Abstract
New prevention strategies for use in developing countries are urgently needed to curb the worldwide HIV/AIDS epidemic. The N-terminally modified chemokine PSC-RANTES is a highly potent entry inhibitor against R5-tropic HIV-1 strains, with an inhibitory mechanism involving long-term intracellular sequestration of the HIV coreceptor, CCR5. PSC-RANTES is fully protective when applied topically in a macaque model of vaginal HIV transmission, but it has 2 potential disadvantages related to further development: the requirement for chemical synthesis adds to production costs, and its strong CCR5 agonist activity might induce local inflammation. It would thus be preferable to find a recombinant analogue that retained the high potency of PSC-RANTES but lacked its agonist activity. Using a strategy based on phage display, we set out to discover PSC-RANTES analogs that contain only natural amino acids. We sought molecules that retain the potency and inhibitory mechanism of PSC-RANTES, while trying to reduce CCR5 signaling to as low a level as possible. We identified 3 analogues, all of which exhibit in vitro potency against HIV-1 comparable to that of PSC-RANTES. The first, 6P4-RANTES, resembles PSC-RANTES in that it is a strong agonist that induces prolonged intracellular sequestration of CCR5. The second, 5P12-RANTES, has no detectable G protein-linked signaling activity and does not bring about receptor sequestration. The third, 5P14-RANTES, induces significant levels of CCR5 internalization without detectable G protein-linked signaling activity. These 3 molecules represent promising candidates for further development as topical HIV prevention strategies.
Keywords: HIV/AIDS, phage display, CCR5, PSC-RANTES
The HIV/AIDS epidemic currently affects an estimated 33 million people, with ≈2.5 million new infections per year (1). Effective prevention strategies must be developed, and approaches involving blockade of HIV transmission via the genital mucosa using topically administered substances (microbicides) (2, 3) are a high priority. The need for promising new microbicide candidates is underscored by disappointing results in recent large-scale vaccine (4) and microbicide (5, 6) trials.
We and others have shown that blockade of CCR5, the major HIV coreceptor used in person-to-person transmission, is a valid strategy for microbicide development (7, 8). Certain analogs of the native chemokine ligands of CCR5 strongly inhibit coreceptor activity (9), the most promising described so far being PSC-RANTES, an analog of the protein RANTES/CCL5 in which several nonnatural, noncoded structures are incorporated into the N-terminal region (10). PSC-RANTES is a highly potent inhibitor of CCR5-dependent HIV entry in vitro (10, 11) and provides full protection from R5-tropic SHIV infection in a macaque vaginal challenge model (7). Despite its high in vitro potency, high concentrations were required for protective activity in macaques (7), as has been seen for other microbicide candidates so far (2, 3). The need for a high dose raised fears that, despite its efficacy, a molecule-like PSC-RANTES, requiring chemical synthesis steps during production, could not be produced at a cost per dose appropriate for use in the parts of the developing world where the need is most urgent (12). If molecules based on PSC-RANTES are to be developed for use of microbicides, analogs must be identified that can be produced more cheaply.
PSC-RANTES acts via an unusual mechanism involving the induction of long-term intracellular sequestration of CCR5 (10, 13). This may be helpful for topical HIV prevention (14) because of prolonged protection of target cells after a single dose and setting a high barrier against the development of resistant viruses. However, like some other chemokine analogs with potent anti-HIV activity (15, 16), PSC-RANTES is a strong CCR5 agonist (17). Thus, events downstream of CCR5 signaling could lead to mucosal inflammation, a phenomenon known to enhance HIV infection (18). The ideal candidate CCR5 inhibitor for microbicide use would show the potency of PSC-RANTES without signaling via CCR5, but it has been suggested (19) that this would be an improbable goal, because (i) the receptor sequestration induced by PSC-RANTES and related molecules is needed for potent HIV inhibition (10), and (ii) receptor activation is an obligatory part of the CCR5 sequestration process (15, 19).
We have used a phage-display strategy to generate fully recombinant chemokine analogs with potent anti-HIV activity. Unlike PSC-RANTES, these could be produced at ultralow cost, as in the multiton production of GMP enzymes for the food and detergent industries (20). During the discovery and optimization process, we paid close attention to 3 key parameters: anti-HIV potency in vitro, capacity to induce CCR5 sequestration, and capacity to elicit G protein-linked signaling via CCR5. This has led to the discovery and initial characterization of a group of promising molecules, including highly potent inhibitors that do not detectably activate G protein-linked signaling.
Results
First Round of Optimization.
We started with two RANTES analogs that had been identified in earlier work (16). Both show anti-HIV activity in the nanomolar range, but are at least 50-fold less potent than PSC-RANTES (Table 1, First round of optimization). Notably, one (1P2-RANTES) behaves like PSC-RANTES, in that it is a strong CCR5 agonist that induces receptor sequestration, whereas the other (1P1-RANTES) shows neither detectable signaling activity on CCR5 nor receptor sequestration (Table 1, First round of optimization; see also ref. 16).
Table 1.
Optimization of anti-HIV chemokines using phage display
| Molecule | N-terminal sequence |
Anti-HIV potency, pM | CCR5 signaling activity, % max | CCR5 sequestration, % control |
|---|---|---|---|---|
| 0–1–2–3–4–5–6–7–8–9 | ||||
| PSC-RANTES | PSC-P-Y-S-S-D-T-T-P | 25 | 100 | 75 |
| Library 1 | X-S-#-X-S-S-X-#-#-# | |||
| 1P1-RANTES | L-S-P-V-S-S-Q-S-S-A | 6,600 | BD | BD |
| 1P2-RANTES | F-S-P-L-S-S-Q-S-S-A | 1,600 | 94 | 68 |
| Second round of optimization | ||||
| Library 2 | X-X-P-X-X-X-Q-#-T-P | |||
| 2P1-RANTES | F-V-P-Q-S-G-Q-S-T-P | 7,900 | BD | BD |
| 2P2-RANTES | L-V-P-Q-P-G-Q-S-T-P | 17,000 | BD | BD |
| 2P3-RANTES | Q-G-P-P-L-M-Q-T-T-P | 650 | BD | BD |
| 2P4-RANTES | M-V-P-Q-S-G-Q-S-T-P | 18,000 | BD | BD |
| 2P5-RANTES | Q-G-P-P-M-M-Q-T-T-P | 5,000 | BD | BD |
| 2P6-RANTES | Q-G-P-P-G-G-Q-T-T-P | 13,000 | 37 | 56 |
| 2P7-RANTES | F-A-P-M-S-Q-Q-S-T-S | 1,400 | 104 | 73 |
| 2P8-RANTES | Q-G-P-L-S-G-Q-S-T-P | 660 | 96 | 71 |
| 2P9-RANTES | Q-G-P-P-G-G-Q-S-T-P | 7,800 | 82 | 62 |
| Third round of optimization | ||||
| Library 5 | Q-G-P-P-L-M-X-X-X-X | |||
| 5P1-RANTES | Q-G-P-P-L-M-W-L-Q-V | 18 | BD | BD |
| 5P2-RANTES | Q-G-P-P-L-M-W-L-Q-S | 29 | BD | BD |
| 5P3-RANTES | Q-G-P-P-L-M-W-M-Q-V | 18 | BD | BD |
| 5P4-RANTES | Q-G-P-P-L-M-W-M-Q-S | 27 | BD | BD |
| 5P5-RANTES | Q-G-P-P-L-M-W-T-Q-V | 21 | BD | BD |
| 5P6-RANTES | Q-G-P-P-L-M-W-T-Q-S | 19 | BD | BD |
| 5P7-RANTES | Q-G-P-P-L-M-A-L-Q-S | 17 | BD | BD |
| 5P8-RANTES | Q-G-P-P-L-M-S-T-Q-S | 26 | BD | 35 |
| 5P9-RANTES | Q-G-P-P-L-M-S-F-Q-S | 16 | BD | 12 |
| 5P10-RANTES | Q-G-P-P-L-M-W-L-Q-T | 21 | BD | BD |
| 5P11-RANTES | Q-G-P-P-L-M-W-R-G-S | 65 | BD | BD |
| 5P12-RANTES | Q-G-P-P-L-M-A-T-Q-S | 28 | BD | BD |
| 5P13-RANTES | Q-G-P-P-L-M-W-L-G-G | 34 | BD | BD |
| 5P14-RANTES | Q-G-P-P-L-M-S-L-Q-V | 26 | BD | 35 |
| 5P15-RANTES | Q-G-P-P-L-M-S-L-S-V | 34 | 43 | 50 |
| 5P16-RANTES | Q-G-P-P-L-M-G-L-S-V | 156 | BD | 18 |
| Fourth round of optimization | ||||
| Library 6 | Q-G-P-○-§-X-X-X-X-X | |||
| 6P1-RANTES | Q-G-P-P-G-G-G-G-L-G | 6,100 | 32 | 49 |
| 6P2-RANTES | Q-G-P-P-G-D-G-G-Q-V | 2,900 | 73 | 63 |
| 6P3-RANTES | Q-G-P-P-G-D-G-G-S-V | 280 | 85 | 66 |
| 6P4-RANTES | Q-G-P-P-G-D-I-V-L-A | 21 | 88 | 70 |
| 6P5-RANTES | Q-G-P-P-G-G-G-G-Q-S | 1,500 | 51 | 65 |
| 6P6-RANTES | Q-G-P-P-G-G-G-G-T-R | 3,000 | 52 | 69 |
| 6P7-RANTES | Q-G-P-P-G-S-W-S-S-V | 30 | 45 | 59 |
| 6P8-RANTES | Q-G-P-P-M-G-G-Q-V-T | 12,000 | 34 | 56 |
| 6P9-RANTES | Q-G-P-P-G-D-T-Y-Q-A | 10,000 | 41 | 55 |
| 6P10-RANTES | Q-G-P-P-G-D-T-V-L-W | 19 | 96 | 70 |
| 6P11-RANTES | Q-G-P-P-G-S-Y-D-Y-S | 79 | 90 | 69 |
| 6P12-RANTES | Q-G-P-P-L-G-A-G-S-S | 1,900 | 15 | 27 |
| 6P13-RANTES | Q-G-P-P-L-G-S-M-G-P | 390 | 23 | 36 |
| 6P14-RANTES | Q-G-P-P-L-D-F-G-G-A | 4,300 | BD | 35 |
| 6P15-RANTES | Q-G-P-P-M-G-G-T-S-A | 1,900 | 26 | 43 |
| 6P16-RANTES | Q-G-P-P-M-Q-G-G-L-S | 290 | BD | BD |
| 6P17-RANTES | Q-G-P-P-M-M-A-G-L-S | 29 | BD | BD |
| 6P18-RANTES | Q-G-P-P-L-Q-A-S-V-T | 1,900 | BD | BD |
| 6P19-RANTES | Q-G-P-P-M-S-G-H-S-T | 840 | 25 | 37 |
| 6P20-RANTES | Q-G-P-P-M-S-A-Y-Q-V | 160 | BD | BD |
N-terminal sequences of libraries and of isolated molecules are indicated. PSC, PSC-RANTES pharmacophore [n-nonanoyl-thioprolyl1-cyclohexyl2]; X, any amino acid; #, Ala, Pro, Ser, or Thr; ○, Gly, Leu, or Pro, [sct, Gly, Leu, or Met. Anti-HIV activity, CCR5 signaling activity, and CCR5 sequestration were screened using the assays described in Methods. BD, below the detection limit for the assay in question (see Methods). The reference compound is PSC-RANTES. Library 1, 1P1-RANTES, and 1P2-RANTES were first described in ref. 16.
Second Round of Optimization.
Given the strong selection for Pro and Gln at positions 2 and 6, respectively, in the first round (Table 1, First round of optimization; see also ref. 16), in the next, we fixed these 2 positions and introduced diversity nearby (Library 2 in Table 1, Second round of optimization). Selection of Library 2 led us to identify 9 target sequences, which were synthesized and screened for anti-HIV activity, capacity to elicit signaling via CCR5, and capacity to induce CCR5 sequestration (Table 1, Second round of optimization). Five of the molecules had the nonsignaling nonsequestering profile of 1P1-RANTES, and 4 had the signaling, sequestering profile of 1P2-RANTES. The highest anti-HIV activity among the nonsignaling, nonsequestering molecules was that of 2P3-RANTES, with potency ≈10-fold higher than that of 1P1-RANTES, but still well below that of PSC-RANTES (Table 1, Second round of optimization). The most potent molecule from the signaling, sequestering group was 2P8-RANTES, which has an IC50 value similar to that of 1P2-RANTES (Table 1, Second round of optimization). Given the predominance of the N-terminal dipeptide sequence Gln0-Gly1 among the selected proteins (including 2P3-RANTES and 2P8-RANTES), we decided to generate further libraries based on these 2 lead molecules.
Third Round of Optimization.
The design of Library 5 was based on the sequence of 2P3-RANTES, the best nonsignaling nonsequestering molecule from Library 2 (Table 1, Second round of optimization). Diversity introduced into positions 6, 7, 8, and 9 (Table 1, Third round of optimization) led to the identification of 16 target sequences on screening. Overall, selection of this library appeared to favor Trp at position 6, Gln at position 8, and Ser or Val at position 9.
All 16 target sequences were synthesized and screened (Table 1, Third round of optimization) and showed anti-HIV potency significantly in excess of that of the parent molecule, 2P3-RANTES. Indeed, 14 of the 16 of the analogs tested showed anti-HIV activity indistinguishable from that of PSC-RANTES (IC50 values 15–35 pM), with only 5P11-RANTES and 5P16-RANTES showing slightly lower potency. Most of the molecules tested resembled 2P3-RANTES, with no detectable CCR5 activation and no detectable CCR5 sequestration. Notably, however, 5P8-RANTES, 5P9-RANTES, and 5P14-RANTES showed high potency and significant sequestering activity, yet no detectable signaling activity.
We chose 2 proteins from this group for further study, 5P12-RANTES as an example of a highly potent anti-HIV molecule with no detectable signaling activity and no detectable sequestering activity, and 5P14-RANTES as an example of a highly potent anti-HIV molecule that combines the capacity to induce significant levels of CCR5 sequestration without eliciting detectable signaling activity.
Fourth Round of Optimization.
The design of Library 6 (Table 1, Fourth round of optimization) was based on the sequence of 2P8-RANTES, the best signaling, sequestering molecule from library 2, with full diversity (all 20 aa) in positions 5, 6, 7, 8, and 9 and limited diversity in positions 3 (Gly, Leu, or Pro) and 4 (Gly, Leu, or Met). Screening produced 20 target sequences (Table 1, Fourth round of optimization) with overwhelming selection for Pro at position 3, and all 3 of Gly, Leu or Met represented at position 4. For positions 5, 6, 7, 8, and 9, the predominant residues selected for were Asp/Gly, Gly/Ala, Gly, Leu/Ser/Gln, and Ala/Ser/Thr/Val, respectively.
Eight of the 20 molecules had improved anti-HIV activity over the parent molecule, 2P8-RANTES, with 4 of them (6P4-RANTES, 6P7-RANTES, 6P10-RANTES, and 6P17-RANTES) having anti-HIV activity indistinguishable from that of PSC-RANTES (IC50 values 15–35 pM). Like 2P8-RANTES, both 6P4-RANTES and 6P10-RANTES showed strong CCR5 signaling and strong receptor-sequestration capacity. 6P7-RANTES showed intermediate signaling and sequestering activity, whereas 6P17-RANTES resembled the nonsignaling, nonsequestering molecules of Library 5, both in terms of activity profile and N-terminal sequence (e.g., 5P12-RANTES; Q0-G-P-P-L-M-A-T-Q-S9, 6P17-RANTES; Q0-G-P-P-M-M-A-G-L-S9). We chose 6P4-RANTES from this library for further study, as an example of a highly potent anti-HIV molecule with strong agonist activity and strong receptor sequestration activity.
Validation of Potent Anti-HIV Activity.
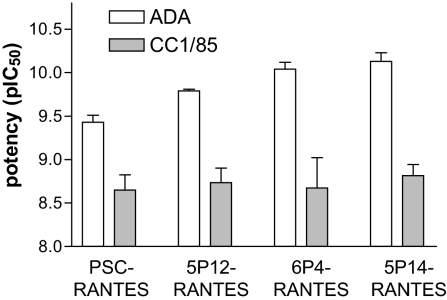
We set out to validate the promising potency of 5P12-RANTES, 5P14-RANTES, and 6P4-RANTES shown in the initial screening assay by using viral replication assays with physiological target cells and relevant R5-tropic viral strains. All 3 molecules had IC50 values indistinguishable from those of PSC-RANTES in replication assays in peripheral blood mononuclear cells (PBMC) using the laboratory R5-tropic strain ADA and the clinical isolate CC1/85 (Fig. 1). Hence, as indicated by the cell fusion assay in the initial screen, 5P12-RANTES, 5P14-RANTES, and 6P4-RANTES are highly potent anti-HIV compounds, with in vitro activity indistinguishable from that of PSC-RANTES.
Fig. 1.
Inhibition of HIV-1 infection of primary PBMC cultures by RANTES analogs. Potency (pIC50) of PSC-RANTES and new inhibitors for blocking viral infection of primary PBMC cultures with HIV-1 ADA, and the primary isolate CC1/85. Data are mean pIC50 ± SE for 3 replicate experiments.
Validation of Inhibitory Mechanism–Receptor Internalization.
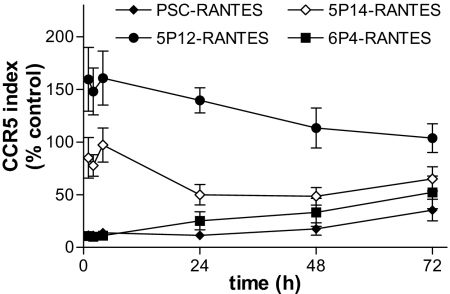
5P12-RANTES, 5P14-RANTES, and 6P4-RANTES had differing capacities to induce CCR5 sequestration in our initial screening assay. Because this assay used an artificial CCR5-expressing cell line, we investigated the capacity of the molecules to cause receptor sequestration in primary CD4+ T blasts (Fig. 2). As noted (10), brief exposure to PSC-RANTES led to prolonged intracellular sequestration of CCR5. 6P4-RANTES induced CCR5 sequestration to a similar extent and duration to PSC-RANTES. 5P12-RANTES caused an initial modest (<2-fold) increase in CCR5 staining, after which the signal rapidly returned near to control levels. 5P14-RANTES led to an intermediate level of CCR5 sequestration, reaching its maximum effect (≈50% of the reference level defined by PSC-RANTES) 24 h after exposure. Overall, these data from physiologically more authentic cells support the observations from the screening assay.
Fig. 2.
CCR5 expression after brief exposure to RANTES analogs. Changes in CCR5 staining of activated primary CD4+ T cells after a 1-h. pulse with PSC-, 5P12-, 5P14-, or 6P4-RANTES. CCR5 was detected with PA12, a monoclonal Ab directed at the N terminus of CCR5 whose binding is not affected by exposure of the receptor to native or modified RANTES compounds. Error bars show ± SE for 6 replicate experiments with different CD4+ T cell donors.
Validation of Inhibitory Mechanism–CCR5 Signaling Activity.
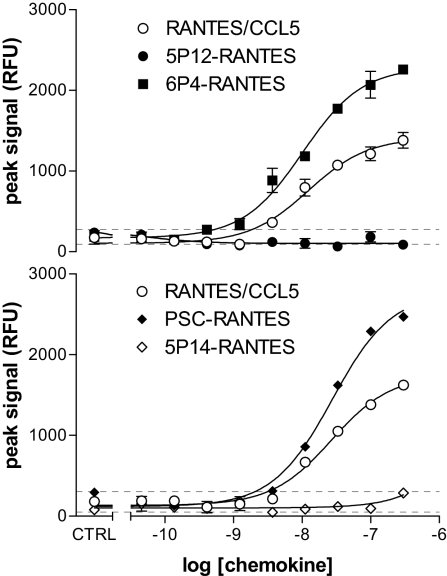
G protein-linked signaling via chemokine receptors has been extensively studied, with general agreement that G protein activation generates intracellular Ca2+ flux resulting from the direct activation of phospholipase C by released G protein βγ subunits, leading to the generation of inositol triphosphate, which triggers release of Ca2+ from intracellular stores (21, 22). Hence, Ca2+ flux can be considered as a reliable indicator of G protein activation through chemokine receptors. To confirm preliminary observations on the capacity of the analogs to elicit G protein-linked signaling in artificial CCR5-expressing cell lines (Table 1), we performed Ca2+ flux assays on CCR5-expressing primary cells, PHA/IL-2-activated T blasts. The results obtained (Fig. 3) agree with those from the initial screen: 6P4-RANTES and PSC-RANTES behave as strong agonists, and 5P12-RANTES and 5P14-RANTES show no detectable G protein-linked signaling activity.
Fig. 3.
Signaling activity of native RANTES and RANTES analogs on CCR5-expressing primary cells. Calcium flux measurements were carried out as described in Methods. Determinations in each of the 2 series shown were in duplicate; error bars indicate minimum and maximum values. Dotted lines indicate limits (min, max) for the negative controls (no chemokine).
Inhibitory Mechanism of 5P12-RANTES–Increased CCR5 Binding Affinity Is Not the Explanation.
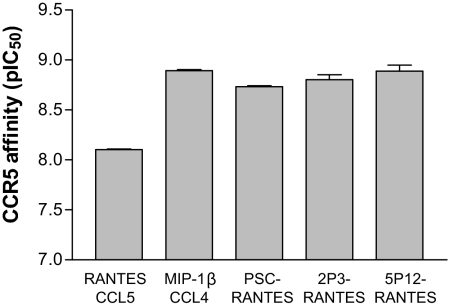
Both receptor occupancy and receptor sequestration can contribute to the inhibitory activity of both chemokines and chemokine analogs (13, 23), and we next tested the hypothesis that potent nonsequestering molecules like 5P12-RANTES owe their enhanced anti-HIV activity to increased binding affinity for CCR5. We performed CCR5 binding affinity studies on 5P12-RANTES and a range of other structurally related chemokine analogs (Fig. 4). In these experiments, the reference chemokines, native RANTES/CCL5, MIP-1β/CCL4, and PSC-RANTES gave apparent affinities in the 1- to 10-nM range, in agreement with published work (10, 17). Notably, despite showing anti-HIV potency greatly in excess of that of 2 other representative nonsignaling, nonsequestering molecules, 2P3-RANTES (23-fold) and 1P1-RANTES (240-fold) (Table 1), 5P12-RANTES has an apparent CCR5 binding affinity (1.6 nM), which is indistinguishable from that of 2P3-RANTES (1.3 nM) and slightly weaker than that determined for 1P1-RANTES in an experiment performed under identical conditions for an earlier study (0.56 nM) (16).
Fig. 4.
CCR5-binding affinity of RANTES analogs. Results of competition binding assays using 125I MIP-1β/CCL4 on CHO−CCR5 cells. The analogs exhibit similar affinity. Bars indicate mean pIC50 values ± SD, determined in 2–8 independent competition binding assays (see Methods for details).
Discussion
By refining a described phage-display-based approach (16) and using several cycles of library design, screening, and evaluation, we succeeded in identifying 3 fully recombinant molecules, 5P12-RANTES, 5P14-RANTES, and 6P4-RANTES, all of which have anti-HIV potency comparable with that of PSC-RANTES.
Structure–Activity Relationships.
In our earlier phage-display study (16), we noted selection for hydrophobic amino acids at the N-terminal position of the protein (i.e., position 0 in Table 1), analogous to the hydrophobic n-nonanoyl chain of the N-terminal extremity of PSC-RANTES. However, further optimization in the present study identified Gln0-Gly1-Pro2 as a favored motif for the N-terminal tripeptide. When Gln is at the N terminus of proteins, it spontaneously cyclizes to a pyroglutamate residue (24) with an increase in hydrophobicity. Presumably, as was the case with our introduction of chemical Nα substituents in our earlier work (e.g., ref. 10), this elimination of the α-NH2 is a better solution for receptor interaction than retaining the α-NH2 and incorporating an amino acid with a hydrophobic side chain.
Selection of 2 next-generation libraries, both carrying the N-terminal Gln0-Gly1-Pro2 motif, led to the isolation of several highly potent anti-HIV molecules, Hence, it would appear that this motif, when placed among appropriate neighboring structures, can adequately substitute for the PSC-moiety (n-nonanoyl-thioprolyl2-cyclohexylglycyl3) of PSC-RANTES (Fig. 5). Substitutions with noncoded amino acids can usually enhance bioactivity beyond the capacity of coded substitutions (e.g., ref. 25), but given the objectives of the present study, we have not pursued the option of using the chemical methods of (10) to seek to further enhance the potency of the new analogs, which is in any case already highly satisfactory.
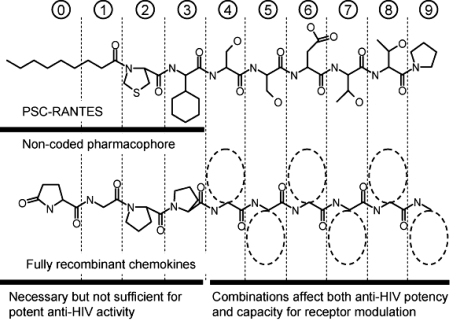
Fig. 5.
Proposed structure–activity relationship for fully recombinant RANTES analogues. Our data suggest that the tetrapeptide Gln-Gly-Pro-Pro can provide a valid replacement for the PSC-moiety of PSC-RANTES (positions 0–3), provided that it is presented in the context of appropriate combinations of downstream structures at positions 4–9. These structures determine not only anti-HIV potency but G protein-linked signaling activity and capacity to induce intracellular receptor sequestration.
Our results suggest that the downstream sequence (i.e., positions 4–9) determines not only anti-HIV potency (e.g., 5P12-RANTES, Q0-[G1-P2-P3-L4-M5-A6-T7-Q8-S9]RANTES is >20-fold more potent than 2P3-RANTES, Q0-[G1-P2-P3-L4-M5-Q6-T7-T8-P9]RANTES; Table 1) but also signaling capacity and inhibitory mechanism (Fig. 5). Hence, although 6P4-RANTES (Q0-[G1-P2-P3-G4-D5-I6-V7-L8-A9]RANTES) is a strong CCR5 agonist and induces profound and prolonged CCR5 sequestration, 5P14-RANTES (Q0-[G1-P2-P3-L4-M5-S6-L7-Q8-V9]RANTES) induces significant receptor sequestration without signaling, and 5P12-RANTES (Q0-[G1-P2-P3-L4-M5-A6-T7-Q8-S9]RANTES) neither sequesters nor signals. Further study of the total of 120 proteins produced and characterized during this study [see supporting information (SI) Table S1] might establish clear links among structure and potency, Ca2+ flux, and CCR5 modulation.
CCR5 Signaling Activity Is Not Required for Highly Potent HIV Entry Inhibition.
Previous work had suggested that the capacity of RANTES analogs to induce intracellular sequestration of CCR5 is key to their ability to potently block R5-tropic HIV entry (10, 17). This capacity was thought likely to depend on signaling activity through CCR5 (15, 19). In this study, however, we identified several analogs, including 5P12-RANTES (Table 1), that elicit neither detectable G protein-linked signaling through CCR5 (Table 1, Fig. 3) nor detectable receptor sequestration (Table 1, Fig. 1). Despite its profile as a nonsignaling, nonsequestering ligand, our data indicate that the anti-HIV mechanism of 5P12-RANTES relates to something other than “classical” receptor antagonism in which coreceptor blockade occurs through competition with virus envelope for a common binding site on CCR5. If this had been the mechanism, increased anti-HIV potency with respect to other structurally related molecules with the same profile (e.g., 1P1-RANTES, 2P3-RANTES) would have required increased CCR5 binding affinity. In reality, despite differences in anti-HIV potency spanning orders of magnitude, these molecules show almost indistinguishable CCR5 binding affinities (Fig. 4; ref. 16).
Dissociation Between CCR5 Signaling and Internalization.
GPCR ligands that sequester their receptor generally have agonist activity, although exceptions have been noted (26), including CCR5 ligands (27, 28). 5P14-RANTES now appears to be a robust example of a CCR5 ligand that does not activate G protein-linked signaling but induces intracellular sequestration (Table 1, Fig. 3). However, neither it nor any of the other nonsignaling sequestering molecules we identified in this study induce receptor sequestration to the same extent as the potent agonists PSC-RANTES and 6P4-RANTES (Table 1, Fig. 2).
Are “Nonsignaling” Molecules Entirely Devoid of Signaling Activity?
Signaling activity independent of G proteins is known for a number of GPCRs (29), and stimulation of G protein-independent signaling pathways has been clearly linked to receptor internalization (29). In addition, high concentrations of RANTES elicit receptor-independent signaling by binding to cell surface proteoglycans (30). Hence it will be necessary to establish a more complete cell signaling profile for these molecules.
Perspectives for Microbicide Development.
There is broad agreement that new, potent, and selective candidates are urgently required for the microbicide pipeline. PSC-RANTES has shown promise in preclinical studies related to this goal (7). However, the high cost of production for a noncoded macromolecule like PSC-RANTES would present a significant obstacle to its distribution at a cost per dose appropriate for use in the regions worst affected by the HIV epidemic (12). We have therefore sought to generate PSC-RANTES analogs that that could be amenable to low-cost production, either via semisynthesis (31) or, in this study, by identifying fully recombinant RANTES analogs (i.e., those containing only natural coded amino acids).
In 5P12-RANTES, 5P14-RANTES, and 6P4-RANTES, we have identified 3 highly promising candidate molecules. All 3 have in vitro anti-HIV potency comparable to that of PSC-RANTES (Fig. 1). Crucially, very recent work (R. Veazey, B. Ling, L. Green, E. Ribka, J. Lifson, et al., unpublished work) has shown that, in line with their in vitro anti-HIV potency, both 5P12-RANTES and 6P4-RANTES, like PSC-RANTES, afford full protection in the highly stringent macaque vaginal challenge model.
6P4-RANTES resembles PSC-RANTES in that it elicits profound and prolonged intracellular sequestration of CCR5 in target cells. Although this property carries theoretical advantages for microbicide development (14), CCR5 agonists could provoke enhanced susceptibility to infection, and in this regard, the nonsignaling molecules, 5P12-RANTES and 5P14-RANTES, may provide better alternatives. Further work in vivo will shed light on the extent to which receptor sequestration is required for efficacy and the extent to which signaling activity has an adverse effect on safety. These studies should enable a choice to be made as to which of these promising molecules is most appropriate for clinical development for urgently needed HIV prevention approaches.
Methods
Chemokines.
Most molecules in this study have only coded amino acid residues and could be made biosynthetically. However, for speed and efficiency, we chose to prepare small batches for initial evaluation by chemical synthesis on a modified ABI 433 peptide synthesizer customized to perform Boc chemistry with in situ neutralization, essentially as in ref. 10 (see also SI Text).
Phage Display.
Libraries were constructed and selected essentially as in ref. 16. For details, see SI Text and Table S2.
Cell Fusion Assay.
The procedure was as described in ref. 10. Each independent experiment involved a full dose-response curve (nine 5-fold serial dilutions from a top concentration of 500 nM) with each dose measurement in triplicate. IC50 values were derived from dose-inhibition curves fitted using Prism software (GraphPad). Each molecule was tested in at least 4 such independent experiments, with PSC-RANTES as a reference compound. Over the course of the study, >100 independent experiments were performed: PSC-RANTES gave a mean IC50 value of 25 pM, compatible with determined values (10, 17).
Viral Replication Assay.
PBMC were isolated as described below (see Cell Isolation and CCR5 Staining) and activated with PHA for 2 days and IL-2 for 3 additional days. Activated PBMC were exposed to half-log serial dilutions (beginning at 100 nM) of PSC-RANTES or the inhibitors for 1 h and triplicate cultures challenged with 100 tissue culture infectious doses of R5 HIV-1 laboratory isolates ADA, BaL, JR-CSF, or the clinical isolate CC1/85 [ref. 32; kindly provided by Shawn Kuhmann and John Moore (Weill Medical College of Cornell University, New York)] in the presence of the same dose of inhibitor. Virus replication was measured after 7–9 days by capsid p24 ELISA (Perkin-Elmer). IC50 values were derived from the data using Prism software (GraphPad).
Steady-State CCR5 Downmodulation Assay.
Screening for CCR5 sequestration capacity was carried out using a multiwell assay as described in ref. 17 (see also SI Text and Fig. S1). The extent of receptor sequestration induced by molecules when added to 30 nM final concentration was measured by the removal of binding sites for the anti-CCR5 mAb 3A9, whose binding is not affected by the presence of chemokines (33). Values were expressed as a percentage of the negative control (no chemokine added) with the detection threshold defined as 10% of the control value. Each molecule was tested in at least 4 independent experiments, with PSC-RANTES at its maximal receptor sequestration as the reference compound.
Cell Isolation and CCR5 Staining.
PBMC were separated from whole blood (obtained after informed consent from coded CCR5-genotyped donors from the Scripps Institute volunteer donor pool) by Ficoll–Hypaque density sedimentation. CCR5 staining was performed as described (ref. 34; see also SI Text).
Ca2+ Flux Assays.
The Ca2+ flux assay was essentially as in ref. 16 by using HeLa-P5L cells loaded with Fluo-4 dye (Molecular Probes). Each molecule was tested in at least 4 independent experiments. For each experiment, molecules were screened (n = 6) at a single concentration, 300 nM, a level at which PSC-RANTES gives a maximal signal. Signaling activity (mean peak relative fluorescence units) was expressed as a percentage of the value obtained for PSC-RANTES in the same experiment (300 nM, n = 6).
CCR5-expressing primary cells were PBMC from buffy coat preparations (Geneva University Hospital Blood Transfusion Service, Geneva) prepared as T blasts by IL-2/PHA activation (see above) for 10–14 days. Cells were loaded with Fluo-4 in 96-well plates with Ca2+ flux assays carried out as described above.
Competition Binding Assay.
Competition binding assays were done on CHO-CCR5 cells with 125I-MIP-1β/CCL4 as a labeled competitor (10, 16, 17). IC50 values were derived from the data using Prism software (GraphPad).
Supplementary Material
Acknowledgments.
This work was supported by the Esperanza Medicines Foundation, AmfAR (Grant #106455-34-RGMC), the Swiss National Science Foundation, the Mintaka Foundation for Medical Research, the La Jolla Foundation for Microbicide Research, IPM, and the National Institutes of Health [Grants RO1A152778 (to D.M.), PO1 A1 51649-01 (to O.H.), and R21A1071935 (to D.M. and O.H.)]. We appreciate crucial additional support from the James B. Pendleton Trust, CONRAD, the World Health Organization, the Fondation BIOS, and the Fondation SIDAIDE.
Footnotes
Conflict of interest statement: O.H. is the inventor on a patent application covering the new chemokine analogs described in this study, which is held by the Mintaka Medical Research Foundation, a nonprofit foundation registered in Geneva, Switzerland. O.H. and R.O. are cofounders of the Mintaka Foundation, with the roles of Chief Scientific Officer and Chief Executive Officer, respectively.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805098105/DCSupplemental.
References
- 1.United Nations AIDS/World Health Organization. AIDS Epidemic Update. Geneva: UN AIDS/World Health Organization; 2007. [Google Scholar]
- 2.Klasse PJ, Shattock RJ, Moore JP. Which topical microbicides for blocking HIV-1 transmission will work in the real world? PLoS Med. 2006;3:1501–1507. doi: 10.1371/journal.pmed.0030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lederman MM, Offord RE, Hartley O. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nat Rev Immunol. 2006;6:371–382. doi: 10.1038/nri1848. [DOI] [PubMed] [Google Scholar]
- 4.HIV vaccine failure prompts Merck to halt trial. Nature. 2007;449:390. doi: 10.1038/449390c. [DOI] [PubMed] [Google Scholar]
- 5.Bolognesi N. AIDS gel's failure calls prevention approach into question. Nat Med. 2007;13:230. doi: 10.1038/nm0307-230b. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J. AIDS research. Microbicide fails to protect against HIV. Science. 2008;319:1026–1027. doi: 10.1126/science.319.5866.1026b. [DOI] [PubMed] [Google Scholar]
- 7.Lederman MM, et al. Prevention of vaginal SHIV transmission in rhesus macaques through inhibition of CCR5. Science. 2004;306:485–487. doi: 10.1126/science.1099288. [DOI] [PubMed] [Google Scholar]
- 8.Veazey RS, et al. Protection of macaques from vaginal SHIV challenge by vaginally delivered inhibitors of virus-cell fusion. Nature. 2005;438:99–102. doi: 10.1038/nature04055. [DOI] [PubMed] [Google Scholar]
- 9.Hartley O, Offord RE. Engineering chemokines to develop optimized HIV inhibitors. Curr Protein Pept Sci. 2005;6:207–219. doi: 10.2174/1389203054065400. [DOI] [PubMed] [Google Scholar]
- 10.Hartley O, et al. Medicinal chemistry applied to a synthetic protein: Development of highly potent HIV entry inhibitors. Proc Natl Acad Sci USA. 2004;101:16460–16465. doi: 10.1073/pnas.0404802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawamura T, et al. PSC-RANTES blocks R5 human immunodeficiency virus infection of Langerhans cells isolated from individuals with a variety of CCR5 diplotypes. J Virol. 2004;78:7602–7609. doi: 10.1128/JVI.78.14.7602-7609.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore JP. Topical microbicides become topical. N Engl J Med. 2005;352:298–300. doi: 10.1056/NEJMcibr043727. [DOI] [PubMed] [Google Scholar]
- 13.Pastore C, et al. Two mechanisms for human immunodeficiency virus type 1 inhibition by N-terminal modifications of RANTES. Antimicrob Agents Chemother. 2003;47:509–517. doi: 10.1128/AAC.47.2.509-517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhmann SE, Hartley O. Targeting chemokine receptors in HIV: A status report. Annu Rev Pharmacol Toxicol. 2008;48:425–461. doi: 10.1146/annurev.pharmtox.48.113006.094847. [DOI] [PubMed] [Google Scholar]
- 15.Oppermann M, Mack M, Proudfoot AE, Olbrich H. Differential effects of CC chemokines on CC chemokine receptor 5 (CCR5) phosphorylation and identification of phosphorylation sites on the CCR5 carboxyl terminus. J Biol Chem. 1999;274:8875–8885. doi: 10.1074/jbc.274.13.8875. [DOI] [PubMed] [Google Scholar]
- 16.Hartley O, et al. Human immunodeficiency virus type 1 entry inhibitors selected on living cells from a library of phage chemokines. J Virol. 2003;77:6637–6644. doi: 10.1128/JVI.77.12.6637-6644.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaertner H, et al. Highly potent HIV inhibition: Engineering a key anti-HIV structure from PSC-RANTES into MIP-1β/CCL4. Protein Eng Des Sel. 2008;21:65–72. doi: 10.1093/protein/gzm079. [DOI] [PubMed] [Google Scholar]
- 18.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol. 2004;2:33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- 19.Vangelista L, Secchi M, Lusso P. Rational design of novel HIV-1 entry inhibitors by RANTES engineering. Vaccine. 2008;26:3008–3015. doi: 10.1016/j.vaccine.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schafer T, et al. Industrial enzymes. Adv Biochem Eng/Biotechnol. 2007;105:59–131. doi: 10.1007/10_2006_039. [DOI] [PubMed] [Google Scholar]
- 21.Thelen M. Dancing to the tune of chemokines. Nat Immunol. 2001;2:129–134. doi: 10.1038/84224. [DOI] [PubMed] [Google Scholar]
- 22.Thelen M, Stein JV. How chemokines invite leukocytes to dance. Nat Immunol. 2008;9:953–959. doi: 10.1038/ni.f.207. [DOI] [PubMed] [Google Scholar]
- 23.Trkola A, et al. Genetic subtype-independent inhibition of human immunodeficiency virus type 1 replication by CC and CXC chemokines. J Virol. 1998;72:396–404. doi: 10.1128/jvi.72.1.396-404.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abraham GN, Podell DN. Pyroglutamic acid. Nonmetabolic formation, function in proteins and peptides, and characteristics of the enzymes effecting its removal. Mol Cell Biochem. 1981;38:181–190. doi: 10.1007/BF00235695. Spec No. [DOI] [PubMed] [Google Scholar]
- 25.Kent S. Novel forms of chemical protein diversity–in nature and in the laboratory. Curr Opin Biotechnol. 2004;15:607–614. doi: 10.1016/j.copbio.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 26.Kenakin T. Collateral efficacy in drug discovery: Taking advantage of the good (allosteric) nature of 7TM receptors. Trends Pharmacol Sci. 2007;28:407–415. doi: 10.1016/j.tips.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Amara A, et al. HIV coreceptor downregulation as antiviral principle: SDF-1 alpha-dependent internalization of the chemokine receptor CXCR4 contributes to inhibition of HIV replication. J Exp Med. 1997;186:139–146. doi: 10.1084/jem.186.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanpain C, et al. Multiple active states and oligomerization of CCR5 revealed by functional properties of monoclonal antibodies. Mol Biol Cell. 2002;13:723–737. doi: 10.1091/mbc.01-03-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: The role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 30.Roscic-Mrkic B, et al. RANTES (CCL5) uses the proteoglycan CD44 as an auxiliary receptor to mediate cellular activation signals and HIV-1 enhancement. Blood. 2003;102:1169–1177. doi: 10.1182/blood-2003-02-0488. [DOI] [PubMed] [Google Scholar]
- 31.Gaertner H, Offord R, Botti P, Kuenzi G, Hartley O. Semisynthetic analogues of PSC-RANTES, a potent anti-HIV protein. Bioconjug Chem. 2008;19:480–489. doi: 10.1021/bc7003044. [DOI] [PubMed] [Google Scholar]
- 32.Connor RI, Sheridan KE, Ceradini D, Choe S, Landau NR. Change in coreceptor use coreceptor use correlates with disease progression in HIV-1−infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu L, et al. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabbe R, et al. Donor- and ligand-dependent differences in C-C chemokine receptor 5 reexpression. J Virol. 2001;75:661–671. doi: 10.1128/JVI.75.2.661-671.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.