Abstract
Background & Aims
A prominent role for inhibitory molecules PD-L1 and PD-L2 in peripheral tolerance has been proposed. However, the phenotype and function of PD-L-expressing cells in human gut remains unclear. Recent studies suggest that intestinal myofibroblasts (CMFs) and fibroblasts are important in the switch from acute inflammation to adaptive immunity. In the normal human colon CMFs represent a distinct population of MHC class II+ cells involved in the regulation of mucosal CD4+ T cell responses.
Methods
PD-L1 and PD-L2 expression on human CMFs was determined using Western Blot, FACS analysis and confocal microscopy. Lymphoproliferation assays and cytokine ELISAs were used to evaluate the role of B7 co-stimulators expressed by CMFs with regard to the regulation of preactivated T helper cell responses.
Results
We demonstrate here the expression of PD-L1/2 molecules by normal human colonic myofibroblasts and fibroblasts in situ and in culture. Both molecules support suppressive functions of CMFs in the regulation of activated CD4+ T helper cell proliferative responses, since blocking this interaction reverses the suppressive effect of CMFs on T cell proliferation and leads to increased production of the major T cell growth factor, IL-2. PD-L1/2-mediated CMF suppressive functions are mainly due to the inhibition of IL-2 production, since supplementation of the co-culture media with exogenous IL-2 led to partial recovery of activated T cell proliferation.
Conclusions
Our data suggest that stromal myofibroblasts and fibroblasts may limit T helper cell proliferative activity in the gut and, thus, might play a prominent role in mucosal intestinal tolerance.
Keywords: mucosal tolerance, immunity, stromal cell, B7 co-stimulatory molecules
Introduction
The induction of tolerance to commensal bacteria and dietary antigens (Ag) is a critically important immunological process in the intestinal mucosa. Many mechanisms have been implicated that explain the defect in CD4+ T cell-dependent immune responses that occur during tolerance, including clonal anergy, clonal deletion, and active regulatory processes. 1 The CD4+ T cell response during tolerance induction is highly orchestrated by their interactions with MHC class II-expressing antigen presenting cells (APCs).2
In addition to a signal delivered following T cell receptor (TCR) engagement of peptide laden MHC class II on APCs, full responsiveness of T cells requires additional signals delivered by B7 family activating and/or inhibitory APC co-stimulatory molecules. The major role of the classic costimulators B7.1 (CD80) and B7.2 (CD86) on APCs, and their ligands CD28 or CTLA-4 on T cells is to positively or negatively regulate T cell responses, respectively, at an early stage of T cell activation.3–4 Recently, novel inhibitory B7 ligands PD-L1 (a.k.a. B7-H1, CD274) and PD-L2 (a.k.a. B7-DC, CD273) and their putative T cell counter receptor Program Death receptor 1, PD-1 (a.k.a. CD279), have been described and a prominent role for PD-L1 and PD-L2 molecules in peripheral tolerance has been proposed.3–6 It has been demonstrated that loss of the PD-L1/2 molecules leads to an increase in the expansion and peripheral homing of T cells.5 Functional studies indicated that PD-L1 and PD-L2 exert overlapping effects on T cell responses. The interaction of both PD-Ls with PD-1 inhibit activated CD4+ and CD8+ T cell proliferation by arresting the T cell cycle in G0/G1.3–4,6 Despite these overlapping effects, it has been suggested that PD-L1 and PD-L2 may have distinct functions in the regulation of Th1-Th2 cell responses.7 The phenotype of PD-L−/− mice suggests that PD-L1 in vivo has a critical negative regulatory role of polarization of Th1 responses, since loss of PD-L1 but not PD-L2 expression leads to the increase in IFN-γ producing CD4+ and CD8+ T cells.8 Moreover, an inadequate Th2 response associated with a reduced number of IL-4 producing cells has been observed in PD-L1−/− mice during Leishmania mexicana infection, while PD-L2−/− mice have much more L. mexicana –specific IgM and IgG2a production.9
The PD-1 receptor is expressed most highly by activated CD4+ and CD8+ effector T cells.3–4 PD-1 ligands have a distinct and different expression pattern, PD-L1 expression appears to occur on hematopoetic and parenchymal cells.4–5,10 PD-L2 expression is mostly restricted to dendritic cells (DCs) and macrophages4–6. The expression of PD-L1 on non-hematopoetic parenchymal cells is particularly intriguing because it suggests that PD-L1 may regulate foreign and self Ag-specific reactive T cell responses in peripheral organs and/or control the extent of pathogenic effector T cell-mediated inflammatory responses within tissues.5–6
Despite significant advances in the knowledge regarding the physiological significance of B7 family negative co-stimulators, the precise role of these molecules in the maintenance of intestinal mucosal tolerance remains unclear. Moreover, knowledge about the phenotype and location of PD-L molecule expressing APCs in human gut remains rudimentary. Studies by our laboratory and others suggest that intestinal stromal cells (myofibroblasts and fibroblasts) are important sentinel cells that play a key role in the immune system in the switch from acute inflammation to adaptive immunity and tissue repair.11–13 Intestinal myofibroblasts are a distinct population of activated fibroblasts that are positive for CD90 (fibroblast/myofibroblast marker) and α-smooth muscle actin (α-SMA), but negative for other hematopoetic and nonhematopoetic professional and non professional APCs cell markers.13 CD90 (e.g., Thy-1) represents a useful fibroblast/myofibroblast marker since, in humans, it is not expressed by T lymphocytes.14 We have recently reported that colonic myofibroblasts (CMFs) in the normal human colonic mucosa represent a distinct and numerous fraction of local MHC class II+ nonprofessional APCs.13 CD90+ stromal cells (fibroblasts and myofibroblasts) are abundant throughout the colonic lamina propria. Myofibroblasts are located directly subjacent to the epithelial basement membrane and form an interface between the epithelium and lamina propria immune cells. Myofibroblasts are connected by cell junctions to fibroblasts that are located deeper in the lamina propria. Studies by our group and others suggested that CMFs may be involved in the regulation of CD4+ T cell responses within the colonic mucosa.13,15–16 In the normal colon, CMFs express rather low levels of B7.1 and B7.2, as compared to professional APCs such as activated DCs and macrophages.13 This suggests that during immune homeostasis (e.g., mucosal tolerance) colonic stromal cells may exert a suppressive function, since the majority of lamina propria CD4+ T lymphocytes are activated and express the B7.1/B7.2 inhibitory ligand CTLA-4.17
Herein we present evidence for a suppressive role of colonic CD90+ myofibroblasts/fibroblasts on activated CD4+ effector T cells suggesting an important role for stromal cells in mucosal tolerance. We have addressed two questions: first, whether normal human myofibroblasts and fibroblasts express negative co-stimulators of the B7 family and, second, whether these stromal cells exert inhibitory functions on activated effector T cells. We demonstrate here the expression of PD-L molecules by myofibroblasts/fibroblasts in vivo and that normal colonic CMFs express PD-L1 and PD-L2 in primary isolates. CMFs are shown to suppress activated CD4+ effector T cell proliferation and IL-2 production via a cell contact dependent mechanism involving PD-L1 and PD-L2 signals. Our data suggest that CMFs may limit CD4+T cell proliferative activity and, thus, might play a prominent role in mucosal tolerance to commensal bacteria and dietary antigens.
Materials and Methods
Antibodies and Reagents
Please see Supplemental information online at www.gastroojournal.org.
Human colonic tissue specimens, acute lamina propria mononuclear cell preparations & primary CMF cultures
For immunohistochemical studies, fresh human tissue samples were obtained from normal colonic biopsies of patients undergoing colonoscopy screening for cancer of the large intestine in compliance with protocols approved by the University of Texas Medical Branch (UTMB) Institutional Review Board (IRB). Surgical specimens from patients undergoing colectomy for colon cancer or prior, inactive diverticulitis (at least 10–15 cm away from the disease affected area) were collected in compliance with protocols approved by the UTMB IRB and used as a source of lamina propria mononuclear cell preparations and for generation of primary colonic myofibroblast cultures.
Colonic lamina propria mononuclear cells were isolated by a modification (omission of dispase) of a protocol kindly provided by Dr. R. Edwards (UC Irvine, Irvine, CA) and performed as described previously.13 The resulting single cell suspension was then processed for immunostaining followed by FACS as described previously.13 Primary cultures of colonic myofibroblasts were generated according to the method described by Mahida et al.18 For more details please see Supplemental information online at www.gastroojournal.org.
Confocal microscopy
Please see Supplemental information at www.gastroojournal.org.
Transfection of siRNA into CMFs
Primary CMF cultures with knockdown expression of PD-Ls molecules were generated in our lab by using siRNA technology. Negative siRNA controls will be included in each experiment. The pool of siRNA probes to the conservative domains of PD-L1, PD-L2 or negative siRNA control was purchased from Ambion® Inc. (Austin, TX). Optimal concentration (0.5 nM) of each siRNA was used for each transfection. Transfection of indicated above primary cells was performed by using Nucleofector™ technology (Amaxa Biosystems, Gaithersburg, MD). CMFs were transfected by using Human Dermal Fibroblast Nucleofector™ kit according to the manufacturer’s instructions and the effect of siRNA on PD-L expression confirmed by flow cytometry.
Western blot analysis
Western blot analysis was performed on 10 μg of protein as previously described13.
Costimulation of T cell response
Purified human naive CD4+ T cells (see Supplemental information at www.gastroojournal.org) were preactivated with anti-CD3, anti-CD28 microbeads provided by T cell Activation/Expansion kit (Miltenyi Biotec) according to the manufacturer’s instructions. Activated or unprimed CD4+ T cells (2×105 cells/well) were plated in triplicate in 96 well plates in the presence or absence of CMFs (5×104 cells/well). Same ratio (4:1) between T cells and CMF were used when tests were performed in 24-well plates (transwell experiments). For transwell experiments CMFs were grown in the bottom of wells, while T cells were added to the 24-well plate filter inserts with a pore size of 0.4 μm (BD Bioscience). Monoclonal antibodies (mAbs) against the studied co-stimulatory molecules and isotype controls were added to the co-cultures (when necessary) at a final concentration 2.5 μg/mL. Co-cultures were incubated for four days maximum at 37°C in 5% CO2. T cell proliferation and IL-2 production were determined as described previously13 (please see Supplemental information at www.gastroojournal.org).
Statistical analysis
Unless otherwise indicated, the results were expressed as the mean ± SE of data obtained from at least three independent experiments done with triplicate sets in each experiment. Differences between means were evaluated by ANOVA using Student’s t-test for multiple comparisons. Values of P <0.05 were considered statistically significant.
Results
Colonic myofibroblasts/fibroblasts express negative co-stimulators PD-L1 and PD-L2 in situ
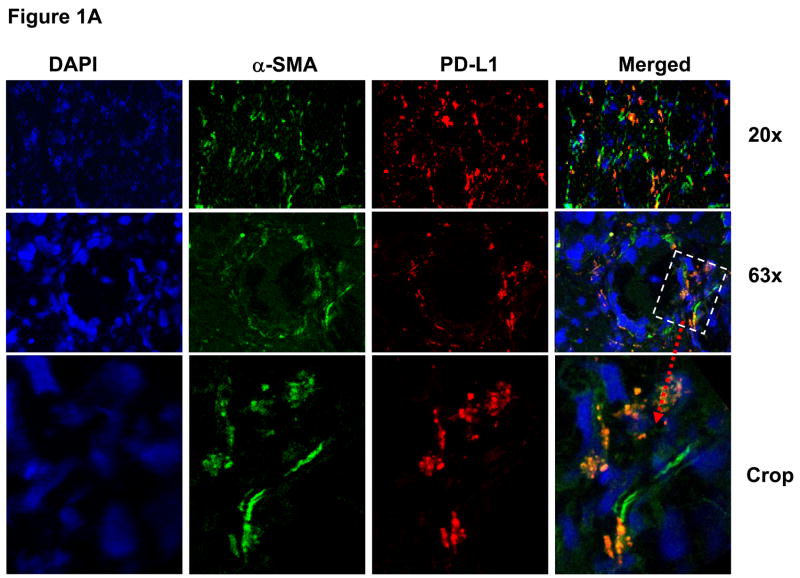
We recently reported that normal, human MHC class II-expressing CMFs, which are located in the lamina propria just beneath the epithelial layer, are novel local nonprofessional APCs.13 CMFs express low levels of B7.1 and B7.2 and, thus, might be local negative regulators of activated CD4+ T cell responses in colonic mucosa. Co-stimulatory signals provided by PD-L1 and PD-L2 molecules on the surface of APC appear to be essential for the negative regulation of activated T cell proliferation. Thus, we first evaluated the expression of PD-L1 and PD-L2 on α-SMA+ myofibroblasts in normal colonic mucosa in situ. PD-L1 and PD-L2 expression in normal colonic frozen tissue section was analyzed by fluorescent immunostaining followed by confocal microscopy analysis. The myofibroblasts were identified in colonic mucosa based on their morphology, subepithelial location and positive immunoreactivity for α-SMA expression as previously reported.13
A significant basal level of PD-L1 (Figure 1A) and PD-L2 (Figure 1B) was observed in the subepithelial pericryptal area in normal colon (shown in red). Importantly, we found that the that this expression of PD-1 ligands was mostly co-localized with cells bearing myofibroblast phenotype (Figure 1, α-SMA+ cells, shown in green, co-localization resulted in the formation of yellow-orange color on merged images). Occasional PD-L1, but not PD-L2 staining of epithelial cells from basolateral side was observed. More consistently, we observed that specific α-SMA− lamina propria cells (myofibroblasts) were expressing PD-1 ligands. High power resolution confocal image analysis was performed in order to differentiate PD-L expression by myofibroblasts from that expressed on basolateral membranes of epithelial cells and other lamina propria cells and confirmed the localization of the PD-1 ligands on the α-SMA+ cells (Figure 1).
Figure 1.
Normal human colonic myofibroblasts express (A) PD-L1 and (B) PD-L2 molecules in situ. The panels show confocal microscopy analysis of multicolor immunofluorescent staining of a representative frozen tissue cross sections of a normal colonic crypt fixed in 2% paraformaldehyde. The myofibroblast cell population was identified in colonic mucosa based on morphology, subepithelial location and positive immunoreactivity for α-SMA. Cell nuclei (in blue) were stained with DAPI. Subepithelial pericryptal myofibroblasts were identified by expression of α-SMA (in green) as detected by AF® 488 conjugated anti-human α-SMA murine mAbs (clone 1A4). PD-L1 or PD-L2 staining (in red) of colonic mucosa was performed using AF® 647 or AF® 546 labeled (A) anti-PD-L1 murine mAbs (clone M1H1) or (B) anti-PD-L2 murine mAbs (clone M1H18). Merged images clearly demonstrate expression of PD-L1 and PD-L2 on α-SMA+ cells (e.g. myofibroblasts, in orange-yellow staining).
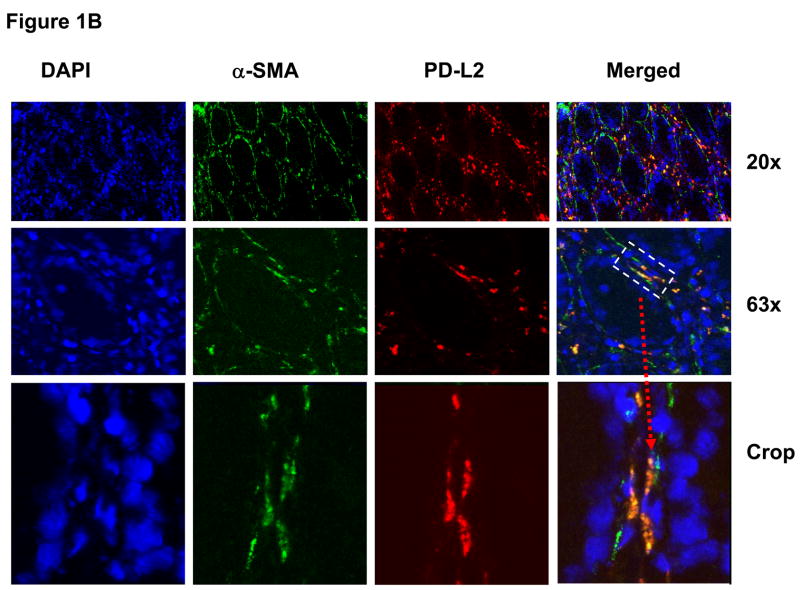
Moreover, since α-SMA-myofibroblasts are a distinct population of activated fibroblasts and CD90 marks the entire population of stromal cells (fibroblast and myofibroblasts), we analyzed the distribution of PD-1 ligands by CD90+ stromal cells. Our data demonstrated that PD-1 ligands were widely distributed on the CD90+ stromal cells (shown in Supplemental information online, Figure s1A–B at www.gastroojournal.org.) Importantly, the PD-1 ligand expressing CD90+ cells were located in both a subepithelial location (myofibroblasts) and deeper in the lamina propria (fibroblasts).
Taken together these data indicate that in the normal colon, PD-L1 and PD-L2 were predominantly expressed by fibroblasts/myofibroblasts and this observations suggest that in normal colon these cells have a “suppressive” phenotype.
CD90+ myofibroblasts/fibroblasts are the major cell population expressing PD-1 ligands in normal colonic lamina propria mononuclear cell preparations
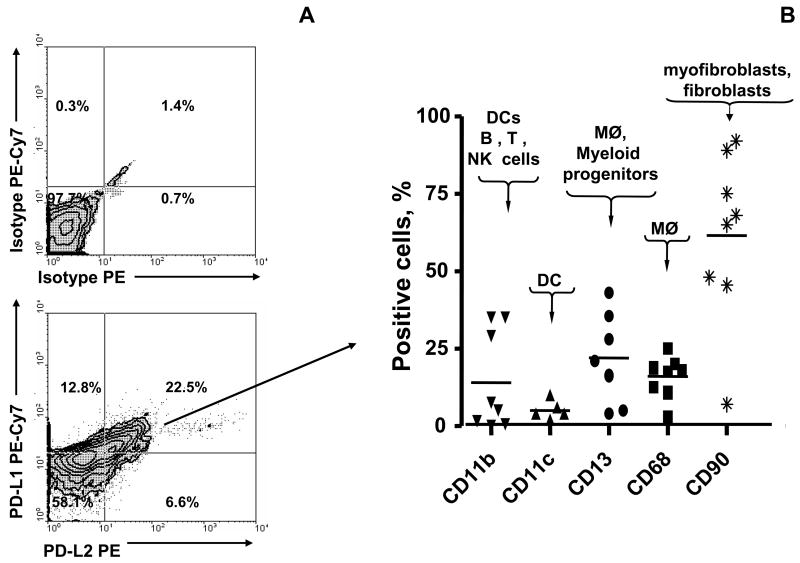
To confirm the confocal microscopy observations on colonic tissue in situ, we analyzed the relative distribution of PD-L molecules between professional immune cells and CD90+ myofibroblast/fibroblast population in freshly isolated single cell suspensions of human colonic lamina propria mononuclear cells (LPMNC). LPMNC were analyzed by multicolor flow cytometry analysis. In addition to lymphocytes, macrophages, and other leukocytes, these preparations also contain resident stromal cells such as myofibroblasts and fibroblasts. LPMNC positive for PD-L1 (~35%), PD-L2 (~29%) or both markers (~22%) were gated and then analyzed for the expression of CD11b (marker of macrophages, DC, B, T and NK cells), CD11c (DC marker), CD13 (marker for macrophages and myeloid cell progenitors, but not expressed by on T, B and NK cells), CD68 (macrophages) and CD90 (myofibroblast/fibroblast marker). Our data indicate that in normal colonic LPMNC CD90+ stromal cells represent about 70% and 50% of PD-L1 and PD-L2 expressing cells, respectively. Moreover, more then 60% of the cells that express both PD-L markers (PD-L1+PD-L2+ phenotype) express CD90 (Figure 2, also see Supplemental information, Table s1 at www.gastroojournal.org).
Figure 2.
The majority of PD-L1+PD-L2+ expressing cells in normal human colonic lamina propria are CD90+ myofibroblasts/fibroblasts. Lamina propria mononuclear cells were subjected to multi-color immunostaining followed by flow cytometry analysis. (A). PD-L1+ PD-L2+ cells representing 24.1±10.49 % (right upper quadrant) of total live isolated cells were gated. One representative experiments of six is shown (n=6). (B) The relative distribution of professional immune cell markers and CD90 was determined in the gated PD-L1+ PD-L2+ live isolated cells. About 70 % of PD-L1+ PD-L2+ cells (gated in R1) express CD90 and, thus, have the myofibroblast/fibroblast phenotype. Results are calculated as the mean value of six experiments ± standard deviation (n=6).
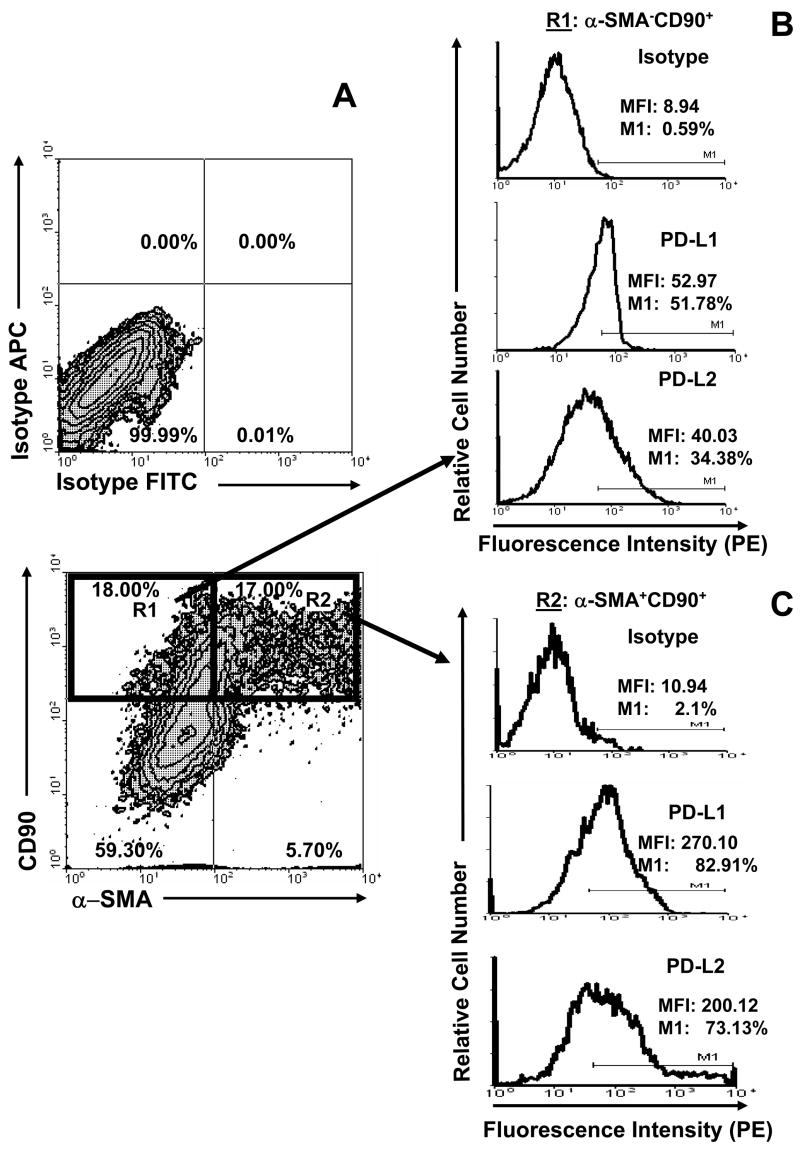
Analysis of intensity of PD-L1 and PD-L2 level expression by CD90+ stromal cells in LPMNC preparations demonstrated that both myofibroblasts (α-SMA+CD90+) and fibroblasts (α-SMA−CD90+) express significant levels of PD-L1 on their surface (Figure 3). PD-L1 expression was higher than that of PD-L2 on both cell types. Moreover, the level of PD-1 ligands expression on myofibroblasts was higher than on fibroblasts. It is clear that CD90+ myofibroblasts/fibroblasts are the major population expressing significant levels of B7 negative co-stimulators, PD-L1 and PD-L2, in normal colonic LPMNC.
Figure 3.
Myofibroblasts and fibroblasts in lamina propria mononuclear cell preparations express B7 negative co-stimulators PD-L1 and PD-L2. Lamina propria mononuclear cells were subjected to multi-color immunostaining followed by flow cytometry analysis. (A) Cells bearing the myofibroblast phenotype (α-SMA+CD90+, R1) represented 16.8 ± 8.37 % of the gated colonic mucosal cells. Expression of PD-L1 and PD-L2 by (B)α-SMA− CD90+ cells (gated in R1) and (C)α-SMA+CD90+ cells (gated in R2). A representative experiment is shown (n=7).
CD90+ stromal cells retain PD-L1+PD-L2+ phenotype in culture
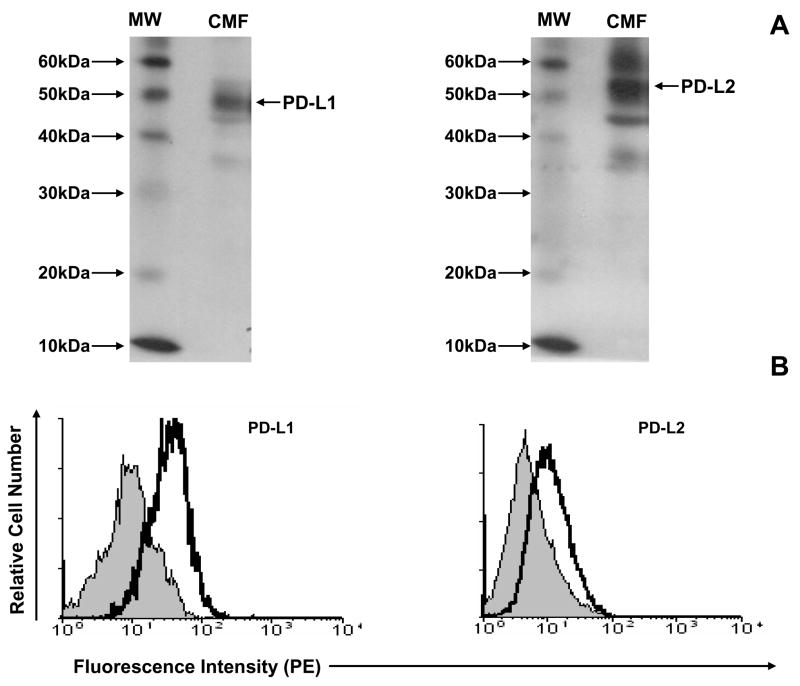
In order to study PD-1 ligand expression and function by colonic CD90+ stromal cells independently of other cell types, we have established primary cultures of these cells from normal human tissue. Since we observed higher expression of PD-1 ligands by myofibroblasts when compared to stromal fibroblasts, we focused our in vitro work mostly on myofibroblasts (CMFs). Western blotting demonstrated significant levels of PD-L1 and PD-L2 expression of the appropriate molecular mass in cell lysates of CMFs (Figure 4A). Surface immunostaining of primary isolates of CMFs, followed by FACS analysis demonstrated the constitutive expression of PD-L1 and, to a lesser extent, PD-L2 on the cell surface of CMFs (Figure 4B). Thus, we established that primary CMF cultures constitutively express PD-L1 and PD-L2.
Figure 4.
Cultured CMFs express B7 negative co-stimulators PD-L1 and PD-L2. (A) Western blotting using antibodies specific for either the anti-PD-L1 or anti-PD-L2blot shows expression of PD-L1 and PD-L2 on 1 week old CMF cultures. Under denaturing conditions, both proteins migrate as subunits of ~50 kDa. CMF- myofibroblast protein extract; MW - molecular weigh marker. (B) Cell-surface expression of PD-L1 and PD-L2 (open histograms) by one-week-old CMF culture was analyzed by immunostaining followed by flow cytometry analysis. Isotype control (filed histogram) was included for each staining. One representative experiment of four is shown for CMF 18Co.
CMFs suppress proliferation of activated CD4+ effector T cells via a cell contact mediated mechanism
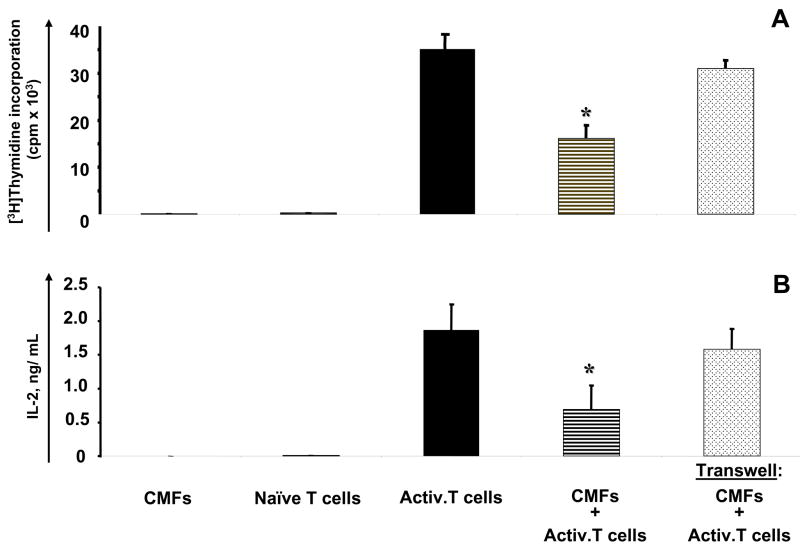
To assess the functional significance of the expression of the B7 family negative co-stimulators by CMFs, we analyzed their effect on the suppression of proliferation of preactivated allogeneic CD45RA+CD4+ T naive helper cells. In these assays CD45RA+CD4+ naive T helper cells were isolated from peripheral blood mononuclear cells of healthy volunteers, preactivated with anti-CD3/anti-CD28 monoclonal antibodies and then incubated in the presence of irradiated CMFs. Proliferation of preactivated CD45RA+CD4+ T cells was significantly decreased in co-culture with CMFs (Figure 5A, also see Supplemental information, Figure s2 at www.gastroojournal.org). However, when CMFs were separated from activated T cells by a filter in a transwell apparatus, no significant suppression of proliferation of activated CD45RA+CD4+ T cells was observed. Similar to the effect of CMFs on T cell proliferation, a significant decrease in IL-2 production (an important indicator of T cell activation 19) was observed in CMF:T cell co-culture, and IL-2 production was restored when CMFs were separated from activated T cells by a filter in the transwell (Figure 5B). Similar results to those shown in Figure 5 (using CMF 9.5N1 culture) were obtained with two different cultured CMF isolates, CMF9.5N2 and CMF10.8N (data not shown). Thus, CMFs suppress proliferative responses of activated T helper cells mainly via a cell contact-mediated mechanism.
Figure 5.
CMFs suppress proliferation of CD3/CD28-activated CD45RA+ CD4+ T cells via a cell contact-mediated mechanism. CD45RA+CD4+ T cells (naïve T cells) were preactivated with anti-CD3/anti-CD28 monoclonal antibody bearing beads (Activ. T cells) by using T cell activation/expansion kit (Miltenyi Biotec). T cells were co-cultured with primary culture of CMFs upon normal or transwell conditions. (A) T cell proliferation was measured after 72-hour co-culture by using [3H]-thymidine incorporation (c.p.m.). Values represent the mean counts per minute (c.p.m.) ± standard error (SE) of triplicate cultures of T cells isolated from one donor. A representative experiment is shown (n=3 donors, three experiment replicate each). *p < 0.01. (B) IL-2 production was measured in the culture supernatant after 48-hour co-culture by using a standard IL-2 specific ELISA kit (Pharmingen). Values are expressed in ng/mL and represent the mean ± standard error (SE) of triplicate cultures of T cells isolated from one donor. A representative experiment is shown (n=3 donors, two experiment replicates each). *p < 0.05.
CMF suppressive effect on activated CD4+ effector T cell proliferation depend on PD-1 ligands
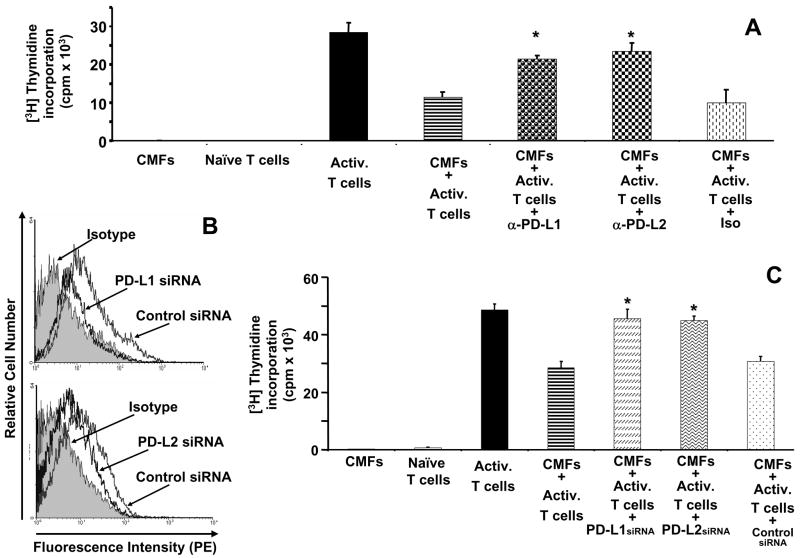
To evaluate the role of the PD-1 ligands in CMF mediated cell contact dependent suppression of activated T cell proliferation and IL-2 production, we performed blocking experiments using monoclonal antibodies to PD-L1 and PD-L2. Blockade of PD-L1 or PD-L2 on CMFs with anti-PD-L1 or anti-PD-L2 blocking mAbs, but not with IgG1κ isotype control, resulted in the rescue of allogeneic CD3/CD28-activated CD45RA+CD4+ T cell proliferation (Figure 6A). We confirmed these results by knockdown of PD-L1 or PD-L2 surface expression with appropriate specific siRNA (Figure 6B), which also resulted in an enhancement of allogeneic CD3/CD28-activated CD45RA+CD4+ T cell proliferation (Figure 6C). No significant reversion of CMF-mediated T cell suppression was observed, however, when CMF transfected with a control siRNA was used, confirming the specificity of the PD-L’s siRNA. Surprisingly, the simultaneous blockade of PD-L1 and PD-L2 did not result in the complete rescue of T cell proliferation observed in T cells activated in the absence of CMFs (data not shown). In conclusion, our data indicate that CMF-mediated suppression of activated CD4+ T cell proliferation is dependent upon PD-1 ligand signals.
Figure 6.
The suppressive effect of colonic myofibroblasts on CD3/CD28-activated CD45RA+CD4+ T cells depends on PD-L1. CD45RA+CD4+ T cells (Naïve T cells) were preactivated with anti-CD3/anti-CD28 monoclonal antibody bearing beads (Activ. T cells) by using T cell activation/expansion kit (Miltenyi Biotec). (A) Activated T cells were co-cultured with primary culture of CMFs in the presence/absence of anti-human PD-L1 (clone M1H1) or anit-PD-L2 (clone M1H18) blocking mAbs. (B) Knockdown of PD-L1 or PD-L2 expression on CMFs was accomplished by using a pool of PD-L1-, PD-L2- specific siRNA or negative siRNA controls. (C) Activated T cells were co-cultured with primary culture of CMFs transfected or not with PD-L1-, PD-L2- specific siRNA or negative siRNA control. T cell proliferation (A, C) was assessed by using [3H]-thymidine incorporation (c.p.m.). Values represent the mean counts per minute (c.p.m) ± standard error (SE) of triplicate cultures of T cells isolated from one donor. A representative experiment is shown (n=4 donors, three experiment replicate each). *p < 0.01.
CMF’s suppressive effect on activated T cell proliferation is partially restored by exogenous IL-2
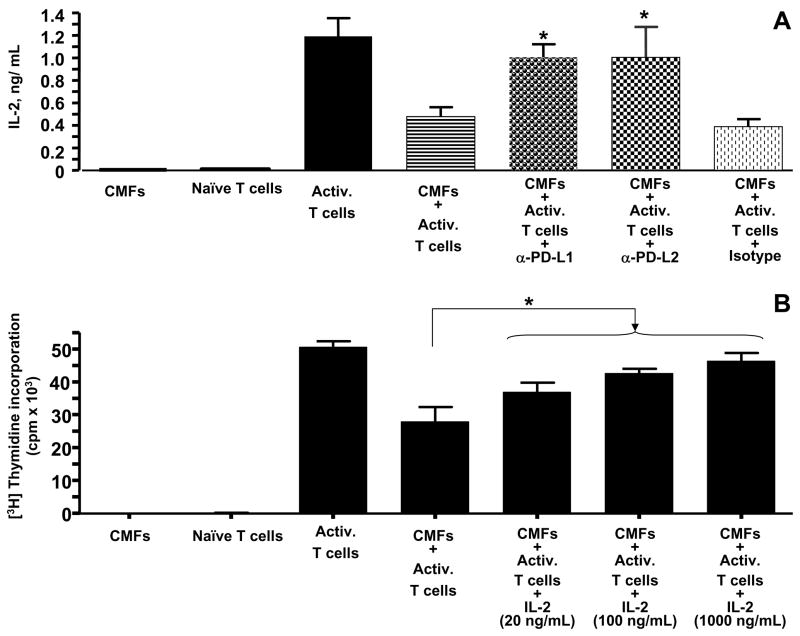
Accumulated evidence indicates that APC-expressed PD-L signals not only regulate T cell proliferation, but also the T cell activation state, in particularly IL-2 production. Therefore, we examined whether PD-Ls expression by CMFs affect production of IL-2 by CD3/CD28-activated CD45RA+CD4+T cells. We demonstrated here with an ELISA analysis that blockade of PD-L1 or PD-L2 on CMFs with anti-PD-L1 or anti-PD-L2 blocking mAbs, but not with an IgG1κ isotype control, resulted in the increased production of IL-2 in 48 h co-culture of CMF:T cells (Figure 7A). We confirmed these results by knockdown of PD-L1 or PD-L2 surface expression with appropriate specific siRNA (data not shown). No additive or synergistic effect between PD-Ls on the rescue of IL-2α production by T cells in the CMF:T cell co-culture was observed (data not shown).
Figure 7.
CMF-mediated suppression of IL-2 production by CD3-/CD28-activated CD45RA+ CD4+ T cells is reversed by blockade of PD-L1 or PD-L2. CD45RA+CD4+ T helper cells (Naïve T cells) were preactivated with anti-CD3/anti-CD28 mAbs bearing beads (Activ T cells). (A) Activated T cells were co-cultured with primary culture of CMFs in the presence/absence of anti-human PD-L1 (clone M1H1) or anit-PD-L2 (clone M1H18) blocking mAbs. IL-2 production was measured in 48-hour culture supernatant using a standard IL-2 specific ELISA kit (Pharmingen). Values were expressed in ng/mL and represent the mean ± standard error (SE) of triplicate cultures of T cells isolated from one donor. A representative experiment is shown (n=4 donors, two experiment replicates each). *p < 0.05. (B) Activated T cells were co-cultured with primary culture of CMFs in the presence/absence of different concentrations of human recombinant IL-2. T cell proliferation was measured 72-hour after co-culture by using [3H]-thymidine incorporation (c.p.m.). Values represent the mean counts per minute (c.p.m.) ± standard error (SE) of triplicate cultures of T cells isolated from four donor, *p < 0.05.
These data suggest that CMF-mediated suppression of activated CD4+ T cell proliferation might be due to the decrease in IL-2 production mediated by PD-L1 and PD-L2. Thus, we analyzed whether addition of exogenous IL-2 in the CMF: T cells co-cultures can rescue the activated T cell proliferation (Figure 7B). Our data demonstrated that supplementation of CMF:T cell co-culture with as little as 20 ng/mL of human recombinant IL-2 led to significant restoration of a CD3/CD28-activated CD45RA+CD4+ T cell proliferation. Increases in the exogenous IL-2 concentration in the co-culture media up to 100–1000 ng/mL led to an increase in the CD3/CD28-activated CD45RA+CD4+ T cell proliferation, but did not completely restore the T cell proliferation level as noted in activated T cell monocultures.
Thus, our data suggest that CMFs in normal colonic mucosa may be major participants in the control of the acute T cell inflammatory responses via PD-Ls mediated negative regulation of activated T effector proliferation mainly via inhibition of IL-2 production.
Discussion
Although the precise mechanisms underlying mucosal tolerance in the gut remain unclear, there is ample evidence that, during homeostasis, the intestinal response to luminal antigens requires strict control to prevent undesirable proinflammatory responses to innocuous dietary antigens and those emanating from commensal microorganisms.1,20
We recently reported that human colonic CD90+ stromal cells, in particularly myofibroblasts, are the major cell phenotype in the normal human colonic lamina propria, which may act as a non professional MHC class II+ APCs.13 Presentation of Ag to T cells in the absence or low B7.1/B7.2 costimulation is believed to result in the induction of tolerance with a concomitant failure of T cell effector functions.5,20–21 Our finding of low levels of B7.1/B7.2 molecules expression on normal human CMFs led us to hypothesize that CMFs might be local “suppressors” of activated T cell responses in the normal colon and may play a role in mucosal tolerance. In this study, we present evidence that CD90+ stromal myofibroblasts/fibroblasts constitutively express PD-L1 and PD-L2 molecules that are major “negative” regulators of the acute proinflammatory responses of activated T helper cells. Moreover, we demonstrate here that PD-L1 and PD-L2 co-stimulatory molecules are major contributors to a CMF-mediated suppressive effect on T cell proliferation and IL-2 production.
Our data indicate that myofibroblasts/fibroblasts represent a major population of the PD-L1- and PD-L2-expressing cells that form a network of interconnected mononuclear cells below the epithelial basement membrane in the normal, non-inflamed human colonic mucosa. Moreover, these cells preserve PD-L1+PD-L2+ phenotype in culture. Although upregulation of the PD-L1 expression on colonic epithelial cells, DC, macrophages, T and B cells has been noted during chronic intestinal inflammation (e.g., inflammatory bowel disease) and cancer, very minor or no surface PD-L1 or L2 expression has been observed by these cells in the normal, non-inflamed human colonic mucosa.22–23 Our results are in agreement with these observations: flow cytometry analysis of the acutely isolated normal human colonic lamina propria mononuclear cells demonstrated that cells positive for CD11b, (markers expressed by DC, B, T and NK cells), CD11c (DC marker), CD13 (aminopeptidase N, which is present on macrophages, monocytes and myeloid cell progenitors, but not on T, B and NK cells) or CD68 (macrophage marker) represent less then 30% of PD-L1/2 expressing cells. The majority of PD-L1+PD-L2+ cells (~ 70 %) were positive for the fibroblast/myofibroblast marker CD90.
While this is the first report demonstrating that human colonic myofibroblasts/fibroblasts express B7 negative co-stimulator in culture and in situ, it is not without precedence for other mesenchymal cells. The constitutive expression of PD-L1 on human normal dermal fibroblast/myofibroblasts24, microglia25 and murine hepatic stellate cells26 has also been recently reported. Although the expression of PD-L1 has been previously reported on organ parenchymal cells and endothelial cells, the expression of PD-L2 in normal conditions has been thought to be predominately restricted to professional APCs such as DCs and macrophages.5,6 Here we report that PD-L2 is also constitutively expressed by fibroblasts/myofibroblasts in the normal colonic mucosa. As has been previously reported for immature human and murine DCs27, we observed lower frequency of PD-L2 expressing in situ and on cultured CMFs when compare to PD-L1. It has been previously hypothesized that level of the expression of PD-L1 and PD-L2 in various tissue and cells may govern the outcome of their interactions with effector T cells.6,25–26 Thus, our data suggest that in the normal colonic mucosa CD90+ stromal cells may control the extent of inflammatory responses mediated by activated effector T cells by expression of PD-L1 and PD-L2. In fact, co-localization of CMFs and T cells within the human intestinal mucosa has been noted previously.28 Herein we demonstrate that cultured CMFs suppress proliferation of CD3/CD28-preactivated T helper cells mostly via mechanisms involving PD-L1 and PD-L2 signals. Since no significant suppression of proliferation of activated CD45RA+CD4+ T cells was observed when CMFs were separated from T cells in a transwell apparatus, we conclude that cell to cell contact is needed for the inhibitory effect of PD-1 ligands. However, these experiments do not exclude the possibility that simply a high concentration of PD-1 ligands, rather that cell to cell contact is required in order to achieve the CMF inhibitory effect on activated T cell proliferation.
Whether PD-L1 and PD-L2 have overlapping or distinct functions is under active investigation. Some studies have suggested that both molecules inhibit T cell proliferation and cytokine production 5–6,22,25, whereas others support stimulatory role for the PD-L1/2.29 We demonstrated here that both molecules support suppressive function of stromal cells in the regulation of activated T helper cell proliferative responses, since blocking this interaction reverses the CMF suppressive effect on T cell proliferation. Our data indicate that PD-L1/2 mediated CMF suppressive functions are mainly due to the suppression of IL-2 production, since supplementation of the co-culture media with exogenous IL-2 led to partial recovery of activated T cell proliferation. This is in agreement with previous findings suggesting that IL-2 levels following the activation of human and murine T cells is critical for determining the outcome of the T cell derived PD-1 engagement by PD-L1 Fc.3,30 Despite the crucial role of the IL-2 in the outcome of PD-1 and PD-L1/2 interactions, our data also indicates that an increase in the IL-2 concentration is insufficient to fully restore proliferation of activated T cells upon PD-1 engagement on T cells by CMF derived PD-L1/2. It has been reported that the level of IL-2Rα expression is reduced on activated CD4+ T cells by co-stimulation with PD-L1.Fc.26 The inability of exogenous IL-2 to fully overcome the CMF suppressive effect may suggest that engagement of its PD-L1/2 by PD-1 on activated T cells led to a decrease in IL-2Rα as well. Moreover, because fibroblasts are reported to produce important levels of the TGFβ1–3, IL-10, PGE2 and indoleamine 2,3-dioxygenase upon activation18,31–34, we do not exclude that interaction between CMF and activated T cells may trigger release of these suppressive molecules by CMFs and these, in turn, may contribute to the CMF suppressive function.
It has been suggested that PD-L1 controls acute inflammatory responses via suppression of ongoing activated T cell responses4–6. Therefore, taking in consideration the recently acquired knowledge about the role of PD-L molecules and the fact that it has been noted that myofibroblasts/fibroblasts can regulate the T cell responses in other inflamed tissues15,35, our data suggests that PD-L1/2 expressing CMFs may be important local contributors to the control of activated T helper cells proliferative response during mucosal tolerance in colon.
Supplementary Material
Acknowledgments
We thank Eugene Knutson and Dr. Thomas Albrecht of the UTMB Optical Imaging Core for their assistance and expertise with the confocal microscopy studies. We thank Mark Griffin of the Gulf Coast Digestive Diseases Center Immunology Core for his assistance with flow cytometry analyses.
Footnotes
Supported by grants from the NIDDK (DK55783), the John Sealy Memorial Endowment Fund, the UTMB Gastrointestinal Research Interdisciplinary Program, the James W. McLaughlin Endowment Fund, Crohn’s & Colitis Foundation of America
No conflicts of interest exist
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedman A, Weiner HL. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc Natl Acad Sci USA. 1994;91:6688–6692. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandtzaeg P. Nature and function of gastrointestinal antigen-presenting cells. Allergy. 2001;56(Suppl 67):16–20. doi: 10.1034/j.1398-9995.2001.00903.x. [DOI] [PubMed] [Google Scholar]
- 3.Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, Collins M, Honjo T, Freeman GJ, Carreno BM. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32:634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 5.Keir ME, Liang SC, Guleria I, Latchman YE, Qipo A, Albacker LA, Koulmanda M, Freeman GJ, Sayegh MH, Sharpe AH. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Latchman YE, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 7.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci USA. 2003;100:5336–5341. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang SC, Greenwald RJ, Latchman YE, Rosas L, Satoskar A, Freeman GJ, Sharpe AH. PD-L1 and PD-L2 have distinct roles in regulating host immunity to cutaneous leishmaniasis. Eur J Immunol. 2006;36:58–64. doi: 10.1002/eji.200535458. [DOI] [PubMed] [Google Scholar]
- 10.Mazanet MM, Hughes CC. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J Immunol. 2002;169:3581–3588. doi: 10.4049/jimmunol.169.7.3581. [DOI] [PubMed] [Google Scholar]
- 11.Andoh A, Bamba S, Fujiyama Y, Brittan M, Wright NA. Colonic subepithelial myofibroblasts in mucosal inflammation and repair: contribution of bone marrow-derived stem cells to the gut regenerative response. J Gastroenterol. 2005;40:1089–1099. doi: 10.1007/s00535-005-1727-4. [DOI] [PubMed] [Google Scholar]
- 12.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277:C1–C9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 13.Saada JI, Pinchuk IV, Barrera CA, Adegboyega PA, Suarez G, Mifflin RC, Di Mari JF, Reyes VE, Powell DW. Subepithelial myofibroblasts are novel nonprofessional APCs in the human colonic mucosa. J Immunol. 2006;177:5968–5979. doi: 10.4049/jimmunol.177.9.5968. [DOI] [PubMed] [Google Scholar]
- 14.Saalbach A, Kraft R, Herrmann K, Haustein UF, Anderegg U. The monoclonal antibody AS02 recognizes a protein on human fibroblasts being highly homologous to Thy-1. Arch Dermatol Res. 1998;290:360–366. doi: 10.1007/s004030050318. [DOI] [PubMed] [Google Scholar]
- 15.Andoh A, Bamba S, Brittan M, Fujiyama Y, Wright NA. Role of intestinal subepithelial myofibroblasts in inflammation and regenerative response in the gut. Pharmacol Ther. 2007;114:94–106. doi: 10.1016/j.pharmthera.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Pinchuk IV, Beswick EJ, Saada JI, Suarez G, Winston J, Mifflin RC, Di Mari JF, Powell DW, Reyes VE. Monocyte chemoattractant protein-1 production by intestinal myofibroblasts in response to staphylococcal enterotoxin a: relevance to staphylococcal enterotoxigenic disease. J Immunol. 2007;178:8097–8106. doi: 10.4049/jimmunol.178.12.8097. [DOI] [PubMed] [Google Scholar]
- 17.Makita S, Kanai T, Oshima S, Uraushihara K, Totsuka T, Sawada T, Nakamura T, Koganei K, Fukushima T, Watanabe M. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J Immunol. 2004;173:3119–3130. doi: 10.4049/jimmunol.173.5.3119. [DOI] [PubMed] [Google Scholar]
- 18.Mahida YR, Beltinger J, Makh S, Goke M, Gray T, Podolsky DK, Hawkey CJ. Adult human colonic subepithelial myofibroblasts express extracellular matrix proteins and cyclooxygenase-1 and -2. Am J Physiol. 1997;273:G1341–G1348. doi: 10.1152/ajpgi.1997.273.6.G1341. [DOI] [PubMed] [Google Scholar]
- 19.Benczik M, Gaffen SL. The interleukin (IL)-2 family cytokines: survival and proliferation signaling pathways in T lymphocytes. Immunol Invest. 2004;33:109–142. doi: 10.1081/imm-120030732. [DOI] [PubMed] [Google Scholar]
- 20.Smith KM, Eaton AD, Finlayson LM, Garside P. Oral tolerance. Am J Respir Crit Care Med. 2000;162:S175–S178. doi: 10.1164/ajrccm.162.supplement_3.15tac7. [DOI] [PubMed] [Google Scholar]
- 21.Mueller DL, Jenkins MK. Molecular mechanisms underlying functional T-cell unresponsiveness. Curr Opin Immunol. 1995;7:375–381. doi: 10.1016/0952-7915(95)80113-8. [DOI] [PubMed] [Google Scholar]
- 22.Nakazawa A, Dotan I, Brimnes J, Allez M, Shao L, Tsushima F, Azuma M, Mayer L. The expression and function of costimulatory molecules B7H and B7-H1 on colonic epithelial cells. Gastroenterology. 2004;126:1347–1357. doi: 10.1053/j.gastro.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Kanai T, Totsuka T, Uraushihara K, Makita S, Nakamura T, Koganei K, Fukushima T, Akiba H, Yagita H, Okumura K, Machida U, Iwai H, Azuma M, Chen L, Watanabe M. Blockade of B7-H1 suppresses the development of chronic intestinal inflammation. J Immunol. 2003;171:4156–4163. doi: 10.4049/jimmunol.171.8.4156. [DOI] [PubMed] [Google Scholar]
- 24.Lee SK, Seo SH, Kim BS, Kim CD, Lee JH, Kang JS, Maeng PJ, Lim JS. IFN-γ regulates the expression of B7-H1 in dermal fibroblast cells. J Dermatol Sci. 2005;40:95–103. doi: 10.1016/j.jdermsci.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Magnus T, Schreiner B, Korn T, Jack C, Guo H, Antel J, Ifergan I, Chen L, Bischof F, Bar-Or A, Wiendl H. Microglial expression of the B7 family member B7 homolog 1 confers strong immune inhibition: implications for immune responses and autoimmunity in the CNS. J Neurosci. 2005;25:2537–2546. doi: 10.1523/JNEUROSCI.4794-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu MC, Chen CH, Liang X, Wang L, Gandhi CR, Fung JJ, Lu L, Qian S. Inhibition of T-cell responses by hepatic stellate cells via B7-H1-mediated T-cell apoptosis in mice. Hepatology. 2004;40:1312–1321. doi: 10.1002/hep.20488. [DOI] [PubMed] [Google Scholar]
- 27.Chen C, Qu QX, Huang JA, Zhu YB, Ge Y, Wang Q, Zhang XG. Expression of programmed-death receptor ligands 1 and 2 may contribute to the poor stimulatory potential of murine immature dendritic cells. Immunobiology. 2007;212:159–165. doi: 10.1016/j.imbio.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Sollid LM. Coeliac disease: dissecting a complex inflammatory disorder. Nat Rev Immunol. 2002;2:647–655. doi: 10.1038/nri885. [DOI] [PubMed] [Google Scholar]
- 29.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett F, Luxenberg D, Ling V, Wang IM, Marquette K, Lowe D, Khan N, Veldman G, Jacobs KA, Valge-Archer VE, Collins M, Carreno BM. Program death-1 engagement upon TCR activation has distinct effects on costimulation and cytokine-driven proliferation: attenuation of ICOS, IL-4, and IL-21, but not CD28, IL-7, and IL-15 responses. J Immunol. 2003;170:711–718. doi: 10.4049/jimmunol.170.2.711. [DOI] [PubMed] [Google Scholar]
- 31.Van Tol EA, Holt L, Li FL, Kong FM, Rippe R, Yamauchi M, Pucilowska J, Lund PK, Sartor RB. Bacterial cell wall polymers promote intestinal fibrosis by direct stimulation of myofibroblasts. Am J Physiol. 1999;277:G245–55. doi: 10.1152/ajpgi.1999.277.1.G245. [DOI] [PubMed] [Google Scholar]
- 32.McKaig BC, Hughes K, Tighe PJ, Mahida YR. Differential expression of TGF-beta isoforms by normal and inflammatory bowel disease intestinal myofibroblasts. Am J Physiol Cell Physiol. 2002;282:C172–C182. doi: 10.1152/ajpcell.00048.2001. [DOI] [PubMed] [Google Scholar]
- 33.Thompson KC, Trowern A, Fowell A, Marathe M, Haycock C, Arthur MJ, Sheron N. Primary rat and mouse hepatic stellate cells express the macrophage inhibitor cytokine interleukin-10 during the course of activation In vitro. Hepatology. 1998;28:1518–1524. doi: 10.1002/hep.510280611. [DOI] [PubMed] [Google Scholar]
- 34.Ghahary A, Li Y, Tredget EE, Kilani RT, Iwashina T, Karami A, Lin X. Expression of indoleamine 2,3-dioxygenase in dermal fibroblasts functions as a local immunosuppressive factor. J Invest Dermatol. 2004;122:953–964. doi: 10.1111/j.0022-202X.2004.22409.x. [DOI] [PubMed] [Google Scholar]
- 35.Buckley CD, Pilling D, Lord JM, Akbar AN, Scheel-Toellner D, Salmon M. Fibroblasts regulate the switch from acute resolving to chronic persistent inflammation. Trends Immunol. 2001;22:199–204. doi: 10.1016/s1471-4906(01)01863-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.