Abstract
Since the first fatty acid amino acid conjugate (FAC) was isolated from regurgitant of Spodoptera exigua larvae in 1997 [volicitin: N-(17-hydroxylinolenoyl)-l-glutamine], their role as elicitors of induced responses in plants has been well documented. However, studies of the biosyntheses and the physiological role of FACs in the insect have been minimal. By using 14C-labeled glutamine, glutamic acid, and linolenic acid in feeding studies of Spodoptera litura larvae, combined with tissue analyses, we found glutamine in the midgut cells to be a major source for biosynthesis of FACs. Furthermore, 20% of the glutamine moiety of FACs was derived from glutamic acid and ammonia through enzymatic reaction of glutamine synthetase (GS). To determine whether FACs improve GS productivity, we studied nitrogen assimilation efficiency of S. litura larvae fed on artificial diets containing 15NH4Cl and glutamic acid. When the diet was enriched with linolenic acid, the nitrogen assimilation efficiency improved from 40% to >60%. In the lumen, the biosynthesized FACs are hydrolyzed to fatty acids and glutamine, which are reabsorbed into tissues and hemolymph. These results strongly suggested that FACs play an active role in nitrogen assimilation in Lepidoptera larva and that glutamine containing FACs in the gut lumen may function as a form of storage of glutamine, a key compound of nitrogen metabolism.
Keywords: volicitin, insect physiology, plant defense, glutamine, insect-produced elicitors
Many plants respond to herbivory by an induced release of volatile organic compounds (VOCs), which are important chemical cues for natural enemies of the herbivores (1, 2). Numerous studies have shown that this ingenious plant defense system is triggered by substances in the regurgitants of the herbivores. The best known of these plant volatile elicitors are the fatty acid amino acid conjugates (FACs) that first were identified from beet armyworm, Spodoptera exigua, larvae (3) but later also found in several other lepidopteran species (4–8) and other insects (9, 10). Of the FACs, volicitin [N-(17-hydroxylinolenoyl)-l-glutamine], is the most active elicitor for seedlings of most cultivars of Zea mays (3, 11, 12). Of the other FACs often found in lepidopteran larvae, N-linolenoyl-l-glutamine is active in Z. mays and in several other species of plants on which it has been tested. FACs with negligible activity are glutamine conjugates with linoleic, oleic, and other minor fatty acids (6–8). Some lepidopteran species also contain glutamic acid conjugates, for example, tobacco hornworm Manduca sexta (11) and tomato hornworm M. quinquemaculata (13).
By tracking radiochemically labeled volicitin in maize leaves, Truitt et al. (14) showed that initiation of plant defenses may be mediated by volicitin-binding protein–ligand interactions. A substance(s) other than volicitin was suggested to serve as a mobile signal within the plant for systemically induced VOC emissions (15). Furthermore, application of volicitin to mechanically wounded leaves specifically induces the transcription of indole-3-glycerol phosphate lyase (Igl) and a specific sesquiterpene cyclase (stc1) in wounded as well as undamaged leaves on the same plant (16, 17).
Although significant research is conducted on the role and function of FACs in induced plant defense, studies of the biosynthesis and physiological role of FACs in the insect have been seriously neglected. It is known that the fatty acid moiety of FACs is derived from the diet of the caterpillars (18). Although the fatty acid composition of FACs approximately mimics the composition of unsaturated fatty acids in the food plant, saturated fatty acids are less preferred as substrates for FAC synthesis (19). The amino acid components of FACs appear to be restricted to glutamine and glutamic acid. Specific incorporation of glutamine into FACs was reported in Spodoptera litura (20), and a coupling enzyme for the glutamine conjugates has been isolated as a membrane-bound protein in gut tissues of M. sexta larvae (21) and in S. litura midgut tissues (22).
Surprisingly, it is still not clear what benefit insects gain from FACs that outweighs the elicitation of strong defensive reactions in plants. In 1974 Collatz and Mommsen (23) suggested that unknown compounds containing fatty acids and amino acids could serve as biosurfactants in the insect gut tract, but is that the main function of FACs? Mori et al. (6) showed that FACs accumulate in the gut lumen and can reach high concentrations (1.3–3.0 nmol/μL in total FACs in gut contents). Furthermore, degradative enzyme(s) in the midgut hydrolyzes FACs to yield free glutamine and fatty acids (5). The quantitative impact of FAC synthesis and degradation on glutamine metabolism raised the question of whether FACs might also function as a way to store glutamine in gut lumen.
We investigated this question by focusing on the glutamine-specific biosynthesis of FACs, its function for the ammonia assimilation, and the glutamine uptake in S. litura larvae. The metabolism of FACs was exploited by tracing 15N-, 13C-, and 14C-labeling components. This report offers both specifics and perspective on FAC function(s) for caterpillar physiology.
Results
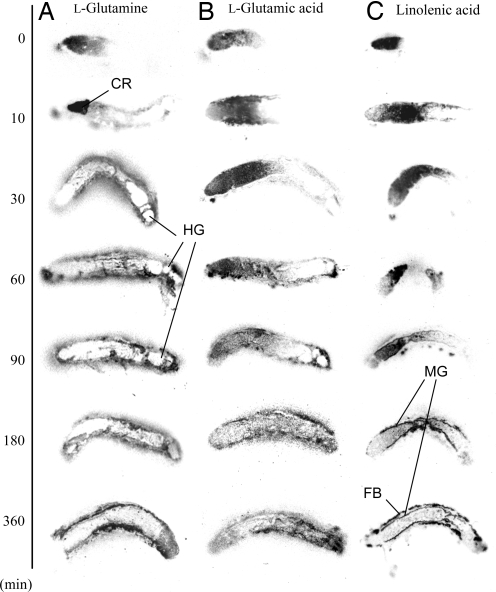
We fed l-[U-14C]glutamine, l-[U-14C]glutamic acid, or α-[1-14C]linolenic acid to caterpillars and observed the movement of labeled compounds through the insect by autoradiography. When larvae were fed [14C]glutamine, it accumulated in the crop during the first 10 min (Fig. 1A). During the following 20 min, the labeled glutamine disappeared almost completely from the gut lumen and spread into the hemolymph and fat body. The blank spot at the hindgut at 30, 60, 90, and 180 min (Fig. 1A) indicated a frass pellet containing negligible radioactivity, suggesting a nearly complete absorption of glutamine. However, radiolabeled glutamic acid appeared to migrate slowly through the gut tract to the midgut (Fig. 1B). Although a trace of radioactivity appeared in the hemolymph and fat body after 30 min, most glutamic acid remained in the gut tract for the duration of the 360-min experiment. Labeled linolenic acid did not appear to spread through the hemolymph but rather to accumulate mainly in fat body tissue (Fig. 1C).
Fig. 1.
Autoradiograms of S. litura larvae, of given times after feeding on an (A) [U-14C]glutamine-, (B) [U-14C]glutamic acid-, or (C) α-[1-14C]linolenic acid-enriched diet. Each image was a longitudinal section of larvae displayed with the cephalic end to the Left. CR, crop; HG, hindgut; MG, midgut; FB, fat body.
The quantitative determination of the fate of labeled glutamine and linolenic acid was accomplished by freezing and dissecting caterpillars 90, 180, or 360 min after feeding followed by scintillation counting. As expected, the distribution of glutamine within the body (hemolymph, fat body; midgut epithelium; gut lumen) did not change after 90 min [supporting information (SI) Fig. S1A]. However, the total radioactivity decreased to ≈50% at the end of the experiment (360 min), which suggests an oxidative consumption in energy metabolism and exhalation as CO2 (24, 25). The linolenic acid count did not decrease for the duration of the experiment (Fig. S1B). However, at 360 min a major absorption into the fat body was observed. TLC analyses approximately determined the composition of linolenic acid metabolites in the fat body as triacyl glycerols, diacyl phospholipids, and free fatty acids (49, 37, and 14%, respectively).
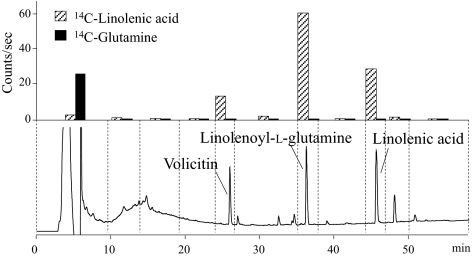
HPLC analyses of gut tissues and contents from larvae fed on labeled compounds were conducted to determine the level of incorporation into FACs. At 180 min after feeding, [14C]linolenic acid radioactivity could be detected in FACs in the gut lumen. At 360 min, almost 67% of the radioactivity in gut lumen was found in FACs, with the rest being mainly free linolenic acid and some minor uncharacterized compounds (Fig. 2). Interestingly, no detectable levels of 14C-labeled glutamine were incorporated into FACs in tissues or gut lumen during the 360-min experiment (Fig. 2). The result was the same with the 14C-labeled glutamate-feeding experiments.
Fig. 2.
Typical HPLC chromatogram and radioactivity of each fraction of gut contents extracted from larva after 360 min of feeding on α-[1-14C]linolenic acid- (striped columns) and [U-14C]glutamine-enriched diet (filled columns).
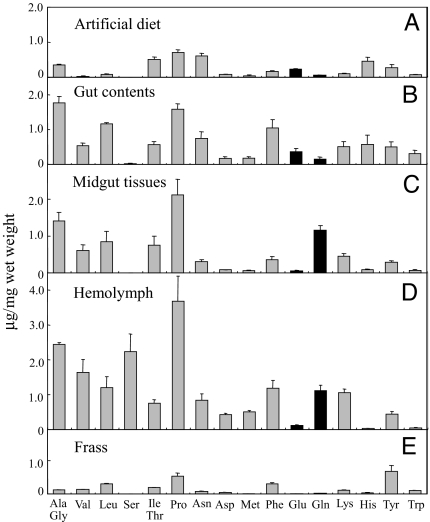
Amino acid analyses of gut contents, midgut tissues, hemolymph, fat body, and frass of S. litura larvae (Fig. 3) showed an inversion of the glutamine/glutamic acid ratio (from 0.40 to 21.5) between gut contents and midgut tissues. Because the larvae were fed on artificial diets with a decent amount of glutamic acid (Fig. 3A), the high content of glutamic acid in the gut content was expected. The lower level of glutamic acid in gut cells and hemolymph was also not surprising considering that glutamic acid generally functions as a neurotransmitter in nerve/muscle junctions and, in locust, this function is impaired if glutamic acid is present in the hemolymph (26). However, the fact that glutamine had the 3rd-highest concentration of amino acids in the midgut tissue (despite a 13th-place component in the diet; Fig. 3 C and A) indicated a conversion from glutamic acid to glutamine in the gut lumen or tissue.
Fig. 3.
Amino acid composition of artificial diet (A), gut contents (B), midgut tissues (C), hemolymph (D), and frass (E). The data were calculated as micrograms per milligram (wet weight) and are shown as mean (± SEM, n = 3).
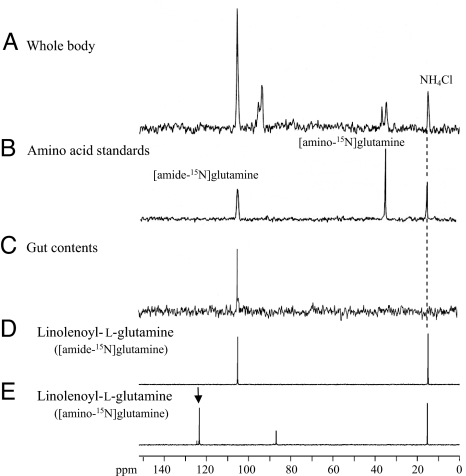
Therefore, the ammonia assimilation into glutamine was further studied by 15N NMR analyses of whole bodies of S. litura larvae (Fig. 4A) that had been fed on [15N]NH4Cl- and glutamic acid-enriched diet. The analyses showed the presence of both amino-15N- and amide-15N-labeled glutamines at 35.6 ppm and 106.7 ppm, respectively (Fig. 4 A and B). Of the labeled glutamines, [amide-15N]glutamine was synthesized directly from 15N-labeled ammonia and glutamic acid by glutamine synthetase (GS) (27).
Fig. 4.
15N NMR spectra of S. litura larval whole body (A), authentic [amino-15N]glutamine and [amide-15N]glutamine (B), gut content extracts (C), N-linolenoyl-l-glutamine synthesized with amide-15N-labeled glutamine (D), and N-linolenoyl-l-glutamine synthesized with amino-15N-labeled glutamine (E), measured at 50.55 MHz. [15N]Ammonium chloride at 15.5 ppm was used for the internal standard.
The ammonia assimilation into FACs was studied by liquid chromatography–mass spectrometry (LCMS) analysis of regurgitant obtained from S. litura larvae treated with artificial diets enriched with [15N]NH4Cl and glutamic acid. The labeling ratios for FACs calculated from area values of extracted ion chromatograms ([M-H]− versus [M-H + 1]− for 15N-labeled) was 21.3 ± 0.7% for N-linolenoyl-l-glutamine, 21.3 ± 0.5% for N-linoleoyl-l-glutamine, 18.1 ± 0.3% for volicitin, and 16.7 ± 0.4% for N-(17-hydroxylinoleoyl)-l-glutamine. These results clearly revealed that a 15N-labeled nitrogen atom was incorporated into volicitin and the other FACs. However, 15N NMR analyses of FACs, partially purified from the gut contents to remove other possible 15N-labeled contamination such as amino acids, gave only one signal at 106.7 ppm (Fig. 4C), which is the same chemical shift with as that of N-linolenoyl-glutamine synthesized with amide-15N-labeled glutamine (both conjugated and free amide-15N-labeled glutamine have signals at the same chemical shift). These results strongly indicate that of the two possible 15N-labeled forms of glutamine, only [amide-15N]glutamine was used for FACs synthesis. This suggested a rapid GS-driven conversion of glutamic acid and [15N]ammonia to [amide-15N]glutamine either in the gut content itself or in the gut tissue and that FACs may be involved in nitrogen assimilation and in the glutamine–glutamic acid conversion.
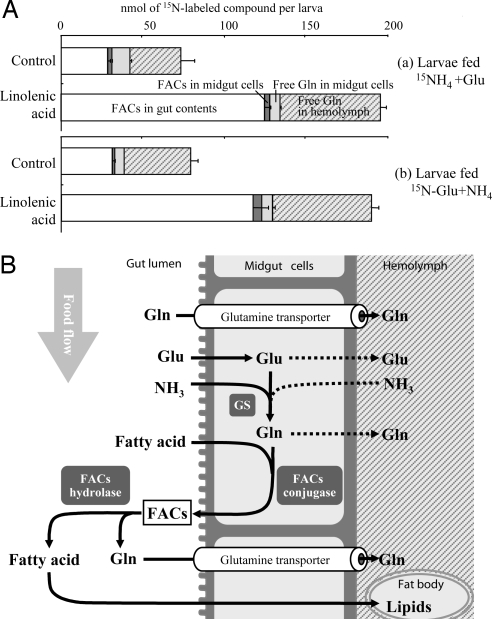
To demonstrate the role of linolenic acid on nitrogen assimilation, S. litura larvae were fed a diet enriched with glutamic acid and ammonium chloride, with or without the addition of linolenic acid. Comparison of the amount of nitrogen in the diet with that excreted in the frass by the larvae showed that larvae on the nitrogen-enriched diet were able to assimilate 37.7 ± 1.2% (n = 3) of its nitrogen contents. However, adding linolenic acid to the diet increased the nitrogen assimilation efficiency to 60.6 ± 0.8% (n = 5). The experiments were repeated with 15N labeling on ammonium chloride or glutamic acid to see in detail how linolenic acid worked for nitrogen assimilation. In both labeling cases, linolenic acid in the diet approximately doubled the total amount of labeled glutamine in the hemolymph (Fig. 5A). Furthermore, amounts of 15N-labeled FACs in the gut contents almost quadrupled with increased linolenic acid. This finding suggests that linolenic acid in some way increased the synthesis of glutamine from ammonia and glutamic acid. However, although labeled glutamine in the hemolymph doubled, even more of newly synthesized labeled glutamine was found incorporated into FACs in the gut lumen (Fig. 5A). This suggests that [15N]glutamine accumulation in hemolymph might be explained by the hydrolysis of 15N-labeled FACs in gut contents as indicated in the putative model in Fig. 5B. These data strongly suggest an active role of fatty acids and FACs in nitrogen metabolism.
Fig. 5.
FAC and glutamine metabolism. (A) 15N-glutamine synthesis and distribution 16 h after feeding artificial diet enriched with [15N]ammonium chloride and glutamic acid (a) or ammonium chloride and [15N]glutamic acid (b), with or without linolenic acid (control). (B) Putative model of FAC and glutamine metabolism in S. litura larval midgut. GS, glutamine synthetase.
To study whether FACs function as storage of glutamine in S. litura larvae, we fed the larvae radioactive 14C-labeled N-linolenoyl-l-glutamine (with labeling on the linolenic acid moiety) to see whether FACs are hydrolyzed and the hydrolytic products are absorbed. After 360 min, ≈60% of the radioactivity had been absorbed into the fat body. Interestingly, no FACs were detected in the fat body or hemolymph, which strongly suggests that only (the labeled) free fatty acids were assimilated after hydrolysis of FACs. The hydrolysis of FACs in larval gut of Heliothis virescens and Helicoverpa zea has been reported (5). The same is the case for S. litura that typically excrete only trace amounts of FACs in their frass pellets when they are feeding on plant leaves. In the labeling experiment, the remaining 40% of the radioactivity was found in the gut contents and frass and was identified as hydroxylated FACs (10%), FACs (14%), and free linolenic acid (16%), which might be an artifact caused by a high concentration of FACs in the diet to detect the radioactivities of the metabolites. After the hydrolysis, free glutamine and fatty acids might be absorbed into hemolymph because only a trace amount of glutamine is usually detectable in frass when feeding on artificial diet (Fig. 3E). This is also supported by the active uptake of glutamine from gut lumen shown in Fig. 1A.
Discussion
Here, we report an active positive role of FACs in caterpillar physiology as a tradeoff against their mostly negative role of inducing plant defensive responses.
Since the report by Pare et al. (18) that glutamine was not derived from diet, the glutamine source for FACs synthesis has been in question, and this was initially the main topic of this work. Surprisingly, it turned out that glutamine in the diet was not immediately used for FACs synthesis, but rather the glutamine originated in the midgut cells. l-Glutamine in the diet was quickly transported from the gut into the hemolymph and fat body (Fig. 1). In the midgut tissue, glutamine had the 3rd-highest concentration among all amino acids; but even more interesting, there was a significant change in the glutamine/glutamic acid proportion between gut contents and midgut tissues (Fig. 3). The 15NH4Cl and glutamic acid feeding experiments strongly suggested this to be caused by a significant conversion of glutamic acid in the food to glutamine as soon as it was absorbed into midgut epithelium cells. Surprisingly, only [amide-15N]glutamine was used for FACs synthesis in the midgut tissues even though [amide-15N]glutamine, [amino-15N]glutamine, as well as [amino-15N]glutamic acid (37.6 ppm) and other metabolites were also found in the insect tissues after the feeding experiment (Fig. 4). Thus, a considerable amount of the glutamine in glutamine–FACs must have been derived from glutamic acid from the diet, and consequently GS conversion of glutamic acid to glutamine must be of importance for FACs synthesis. Conversion of glutamic acid to glutamine by GS has been reported to occur in malpighian tubes and gut epithelium (28). In addition, GS activity has also been reported in fat body and several insect tissues such as muscles, the nervous system (29), and salivary glands (27).
Experiments with N-linolenoyl-l-glutamine containing radioactively labeled linolenic acid revealed hydrolysis of these FACs and absorption of the released linolenic acid mainly into fat body. It took 360 min for half of the FACs to be hydrolyzed and the radioactive linolenic acid to be absorbed into fat body, whereas free glutamine from the diet was rapidly (within 30 min) absorbed into hemolymph as mentioned above. Furthermore, some radioactivity was also observed in the frass, which was expected because fatty acids are usually detected in caterpillar frass. It is widely accepted that GS, which catalyzes the condensation of glutamic acid and ammonia, is one of the most important enzymes in nitrogen metabolism for most heterotrophs. That is true especially for herbivorous caterpillars who constantly suffer from deficiency of nitrogen nutrients. Furthermore, glutamic acid seemed to be less absorbable than glutamine (Fig. 1 A and B). This could be because glutamic acid generally functions as a neurotransmitter in hemolymph, and too much glutamic acid might cause cytotoxicity in insects (26). Disorder in GS function and glutamine shortage can easily cause death (30). Therefore, we wondered whether FACs with an obvious active role in nitrogen metabolism might even improve GS productivity in insects. As it turned out, enriching the diet with linolenic acid (2.4 times as much as original fatty acid contents) enabled larvae to assimilate more than twice as much ammonia and glutamic acid from their food (Fig. 5A) and greatly improved the total nitrogen assimilation efficiency from 40% up to >60%. Our previous article (22) suggested that S. litura larvae accumulates almost 5 times more glutamine as free amino acids in hemolymph than as FACs in gut lumen when feeding on artificial diet. However, as shown in Fig. 5A, the newly synthesized glutamine was coupled to fatty acid and secreted into the gut lumen as FACs rather than absorbed into hemolymph. FACs might function not only as a sink for glutamine by depleting glutamine in midgut cells to change the equilibrium to favor glutamine synthesis but also as a primary storage of glutamine synthesized by GS.
From our results, it is obvious that FACs have an active and much more multifaceted role in insect physiology than earlier thought. Recent data on FAC conjugase from the Tumlinson laboratory (C. Lait, M. Lobaido, A. Wiester, and J.H.T., unpublished data) together with previous hydrolysis data (5, 11) indicate that biosynthesis and hydrolysis of FACs may be regulated at different rates in 3 lepidopteran species, H. virescens, H. zea, and M. sexta. The biosynthesis, hydrolysis, and relatively rapid turnover of FACs, in combination with a very rapid assimilation of free glutamine, suggest that FACs in the gut lumen could also be considered as stored forms of glutamine, one of the key compounds for nitrogen metabolism in insects. Taken all together, we propose a putative scheme of biosynthesis of FACs in midgut tissues, as shown in Fig. 5B.
In general, lepidopteran species benefit from maximizing the growth rate. Shortening of the total larval stage might diminish the risk of parasitism (31), minimize exposure to plant defensive substances such as phenol oxidase or proteinase inhibitor, which reduce food nutrition or prevent digestion in caterpillars (32–34). Although elicitation of induced plant defenses and attraction of natural enemies by FACs are obvious tradeoffs, the positive effect on nitrogen assimilation might be a strong enough incentive.
Whether this holds true across a broad spectrum of species can be assessed by the fact that FACs appear to be significantly prevalent among lepidopteran species (N.Y., R.N., H.T.A., J.H.T., and N.M., unpublished data). However, there are some large-sized species that do not synthesize FACs. Consequently, FACs do not appear to be physiologically necessary in all species, nor is it appropriate to suggest that FACs are the only factors that affect weight gain.
FACs are not limited to Lepidoptera only. We have already reported glutamic acid and glutamine conjugates identified in crickets, larval fruit flies (10), and in katydids (9). In general, in most of the insects outside of Lepidoptera studied thus far, glutamic acid conjugates are dominant with only trace amounts of glutamine conjugates. However, the katydid is an exception. We have obviously only just initiated the study of the physiological role of FACs in insects, but in addition to the potentially important role as emulsifiers, FACs appear to have more advantageous functions worth the risk of eliciting plant defense reaction. Our results in this work set the stage for future studies that will determine the function(s) of FACs, other than as elicitors of plant defenses.
Materials and Methods
Chemicals.
Amino-15N-, amide-15N-labeled glutamine, [amino-15N]glutamic acid and [15N]ammonium chloride were obtained from Cambridge Isotope Laboratories. α-[1-14C]Linolenic acid, [U-14C]glutamine, and [U-14C]glutamic acid were purchased from American Research Chemicals.
Caterpillar Source and Rearing.
Colonies of S. litura were reared in the laboratory on an artificial diet (Insecta-LFS; Nihon Nosan Kogyo Ltd.) at 24 °C under a 16-h light/8-h dark day.
Autoradiography and Tracing Experiments Using 14C-Labeled l-Glutamine, l-Glutamic acid, and α-Linolenic Acid.
Small pieces of the artificial diet (≈10 mg) were enriched with 5 μL of l-[U-14C]glutamine, l-[U-14C]glutamic acid, or α-[1-14C]linolenic acid (1.85 MBq/500 μL of water or ethanol solution). After evaporation of the solvent, pieces of the dried diet were moistened and then were consumed completely by last instar of S. litura larvae. The caterpillars were frozen at −24 °C immediately or transferred to a piece of normal diet. After 10, 30, 60, 90, 270, or 360 min of feeding, they were also frozen. Each frozen caterpillar was sliced in half longitudinally. Autoradiograms were made with an Imaging Plate (IP) (Fujifilm) and developed after 16 h of exposure under −24 °C. IP was processed with a plate reader BAS2000 (Fujifilm), and the scanned images were obtained with image processing software BASstation version 3.0 (Fujifilm).
In the same way, larvae treated with 5 μL of l-[U-14C]glutamine and α-[1-14C]linolenic acid were frozen after 90, 180, and 360 min of feeding. The caterpillars were transected after the 3rd, 6th, and 9th segments, dividing into 4 parts: foregut–crop, midgut anterior, midgut posterior, and hindgut. Each part was further separated to gut contents, gut tissues, and hemolymph-fat body parts and then homogenized with ≈200 μL of water and/or methanol. Each portion was added to 3 mL of emulsifying liquid scintillation mixture Aquasol-2 (PerkinElmer) and analyzed with a scintillation counter LSC-1000 (Aloka).
Gut contents and frass of the caterpillars were extracted with 50% water/acetonitrile solution (vol/vol) and then analyzed by HPLC with UV detection at 200 nm (LC-10ADvp pump and SPD-M10Avp detector; Shimadzu). A reversed-phase column (YMC-Pack ODS-AMQ, 250 × 4.6 mm inner diameter; YMC) was eluted (1 mL/min) with a solvent of 40–95% acetonitrile containing 0.08% acetic acid, in water containing 0.05% acetic acid over 40 min, and then 10 min with 95/5 acetonitrile/water. The column temperature was maintained at 40 °C (CTO-10Avp column oven; Shimadzu). All peaks and fractions collected were analyzed with a liquid scintillation counter, as described above. Volicitin and N-linolenoyl-l-glutamine were identified by comparing peak retention times with those of authentic samples.
Further experiments on linolenic acid metabolites were conducted with the larvae fed α-[1-14C]linolenic acid and then dissected and analyzed after 360 min. Fat body was homogenized with acetonitrile and centrifuged at 9,000 × g for 10 min, then the residue was again extracted with ethanol/ether (4/1, vol/vol). These fractions were analyzed with TLC Wakogel F254 (Wako Pure Chemical Industries) by using hexane/ether/acetic acid (2/2/0.08) as developing solvent for simple lipids, and chloroform/methanol/water (65/25/4) for phospholipids. After iodine staining, each spot was identified with authentic standards such as monoacyl glycerol (Rf value, 0.14), diacyl glycerol (Rf value, 0.41), linolenic acid (Rf value, 0.60), triacyl glycerol (Rf value, 0.97), dilinolenoyl phosphatidylethanolamine (Rf value, 0.43), and dilinolenoyl phosphatidylcholine (Rf value, 0.13). Phospholipids were also stained with Dittmer–Lester reagent. Collected spots were analyzed with scintillation counting, as described.
14C-labeled N-linolenoyl-l-glutamine was prepared as follows. Gut contents of the caterpillars, fed on α-[1-14C]linolenic acid-enriched diets, were extracted with acetonitrile, and the radiochemically labeled N-linolenoyl-l-glutamine was purified with HPLC, as described above. A portion of the 14C-labeled N-linolenoyl-l-glutamine fraction was added to pieces of artificial diet and fed to caterpillars, and then the caterpillars were transferred onto normal diets. After 360 min, the caterpillars were frozen and dissected to obtain fat body, gut contents, and gut tissues. These parts and frass were extracted and analyzed by HPLC, as described above.
Amino Acid Analyses.
The amino acid analyses were conducted by the same method as described in ref. 22. The amino acid extracts were loaded on a cation exchange cartridge Oasis MCX (6 mL; Waters) and extracted with 4 N ammonia solution (50% water/methanol). After evaporation and dilution in 100 μL of water, the amino acid sample was mixed with 80 μL of ethanol/pyridine (4/1) mixture and 10 μL of ethyl chloroformate and gently shaken for 5 min to derivatize each amino acid by the method of Silva et al. (35). The reaction solution was extracted with 200 μL of dichloromethane. Then, aliquots (1.0 μL) of each sample were analyzed by gas chromatography–mass spectrometry (GCMS) (HP-5890 plus series II gas chromatograph with a 30 m × 0.32 mm, 0.33-μm film thickness, HP-5MS capillary column, interfaced to an HP-5989B mass spectrometer; Hewlett–Packard). The column temperature was held at 100 °C for 5 min after injection and then programmed at 10 °C/min to 290 °C.
For quantitative analyses of amino acids, the samples were analyzed by GC (HP-6850 gas chromatograph with a 25 m × 0.2 mm, 0.33-μm film thickness, HP-5 capillary column; Hewlett–Packard) under the same analytical conditions as those used for GCMS analysis.
Ammonia Assimilation Assay.
The last instar of S. litura larvae was transferred to artificial diets enriched with glutamic acid and [15N]ammonium chloride (26 mg and 14 mg per 3 g of diet, respectively). After 16 h of feeding on the enriched diets, some caterpillars were frozen at −24 °C. The icy gut contents were removed to plastic tubes and boiled immediately for 20 min to avoid enzymatic decomposition of FACs. Each gut content was added with 500 μL of 50% water/acetonitrile solution (vol/vol) containing 10 μg of N-palmitoleoyl-l-glutamine as an internal standard, then homogenized and centrifuged to obtain the supernatants for LCMS analyses. Negative ESI mass spectral measurements were carried out by an LCMS-2010A instrument (Shimadzu) combined with an HPLC system (LC-10ADvp pump, CTO-10ACvp column oven, and SCL-10AVvp system controller; Shimadzu). A reversed-phase column (Cosmosil 5C18-AR-II, 50 × 2.0 mm inner diameter; Nacalai tesque) was eluted (0.2 mL/min) with a solvent gradient of 40–95% acetonitrile containing 0.08% acetic acid, in water containing 0.05% acetic acid, over 15 min. The column temperature was maintained at 40 °C (CTO-10Avp column oven; Shimadzu). Calculation of the incorporation percentages of the labeled glutamine for volicitin was accomplished as follows: {[(m + 1)/m]sample − [(m + 1)/m]control} × 100, where (m + 1)/m is the area ratio m/z 422 to m/z 421 for volicitin, as reported in ref. 20. The “sample” represents the isotropically enriched conjugates (m + 1)/m, and the “control” is the ratio (m + 1)/m obtained for control oral secretion. The calculated results are presented as mean ± SEM of 3 replications. Incorporation percentages of the labeled glutamine for the other FACs were obtained in a similar way.
The other caterpillars were kept feeding on the enriched diets for 16–22 h and frozen for NMR analysis. The FACs fraction was obtained from 15 caterpillar gut contents and purified with ODS Sep-Pak (Waters) to remove other possible 15N-labeled contamination such as amino acids.
15N NMR Analysis.
Whole larval body was placed in a sample tube of 10-mm diameter, and NMR spectra were recorded at the parameter of inverse gated 1H decoupling with a JNM-ECX400 spectrometer (JEOL) operating at 50.55 MHz. The spectral conditions were as follows: x 90 width, 30 μs; x acquisition time, 0.4 s; x angle, 45°; x pulse, 15 μs; relaxation delay, 0.5 s. Ammonia was the external reference (0 ppm). The signal at 15.5 ppm was identified as [15N]ammonium chloride compared with an authentic standard, and this chemical shift value was used for the internal standard in the following NMR analysis.
The gut extracts concentrated and diluted with CD3OD were also analyzed in a 5-mm sample tube by single-pulse decoupling with an ECP 500 spectrometer (JEOL), with following minor changes in parameters: x 90 width, 24.8 μs; x acquisition time, 1.1 s; x angle, 90°; x pulse, 5 μs; relaxation delay, 1 s. Linolenoyl-l-glutamine with [amino-15N]glutamine and [amide-15N]glutamine were synthesized as described by Koch et al. (36). Both compounds were analyzed in the same way: ≈20 mg of each compound along with [15N]ammonium chloride (30 mg) as an internal standard was dissolved in 600 μL of water/methanol (1/5, vol/vol).
Nitrogen Assimilation Assay.
Low-nitrogen diets were used in this experiment. The low-nitrogen diets were prepared with 300 mg of artificial diet powder, Insecta LF (Nihon Nosan Kogyo Ltd.), 200 mg of agar powder, and 16.4 g of distilled water. This diet was enriched with 86.7 mg of glutamic acid and 32 mg of ammonium chloride (in total twice amount of original nitrogen contents), and then half of the diet was further enriched with 20 mg of linolenic acid (2.4 × original fatty acid amount). Caterpillars were fed on these two types of diets for 12 h as preconditioning, and frass was discarded. Then, the experiment for nitrogen assimilation assay was started, and after another 16 h, frass was collected for analysis. After recording the weight of frass pellets excreted and of the diet eaten by caterpillars, diet pieces and frass pellets were thoroughly dried and analyzed with C/N analyzer vario III (Elementar). The nitrogen assimilation efficiency (%) was calculated as follows: (1 − excreted frass nitrogen contents/ingested food nitrogen contents) × 100.
The labeling experiments were conducted basically in the same way but using either [15N]ammonia or [15N]glutamic acid instead of authentic nonlabeled compounds. The half-portion of these differently labeled diets were, respectively, further enriched with linolenic acid at the same concentration above. After 16 h of feeding, caterpillars were frozen and dissected into gut contents, gut tissues, and fat body with hemolymph. After recording net weight and volume, each part was prepared for FACs analysis by LCMS and amino acid analysis by GCMS as described.
Supplementary Material
Acknowledgments.
We thank Dr. Y. Nakagawa and Dr. M. Yamasaki for technical help and Dr. Irmgard Seidl-Adams for critical reading of the manuscript. This work carried out in part at the Radioisotope Research Center of Kyoto University and was supported by Grants-in-Aid for Scientific Research 15580090, 18580053, and 19580122; by the 21st-century COE program for Innovative Food and Environmental Studies Pioneered by Entomomimetic Sciences from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; and by Grant 2006-35607-16950 from National Research Initiative, Cooperative State Research, Education, and Extension service, U.S. Department of Agriculture.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809623105/DCSupplemental.
References
- 1.Turlings TCJ, Tumlinson JH, Lewis WJ. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;250:1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- 2.Kessler A, Baldwin IT. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- 3.Alborn HT, et al. An elicitor of plant volatiles from beet armyworm oral secretion. Science. 1997;276:945–949. [Google Scholar]
- 4.De Moraes CM, Mescher MC. Biochemical crypsis in the avoidance of natural enemies by an insect herbivore. Proc Natl Acad Sci USA. 2004;101:8993–8997. doi: 10.1073/pnas.0403248101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mori N, Alborn HT, Teal PEA, Tumlinson JH. Enzymatic decomposition of elicitors of plant volatiles in Heliothis virescens and Helicoverpa zea. J Insect Physiol. 2001;47:749–757. doi: 10.1016/s0022-1910(00)00171-2. [DOI] [PubMed] [Google Scholar]
- 6.Mori N, et al. Identification of volicitin-related compounds from the regurgitant of lepidopteran caterpillars. Biosci Biotechnol Biochem. 2003;67:1168–1171. doi: 10.1271/bbb.67.1168. [DOI] [PubMed] [Google Scholar]
- 7.Turlings TCJ, Alborn HT, Loughrin JH, Tumlinson JH. Volicitin, an elicitor of maize volatiles in oral secretion of Spodoptera exigua: Isolation and bioactivity. J Chem Ecol. 2000;26:189–202. [Google Scholar]
- 8.Pohnert G, Jung V, Haukioja E, Lempa K, Boland W. New fatty acid amides from regurgitant of lepidopteran (Noctuidae, Geometridae) caterpillars. Tetrahedron. 1999;55:11275–11280. [Google Scholar]
- 9.Alborn HT, et al. Disulfooxy fatty acids from the American bird grasshopper Schistocerca americana, elicitors of plant volatiles. Proc Natl Acad Sci USA. 2007;104:12976–12981. doi: 10.1073/pnas.0705947104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshinaga N, et al. Fatty acid amides, previously identified in caterpillars, found in the cricket Teleogryllus taiwanemma and fruit fly Drosophila melanogaster larvae. J Chem Ecol. 2007;33:1376–1381. doi: 10.1007/s10886-007-9321-2. [DOI] [PubMed] [Google Scholar]
- 11.Alborn HT, Brennan MM, Tumlinson JH. Differential activity and degradation of plant volatile elicitors in regurgitant of tobacco hornworm (Manduca sexta) larvae. J Chem Ecol. 2003;29:1357–1372. doi: 10.1023/a:1024209302628. [DOI] [PubMed] [Google Scholar]
- 12.Sawada Y, et al. Absolute configuration of volicitin from the regurgitant of lepidopteran caterpillars and biological activity of volicitin-related compounds. Biosci Biotechnol Biochem. 2006;70:2185–2190. doi: 10.1271/bbb.60133. [DOI] [PubMed] [Google Scholar]
- 13.Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol. 2001;125:711–717. doi: 10.1104/pp.125.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Truitt CL, Wei HX, Pare PW. A plasma membrane protein from Zea mays binds with the herbivore elicitor volicitin. Plant Cell. 2004;16:523–532. doi: 10.1105/tpc.017723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Truitt CL, Pare PW. In situ translocation of volicitin by beet armyworm larvae to maize and systemic immobility of the herbivore elicitor in planta. Planta. 2004;218:999–1007. doi: 10.1007/s00425-003-1173-6. [DOI] [PubMed] [Google Scholar]
- 16.Frey M, et al. An herbivore elicitor activates the gene for indole emission in maize. Proc Natl Acad Sci USA. 2000;96:14801–14806. doi: 10.1073/pnas.260499897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen B, Zheng Z, Dooner HK. A maize sesquiterpene cyclase gene induced by insect herbivory and volicitin: Characterization of wild-type and mutant alleles. Proc Natl Acad Sci USA. 2000;97:14807–14812. doi: 10.1073/pnas.240284097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pare PW, Alborn HT, Tumlinson JH. Concerted biosynthesis of an insect elicitor of plant volatiles. Proc Natl Acad Sci USA. 1998;95:13971–13975. doi: 10.1073/pnas.95.23.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aboshi T, Yoshinaga N, Noge K, Nishida R, Mori N. Efficient incorporation of unsaturated fatty acids into volicitin-related compounds in Spodoptera litura (Lepidoptera: Noctuidae) Biosci Biotechnol Biochem. 2007;71:607–610. doi: 10.1271/bbb.60546. [DOI] [PubMed] [Google Scholar]
- 20.Yoshinaga N, Sawada Y, Nishida R, Kuwahara Y, Mori N. Specific incorporation of l-glutamine into volicitin in the regurgitant of. Spodoptera litura. Biosci Biotechnol Biochem. 2003;67:2655–2657. doi: 10.1271/bbb.67.2655. [DOI] [PubMed] [Google Scholar]
- 21.Lait CG, Alborn HT, Teal PEA, Tumlinson JH. Rapid biosynthesis of N-linolenoyl-l-glutamine, an elicitor of plant volatiles, by membrane-associated enzyme(s) in Manduca sexta. Proc Natl Acad Sci USA. 2003;100:7027–7032. doi: 10.1073/pnas.1232474100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshinaga N, Morigaki N, Matsuda F, Nishida R, Mori N. In vitro biosynthesis of volicitin in Spodoptera litura. Insect Biochem Mol Biol. 2005;35:175–184. doi: 10.1016/j.ibmb.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Collatz KG, Mommsen T. The structure of the emulsifying substances in several invertebrates. J Comp Physiol. 1974;94:339–352. [Google Scholar]
- 24.Briegel H. Mosquito reproduction: Incomplete utilization of the blood meal protein for oogenesis. J Insect Physiol. 1985;31:15–21. [Google Scholar]
- 25.Zhou G, et al. Metabolic fate of 13C-labeled meal protein amino acids in Aedes aegypti mosquitoes. J Insect Physiol. 2004;50:337–349. doi: 10.1016/j.jinsphys.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Chapman RF. The Insects: Structure and Function. 4th Ed. Cambridge, UK: Cambridge Univ Press; 1998. p. 64. [Google Scholar]
- 27.Hirayama C, Konno K, Shinbo H. Utilization of ammonia as a nitrogen source in the silkworm. J Insect Physiol. 1996;42:983–988. [Google Scholar]
- 28.Levenbook L, Kuhn J. Properties and distribution of glutamine synthetase in the southern armyworm. Biochim Biophys Acta. 1962;65:219–232. doi: 10.1016/0006-3002(62)91041-7. [DOI] [PubMed] [Google Scholar]
- 29.Botham RP, Beadle DJ, Hart RJ, Potter C, Wilson RG. Synaptic vesicle depletion and glutamate uptake in a nerve–muscle preparation of the locust, Locusta migatoria L. Experientia. 1978;34:209–210. [Google Scholar]
- 30.Kutlesa NJ, Cavebey S. Insecticidal activity of glufosinate through glutamine depletion in a caterpillar. Pest Manage Sci. 2001;57:25–32. doi: 10.1002/1526-4998(200101)57:1<25::AID-PS272>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 31.Loader C, Damman H. Nitrogen content of food plants and vulnerability of Pieris rapae to natural enemies. Ecology. 1991;72:1586–1590. [Google Scholar]
- 32.Felton GW, Donato K, Del Vecchio RJ, Duffey SS. Activation of plant foliar oxidases by insect feeding reduces nutritive quality of foliage for noctuid herbivores. J Chem Ecol. 1989;15:2667–2694. doi: 10.1007/BF01014725. [DOI] [PubMed] [Google Scholar]
- 33.Constabel CP, Bergey DR, Ryan CA. Systemin activates synthesis of wound-inducible tomato leaf polyphenoloxidase via the octadecanoid defense signaling pathway. Proc Natl Acad Sci USA. 1995;92:407–411. doi: 10.1073/pnas.92.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farmer EE, Ryan CA. Interplant communication: Airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc Natl Acad Sci USA. 1990;87:7713–7716. doi: 10.1073/pnas.87.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva BM, et al. Development and evaluation of GC/FID method for the analysis of free amino acids in quince fruit and jam. Anal Sci. 2003;19:1285–1290. doi: 10.2116/analsci.19.1285. [DOI] [PubMed] [Google Scholar]
- 36.Koch T, Krumm T, Jung V, Engelberth J, Boland W. Differential induction of plant volatile biosynthesis in the lima bean by early and late intermediates of the octadecanoid-signaling pathway. Plant Physiol. 1999;121:153–162. doi: 10.1104/pp.121.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.