Abstract
Replication-defective adenovirus vectors, primarily developed from serotype 5 (Ad5) viruses, have been widely used for gene transfer and vaccination approaches. Vectors based on other serotypes of adenovirus could be used in conjunction with, or in place of, Ad5 vectors. In this study, Ad41, an enteric adenovirus usually described as ‘non-cultivable’ or ‘fastidious,’ has been successfully cloned, rescued and propagated on 293-ORF6 cells. The complementation capabilities of this cell line allow generation of Ad41 vectors at titers comparable to those obtained for Ad5 vectors. Mice immunized with an Ad41 vector containing an HIV envelope (Env) gene mounted anti-Env cellular and humoral immune responses. Ad41-Env vectors appear to be particularly attractive when used in heterologous prime-boost regimens, where they induce significantly higher cellular immune responses to HIV-Env than Ad5-based regimens. Ad41-based constructs are attractive vaccine vectors alone or in combination with Ad5 adenovectors, since each vector type can provide circumvention of pre-existing immunity to the other.
Keywords: Adenovirus, Vaccine vector, Human Immunodeficiency Virus
1. Introduction
Adenovirus (rAd) vectors have long been studied as candidates for gene-based vaccines and therapies. There are many attractive characteristics of adenovirus including growth to high titer, manufacturability, and adequate space in the genome for gene insertions. Moreover, these viruses have been used safely as vaccines for Acute Respiratory Syndrome (ARS) and adenovirus vectors are being tested for efficacy in vaccine clinical trials [1–3]. The oral ARS vaccines consisted of enteric-coated, live, replication-competent Ad4 and Ad7. The viruses replicated in the intestine and induced significant protection against respiratory disease [4,5]. Oral immunization targets the intestinal epithelium, a site where induction of mucosal and systemic immunity is well documented (reviewed in [6,7]). Mucosal immunity is considered important for protection against many infectious diseases since the host first encounters most pathogens at a mucosal surface. Although there has been progress, mucosal immunity has been difficult to stimulate [8]. The development of adenovirus vectors based on serotypes that naturally target the intestinal epithelium could lead to substantially higher mucosal immune responses.
The enteric adenoviruses, Ad40 and Ad41, are remarkable human adenoviruses for their gut tropism, capsid structure, and difficulty to culture. These species F adenovirus are associated with gastrointestinal disease and do not cause disease outside of the gastrointestinal tract [9,10]. The molecular basis for their specific pathogenicity is not defined, but may be related to the capsid structure [11]. Unique features of the Ad41 capsid are the presence of two fiber proteins, Long and Short [12,13], and the absence of the integrin-binding motif RGD in the penton base protein [14]. The Long fiber protein has been demonstrated to interact with the Coxsakie and Adenovirus Receptor whereas function of the Short fiber protein has not been determined [15,16]. The Ad41 capsid appears to be resistant to low pH, unlike respiratory adenoviruses [11]. The gut tropism of Ad41 involves more than the structural components of the capsid. Ad41 has been shown to efficiently infect many cell types in culture, however, unlike most serotypes of human adenovirus, Ad41 was originally described as ‘fastidious’ because historically it has been very difficult to grow in the laboratory [17]. These novel attributes stimulated our interest in Ad41 for gene transfer applications. As a first step we sought to generate a system for efficient growth of wild type and E1-deleted viruses that would enable the study and potential vaccine applications of this distinctive enteric Ad.
This article describes the development of the first E1-deleted Ad41 gene transfer vectors. The study had two aims. First, to determine if Ad41 and E1-deleted Ad41 vectors (rAd41) were efficiently propagated on an Ad5-based complementing cell line. The 293-ORF6 packaging cell line for the propagation of E1- and E4-deleted Ad5 vectors has been described [18]. This cell line allows efficient propagation of E1-deleted vectors based on Ad7, a species B adenovirus [19,20]. These results prompted us to investigate the complementation capabilities of 293-ORF6 cells for wild type Ad41 and E1-deleted Ad41 vectors. In this paper, we report a requirement for a serotype-specific interaction between E1B 55K and E4 ORF6 34K proteins for the rescue, replication and growth of Ad41 viruses and vectors. Second, we show that these newly developed adenovirus vectors were immunogenic. We also investigated the possibility of using combinations of adenovirus vectors to induce a high immune response to the transgene despite the presence of pre-existing neutralizing antibody. Since the seroprevalance of neutralizing antibody to Ad41 in the human population has been reported to be approximately 50% [21–24] it was important to begin to understand the effect of pre-existing adenovirus immunity on rAd41 and rAd5 vaccine vectors.
2. Material and methods
2.1 General methods
All DNA manipulations were performed following published procedures[25]. E. coli DH10B (InVitrogen, USA) was routinely used for plasmid DNA amplification. Bacteria were routinely grown in Luria Broth (LB). Ampicillin, kanamycin, and Zeocin were used at a final concentration of 100, 50 and 50 ug/mL, respectively. E1-deleted Ad41 vectors were built by the AdFast method [26] following recombination between a base plasmid and a shuttle plasmid in E. coli strain BJDE3. Cosmid DNA molecules were packaged in vitro following manufacturer’s (Stratagene, USA) recommendations. HEK-293 [27] and 293-ORF6 [18] cells were maintained as adherent cultures in DMEM with 5% fetal bovine serum. E4 ORF6 34K protein expression in 293-ORF6 cells was induced at the time of plasmid DNA transfection or viral infection by the addition of ZnCl2 (100 μM final; [18]) to the culture medium.
2.2 Construction of recombinant adenovirus vectors
Wild type Ad41 virus, strain Tak [17], was obtained from ATCC, and plaque purified on 293-ORF6 cells. Lysates prepared from plaque-purified viruses were used to isolate viral DNA following manufacturer’s recommendations (#1-858-74, Roche Diagnostics, USA). Recombinant adenovirus vectors were rescued from plasmid DNA by linearization with PmeI, and transfection of cells using PolyFect following manufacturer’s recommendations (Qiagen, Inc., USA). Three to 5 days after transfection the cells were subjected to three cycles of freeze-thaw and the resulting viral lysates were serial passaged onto fresh monolayers of cells until full cytopathic effect was observed, typically 2 passages post-transfection. Lysates were analyzed for infectious adenoviral particles by fluorescence focusing (FFU) assay with a polyclonal antibody against the Ad2 hexon protein. Infectious titers of Ad5 viruses were determined in an FFU assay with monoclonal antibody 38-2 against the Ad5 DNA binding protein [28]. The genetic identity of the viral preparations was confirmed by PCR analysis and transgene expression was confirmed by fluorescence microscopy (GFP vectors) or western blot (gp140 vectors). Virus purification was accomplished by three rounds of cesium chloride isopycnic gradient centrifugation following standard procedures. Total particle unit titer was determined by absorbance in the presence of SDS [29].
2.3 Verification of Env transgene expression
293-ORF6 cells were infected with 1000 particles per cell (between 0.5 and 2 FFU per cell, depending on the individual preparation) and the cell-associated protein was extracted with RIPA buffer at 25 h.p.i. Samples were analyzed by SDS-PAGE and western blotting using HIV-IG (AIDS Research and Reference reagent Program, cat. #3957) as a primary antibody and a goat anti-human IgG-HRP conjugate (Chemicon, Cat. #AP309P) as the secondary antibody.
2.4 Animals
Female BALB/c (H-2d) mice 8 to 10 weeks of age were purchased from Charles River Laboratories (Wilmington, Mass.). They were housed at the Vaccine Research Center animal facility in filter-topped cages on standard rodent diet and allowed to acclimate for at least one week prior to the study. The animals were cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.5 Immunizations
Mice were immunized intramuscularly (im) with adenoviral vectors (1010 particles) diluted in PBS. Mice (n = 5) were injected with 200 μl of vaccine divided equally into both quadriceps. At 14 days after each immunization, blood samples were collected and sera were isolated and frozen, the mice were sacrificed and spleens were harvested for cellular immunity assessment.
2.6 HIV-1-env specific immunoglobulin G (IgG) analysis
The enzyme-linked immunosorbent assay (ELISA) used for detecting antibodies against HIV-1 Env proteins in immunized mice sera was described elsewhere [30]. Briefly, Elisa microplates were coated with 100 μl of Galanthus nivalis lectin (Sigma, St. Louis, Mo)(10 μg/ml) per well overnight at 4°C. The lectin solution was removed and wells were blocked with 200 μl of PBS containing 10% fetal bovine serum for 2 h at room temperature. The plates were washed twice with PBS containing 0.2% Tween 20 (PBS-T). One hundred microliters of supernatant from 293 cells transfected with pVRC2801 (R5 gp140 CFI-clade B) was added to each well, and wells were incubated for an hour at room temperature. The plates were washed with PBS-T five times. Mouse sera were diluted 1:100 in PBS, 1% fetal bovine serum, and 0.02% Tween 20, were added to the HIV-1-env-containing plates and incubated for one hour at room temperature. Plates were then washed five times with PBS–T and incubated with 100 μl of horseradish peroxidase-conjugated goat anti-mouse IgG (1:5,000; Chemicon International Inc., Temecula, Calif.) for 1 h at room temperature. Plates were washed five times, and 100 μl of OPD peroxidase substrate (Sigma) was added to each well. The reaction was stopped after 30 min by addition of 50 μl of 2N H2SO4. The plates were read on an enzyme-linked immunosorbent assay (ELISA) reader at 490 nm.
2.7 Cytokine secretion analysis
Spleens from treated animals were removed aseptically, and unicellular suspensions were prepared by passing the spleens through a metal mesh. Red blood cells were lysed by suspending the splenocytes in Pharmlyse buffer (Pharmingen). Cells were then washed, and 106 cells were stimulated using HIV-1 peptide pools (15-mer peptides covering the whole Env (158 peptides) protein from HIV-1 subtype B; 11 residues overlap between each peptide (2.5-μg/ml of each peptide). Stimulation was performed in the presence of anti-CD28 and anti-CD49D antibodies (BD Pharmingen) at 1 μg/ml. Positive control stimulation was performed by using phorbol myristate acetate at 25 ng/ml (Sigma) and ionomycin at 1 μg/ml (Sigma) as stimulants. Negative controls consisted of unstimulated cells and cells stimulated with an irrelevant peptide pool (Ebola virus glycoprotein-specific peptide). Stimulation was first performed at 37°C for 1 h. Brefeldin A (10 μg/ml) (Sigma) was then added to inhibit the cytokine secretion, and stimulation was completed by incubation at 37°C for five more hours.
For the staining, stimulation medium was discarded and cells (106) were treated with anti-CD16/CD32 antibody (5 μg/ml) (BD Pharmingen) for 15 min at 4°C. Cells were centrifuged at 900 x g, 4°C, for 3 min, and cell pellets were resuspended in 150 μl of Cytofix/Cytoperm (BD Pharmingen) and incubated at room temperature for 20 min. Cells were washed in PBS–0.1% saponin (Sigma) and resuspended in 100 μl of PBS–0.1% saponin containing 0.15 μg of anti-CD3 phycoerythrin, 0.3 μg of anti-CD4 peridin chlorophyll-a protein (PerCP), 0.6 μg of anti-CD8 allophycocyanin, 0.375 μg of anti-gamma interferon (IFN-γ) fluorescein isothiocyanate, and 0.188 μg of anti-tumor necrosis factor alpha (TNF-α) fluorescein isothiocyanate. Cells were incubated with these antibodies for 25 min at 4°C. Cells were then washed and analyzed by flow cytometry for intracellular IFN-γ and TNF-α staining.
3. Results
3.1 Generation of recombinant Ad41 vectors
Wild type Ad41 (Ad41), obtained from the ATCC (#VR-930), was rendered replication incompetent by deletion of the E1 region. To generate rAd41 the Ad41 genome was rescued into a plasmid by homologous recombination (HR) in Rec+ E. coli with a small plasmid (Fig. 1, steps 1 & 2). The plasmid DNA from the Rec+ E. coli was packaged into lambda phage and used to transduce Rec- E. coli (DH10B) since transformation of CaCl2–competent cells was not suitably efficient. The complete Ad41 genome sequence was submitted to GenBank (#DQ315364). To introduce the E1 deletion and transgene expression cassette (Fig. 1, steps 4 – 8), a dual positive / negative selection cassette, BNot [26], was inserted into the E1 region by HR. The Ad41 genome plasmid was linearized by partial digestion with Cla I (Fig. 1, step 4) and recombined with the linearized BNot plasmid (Fig. 1, step 5) in E. coli by co-transformation of BJDE3 bacteria [26]. The BNot cassette was then replaced with the desired E1 deletion and expression cassette by HR. The Ad41-BNot plasmid was linearized with Not I and E. coli were transformed with this plasmid along with the appropriate shuttle plasmid to generate rAd41 vectors genomes designed to express GFP or HIV gp140 dV1V2, clade B (gp140(B)) [31] (pAd41-GFP and pAd41-gp140(B), respectively).
Figure 1. Derivation of rAd41 E1-deleted adenovectors.
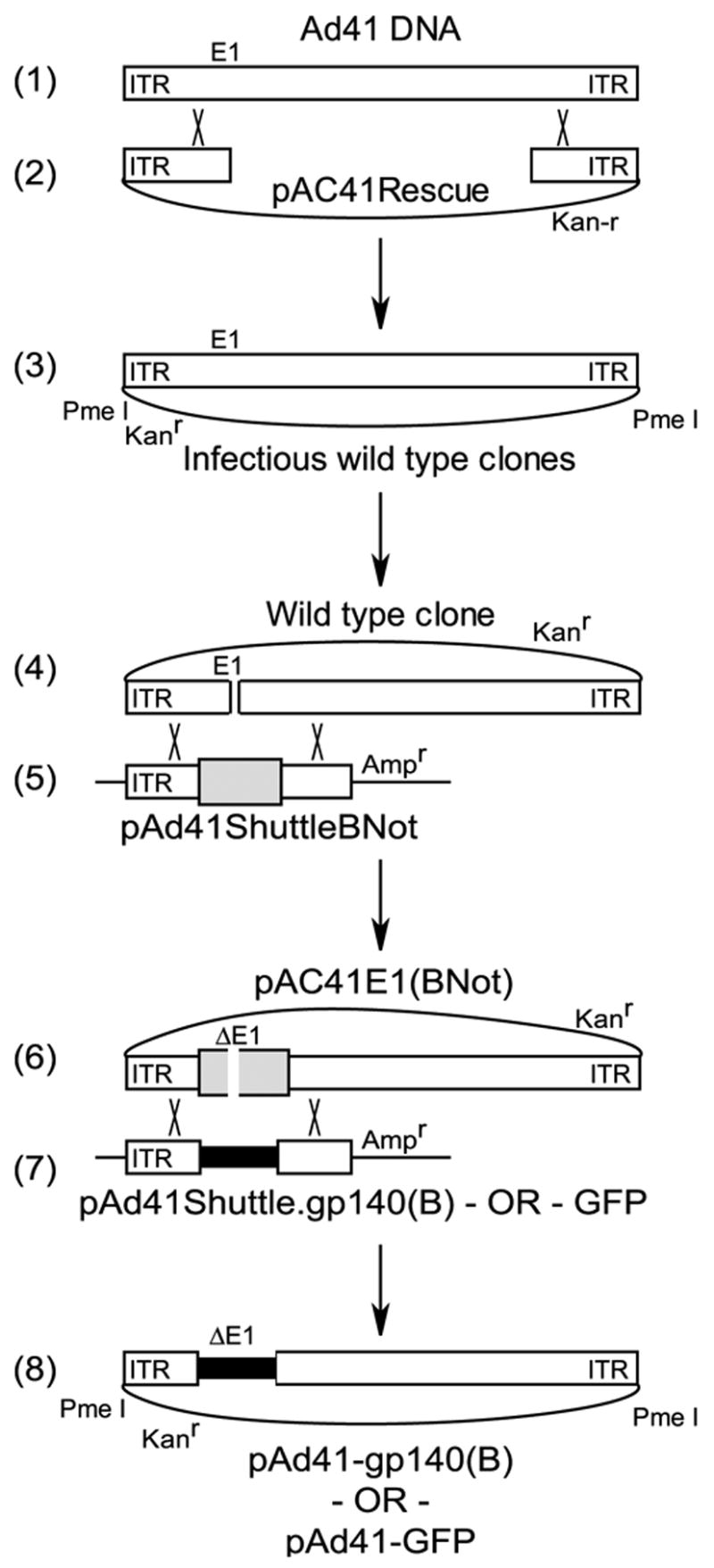
Ad41 genomic DNA (1) was recombined with a linearized small rescue plasmid (2) via homology regions of 400 bp in length to generate Ad41 wild type plasmid clones (3). Wild type plasmid clone, linearized in the E1 region by restriction enzyme digestion (4), was recombined with an Ad41 shuttle (5) with a unique Not I restriction enzyme site and a bacterial selection cassette (large gray bar). Linearization of this plasmid with Not I (6) and recombination with a transgene cassette-containing shuttle (7) yielded rAd41 vector genomes in plasmid form. (8) The rAd41 vector genomes were liberated from the plasmid backbone by Pme I digestion prior to transfection of the complementing cell line.
Since it has been previously demonstrated that Ad41 does not replicate consistently in culture, we utilized a stringent viral rescue assay to determine the viability of our plasmid clones. Cells were transfected with linearized genomic plasmids, harvested at 5 days post-transfection and passaged as freeze-thawed lysates to fresh cells for two serial passages at 5-day intervals. Infectious progeny in each lysate was determined by the FFU assay. The rescue of wild type Ad41 was efficient in 293-ORF6 cells as demonstrated by generation of high titers (1 x109 FFU/mL) at one or two passages post-transfection (Fig. 2A). Rescue was most efficient by the inclusion of exogenous Zn2+ in the culture medium, which induces the expression of the Ad5 E4 ORF6 gene. Ad41 was rescued on the 293 cell line, although nearly 100-fold less efficiently, reaching a titer of 1.6 x 107 FFU/mL. Therefore the Ad41 genome in plasmid form was infectious. The rescue of the E1-deleted rAd41, with a deletion of bases 360–3100, was dissimilar to wild type Ad41. The only culture condition resulting in efficient rescue was 293-ORF6 cells with Zn2+ induction that produced a titer of 1.8 x 108 FFU/mL (Fig. 2B). 1000-fold less rAd41 viral progeny was detected in those 293-ORF6 lysates without E4 ORF6 induction. Low efficiency rescue and replication probably resulted from low-level expression of the Ad5 E4 ORF6 gene due to heavy metal ions in the fetal bovine serum added to the culture medium. In contrast, viral progeny were not detected in lysates from 293 cells. Recombinant Ad41 vectors with different E1 deletions were tested in the rescue assay. Ad41 vectors with E1 deletions of 480–3100 and 1417–3100 (deletion of E1B only) were successfully rescued on 293-ORF6 cells (data not shown). E1-deletions 360–3165 and 480–3165 were rescued but the expression cassettes contained spontaneous deletions. In contrast, the E1 deletion 480–1337 (deletion of E1A only) was not rescued, nor was a rAd41 with the 360–3165 deletion with the CMV cassette in the left-to-right orientation (data not shown). All subsequent experiments were performed with 360–3100 deleted rAd41 vectors.
Figure 2. Virus rescue and initial propagation in different host cells.
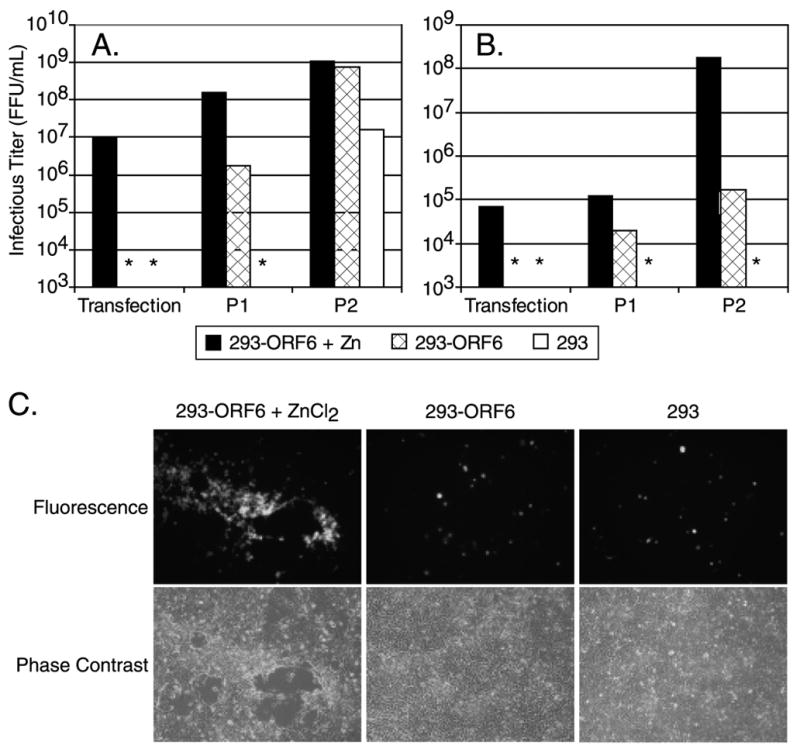
The Ad5 complementing cell lines 293-ORF6 and 293 were transfected with linearized plasmid DNA, lysates (equal volumes) were passaged to fresh monolayers at 5 days or upon the appearance of full cytopathic effect and infectious titers were determined at each passage. (A) Wild type Ad41. (B) E1-deleted rAd41. (C) Representative photomicrographs of cells from (B) 5 days post-transfection showing cytopathic effect and GFP-foci only on Zn-induced 293-ORF6. * = below level of detection.
Confirmation of rAd41 virus viability and host-cell restriction was provided by the appearance of localized cytopathic effect coincident with GFP-foci exclusively on monolayers of 293-ORF6 cells induced with Zn2+ (Fig. 2C). In contrast, the monolayers of 293 or 293-ORF6 without induction of E4 ORF6 expression showed only single GFP-positive cells resulting from transfection with the GFP expression cassette without virus replication and spread. Thus, E1-deleted rAd41 replicated on the Ad5 complementing cell line 293-ORF6 and replication was restricted to these cells.
To determine the growth characteristics of rAd41, a single burst productivity assessment was performed on 293-ORF6 cells. The FFU titers of the E1-deleted rAd41 infected cell lysates and the E1-, E3-, E4-deleted rAd5, which is also dependent on E1 and E4 complementation for growth, were determined by an FFU assay using an Ad2 hexon antiserum. The particle to FFU ratio of the rAd41 and rAd5 stocks was 39 and 16, respectively. Thus, the input viral particles (pu) of rAd41 was 3.9 pu/cell at 0.1 MOI (FFU/cell), 39 pu/cell at 1.0 MOI, and 195 pu/cell at 5.0 MOI, while input rAd5 was 80 pu/cell at 5 MOI. The production of infectious viral progeny was comparable at similar input viral particles for rAd41 and rAd5 (Fig. 3A). The FFU titer of the E1-, E3-, E4-deleted rAd5 samples was then determined with an Ad5 DBP monoclonal antibody instead of the Ad2 hexon antiserum, along with 293-ORF6 infected-cell lysates of first-generation Ad5 vector (E1-, E3-deleted) and wild type Ad5 virus. The productivity of the two rAd5 vectors was comparable to wild type Ad5, regardless of the E1 and E4 deletions (Fig 3B). This wild-type level of productivity of the E1-, E3-deleted and E1-, E3-, E4-deleted rAd5 vectors was consistent with previously reported data [18]. The kinetics of the release of progeny virions to the culture medium was dramatically different. Substantial proportions of wild type and rAd5 progeny was detected in the culture medium whereas rAd41 remained nearly 100% associated with the cells out to 5 d.p.i. (Fig. 3C). Thus, rAd41 vectors efficiently replicate in the 293-ORF6 cell line and yet remain associated with the cells significantly longer than even an E3-, E4 deleted Ad5 vector.
Figure 3. Growth characteristics of E1-deleted Ad41 in cell culture.
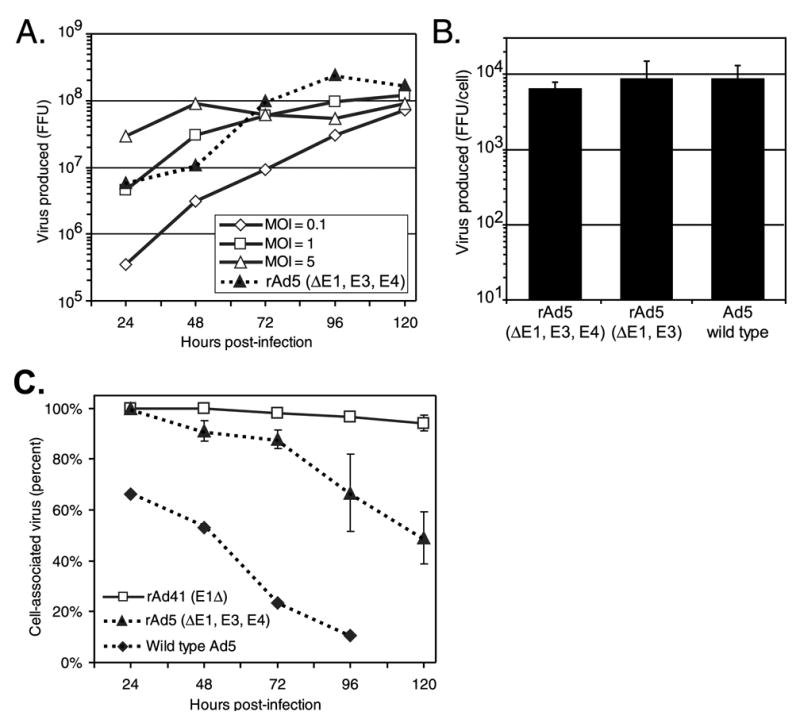
293-ORF6 cells were infected and induced with ZnCl2. Infectious viral progeny were determined in freeze-thaw lysates of total culture, cell pellets, or cell culture supernatant at the times indicated by fluorescent-focus forming unit assays. (A) Total productivity of rAd41 is comparable to E1-, E3-, E4-deleted rAd5 at an MOI = 5 as determined by an anti-Ad2 hexon antibody ffu assay. (B) Total productivity of the E1-, E3-, E4-deleted rAd5 in (A) is equivalent to wild type Ad5 and a first generation, E1-, E3- Ad5 vector. Cells were infected with an MOI = 5, harvested at 72 h.p.i., and titers were determined with an anti-Ad5 DNA-binding protein monoclonal antibody. (C) Ad41 adenovector progeny virions remain associated with the cells to 5 days post-infection.
3.2 Genetic stability of the Ad41 right-hand end
Restriction digestion analysis and DNA sequencing of two wild type Ad41 genomic plasmid clones revealed additional, unexpected, ITR sequences at the right hand end (r.h.e.) of the genome (Fig. 4A & B) although the rest of the genome matched the wild type Ad41 sequence predicted from restriction digestion analysis and sequencing of extracted wild type Ad41 DNA (data not shown). This was unexpected, since in our experience Ad5 viruses and vectors have been genetically stable. To determine if the genomic plasmid clones had authentic Ad41 genomes or were an artifact of the homologous recombination method of plasmid rescue, a restriction digestion analysis and Southern blot specific for the r.h.e. was used. The sequence of Ad41 predicts digestion with Swa I will produce a single r.h.e. fragment of 438 bp. When the two rAd41 plasmid clones were digested with Swa I single fragments were released from each that were larger, 555 bp or 1992 bp (Fig. 4C, lanes 2 and 3). The source of Ad41 DNA for the homologous recombination reactions was wild type Ad41 that had been expanded from a plaque. Swa I digestion of this DNA revealed that this genome pool was heterogeneous (Fig. 4C, lane 1). Likewise, we analyzed the wild type Ad41 viruses rescued from the plasmid clones and purified by gradient centrifugation. Swa1 digestion of these DNAs revealed a large number of bands rather than the expected single band (Fig. 4C, lanes 4 & 5). Interestingly, the size of the r.h.e. Swa I fragment both increased and decreased relative to the starting plasmid clones in these rAd41 virus populations. Taken together, this data supports the hypothesis that the r.h.e. sequences cloned into the viral genomic plasmids resulted from viral genomes present in the wild type Ad41 DNA preparation and not from an artifact of the homologous recombination method. Extensive restriction digestion analysis with a panel of enzymes did not reveal genetic variation in other regions of the virus genomes, including the left-hand end (data not shown). Similarly, PCR analysis of the E1 region and expression cassette using primers that flanked the region did not detect genome deletions (data not shown). We interpret these results to indicate that Ad41 is genetically unstable at the r.h.e. resulting in various numbers of sequence repeats.
Figure 4. The right hand end (r.h.e.) of Ad41 is unstable.
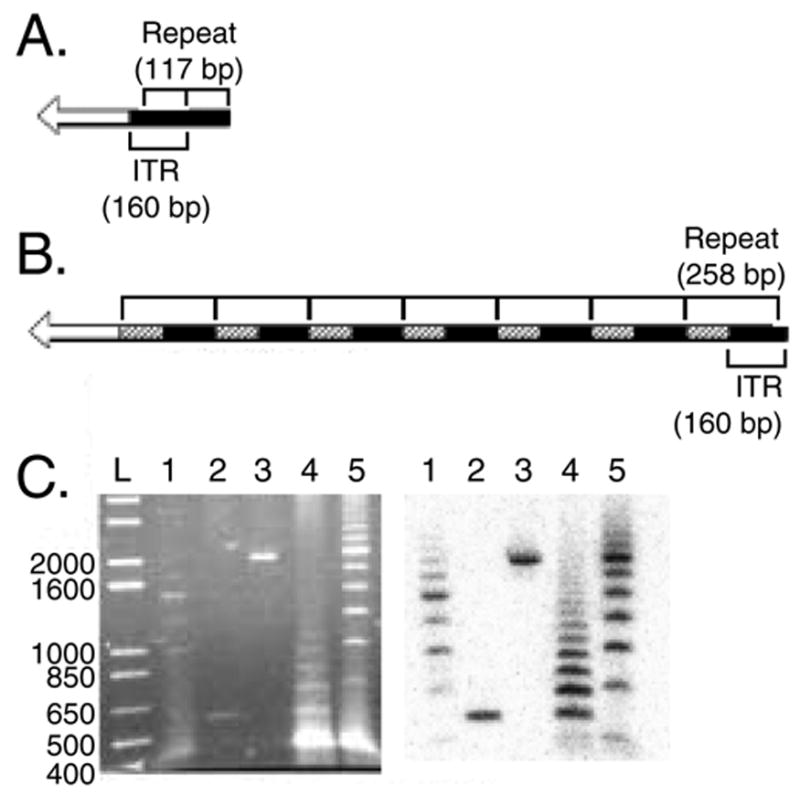
(A & B) Schematic of the repeat structure of the r.h.e. of two independently derived genomic clones. (A) The terminal 117 bp of the ITR is repeated one time. (B) The terminal 21 bp does not repeat; the adjoining 138 bp (ITR) along with 118 bp of adjoining non-ITR sequence is repeated seven times. Filled bars = ITR sequences; hatched bars = repeated non-ITR sequences; open bars = non-ITR, non-repeated sequence. (C) Restriction digestion analysis. Ethidium bromide stained gel (left panel) and Southern blot probed with a non-ITR r.h.e. sequence (right panel) of Swa I digested DNA. L = 1 kb-plus ladder; 1 = Ad41 genome pool used to derive the clones shown in (A & B); 2 = plasmid clone of Ad41 viral genome represented in (A); 3 = plasmid clone of Ad41 viral genome represented in (B); 4 = virus at three passages following rescue from the plasmid in lane 2; 5 = virus at three passages following rescue from the plasmid in lane 3. The plasmid clones were also digested with Pme I to liberate the linear viral genome from the plasmid backbone. Numbers on the left are in base pairs.
3.3 Heterologous rAd prime-boost protocols induce higher CD4+ and CD8+ responses to HIV-Env than homologous protocols using rAd5 or rAd41
After verifying that both rAd5 and rAd41 vectors expressed the HIV Env protein gp140 (B)antigen at similar levels (Fig. 5A), comparative immunogenicity of rAd5 and rAd41 vectors was analyzed in mice using various prime/boost combinations. Immune responses were analyzed by intracellular flow cytometry for IFN-γ and TNF-α with pools of peptides from HIV-1 Env. After one priming mice with rAd5, boosting with the heterologous rAd41 vector induced cellular CD4+ immune responses on average more than twice as high than those induced with a homologous boost with rAd5 (p<0.001; Fig. 5B). The heterologous boost also induced a significantly higher CD8+ response, of about 5-fold, than boosting with rAd5 (p<0.001; Fig. 5C).
Figure 5. Immunization of mice with a prime/boost strategy using heterologous viruses (Ad5, Ad41) significantly improves the CTL response compared to the homologous Ad5/Ad5 protocol.
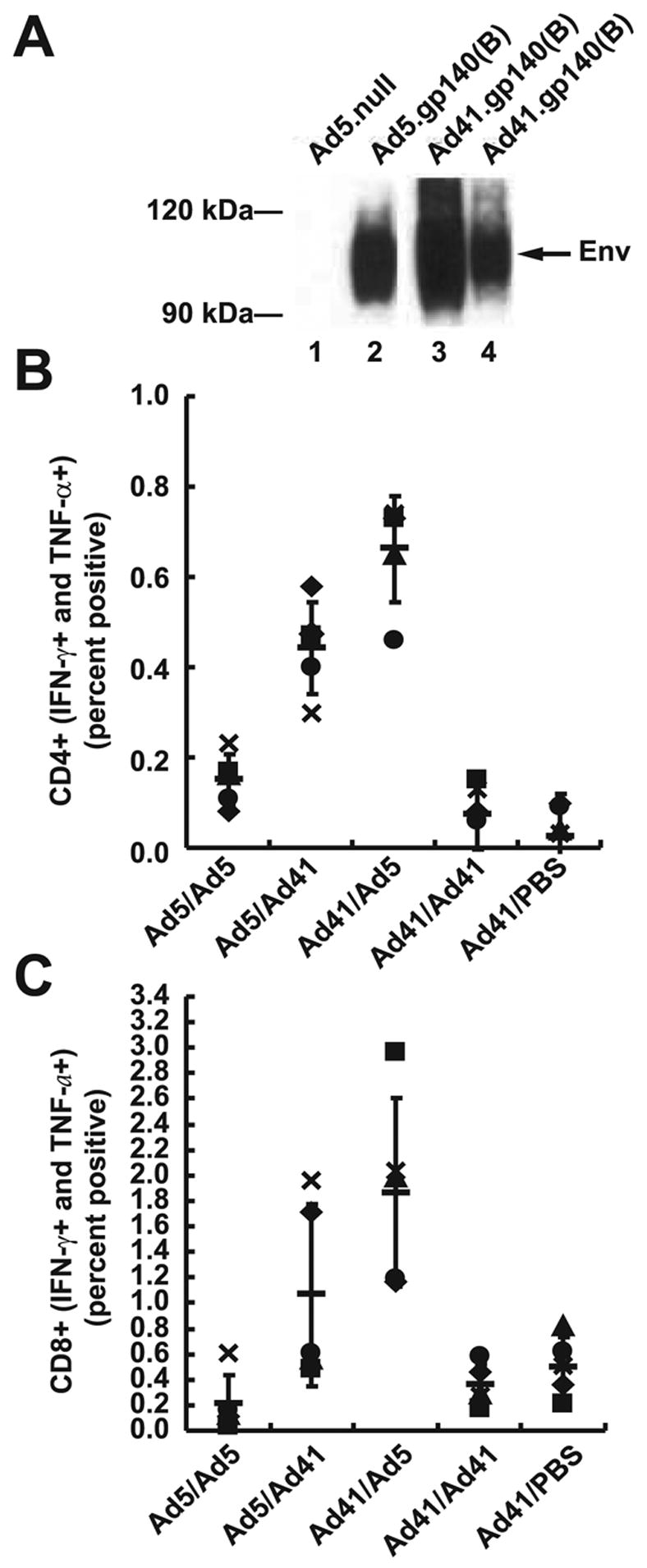
(A) The HIV Env protein gp140 (B) is expressed at similar levels from a rAd41 vector as rAd5 as determined by Western blot. Lane 1, Ad5.null; lane 2, Ad5.gp140(B);lane 3, Ad41.gp140(B); lane 4, Ad41.gp140(B) with one-fifth the quantity of protein extract of lanes 1 through 3. Balb/c mice were immunized with 1010 vp Ad5 .gp140 and/or Ad41 .gp140(B). Env-specific CD4 (B) and CD8 (C) cellular immune responses were assessed by ICS as previously described. Data shown represent individual animal data for the five mice used in each group (one symbol per animal), along with group averages (horizontal bars) and standard deviations.
Improvements of both CD4+ and CD8+ responses were even more dramatic when a heterologous prime/boost immunization was conducted using first rAd41 followed by rAd5 boost. CD4+ and CD8+ responses were 3- and 6-fold higher (p<0.001) than the rAd5/rAd5 protocol (Fig. 5B & C). The advantage provided by a heterologous prime/boost protocol compared to the homologous combination of vaccines was confirmed by comparison to homologous immunization with rAd41; CD4+ and CD8+ immune responses were significantly lower than those induced by heterologous protocols (p<0.001), and comparable to those obtained with the rAd5/rAd5 protocol (p=0.119 for CD4 (Fig. 5B) and p=0.2807 for CD8 (Fig. 5C)). Interestingly, the rAd41/rAd41 and rAd5/rAd5 responses were similar to those induced by a single injection of rAd41, consistent with elimination of Ad particles by anti-vector immunity induced by the prime.
3.4 Humoral responses in immunized mice
The systemic humoral immune response was analyzed by ELISA to characterize the HIV-1-Env specific IgG response. After a single injection, recombinant Ad5 induced significantly higher IgG immune response in sera than rAd41 vectors, which induced responses only slightly higher than negative control sera taken prior to immunization (Fig. 6A). Surprisingly, using rAd41 as a boost after Ad5 prime induced as good, but not better IgG response than the homologous rAd5 prime/boost protocol. After an Ad41 prime, boosting with rAd5 or with rAd41 significantly increased IgG immune responses, but these responses remained lower than those obtained by the protocols using Ad5 for the prime (p= 0.0168).
Figure 6. Immunogenicity of Ad5 .gp140(B) and Ad41 .gp140(B) in mice sera.
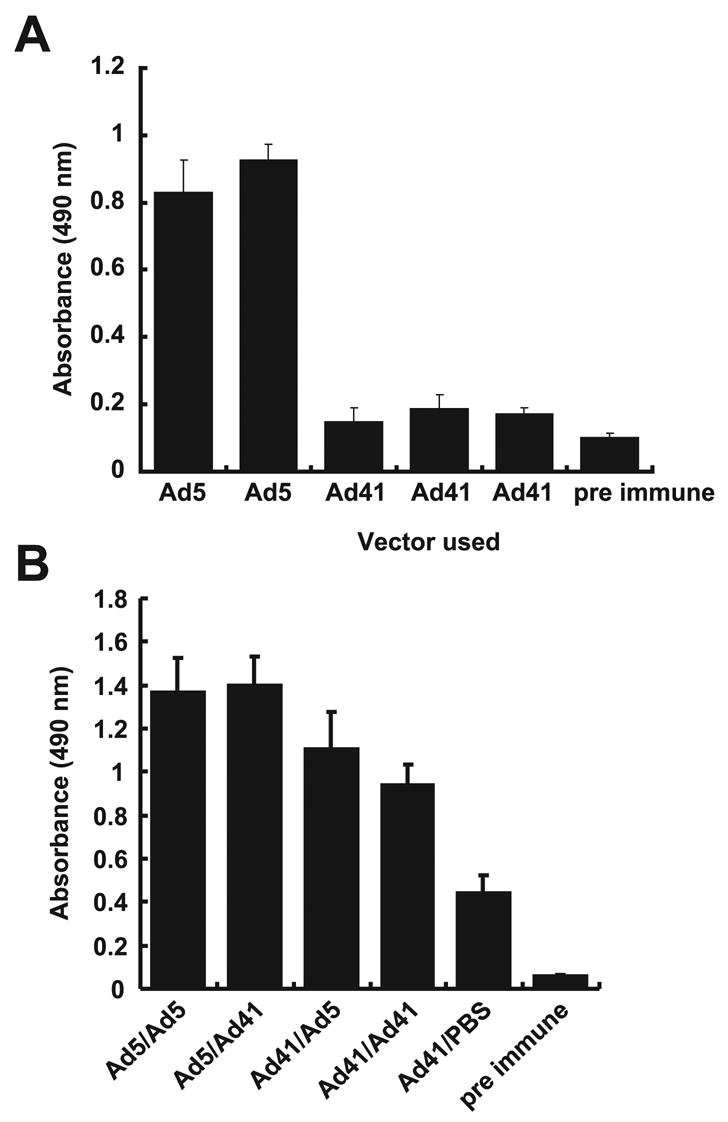
Balb/c mice were immunized with 1010 vp Ad5 .gp140(B)and/or Ad41 .gp140(B). Env-specific IgG responses in sera were assessed by Elisa as previously described at 14 days after the prime (A) and 14 days after the boost (B). Data shown are averages of the absorbances obtained for the five individual animals in each group, along with standard deviations.
Interestingly, mice that received a single Ad41 injection showed a humoral immune response that doubled with no boost over time compared to its level after the prime, suggesting a delayed induction of anti-HIV immune response by Ad41 vectors. For this reason, the improved immune responses observed with different boosts could be due in part to the delayed response to the prime; however, the response to rAd41 with no boost remained significantly below that of two rAd41 injections.
3.5 Mice develop anti-vector neutralizing antibodies after a single injection of rAd41
To verify that mice readily developed anti-Ad41 neutralizing antibodies after the first rAd41 immunization, an in vitro assay was performed using Ad41-GFP as the target virus. Ad41-GFP was used to detect the amount of neutralization by quantifying the reduction of fluorescence obtained in cells infected with this virus after preincubation with the mice sera. Preincubation of Ad41-GFP sera from mice injected with Ad41 showed a strong neutralization of the Ad41 vector. This neutralization, as shown by the reduction of fluorescence in Ad41-GFP infected cells, was seen only at low serum dilution (1:10; Fig. 7). Serum dilution of 1:100 induced an average of 50% neutralization of Ad41. Thus, rAd41 induced anti-vector antibodies in these mice. While it doesn’t affect the interpretation of the results, the apparent increase in transduction efficiency of pre-immune serum-treated rAd41 compared to rAd41 in PBS may have been mediated by serum factors as reported previously [32].
Figure 7. Ad41-specific Nab titers in sera from mice immunized once with Ad41 .gp140(B).
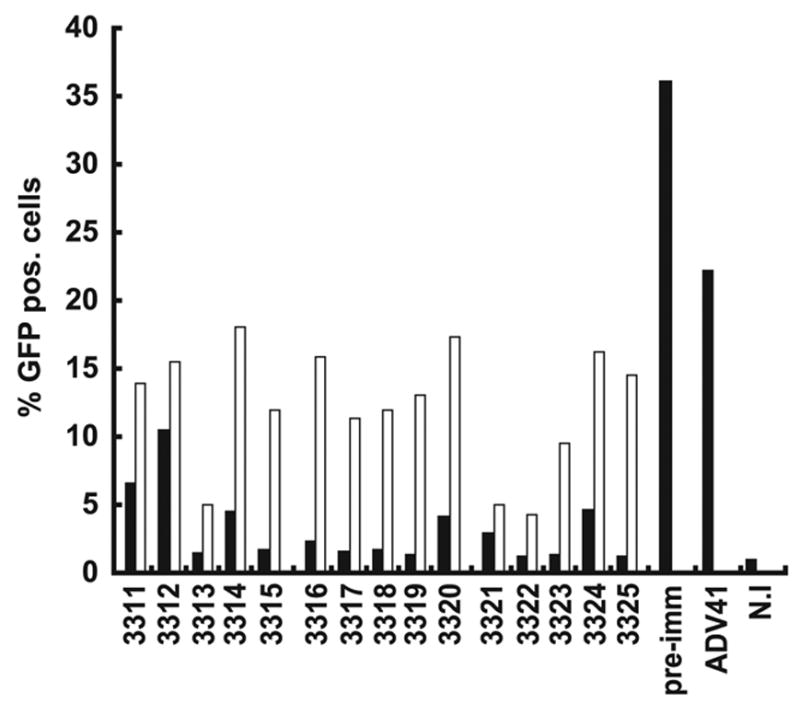
Balb/c mice were immunized with 1010 vp Ad41 .gp140(B). Sera collected 14 days after the immunization were used to test the neutralization activity against Ad41-GFP. Ad41-GFP was incubated for 30 minutes at room temperature with each diluted serum, then used to infect A549 cells (2000 vp/cell) in RPMI 2%FBS. GFP-positive cells were analyzed after 48 hours by flow cytometry. Black bars, serum diluted 1:10; white bars, serum diluted 1:100. ADV41= Ad41 incubated on cells with no serum; pre-imm = Ad41 incubated on cells after contact with preimmune mice sera.
4. Discussion
The immunogenicity of rAd41 vaccines and the extent of immunologic cross-reactivity between Ad5 and Ad41 have not previously been determined. Here, we have performed the construction of a novel Ad41-based vector and we have investigated the impact of anti-Ad5 and anti-Ad41 immunity on the immunogenicity of rAd5 and rAd41 vaccines expressing HIV Env in mice. Recombinant adenovirus type 5 (rAd5) vector-based vaccines have been shown to elicit high frequency cellular immune responses in animal models [1,2]. Candidate rAd5 vaccines for HIV-1 and other pathogens are therefore being advanced into large-scale clinical trials. Several other adenoviruses of human [33,34] and non-human origin [35–37] are being developed for gene transfer applications including vaccines. All of these serotypes are expected to induce anti-vector immunity upon administration to humans, although the prevalence of pre-existing immunity from natural exposure to the viruses may be different. The effect of this pre-existing immunity on the efficacy of rAd vaccines will not be known until human efficacy trials are completed. However, anti-vector immune responses have been found to reduce, depending on the administration route, the immunogenicity of rAd5 vaccines in animal models [38–41]. The development of non-type 5 adenovirus vector systems that elicit potent antigen-specific immune responses is therefore an important research priority. Vaccine vectors based on serologically distinct adenoviruses could allow successful repeat immunizations [42–44]. Antibodies to Ad41 have been detected in approximately 50% of people from different parts of the world but the United States population has not been assayed [21–24]. While we believe the determination of Ad41 antibody seroprevalance in more areas of the world is important it was beyond the scope of this study.
Ad41 of species F has been described as fastidious or noncultivable [17]. The growth of wild-type Ad41 on cultured cell lines has been successful to varying degrees [45,46,47,48]. The growth of Ad41 Tak on the 293 cell line has been shown to generate infectious progeny but of poor yield and quality: with a capsid assembly defect, productivity of only 1 to 15 infectious units per cell, and high particle to infectious unit ratios [47,48]. The work here demonstrated efficient rescue of both wild-type Ad41 and E1-deleted rAd41 from naked DNA using the 293-ORF6 cell line. Rescue and replication were dependent on the expression of the Ad5 E1 products and the Ad5 E4 ORF6 34K protein. The rescue of infectious Ad41 viral progeny from naked plasmid DNA was a stringent test of infectivity. The initial rescue of the genome occurred in the absence of exogenous Ad41 products. Thus, the rescue and subsequent amplification of viral progeny from serial passaging is entirely consistent with complete functional complementation by the 293-ORF6 cell line. Additionally, the number of wild type Ad41 viral progeny from 293-ORF6 cells was similar to that of wild type Ad5 (ARM, ATCC VR-1516). Despite the efficient growth of Ad41, we observed genetic instability of the right hand end of the genome. Due to the observed genetic instability it is unlikely that the specific rAd41 vectors in this study would be suitable for commercial development since several serial passages of the vector would be required. However, initial clinical testing could be feasible since the E1 region and expression cassette were stable in both the GFP and HIV Env rAd41 vectors. The cause of the right-hand end instability is unknown and we are investigating improvements to genetic stability.
Ad41 vectors with deletion of E1A and E1B were efficiently complemented by 293-ORF6 cells. The maximum productivity, approximately 100 FFU per cell, and growth kinetics of rAd41 was similar to that of rAd5 vectors (Figure 3). The infectious unit assay used here was insensitive, as it was based on an antiserum to a late protein product. Even so, there was evidence of efficient production of active particles; the average particle to FFU ratio of cesium chloride gradient purified stocks was 600 (n = 6, data not shown). The rAd5 vectors presented here were high-yielding as compared to wild type Ad5, further substantiating the efficient complementation of E1-deleted rAd41 vectors by the 293-ORF6 cell line. Furthermore, to the best of our knowledge, this is the first time a direct comparison of viral productivity of wild type Ad5, E1-, E3-deleted rAd5 and E1-, E3-, E4-deleted rAd5 has been presented. A notable difference between Ad41 and Ad5 was that the rAd41 vector was primarily cell-associated, as was reported for wild type Ad41 [47]. The E3 region of Ad41 does not encode a homolog of the adenovirus death protein E3 11.6k [49,50], thus it is likely that virions remain intracellular in these culture conditions. The method of egress and subsequent viral spread in vivo remains to be elucidated, it is possible that Ad41 does not have an active cytolytic gene product. Of note, lateral spread in culture was sufficient to generate localized areas of c.p.e. in monolayers of 293-ORF6 cells in less than 5 days. These observations of efficient complementation of Ad41 and rAd41 by the combination of Ad5 E4 ORF6 34K protein and the commonly available 293 cell line should facilitate the study of this fastidious virus. The expression of Ad5 E4 ORF6 either in trans as described here or by expression from a rAd41 vector should be equally effective for the propagation of Ad41 viruses with Ad5 E1-complementing cell lines.
Analysis of the immune response demonstrated that the rAd41-Env vaccine administered systemically elicited potent cellular and humoral immune responses that were not detectably suppressed by previous exposure to a rAd5-Env vaccine. These data suggest that Ad41 as a vaccine vector may stimulate immune responses to the transgene product, even in the presence of pre-existing anti-Ad5 immunity. In naive mice, however, rAd41-Env elicited slightly lower cellular immune responses and substantially lower humoral immune responses, even after homologous boost, compared to Ad5-Env, when measured 14 days post-immunization. These differences in immunogenicity are similar to findings from others using vectors based on serotype 35 [33].
As a first step toward developing a new vaccine vector system, the present study explored the immunogenicity of rAd41 following intramuscular injection. It is unlikely that the full potential of rAd41 would be realized by intramuscular injection. For pathogens such as HIV that are transmitted mainly through mucosal surfaces, rAd41 vectors may be of particular interest. Although several human adenovirus serotypes can replicate in the gut it has been proposed that species F adenoviruses have a unique mucosal tropism, possibly mediated by the two fiber proteins [11,51]. This natural biology may thus provide a vaccine vector system that is resistant to the hostile gut environment. Ad41 vectors therefore present a potentially significant advantage over Ad5 vectors by offering the possibility to be administered orally without enteric encapsulation and induce mucosal immunity through infection of the intestine, thereby decreasing the likelihood of HIV transmission by via the mucosa. Oral immunization may have the additional advantage of providing a means of delivering the vaccine vector in the presence of pre-existing vector immunity, whether either maternal, from natural infection, or from previous vector exprosure [52]. We are actively investigating the mucosal immune response generated by rAd41 vectors.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, Vaccine Research Center, NIAID. The authors would like to thank Yan Chen for excellent technical assistance and Toni Miller for figure preparation. J.G.D.G, D.E.B., and C.R.K. are employees of GenVec, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sullivan NJ, Sanchez A, Rollin PE, Yang ZY, Nabel GJ. Development of a preventive vaccine for Ebola virus infection in primates. Nature. 2000;408(6812):605–609. doi: 10.1038/35046108. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan NJ, Geisbert TW, Geisbert JB, Xu L, Yang ZY, Roederer M, et al. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature. 2003;424(6949):681–684. doi: 10.1038/nature01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiver JW, Emini EA. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu Rev Med. 2004;55:355–372. doi: 10.1146/annurev.med.55.091902.104344. [DOI] [PubMed] [Google Scholar]
- 4.Couch RB, Chanock RM, Cate TR, Lang DJ, Knight V, Huebner RJ. Immunization with Types 4 and 7 Adenovirus by Selective Infection of the Intestinal Tract. Am Rev Respir Dis. 1963;88 doi: 10.1164/arrd.1963.88.3P2.394. SUPPL 394–403. [DOI] [PubMed] [Google Scholar]
- 5.Top FH, Jr, Dudding BA, Russell PK, Buescher EL. Control of respiratory disease in recruits with types 4.7 adenovirus vaccines. Am J Epidemiol. 1971;94(2):142–146. doi: 10.1093/oxfordjournals.aje.a121306. [DOI] [PubMed] [Google Scholar]
- 6.Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7(4):265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- 7.Mestecky J, Michalek SM, Moldoveanu Z, Russell MW. Routes of immunization and antigen delivery systems for optimal mucosal immune responses in humans. Behring Inst Mitt. 1997;(98):33–43. [PubMed] [Google Scholar]
- 8.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6(2):148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 9.Christensen ML. Human viral gastroenteritis. Clin Microbiol Rev. 1989;2(1):51–89. doi: 10.1128/cmr.2.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uhnoo I, Wadell G, Svensson L, Johansson ME. Importance of enteric adenoviruses 40 and 41 in acute gastroenteritis in infants and young children. J Clin Microbiol. 1984;20(3):365–372. doi: 10.1128/jcm.20.3.365-372.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Favier AL, Burmeister WP, Chroboczek J. Unique physicochemical properties of human enteric Ad41 responsible for its survival and replication in the gastrointestinal tract. Virology. 2004;322(1):93–104. doi: 10.1016/j.virol.2004.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh HY, Pieniazek N, Pieniazek D, Gelderblom H, Luftig RB. Human adenovirus type 41 contains two fibers. Virus Res. 1994;33(2):179–198. doi: 10.1016/0168-1702(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 13.Pieniazek NJ, Slemenda SB, Pieniazek D, Velarde J, Jr, Luftig RB. Human enteric adenovirus type 41 (Tak) contains a second fiber protein gene. Nucleic Acids Res. 1990;18(7):1901. doi: 10.1093/nar/18.7.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albinsson B, Kidd AH. Adenovirus type 41 lacks an RGD alpha(v)-integrin binding motif on the penton base and undergoes delayed uptake in A549 cells. Virus Res. 1999;64 (2):125–136. doi: 10.1016/s0168-1702(99)00087-8. [DOI] [PubMed] [Google Scholar]
- 15.Roelvink PW, Lizonova A, Lee JG, Li Y, Bergelson JM, Finberg RW. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol. 1998;72(10):7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schoggins JW, Gall JG, Falck-Pedersen E. Subgroup B and F fiber chimeras eliminate normal adenovirus type 5 vector transduction in vitro and in vivo. J Virol. 2003;77 (2):1039–1048. doi: 10.1128/JVI.77.2.1039-1048.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Jong JC, Wigand R, Kidd AH, Wadell G, Kapsenberg JG, Muzerie CJ. Candidate adenoviruses 40 and 41: fastidious adenoviruses from human infant stool. J Med Virol. 1983;11(3):215–231. doi: 10.1002/jmv.1890110305. [DOI] [PubMed] [Google Scholar]
- 18.Brough DE, Lizonova A, Hsu C, Kulesa VA, Kovesdi I. A gene transfer vector-cell line system for complete functional complementation of adenovirus early regions E1 and E4. J Virol. 1996;70(9):6497–6501. doi: 10.1128/jvi.70.9.6497-6501.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abrahamsen K, Kong HL, Mastrangeli A, Brough D, Lizonova A, Crystal RG, et al. Construction of an adenovirus type 7a E1A- vector. J Virol. 1997;71(11):8946–8951. doi: 10.1128/jvi.71.11.8946-8951.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nan X, Peng B, Hahn TW, Richardson E, Lizonova A, Kovesdi I, et al. Development of an Ad7 cosmid system and generation of an Ad7deltaE1deltaE3HIV(MN) env/rev recombinant virus. Gene Ther. 2003;10(4):326–336. doi: 10.1038/sj.gt.3301903. [DOI] [PubMed] [Google Scholar]
- 21.Jarecki-Khan K, Unicomb LE. Seroprevalence of enteric and nonenteric adenoviruses in Bangladesh. J Clin Microbiol. 1992;30(10):2733–2734. doi: 10.1128/jcm.30.10.2733-2734.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shinozaki T, Araki K, Ushijima H, Fujii R. Antibody response to enteric adenovirus types 40 and 41 in sera from people in various age groups. J Clin Microbiol. 1987;25(9):1679–1682. doi: 10.1128/jcm.25.9.1679-1682.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saderi H, Roustai MH, Sabahi F. Antibodies to enteric adenoviruses (Ad40 and Ad41) in sera from Iranian children. J Clin Virol. 2000;16(2):145–147. doi: 10.1016/s1386-6532(99)00071-2. [DOI] [PubMed] [Google Scholar]
- 24.Kidd AH, Banatvala JE, de Jong JC. Antibodies to fastidious faecal adenoviruses (species 40 and 41) in sera from children. J Med Virol. 1983;11(4):333–341. doi: 10.1002/jmv.1890110409. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2001. [Google Scholar]
- 26.McVey D, Zuber M, Ettyreddy D, Brough DE, Kovesdi I. Rapid construction of adenoviral vectors by lambda phage genetics. J Virol. 2002;76(8):3670–3677. doi: 10.1128/JVI.76.8.3670-3677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham FL, Smiley J, Russell WC, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 28.Cleghon V, Piderit A, Brough DE, Klessig DF. Phosphorylation of the adenovirus DNA-binding protein and epitope mapping of monoclonal antibodies against it. Virology. 1993;197(2):564–575. doi: 10.1006/viro.1993.1630. [DOI] [PubMed] [Google Scholar]
- 29.Mittereder N, March KL, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70(11):7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong WP, Huang Y, Yang ZY, Chakrabarti BK, Moodie Z, Nabel GJ. Immunogenicity of multiple gene and clade human immunodeficiency virus type 1 DNA vaccines. J Virol. 2003;77(23):12764–12772. doi: 10.1128/JVI.77.23.12764-12772.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang ZY, Chakrabarti BK, Xu L, Welcher B, Kong WP, Leung K. Selective modification of variable loops alters tropism and enhances immunogenicity of human immunodeficiency virus type 1 envelope. J Virol. 2004;78(8):4029–4036. doi: 10.1128/JVI.78.8.4029-4036.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shayakhmetov DM, Gaggar A, Ni S, Li ZY, Lieber A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol. 2005;79(12):7478–7491. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barouch DH, Pau MG, Custers JH, Koudstaal W, Kostense S, Havenga MJ. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J Immunol. 2004;172(10):6290–6297. doi: 10.4049/jimmunol.172.10.6290. [DOI] [PubMed] [Google Scholar]
- 34.Casimiro DR, Bett AJ, Fu TM, Davies ME, Tang A, Wilson KA. Heterologous human immunodeficiency virus type 1 priming-boosting immunization strategies involving replication-defective adenovirus and poxvirus vaccine vectors. J Virol. 2004;78(20):11434–11438. doi: 10.1128/JVI.78.20.11434-11438.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fitzgerald JC, Gao GP, Reyes-Sandoval A, Pavlakis GN, Xiang ZQ, Wlazlo AP. A simian replication-defective adenoviral recombinant vaccine to HIV-1 gag. J Immunol. 2003;170(3):1416–1422. doi: 10.4049/jimmunol.170.3.1416. [DOI] [PubMed] [Google Scholar]
- 36.Bangari DS, Shukla S, Mittal SK. Comparative transduction efficiencies of human and nonhuman adenoviral vectors in human, murine, bovine, and porcine cells in culture. Biochem Biophys Res Commun. 2005;327(3):960–966. doi: 10.1016/j.bbrc.2004.12.099. [DOI] [PubMed] [Google Scholar]
- 37.Loser P, Hofmann C, Both GW, Uckert W, Hillgenberg M. Construction, rescue, and characterization of vectors derived from ovine atadenovirus. J Virol. 2003;77(22):11941–11951. doi: 10.1128/JVI.77.22.11941-11951.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casimiro DR, Chen L, Fu TM, Evans RK, Caulfield MJ, Davies ME. Comparative immunogenicity in rhesus monkeys of DNA plasmid, recombinant vaccinia virus, and replication-defective adenovirus vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol. 2003;77(11):6305–6313. doi: 10.1128/JVI.77.11.6305-6313.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barouch DH, McKay PF, Sumida SM, Santra S, Jackson SS, Gorgone DA. Plasmid chemokines and colony-stimulating factors enhance the immunogenicity of DNA priming-viral vector boosting human immunodeficiency virus type 1 vaccines. J Virol. 2003;77(16):8729–8735. doi: 10.1128/JVI.77.16.8729-8735.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lemiale F, Kong WP, Akyurek LM, Ling X, Huang Y, Chakrabarti BK. Enhanced mucosal immunoglobulin A response of intranasal adenoviral vector human immunodeficiency virus vaccine and localization in the central nervous system. J Virol. 2003;77(18):10078–10087. doi: 10.1128/JVI.77.18.10078-10087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang ZY, Wyatt LS, Kong WP, Moodie Z, Moss B, Nabel GJ. Overcoming immunity to a viral vaccine by DNA priming before vector boosting. J Virol. 2003;77(1):799–803. doi: 10.1128/JVI.77.1.799-803.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mack CA, Song WR, Carpenter H, Wickham TJ, Kovesdi I, Harvey BG. Circumvention of anti-adenovirus neutralizing immunity by administration of an adenoviral vector of an alternate serotype. Hum Gene Ther. 1997;8(1):99–109. doi: 10.1089/hum.1997.8.1-99. [DOI] [PubMed] [Google Scholar]
- 43.Mastrangeli A, Harvey BG, Yao J, Wolff G, Kovesdi I, Crystal RG. “Sero-switch” adenovirus-mediated in vivo gene transfer: circumvention of anti-adenovirus humoral immune defenses against repeat adenovirus vector administration by changing the adenovirus serotype. Hum Gene Ther. 1996;7(1):79–87. doi: 10.1089/hum.1996.7.1-79. [DOI] [PubMed] [Google Scholar]
- 44.Kass-Eisler A, Falck-Pedersen E, Alvira M, Rivera J, Buttrick PM, Wittenberg BA. Quantitative determination of adenovirus-mediated gene delivery to rat cardiac myocytes in vitro and in vivo. Proc Natl Acad Sci U S A. 1993;90(24):11498–11502. doi: 10.1073/pnas.90.24.11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pieniazek D, Pieniazek NJ, Macejak D, Coward J, Rayfield M, Luftig RB. Differential growth of human enteric adenovirus 41 (TAK) in continuous cell lines. Virology. 1990;174(1):239–249. doi: 10.1016/0042-6822(90)90072-y. [DOI] [PubMed] [Google Scholar]
- 46.Takiff HE, Straus SE, Garon CF. Propagation and in vitro studies of previously non-cultivable enteral adenoviruses in 293 cells. Lancet. 1981;2(8251):832–834. doi: 10.1016/s0140-6736(81)91104-1. [DOI] [PubMed] [Google Scholar]
- 47.Brown M, Wilson-Friesen HL, Doane F. A block in release of progeny virus and a high particle-to-infectious unit ratio contribute to poor growth of enteric adenovirus types 40 and 41 in cell culture. J Virol. 1992;66(5):3198–3205. doi: 10.1128/jvi.66.5.3198-3205.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Favier AL, Schoehn G, Jaquinod M, Harsi C, Chroboczek J. Structural studies of human enteric adenovirus type 41. Virology. 2002;293(1):75–85. doi: 10.1006/viro.2001.1235. [DOI] [PubMed] [Google Scholar]
- 49.Yeh HY, Pieniazek N, Pieniazek D, Luftig RB. Genetic organization, size, and complete sequence of early region 3 genes of human adenovirus type 41. J Virol. 1996;70 (4):2658–2663. doi: 10.1128/jvi.70.4.2658-2663.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tollefson AE, Scaria A, Hermiston TW, Ryerse JS, Wold LJ, Wold WS. The adenovirus death protein (E3-11.6K) is required at very late stages of infection for efficient cell lysis and release of adenovirus from infected cells. J Virol. 1996;70(4):2296–2306. doi: 10.1128/jvi.70.4.2296-2306.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Croyle MA, Stone M, Amidon GL, Roessler BJ. In vitro and in vivo assessment of adenovirus 41 as a vector for gene delivery to the intestine. Gene Ther. 1998;5(5):645–654. doi: 10.1038/sj.gt.3300645. [DOI] [PubMed] [Google Scholar]
- 52.Xiang ZQ, Gao GP, Reyes-Sandoval A, Li Y, Wilson JM, Ertl HC. Oral vaccination of mice with adenoviral vectors is not impaired by preexisting immunity to the vaccine carrier. J Virol. 2003;77(20):10780–10789. doi: 10.1128/JVI.77.20.10780-10789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


