic and 7.7 for Garlicin. It is higher for blended garlic than Garlicin because the thiosulfinates were formed long before analysis, upon blending, and because allyl trans-1-propenyl thiosulfinate is much less stable than its isomer. Hence, the relative response factors for the total allyl trans-1-propenyl thiosulfinates was 1.69 (4040/2395) for blended fresh garlic and 1.61 (1.69 × 7.7/8.7 + 1/8.7) for Garlicin.
Alliin and Arginine
Alliin and arginine (a stable non-sulfur marker and most abundant free amino acid in garlic) were analyzed by C18 HPLC analysis at 337 nm after derivatization with o-phthaldialdehyde and tert-buthyl thiol, according to the method of Ziegler and Sticher (1989).(21) Garlicin and Kyolic were extracted with 10 mM carboxymethoxylamine (alliinase inhibitor) at 40 mL/g pulverized tablet, while blended garlic was extracted at 20 mL/g blendate. This method widely separates the natural L(+)-isomer from the L(−)-isomer that forms to a modest extent (7–15% of total) during garlic powder manufacture. The L(−)-isomer is a less effective, but sufficiently effective, substrate for alliinase-generation of allicin. Identity of the alliin peak in Kyolic was verified by disappearance of the peak upon addition of crude alliinase, prepared as previously described.(2) This was necessary due to the lack of active alliinase in this product.
Cysteines, γ-Glutamylcysteines, and γGlutamylphenylalanine
These compounds were analyzed by C18 HPLC of the aqueous extracts of ground tablets (30 mL/g, vortexed 10 sec, rotated 20 min) and blended garlic (10 mL/g), upon elution with 0.05 M KH2PO4/MeOH (97/3) at 220 nm, as previously described.(19) γ-Glutamyl-S-trans-1-propenylcysteine, γ-glutamyl-S-cis-1-propenylcysteine, and S-trans-1-propenylcysteine were quantified based on the relative extinction coefficients of 2.1, 3.2, and 3.0, respectively, compared to those of the appropriate S-allyl standards.(19) γ-Glutamylphenylalanine was identical with peak U of a previous report.(19) The S-allylcysteine content was verified by an additional HPLC method that gave improved resolution for the Kyolic samples: H2O/acetonitrile/trifluoroacetic acid (95/5/0.1) for 7 min, followed by a 10 min wash with H2O/acetonitrile/trifluoroacetic acid (88/12/0.1).
Allyl sulfides
Diallyl trisulfide, diallyl disulfide, diallyl sulfide, allyl methyl trisulfide, and allyl methyl disulfide are the main transformation products of allicin and the allyl methyl thiosulfinates (Figure 1).(22,23) For blended garlic, extracted with 10 volumes of 50% acetonitrile/water, these compounds were analyzed by C18 HPLC at 240 nm, upon elution with 72% methanol/water at 1.6 mL/min, similar to previous work.(23) Due to much lower abundance and interference by non-sulfur compounds, the allyl sulfides of Garlicin and Kyolic were analyzed by GC, using a sulfur-selective FPD detector (Agilent Technologies, Wilmington, DE). The separation was performed with an HP-1 (cross-linked methyl siloxane) capillary column, 30 m × 0.25 mm × 0.25 µm film, at a flow rate of 5 mL/min (nitrogen) and a split ratio of 10:1. The oven was operated at 70°C for 3 min, then elevated to 140°C at 3°/min. The detector was operated at 250°C. Ground Garlicin tablets were suspended at 10 mL/g in 10 mM carboxymethoxylamine (alliinase inhibitor), followed by extraction with one volume of dichloromethane. Alliinase inhibitor was used to prevent allicin formation, because allicin rapidly breaks down to a variety of sulfides in a heated GC.(24,25) Ground Kyolic tablets were suspended at 10 mL/g in water (due to absence of allicin), followed by extraction with one volume of dichloromethane. Standards for allyl methyl di- and trisulfides were not available, but they were identified based on a logarithmic plot of absolute retention time vs. number of sulfur atoms and allyl methyl sulfide standard (Aldrich, Milwaukee, WI).(23) They were quantified based on relative extinction coefficients compared to diallyl disulfide.(23)
Dissolution allicin release
The formation and release of allicin and other allyl thiosulfinates from the Garlicin tablets under simulated gastrointestinal conditions was determined according to the USP-NF dissolution method for delayed-release garlic tablets and as previously described.(13,15) Using a model VK 700 dissolution apparatus (VanKel Technology Group, Cary, NC) equilibrated at 37°C, one tablet was placed into each of six covered 1-L round bottom glass vessels containing 750 mL of 0.1N HCl and paddle-stirred at 100 rpm for 2 h, after which 250 mL of 0.2 M Na3PO4 was added and the pH slightly adjusted if necessary, giving 1000 mL at pH 6.80 ± 0.05. After stirring for an additional 60 min, 1 mL of medium was added to 0.05 mL of 210 mM (final 10 mM) carboxymethoxylamine (Sigma, St. Louis MO) alliinase inhibitor, followed by HPLC analysis of allyl thiosulfinates. The time to achieve complete disintegration was determined by observation during the dissolution test.
Stability of allyl thiosulfinates in condiments
The stability of the thiosulfinates in a variety of condiment-blended garlic mixtures was determined at 4°C. Blended garlic (35g) was thoroughly mixed with the condiments at a ratio of 1 g blendate per 2.75 g of condiment. After mixing, portions were placed at 4°C and at −80°C (Day 0 control). At 24, 48, and 72 h, triplicate aliquots of each mixture placed at 4°C were weighed and placed at −80°C. After thawing, the mixtures were extracted with 25% acetonitrile/water at a ratio of 4 mL/g (shaken by hand for 30 sec, then rotated for 10 min), followed by addition of 1 volume of 75% acetonitrile/water (final, 50%) and HPLC analysis. The HPLC conditions used were the same as was described for the thiosulfinates, except that the column was eluted with a higher percent of acetonitrile (60%) to allow for analysis of the typical allicin transformation compounds (diallyl sulfides, ajoene, vinyl dithiins). Unmixed portions of the condiments were also analyzed to determine the possible presence of compounds from the condiments that would interfere with the allyl thiosulfinates.
Allyl thiosulfinate bioavailability
The bioavailability of the allyl thiosulfinates was determined by measuring the breath content of allyl methyl sulfide, the main metabolite of the allyl group of allyl thiosulfinates, over a 32-h period after consuming single doses of blended garlic or Garlicin tablets with a standard meal (tuna and mayonnaise sandwich, containing 25 g of pressed tuna and 13 g of protein), and measuring the area under the 32-h elimination curve (GraphPad Prism 3.0, San Diego, CA), similar to previous descriptions.(13,16)
Whole breath samples were collected in 1.2-L Tedlar bags (Alltech, Deerfield, IL) every hour for the first 8 h, then every 2 h, except during sleep. Participants were restricted from consuming significant amounts of garlic for 24 h prior to a test. Onion (raw and cooked) and mustard were also restricted, due to the presence of compounds that eluted closely to and interfered with the analysis of allyl methyl sulfide. Tests were conducted at least 3 days apart. Each of the four participants was tested two times with blended garlic and three times with Garlicin. Breath samples (5 mL) were injected once directly into a gas chromatograph fitted with a model 5380 sulfur-selective pulsed flame photometric detector (OI Analytical, College Station, TX) and a 30 m × 0.32 mm × 4 µm SPB-1 Sulfur (bonded polydimethylpolysiloxane) capillary column (Supelco, Bellefonte, PA). Helium was the carrier gas (1.6 mL/min) and air the make-up gas. The column temperature was programmed from 45°C (0.2 min) to 200 °C (1.2 min) at 50°/min, giving a retention time of 3.8 min and a run time of 12 min. The injection port was operated at 175°C in the splitless mode, with a purge flow of 45 mL/min for 0.8 min. The detector was operated at 250°C. The peak area is the square root of the detector response. This sulfur-selective detector gave at least 15 times greater sensitivity (6 ng/L or 2 ppb at s/n =2, giving a minimum AUC of 15 ng•h/L) than the FID detector used in prior allicin bioavailability studies, which made it possible to measure the AUC after consuming the smaller amounts of allyl thiosulfinates present in this study than the 3-fold larger amounts consumed in the prior studies.(13,16)
The allyl methyl sulfide (98% pure) vapor standard (266 ng/L) was prepared by adding 13.0 µL of a solution of 88 µg allyl methyl sulfide/mL in methanol in triplicate to 4.3-L glass jugs of nitrogen with taped lid holes, allowing 1.5 h for complete vaporization. The concentration in the jugs remained stable for 18–36 h. Dilutions of this standard gave a linear response down to 20 ng/L. The concentrations for breaths not analyzed within 5 h (the evening breaths) were corrected for predetermined bag-specific losses of allyl methyl sulfide (0.06 – 0.5% loss/h).
Statistical analysis
Statistical analyses were conducted using Microsoft Excel software. Data were examined for homogeneity among variances. Differences between groups were analyzed by Student’s t-test (2-tail). P-values <0.05 were considered to be significant. Data are presented as means ± SD.
RESULTS AND DISCUSSION
Composition of the garlic products used in the clinical trial
The content of sulfur compounds and selected non-sulfur compounds in the garlic products consumed during the clinical trial, at the daily dose, are given in Table 1. The daily dose values can be converted to mg/g values by multiplying by the following conversion factors: blended garlic × 0.2465 (mg/g fresh wt) or × 0.6521 (mg/g dry wt), Garlicin × 0.3655, Kyolic × 0.333. The daily doses contained 1535, 1400, and 1800 mg of garlic material (dry wt) for blended garlic, Garlicin, and Kyolic, respectively. Structures of the compounds are given in Figure 1. For Garlicin and Kyolic tablets, the thiosulfinate values represent the thiosulfinate potential, which is the yield of thiosulfinates found after pulverized tablets are mixed with water, which activates active alliinase, for sufficient time to achieve maximum thiosulfinate formation.(13) Alliin was undetectable in the blended garlic, demonstrating that the blending procedure allowed for complete formation of allyl thiosulfinates.
Table 1.
Analysis of the Three Garlic Products Consumed (Amount per Daily Dose)
| Compound | Blended fresh garlica (mg/4.06 g garlic = mg/1.54 g dgmb) n=8-16c |
Garlicina (mg/4 tablets = mg/1.40 g dgm) n=6c |
Kyolic 100a (mg/6 tablets = mg/1.80 g dgm) n=6c |
|---|---|---|---|
| Sulfur compounds – derived from alliin | |||
| Alliin | ndd (<0.06) | 36.4 ± 2.3e | 0.102 ± 0.021e |
| Allicin (diallyl thiosulfinate) | 12.6 ± 0.45 | 15.3 ± 0.45 | nd (<0.001) |
| Allyl methyl thiosulfinates | 4.88 ± 0.21 | 2.95 ± 0.09 | nd (<0.001) |
| Allyl trans-1-propenyl thiosulfinates | 1.25 ± 0.09 | 0.68 ± 0.03 | nd (<0.001) |
| Total allyl thiosulfinates | 18.7 ± 0.65 | 18.9 ± 0.56f | --- |
| Total allyl thiosulfinates (µmol allyl) | 198 ± 7.5 | 214 ± 6.0 | --- |
| Allyl sulfidesg | 0.63 ± 0.07 | 0.061 ± 0.004 | nd (<0.018) |
| Sulfur compounds – not derived from alliin | |||
| γ-Glutamyl-S-allylcysteine | 20.5 ± 1.2 | 8.44 ± 0.68 | 2.39 ± 0.03 |
| γ-Glutamyl-S-trans-1-propenylcysteine | 14.7 ± 0.6 | 7.86 ± 0.64 | 0.87 ± 0.02 |
| γ-Glutamyl-S-cis-1-propenylcysteine | 0.24 ± 0.01 | 1.11 ± 0.09 | 0.23 ± 0.01f |
| S-Allylcysteine | 0.25 ± 0.01 | 1.06 ± 0.08 | 1.81 ± 0.04 |
| S-Trans-1-propenylcysteine | nd (<0.08) | 0.12 ± 0.01 | 0.76 ± 0.01 |
| Non-sulfur compounds | |||
| γ-Glutamylphenylalanine | 4.56 ± 0.33 | 3.72 ± 0.28 | 0.71 ± 0.02 |
| Arginine | 18.8 ± 1.7 | 36.5 ± 1.7 | 3.90 ± 0.09 |
The average daily dose of blended garlic consumed was 5.68 g (4.06 g fresh garlic). Peeled fresh cloves contained 37.8 ± 0.2% dry matter. Garlicin tablets weighed 0.684 ± 0.002 g and contained 0.350 g (label claim) of whole garlic powder. Kyolic tablets weighed 0.5005 ± 0.0008 g and contained 0.300 g (label claim) of dry aged garlic extract.
dgm = dry garlic matter, based on the dry weight of the fresh garlic and the label claims for Garlicin and Kyolic.
Assay replicates. For blended fresh garlic, n= 16 for the thiosulfinates or n=8 for all other compounds. Four aliquots were randomly removed from each of four 19-liter pails, in which the blended garlic was originally prepared and temporarily stored. For Garlicin and Kyolic tablets, the n-value represents separate grindings of 15 tablets from six randomly selected bottles of the same batch number.
nd = not detected, followed by limit of detection
Alliinase inhibited with 10 mM carboxymethoxylamine.
Not significantly different from blended fresh garlic. All other pair-wise differences between the three garlic products were significant (P < 0.01).
For blended garlic the individual sulfide values (µg/dose) were diallyl disulfide 360, diallyl trisulfide 220, and allyl methyl trisulfide 50, while diallyl sulfide and allyl methyl disulfide were undetectable (<15). For Garlicin the values (µg/dose) were diallyl disulfide 20, diallyl trisulfide 20, diallyl sulfide 11, allyl methyl trisulfide 5.5, and allyl methyl disulfide 4.9.
The qualitative differences between fresh garlic and Kyolic aged extract mainly represent differences caused by the aging and extraction procedures.(10,26) The qualitative compositional differences between fresh garlic and Garlicin represent differences caused by natural variation.(20,26) For the purposes of the clinical trial, the content of total allyl thiosulfinates for blended fresh garlic and the yield of total allyl thiosulfinates from Garlicin were intentionally designed to be similar. The higher amount of allyl sulfides found in blended garlic represents partial transformation of the moderately unstable allyl thiosulfinates during the lengthy parceling procedure. Garlicin alliin consists of both the L(+)-isomer and the L(−)-isomer, at a ratio of 5.6:1, which is typical for garlic powders. Both isomers are rapidly transformed to allyl thiosulfinates by garlic alliinase;(27) however, the L(−)-isomer is absent in unprocessed garlic.(6) Free arginine and γ-glutamylphenylalanine were selected to represent non-sulfur compounds because of their uniquely high abundance in garlic and because of the known stability of arginine when garlic is aged.(10,26,28)
To determine if the composition of the garlic products used in the clinical trial falls within the range of typical samples, a comparison for the main allyl sulfur compounds was made to published values for fresh garlic and to 3–4 other sample lots for Garlicin and Kyolic (Table 2). All study compounds fell within 1.3 S.D. units of the composition of other samples, indicating that the garlic products used in the trial are typical.
Table 2.
Comparison of Study Samples to Other Samples for the Main Allyl Sulfur Compounds
| Product/compound | Study sample | Other samples (mean ± SD, range)a |
|---|---|---|
| (mg/g fresh wt or mg/g tablet) | ||
| Blended fresh garlic | ||
| Allicin | 3.1 | 4.4 ± 1.3 (n=21), 2.3 – 6.6 |
| Allyl methyl thiosulfinates | 1.20 | 1.0 ± 0.5 (n=21), 0.4 – 2.1 |
| γ-Glutamyl-S-allylcysteine | 5.1 | 3.8 ± 1.7 (n = 27), 0.9 – 6.8 |
| Garlicin | ||
| Allicin | 5.6 | 5.2 ± 0.3 (n=3), 5.0 – 5.5 |
| Allyl methyl thiosulfinates | 1.1 | 1.2 ± 0.1 (n=3), 1.2 – 1.3 |
| γ-Glutamyl-S-allylcysteine | 3.1 | 3.3 ± 1.2 (n=3), 2.6 – 4.7 |
| Dissolution allicin release | >95% | all >95% (n=3) |
| Kyolic | ||
| S-allylcysteine | 0.60 | 0.60 ± 0.11 (n=4), 0.50 – 0.75 |
| γ-Glutamyl-S-allylcysteine | 0.80 | 0.82 ± 1.0 (n= 4), 0.16 – 2.3 |
Values of other fresh garlic samples were previously published for thiosulfinates and γ-glutamyl-S-allylcysteine.(20,26) Values of other samples of Garlicin and Kyolic were determined in June 2002 for additional lots purchased at local stores, with expiration dates ranging from January 2004 to May 2004 for Garlicin and from December 2004 to March 2006 for Kyolic.
Stability of allyl thiosulfinates in condiments (4°C)
The consumption of blended fresh garlic was made palatable by mixing it with a condiment and placing the mixture in a sandwich. However, because the stability of allyl thiosulfinates is known to be dependent upon their environment, especially in the presence of oils (triglycerides) or cysteine (protein), their stability in a variety of possible mixtures of condiments with the blended garlic was determined for up to 3 days at 4°C, the same temperature and maximum time at which the study sandwiches are stored between preparation and consumption, and at the same garlic/condiment ratio used in the sandwiches. To reduce the possible instability of the thiosulfinates, only fat free or low fat condiments were tested. Of the 11 condiments tested, the thiosulfinates were shown to be adequately stable (≤10% loss in 3 days) in five (the upper half of Table 3). These five condiments were used to prepare the study sandwiches.
Table 3.
Stability of Allyl Thiosulfinates in Blended Garlic Mixed with Condimentsa
| Condiment | Allicin | Allyl methyl thiosulfinates | Allyl trans-1-propenyl thiosulfinates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days at 4°C: | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 |
| Mayonnaise, fat free | 101 | 100 | 98 | 95 | 100 | 101 | 100 | 99 | 100 | 99 | 97 | 96 |
| Yogurt, strained, fat free | 96 | 93 | 92 | 91 | 97 | 94 | 90 | 93 | 99 | 101 | 98 | 96 |
| Creamy horseradishb | 98 | 97 | 94 | 93 | 101 | 103 | 102 | 101 | 101 | 102 | 100 | 99 |
| Sour cream, fat free | 100 | 101 | 99 | 99 | 97 | 101 | 100 | 101 | 100 | 105 | 109 | 111 |
| LaVictoria salsa | 97 | 98 | 97 | 98 | 100 | 100 | 98 | 99 | 102 | 102 | 102 | 101 |
| Salsa verdec | 71 | 1 | <1 | <1 | 86 | <1 | <1 | <1 | 83 | 20 | 19 | 18 |
| Honey mustard | 91 | 81 | 76 | 71 | 89 | 82 | 80 | 82 | 100 | 93 | 91 | 96 |
| Dijon mustard + mayonnaise, fat free (1:3) | 70 | 64 | 58 | 53 | 77 | 75 | 73 | 71 | 66 | 62 | 58 | 57 |
| Cucumber, diced | 86 | 80 | 78 | 78 | 87 | 83 | 82 | 82 | 80 | 74 | 72 | 70 |
| Mango chutney | 29 | 36 | 30 | 33 | 45 | 54 | 46 | 51 | 26 | 33 | 27 | 30 |
Values are means for 3 determinations, given as percent of the value found at day 0 for blended garlic in the absence of condiment. The condiments in the bottom half of the table were not used in the trial.
Non-commercial preparation, prepared by mixing fat-free mayonnaise (12 vol), diced dill pickle (3 vol), horseradish (1 vol), and ketchup (1 vol).
Salsa verde is made from green tomatoes.
The importance of conducting this stability test is highlighted by the instability of the thiosulfinates in the remainder of the condiments. Except for salsa verde, there tended to be an immediate partial loss of the thiosulfinates at the Day 0 time point (the mixtures were at room temperature for about an hour before being placed at −80°C), followed by fairly level values throughout the following three days. This indicates that something in the condiments reacted rapidly with the thiosulfinates until the reactant was consumed, a reaction that is typical for cysteine or other thiol-containing compounds. The most dramatic loss of thiosulfinates occurred with salsa verde, a green tomato product. Uniquely, the loss of allyl thiosulfinates in salsa verde was accompanied by a nearly quantitative increase in diallyl disulfide. It is well known that alkaline medium (≥pH 10) causes rapid transformation of allicin to diallyl disulfide,(29,30) but the pH of salsa verde was 4.1, leaving the reason for diallyl disulfide formation unknown. Neither diallyl disulfide, nor any other known transformation compound of allicin, were found in the other condiment-garlic mixtures, but for honey mustard and cucumber there were substantial increases in compounds of unknown identity that were less polar than diallyl trisulfide. Significant interference with the thiosulfinates by compounds present in the unmixed condiments was found only in the honey mustard, but this interference was eliminated by decreasing the amount of acetonitrile in the HPLC eluant. Detectable amounts of the allyl thiosulfinates were not found in any of the 11 condiments prior to mixing. A test of the stability of the thiosulfinates of crushed garlic at 4°C in the absence of condiment revealed no significant decrease in any of the thiosulfinates at 12 days (not shown).
Long-term stability of the garlic products
Because of the length of the clinical trial (3 years between the start of the first group of participants to the end of the 6-month protocol for the last group) and the known instability of allyl thiosulfinates,(26,31) the allyl thiosulfinate content of the frozen blended fresh garlic was measured periodically over two years (Table 4). No loss of any thiosulfinate was found between 3 and 24 months, demonstrating that they were adequately stabilized upon storage at −80 °C. Hence, little loss would be expected by 3 years, thus validating the storage conditions for the entire length of the clinical trial. The pre-start values were measured immediately before the several-day long parceling period, during which period the blended garlic was at refrigeration and room temperatures for various lengths of time prior to cryofreezer storage, which resulted in a 9% loss of thiosulfinates. The ability of Garlicin tablets to form allicin and total allyl thiosulfinates also did not change over the two-year period, indicating that alliin was stable at 4°C and that alliinase activity remained high. The content of S-allylcysteine in the Kyolic tablets was found to be stable at room temperature for 12 mo but to decline significantly by 12% in 24 mo; however, the content of γ-glutamyl-S-allylcysteine was stable for 24 mo (Table 5). This product was stored at room temperature because pure S-allylcysteine was previously reported to be stable for at least 4 years at 25°C. (32) It appears that other compounds present in the tablets decrease the stability of S-allylcysteine. Starting at 25 months, the Kyolic tablets were stored at 4°C to improve stability.
Table 4.
Stability of the Thiosulfinate Content (Blended Garlic) or Thiosulfinate Potential (Garlicin)
| Blended fresh garlic, stored at −80°C (mg/4.06 g garlic) (n=16) | Garlicin, stored at 4°C (mg/4 tablets) (n=6) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre-starta | 3 mo | 12 mo | 18 mo | 24 mo | Start | 12 mo | 24 mo | |
| Allicin | 13.5 ± 0.27b | 12.6 ± 0.45 | 12.5 ± 0.12 | 12.6 ± 0.26 | 12.7 ± 0.29 | 15.3 ± 0.45 | 15.4 ± 0.13 | 15.4 ± 0.18 |
| Allyl methyl thiosulfinates | 5.30 ± 0.14b | 4.88 ± 0.21 | 4.90 ± 0.07 | 4.97 ± 0.33 | 4.88 ± 0.13 | 2.95 ± 0.09 | 3.14 ± 0.06b | 3.08 ± 0.02b |
| Allyl trans-1-propenyl thiosulfinates | 1.83 ± 0.13b | 1.25 ± 0.09 | 1.16 ± 0.07 | 1.17 ± 0.10 | 1.14 ± 0.06 | 0.68 ± 0.03 | 0.59 ± 0.05b | 0.61 ± 0.03b |
| Total allyl thiosulfinates | 20.6 ± 0.44b | 18.7 ± 0.65 | 18.5 ± 0.30 | 18.7 ± 0.28 | 18.7 ± 0.38 | 18.9 ± 0.56 | 19.1 ± 0.12 | 19.2 ± 0.27 |
| Total allyl thiosulfinates (µmol allyl) | 216 ± 4.0b | 198 ± 7.5 | 197 ± 3.6 | 199 ± 6.2 | 199 ± 4.0 | 214 ± 6.0 | 217 ± 2.4 | 217 ± 3.0 |
The pre-start value was measured immediately before the several-day long parceling period.
Significantly different (P<0.05) from the 3-month value (blended garlic) or the start value (Garlicin).
Table 5.
Stability of Allyl Compounds in Kyolic Tablets Stored at Room Temperature
| mg/6 tablets (n=6) | |||
|---|---|---|---|
| Start | 12 mo | 24 mo | |
| S-Allylcysteine | 1.81 ± 0.04 | 1.76 ± 0.13 | 1.59 ± 0.06a |
| γ-Glutamyl-S-allylcysteine | 2.39 ± 0.03 | 2.43 ± 0.09 | 2.37 ± 0.11 |
Significantly different (P<0.01) from the start value.
Dissolution release of allyl thiosulfinates from Garlicin
Simulated gastrointestinal conditions for release of allicin from garlic powder tablets have been defined in the U.S. Pharmacopeia/National Formulary(14,15) and provide a rapid in vitro method to estimate formation of thiosulfinates from garlic tablets in the body. Under these dissolution conditions, the Garlicin tablets were found to release, in 51–52 min, essentially all (>95%) of the allicin and allyl trans-1-propenyl thiosulfinates that they are capable of producing (Table 6), and this ability did not decrease when the tablets were stored at 4°C for two years. The formation of allyl methyl thiosulfinates, however, was only about 70% complete, but because the allyl methyl thiosulfinates represent only 16% of the total allyl thiosulfinates of Garlicin, the total amount of allyl thiosulfinates released was still over 90%. When the dissolution tests were extended another 20 min in buffer (not shown), the release of allyl methyl thiosulfinates reached 85%. Formation of allyl methyl thiosulfinates is known to be significantly slower and more sensitive to alliinase inhibition than formation of allicin.(9)
Table 6.
Dissolution Release of Allicin and Other Allyl Thiosulfinates from Garlicin Tablets Stored at 4°C
| Start | 12 mo | 24 mo | |
|---|---|---|---|
| (% of potential)a | |||
| Allicin | >95% | >95% | > 95% |
| Allyl methyl thiosulfinates | 73% | 69% | 71% |
| Allyl trans-1-propenyl thiosulfinates | >95% | >95% | >95% |
| Total allyl thiosulfinates | >92% | >91% | >91% |
| Disintegration time (buffer) | 51 min | 52 min | 52 min |
Values indicate the average % of potential reached at 1 h in the buffer stage for six tablets tested individually. The USP/NF 2003 protocol for allicin release from enteric-coated garlic tablets requires that >80% of the allicin potential be released after 2 h in acid and 1 h in buffer.(15)
Bioavailability of allyl thiosulfinates from Garlicin
The ability of Garlicin tablets, which contain alliin and alliinase but no thiosulfinates, to form maximum possible amounts of allyl thiosulfinates in the gastrointestinal tract was determined by comparing the bioavailability of allyl thiosulfinates from Garlicin to the bioavailability of allyl thiosulfinates from blended fresh garlic, which contains preformed allyl thiosulfinates but no alliin. Both were consumed at similar amounts of allyl thiosulfinate content (fresh garlic) or allyl thiosulfinate potential (Garlicin). The same amounts of the same batches of the products used for the clinical trial were also used in the bioavailability tests. Breath allyl methyl sulfide content is the only established method for determining the bioavailability of allicin or allyl thiosulfinates, as neither allicin nor its known metabolites have yet been found in blood or urine.(10, 16) Although breath allyl methyl sulfide can also be produced by consumption of S-allylmercaptocysteine, ajoene, allyl mercaptan, and the allyl sulfides (Table 1),(16) only the allyl sulfides are present in the fresh garlic and Garlicin, and their abundance accounts for only 4% and 0.3%, respectively, of the allyl methyl sulfide that can be produced from the allyl thiosulfinates. AUC32 plots for exhalation of allyl methyl sulfide revealed no difference between Garlicin and blended fresh garlic (Table 7), demonstrating that the batch of Garlicin tablets being used in the clinical trial is as effective as blended fresh garlic in delivering allyl thiosulfinates to the body. Although Garlicin tablets have previously been shown to provide high allicin bioavailability when consumed at a 3-fold higher dose,(13) it was considered important to verify that the particular batch of Garlicin being used in the mentioned clinical trial also gives high bioavailability when consumed at the trial dose. Significant variation in allicin release among different batches of the same brand of garlic powder tablets is known to occur.(2)
Table 7.
Effects of Consuming Blended Garlic and Garlicin on Breath Allyl Methyl Sulfide, the Main Metabolite of Allicina
| Blended fresh garlic (4.06 g garlic) |
Garlicin (4 tablets) |
|
|---|---|---|
| allyl thiosulfinates (µmol allyl) | 198 (content) | 214 (potential) |
| AUC32h (ng•h/L) | 1480 ± 400 | 1440 ± 450 |
| Cmax (ng/L) | 174 ± 35 | 207 ± 47 |
| Tmax (h) | 2.9 ± 0.3 | 6.0 ± 1.4b |
| % of Cmax at 32h | 0.2 ± 1.8 | 0.6 ± 0.8 |
Abbreviations: AUC32 = area under the 32-hour curve; Cmax = maximum concentration reached; Tmax = time after consumption to reach maximum concentration.
Values are means ± standard deviation for the same four persons.
Different from blended fresh garlic: P <0.05. No other differences are significant (P > 0.3).
The maximum breath concentrations of allyl methyl sulfide (Cmax) were also not significantly different between the two garlic products. However, the time to reach the maximum breath concentrations (Tmax) was significantly greater by 3.1 h for Garlicin, reflecting the amount of time required for these enteric-coated tablets to disintegrate. The concentration of allyl methyl sulfide in the breath at 32 h after consumption of the garlic products was only 0.2–0.6% of the maximum concentrations, indicating that 32 h was a sufficient amount of time to obtain a reliable AUC. A slight, but insignificant, trend (P = 0.25) toward higher within-person variation (not shown) for the AUC32 was found for Garlicin (CV% = 20.9 ± 12.2) than for fresh garlic (12.3 ± 5.5). Hence, Garlicin was tested three times on each person rather than two times. However, the third set of tests for Garlicin changed the mean AUC32 by only 0.6% and decreased the standard deviation by only 18%. Greater within-person variation would be expected for alliinase-dependent Garlicin, because the activity of alliinase is subject to conditions in the intestinal tract that are variable, such as the presence of other food components and concentrations of proteolytic enzymes.
As a control to determine if consumption of alliinase-inactivated Garlicin would also result in production of allyl methyl sulfide, four pulverized tablets were suspended in 0.5N HCl to inactivate alliinase, followed by pH neutralization with NaOH. Only trace amounts of allicin were produced (0.03% of the value found when pulverized tablets were suspended in water, indicating little alliin loss). After consumption of the suspension (one person), a small amount of allyl methyl sulfide was found in the breath, giving an AUC32 that accounted for 3.2% of the value found when undisturbed tablets were consumed. The production of small amounts of allyl methyl sulfide after consuming acid-inactivated Garlicin indicates that there is some alliinase activity in the body. Indeed, Japanese researchers showed in the 1960s that B. subtilis and E. coli, common microbes of the intestinal tract, possess alliinase activity.(33,34) The failure of a previous study to find allyl methyl sulfide in the breath after consuming garlic that had been inactivated by microwave cooking probably reflects the lower sensitivity of the FID detector used in that study.(13,16)
Acknowledgments
SUPPORT. This work was supported by a grant from the National Institutes of Health/ National Center for Complementary and Alternative Medicine.
LITERATURE CITED
- 1.Ackermann R, Mulrow C, Ramirez G, Gardner C, Morbidoni L, Lawrence V. Garlic shows promise for improving some cardiovascular risk factors. Arch.Intern.Med. 2001;161:813–824. doi: 10.1001/archinte.161.6.813. [DOI] [PubMed] [Google Scholar]
- 2.Lawson LD, Wang ZJ, Papadimitriou D. Allicin release under simulated gastrointestinal conditions from garlic powder tablets employed in clinical trials on serum cholesterol. Planta Med. 2001;67:13–18. doi: 10.1055/s-2001-10624. [DOI] [PubMed] [Google Scholar]
- 3.Neil HAW, Silagy CA, Lancaster T, Hodgeman J, Vos K, Moore JW, Jones L, Cahill J, Fowler G. Garlic powder in the treatment of moderate hyperlipidaemia: a controlled trial and meta-analysis. J.Roy.Coll.Physicians London. 1996;30:329–334. [PMC free article] [PubMed] [Google Scholar]
- 4.Stevinson C, Pittler MH, Ernst E. Garlic for treating hypercholesterolemia: a meta-analysis of randomized clinical trials. Ann.Intern.Med. 2000;133:420–429. doi: 10.7326/0003-4819-133-6-200009190-00009. [DOI] [PubMed] [Google Scholar]
- 5.Alder R, Lookinland S, Berry JA, Williams M. A systematic review of the effectiveness of garlic as an anti-hyperlipidemic agent. J.Am.Acad.Nurse Practitioners. 2003;15:120–129. doi: 10.1111/j.1745-7599.2003.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 6.Stoll A, Seebeck E. Chemical investigations on alliin, the specific principle of garlic. Adv.Enzymol. 1951;11:377–400. doi: 10.1002/9780470122563.ch8. [DOI] [PubMed] [Google Scholar]
- 7.Jansen H, Müller B, Knobloch K. Characterization of an alliin lyase preparation from garlic (Allium sativum) Planta Med. 1989;55:434–439. doi: 10.1055/s-2006-962059. [DOI] [PubMed] [Google Scholar]
- 8.Blania G, Spangenberg B. Formation of allicin from dried garlic (Allium sativum): A simple HPTLC method for simultaneous determination of allicin and ajoene in dried garlic and garlic preparations. Planta Med. 1991;57:371–375. doi: 10.1055/s-2006-960120. [DOI] [PubMed] [Google Scholar]
- 9.Lawson LD, Hughes BG. Characterization of the formation of allicin and other thiosulfinates from garlic. Planta Med. 1992;58:345–350. doi: 10.1055/s-2006-961482. [DOI] [PubMed] [Google Scholar]
- 10.Lawson LD. Garlic: a review of its medicinal effects and indicated active compounds. In: Lawson LD, Bauer R, editors. Phytomedicines of Europe: chemistry and biological activity. Washington, DC: American Chemical Society; 1998. pp. 176–209. [Google Scholar]
- 11.Cavallito CJ, Bailey JH. Allicin, the antibacterial principle of Allium sativum. I. Isolation, physical properties and antibacterial action. J.Am.Chem.Soc. 1944;66:1950–1951. doi: 10.1021/ja01207a039. [DOI] [PubMed] [Google Scholar]
- 12.Cavallito CJ, Bailey JH, Buck JS. The antibacterial principle of Allium sativum. III. Its precursor and "essential oil of garlic". J.Am.Chem.Soc. 1945;67:1032–1033. [Google Scholar]
- 13.Lawson LD, Wang ZJ. Low allicin release from garlic supplements: a major problem due to the sensitivities of alliinase activity. J.Agric.Food Chem. 2001;49:2592–2599. doi: 10.1021/jf001287m. [DOI] [PubMed] [Google Scholar]
- 14.United States Pharmacopeial Convention. United States Pharmacopeia. Rockville, MD: United States Pharmacopeial Convention, Inc.; 2000. 724 Drug release; delayed-release (enteric-coated) articles - general drug release standard; p. 1947. [Google Scholar]
- 15.United States Pharmacopeial Convention. United States Pharmacopeia 26. Rockville, MD: United States Pharmacopeial Convention, Inc.; 2003. Garlic delayed-release tablets; pp. 2752–2754. [Google Scholar]
- 16.Lawson LD, Wang ZJ. Allicin and allicin-derived garlic compounds increase breath acetone through allyl methyl sulfide: use in measuring allicin bioavailability. J.Agric.Food Chem. 2005;53:1974–1983. doi: 10.1021/jf048323s. [DOI] [PubMed] [Google Scholar]
- 17.Kannar D, Wattanapenpaiboon N, Savige GS, Wahlqvist ML. Hypocholesterolemic effect of an enteric-coated garlic supplement. J.Am.Coll.Nutr. 2001;20:225–231. doi: 10.1080/07315724.2001.10719036. [DOI] [PubMed] [Google Scholar]
- 18.Wakunaga Kyolic Aged Garlic Extract Home Page. [accessed Oct 2004]. http://www.kyolic.com.
- 19.Lawson LD, Wang ZYJ, Hughes BG. Gamma-glutamyl-S-alkylcysteines in garlic and other Allium SPP: precursors of age-dependent trans-1-propenyl thiosulfinates. J.Nat.Prod. 1991;54:436–444. [Google Scholar]
- 20.Lawson LD, Wood SG, Hughes BG. HPLC analysis of allicin and other thiosulfinates in garlic clove homogenates. Planta Med. 1991;57:263–270. doi: 10.1055/s-2006-960087. [DOI] [PubMed] [Google Scholar]
- 21.Ziegler SJ, Sticher O. HPLC of S-alk(en)yl-L-cysteine derivatives in garlic including quantitative determination of (+)-S-allyl-L-cysteine sulfoxide (alliin) Planta Med. 1989;55:372–378. doi: 10.1055/s-2006-962031. [DOI] [PubMed] [Google Scholar]
- 22.Vernin G, Metzger J, Fraisse D, Scharff C. GC-MS (EI, PCI, NCI) computer analysis of volatile sulfur compounds in garlic essential oils. Application of the mass fragmentometry SIM technique. Planta Med. 1986;52:96–101. [Google Scholar]
- 23.Lawson LD, Wang ZJ, Hughes BG. Identification and HPLC quantitation of the sulfides and dialk(en)yl thiosulfinates in commercial garlic products. Planta Med. 1991;57:363–370. doi: 10.1055/s-2006-960119. [DOI] [PubMed] [Google Scholar]
- 24.Brodnitz MH, Pascale JV, Van Derslice L. Flavor components of garlic extract. J.Agric.Food Chem. 1971;19:273–275. [Google Scholar]
- 25.Block E, Putnam D, Zhao SH. Allium chemistry: GC-MS analysis of thiosulfinates and related compounds from onion, leek, scallion, shallot, chive, and Chinese chive. J.Agric.Food Chem. 1992;40:2431–2438. [Google Scholar]
- 26.Lawson LD. The composition and chemistry of garlic cloves and processed garlic. In: Koch HP, Lawson LD, editors. Garlic: the science and therapeutic application of Allium sativum L. and related species. Baltimore: Williams & Wilkins; 1996. pp. 37–108. [Google Scholar]
- 27.Rybak ME, Calvey EM, Harnly JM. Quantitative determination of allicin in garlic: supercritical fluid extraction and standard addition of alliin. J.Agric.Food Chem. 2004;52:682–687. doi: 10.1021/jf034853x. [DOI] [PubMed] [Google Scholar]
- 28.Ueda Y, Kawajiri H, Miyamura N, Miyajima R. Content of some sulfur-containing components and free amino acids in various strains of garlic. Nippon Shokuhin Kogyo Gakkaishi (J.Jpn.Soc.Food Sci.Technol.) 1991;38:429–434. [Google Scholar]
- 29.Kice JL, Rogers TE. Mechanisms of the alkaline hydrolysis of aryl thiolsulfinates and thiolsulfonates. J.Am.Chem.Soc. 1974;96:8009–8015. [Google Scholar]
- 30.Müller B. Analytische Bewertung von Knoblauchpräparaten. Dtsch.Apoth.Ztg. 1989;129:2500–2504. [Google Scholar]
- 31.Sreenivasamurthy V, Sreekantiah KR, Johar DS. Studies on the stability of allicin and alliin present in garlic. J.Sci.Ind.Res. 1961;20C:292–295. [PubMed] [Google Scholar]
- 32.Kodera Y, Suzuki A, Imada O, Kasuga S, Sumioka I, Kanezawa A, Taru N, Fujikawa M, Nagae S, Masamoto K, Maeshige K, Ono K. Physical, chemical, and biological properties of S-allylcysteine, an amino acid derived from garlic. J.Agric.Food Chem. 2002;50:622–632. doi: 10.1021/jf0106648. [DOI] [PubMed] [Google Scholar]
- 33.Saari JC, Schultze MO. Cleavage of S-(1,2-dichlorovinyl)-L-cysteine by Escherichia coli B. Arch.Biochem.Biophys. 1965;109:595–602. doi: 10.1016/0003-9861(65)90405-4. [DOI] [PubMed] [Google Scholar]
- 34.Murakami F. Studies on the nutritional value of Allium plants. XXXVII. Decomposition of alliin homologues by acetone-powdered enzyme preparation of Bacillus subtilis. Bitamin [Kyoto] 1960;20:131–135. [Google Scholar]