Abstract
We found exaggerated chemotaxis in plasma treated with EDTA and thought that the EDTA might itself be inhibiting a tonic inhibitor(s) of chemotaxis. Our plasma fractionations suggested that evidence should be sought for a lipid moiety carrying this activity, and on spectrometry (LC-MS-MS together with GC-MS analyses), the biologically active but not the inactive fraction contained oleic and arachidonic acids. Because fatty acids are largely protein bound, we flooded plasma preparations with delipidated albumin, reasoning that it would bind enough fatty acids, including inhibitory ones, to counter their tonic inhibition. Indeed, we observed dramatic increases in chemotaxis. Hence, adding delipidated albumin to plasma has a similar effect to that of adding EDTA—amplification of the chemotactic response. Oleic acid in physiologic concentrations diminishes the magnifying effects of both EDTA and of delipidated albumin, and in fact diminishes the chemotactic response even without the presence of the amplifiers of chemotaxis. In contrast, arachidonic acid amplifies further the effect of EDTA but not of delipidated albumin, and this augmentation appears to be caused by an EDTA-dependent enrichment of the chemotactic gradient with leukotriene B4 (LTB4). We conclude that oleic acid, the blood levels of which vary among individuals, is at least one tonic inhibitor of chemotaxis in plasma.
Keywords: fatty acids, oleic acid, neutrophils
This work began with the videomicroscopic observation that polymorphonuclear leukocytes (PMNs) from blood anticoagulated with EDTA, but not with heparin, segregate into groups, usually around a monocyte (1, 2). The EDTA effect occurred in serum as well as in plasma (2). When the monocytes became less adhesive and moved from the center of the field, the PMNs did not follow. Rather, they ignored the monocyte, remaining focused on the place where it used to be, and often continuing to arrive in large numbers; they appeared to have taken over the generation of a chemotactic gradient, replacing the one initially provided by the adherent monocyte (2). Moreover, this prolonged chemoattraction did not depend on triggering by monocytes; we saw large aggregates accrue around PMNs containing ingested material, damaged (motionless, mummified appearance) PMNs, or detritus in the slide preparation.
To pursue the phenomenon of prolonged chemotaxis in EDTA/plasma in a more systematic approach, we used the chemoattractant gradient established by an erythrocyte destroyed by laser microirradiation (3, 4). With videomicroscopy, one can observe directly and continuously the behavior of PMN in real time before, during, and after establishment of a chemotactic gradient. Within several seconds of the laser flash, PMNs in the area turn in the direction of the newly created chemotactic target. PMNs then move to the target, and surround it for several minutes, before departing cells begin to outnumber arriving ones, presumably mirroring a decline in the exudation of chemoattractant. As in the case of chemotactic gradients initiated by other PMN-activating conditions, aggregates of PMNs in EDTA/plasma also tended to be outsized. PMNs continued to accumulate after the time corresponding to the largest accumulation of PMNs in non-EDTA controls.
Our working hypothesis was that the effects observed represent the loss of normal modulation in plasma by tonic factor(s) designed to keep the chemotactic response from getting out of hand. In this paradigm, chelation of divalent cations by EDTA would prevent the inhibitor from functioning normally. The result is a prolonged ingress of PMNs, that is, an amplified chemotactic response. This manuscript describes the search for the source of that inhibitory activity in human plasma.
Results
The Elusive “Protein.”
With the initial assumption that the inhibitor(s) was a protein, we used an approach that had been successful in isolating, for example, human IL-1 and IL-6, as well as IL-8 and NAP-2, from white blood cells (5, 6). With buffy coats from 10 L of donor blood, leukocytes are separated by standard means, and incubated for 2 days in serum-free media (RPMI) ± 10% FCS with stimulus (e.g., LPS). This treatment provides conditioned media replete with both known and as yet unidentified cytokines and chemokines, as well as, presumably, any tonic inhibitor(s). The media is concentrated and purified by selective adsorption to glass beads or by affinity chromatography employing antibodies made against the contents of such conditioned media. (For example, in this system, 10–100 ng/ml of IL-8 can be enriched to 10–100 μg.) Subsequently, these concentrates are fractionated by HPLC to obtain pure factor(s), which are then visualized by SDS/PAGE and identified by amino acid sequence analysis.
Initially in conditioned media containing FCS, the tonic inhibitor of chemotaxis, assayed by videomicroscopy, copurified with other cytokines and chemokines using controlled pore glass (CPG) chromatography. However, in a further purification step based on selective affinity for heparin, unlike chemokines, the factor showed only weak binding upon heparin-Sepharose affinity chromatography. Indeed, the biological activity eluted at 0.2- 0.6 M in the NaCl gradient, whereas chemokines did not elute from the column before 1 M NaCl was reached. In subsequent fractions, the tonic inhibitor was also present in serum-free media.
In serum-free conditioned media, proteins can usually be purified to homogeneity in a 3- rather than 5-step procedure. However, in our assay no biological activity could be recovered upon cation-exchange chromatography (elution at pH 4.0), which was used as a third purification step after adsorption to CPG chromatography and heparin-Sepharose affinity chromatography. The possibility that the factor was acid labile was excluded because the tonic inhibitor could be recovered after pH 2.0 treatment. Loss of biological material because of dialysis was unlikely because activity was still found upon dialysis of material eluted from the CPG.
In a subsequent approach to further purify the tonic inhibitor(s), biologically active fractions from the heparin-Sepharose column were submitted to reversed-phase high-pressure liquid chromatography (RP-HPLC). In none of the RP-HPLC eluate fractions could any biological activity be recovered, nor could activity be restored through recombination of fractions. Mass spectrometry analysis of the fractions was unrevealing.
Thus, the inhibitory activity was not eluting from heparin-Sepharose where a chemokine would elute and was not concentrated by the CPG and heparin-Sepharose fractionations as most bound proteins would be. Biologically active heparin-Sepharose fractions subjected to proteolysis using either trypsin or the endoproteinase Asp-N were not broken down, in contrast to control BSA. Hence, either the presence of protease inhibitors in the column fractions at this stage of the purification was preventing inactivation, or the biologic activity under investigation was not due to a protein component.
Evidence for an Active Lipid Inhibitor(s).
We turned to the possibility that our unusually behaving (for a protein) twice-chromatographed (CPG and heparin-Sepharose) positive fractions contained an active lipid. We chose a positive and a negative fraction from a heparin-Sepharose eluate in which, of 21 fractions, only 2 contiguous fractions (nos. 6 and 7) were positive. We tested fractions 6 (positive) and 4 (negative); both eluted before the main protein peak.
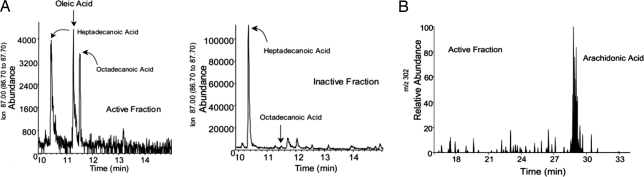
For both fractions, we carried out liquid chromatography tandem mass spectrometry (LC-MS-MS) analyses and in parallel derivatized a portion of each fraction for gas chromatography-mass spectrometry (GC-MS) based lipidomic analyses, using published methodology (7–9). These analyses focused on identifying known fatty acids as well as novel compounds derived from them (7–9). The results were clear: The LC-MS-MS analyses indicated that there were apparently no known eicosanoids (present as leukotrienes or lipoxins) in appreciable amounts or potential other novel oxygenated lipid-derived products in either of these fractions (Fig. 1). If novel products were present, they were apparently below limits of detection (≈25 picograms/sample). Of interest, the active fraction 6 contained native arachidonic acid. This was not observed in the inactive fraction.
Fig. 1.
Identification of oleic and arachidonic acid in active fractions. (A) GC/MS selected ion monitoring for m/z 87 = R-CH2-CH2-COO-Me, the common ion for PUFA, of active and inactive fractions. Samples were treated with BSTFA to produce Me3Si derivatives before injection. (B) LC/MS/MS selected ion chromatogram of m/z 302 = M-H shows the presence of arachidonic acid in the active fraction, which co-elutes with authenticated standards.
After derivativization of the same fractions and conversion to methyl esters and potential O-trimethysilyl derivatives, it was possible to discern the presence of other unsaturated fatty acids. The major component identified was oleic acid C18:1 (Fig. 1). Again, this was identified and present only within the active fraction 6. A number of other major materials were present in the total ion chromatogram corresponding to usual contaminants present for this derivatization procedure. In addition, in other spectra we identified cholesterol and obtained hints of the presence of potential monohydroxy acids within the active fraction. However, these were not present in substantial amounts, so that we could dismiss mono-, di-, or trihydroxy fatty acids (eicosanoids, or new compounds) as potential tonic inhibitors.
In short, the results indicated the presence of unoxygenated fatty acids, specifically oleic and arachidonic acids, in the bioactive fraction. They therefore became prime suspects as endogenous inhibitors of chemotaxis in plasma. There is a report of inhibition by oleic acid of chemotaxis of human neutrophils toward zymosan-activated serum and of C5a-induced myeloperoxidase release (10). A more recent study indicated inhibition of chemotaxis by both oleic and arachidonic acids, of mature rat intestinal dendritic cells toward the chemokine CC chemokine ligand 21 (11). We began by examining the effect of added oleic acid on the amplified chemotactic response induced by EDTA.
Reversal by Oleic Acid of the Outsized Chemotaxis Induced by EDTA.
In all of 4 initial experiments, one of which is shown in Fig. 2, EDTA (5–6 mM) controls induced an outsized chemoctactic response, and oleic acid (125–250 μM) suppressed the EDTA effect. This suppression was the first evidence that at least 1 fatty acid, oleic, in its known physiologic range in human blood (72.8–400.5 μM) (12), reversed the EDTA effect.
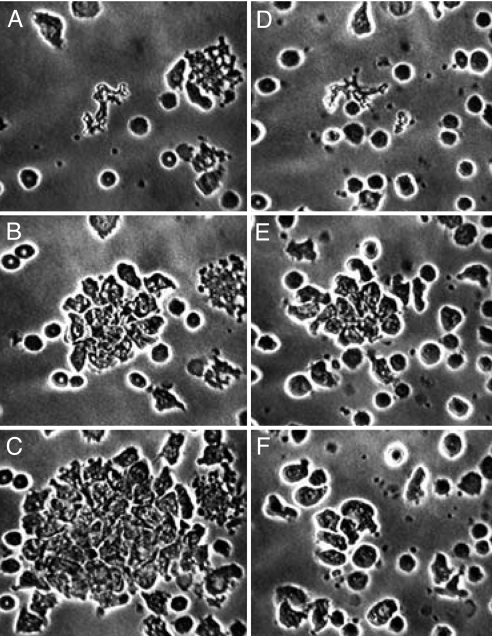
Fig. 2.
Reversal by oleic acid of the outsized chemotaxis induced by EDTA. Both preparations contain EDTA. (A–C) EDTA control cells, having received only the solvent for oleic acid (EtOH); (D–F) cells that received oleic acid. (A and D) Fields just after central erythrocytes have been destroyed by ruby laser microirradiation, creating a chemotactic gradient. (B and E) 15 min later: with EDTA alone (B), neutrophils continue to arrive; with EDTA + oleic acid (E), maximum aggregation has occurred and cells have begun to disperse (as normal cells do without EDTA). (C and F) After an additional 21.5 min, with EDTA alone (C), neutrophils are still arriving; with EDTA + oleic acid (F), neutrophil locomotion is normal but direction is random. EDTA, 5 mM; oleic acid, 125 μM; EtOH, 0.2%.
Subsequently, we noted that oleic acid reversed the EDTA effect even with the latter at 10 mM, which became our standard concentration (it had been used in earlier work when we wanted to reduce divalent cations to <1 nM) (1, 2). For example, 250 μM oleic acid diminished the outsized chemotactic response induced by 10 mM EDTA, with very high statistical significance: control, 8.88 cm ± 0.80 (SEM); oleic, 5.80 cm ± 0.62 (SEM); n = 10, P = 0.003. This diminution occurred without suppression of non-directed motile function of the PMNs.
With 10 mM EDTA, there was a trend toward an effect of oleic acid at 100 μM that became significant when many experiments had been done: control (mean of 2 for each experiment), 9.91 cm ± 0.41 (SEM); oleic, 8.70 cm ± 0.54 (SEM); n = 21, P = 0.04. The 2 controls did not differ significantly from each other: C1, 9.93 ± 0.47; C2, 9.88 ± 0.46; n = 21, P = 0.94.
Arachidonic Acid Apparently at Odds with Oleic Acid.
Employing as a control the other suspect (from the spectrometric results) for tonic inhibition of chemotaxis in plasma, we were surprised to find the opposite: Instead of suppressing, arachidonic acid amplified the EDTA effect (Fig. 3). The results of 12 such experiments are seen in Table 1.
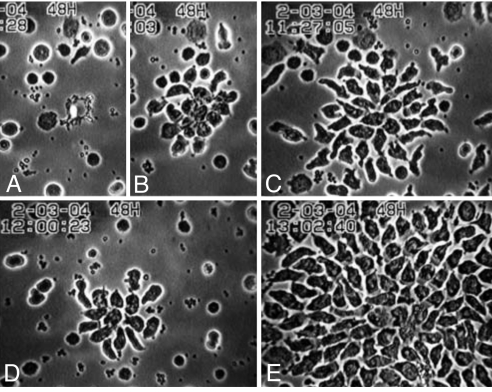
Fig. 3.
Amplification by arachidonic acid of the outsized chemotaxis induced by EDTA. All preparations contain EDTA. (A–C) EDTA control cells, having received only the solvent for the fatty acids (EtOH); D has received oleic acid and E arachidonic acid. (A–C) EDTA control at laser flash (A), 7.6 min later (B), and after a total of 27.6 min (C) when the cell aggregate is at its maximum. (D) EDTA + oleic acid, maximum aggregation, 15.7 min after the laser flash. (E) EDTA + arachidonic acid, maximum aggregation, 32.6 min after the laser flash. EDTA, 10 mM; oleic acid, 250 μM; arachidonic acid, 400 μM; EtOH, 0.2%.
Table 1.
Arachidonic acid apparently at odds with oleic acid when EDTA is employed to amplify chemotaxis in plasma
| Mean diameter ± SEM, cm | n | P (vs. C) | |
|---|---|---|---|
| EDTA control (C), 10 mM | 9.46 ± 0.56 | 12 | — |
| Oleic acid, 250 μM | 5.73 ± 0.39 | 12 | 0.0001 |
| Arachidonic acid, 400 μM | 11.54 ± .59 | 12 | 0.006 |
Unlike oleic acid, which is inhibitory, arachidonic acid, a minor component of plasma free fatty acids, here employed in supraphysologic concentration, augmented the outsized chemotactic response induced by EDTA. Neither fatty acid affected the non-directed motile function of the PMN.
In those experiments, oleic acid (250 μM), the major free fatty acid in plasma (26–45%) (12), is well within the physiologic range (72.8–400.5 μM); 400 μM arachidonic is well above its usual concentration (16.8–53.4 μM; ≈6% of total fatty acids) (12). Therefore we did a series of experiments with a physiologic concentration of arachidonate (50 μM). In 8 experiments, the results were again significant, but not markedly so: EDTA control, 9.75 ± 0.80 (SEM) cm; arachidonate, 11.13 ± 1.19 cm; P = 0.04.
Delipidated Human Serum Albumin (DHSA), an Independent Amplifier of Chemotaxis.
EDTA, by augmenting the chemotactic response, increases the signal-to-noise ratio when inhibitors of chemotaxis are being sought in a series of plasmas that may vary considerably in their content of particular endogenous fatty acids. Its addition is therefore an excellent way to screen for inhibitory effects of added fatty acids alone or in combination. However, the mechanism of action of EDTA in this system is as yet unknown, and so we sought another way of increasing the dynamic range of the chemotactic response that was more easily explained.
Because fatty acids bind to albumin, we reasoned that the addition of sufficient amounts of DHSA to plasma would bind enough fatty acids, including inhibitory ones, to counter their tonic inhibition. In several preliminary experiments, the results were dramatic. Delipidated albumin added to plasma (room temperature, 1 h) before PMNs were added induced chemotactic responses that were at least as rapid as those of controls and resulted in accumulations of neutrophils that were often very large. Results with 300 mg/ml DHSA are seen in Table 2(DHSA vs. No DHSA): DHSA magnified the chemotactic response in a highly significant manner. An equal concentration of normal serum albumin did not result in increased chemotaxis (2 experiments; data not shown).
Table 2.
Delipidated albumin (DHSA), an independent amplifier of chemotaxis in plasma
| Mean diameter ± SEM, cm | n | P (vs. DHSA.) | ||
|---|---|---|---|---|
| 1 | DHSA control, 300 mg/ml | 8.08 ± 0.42 | 10 | — |
| 2 | DHSA + oleic acid, 250 μM | 5.33 ± 0.39 | 10 | 0.0002 |
| 3 | DHSA + arachidonic acid, 50 μM | 8.05 ± 0.59 | 10 | 0.97 |
| 4 | No DHSA | 4.70 ± 0.26 | 10 | 0.0001 |
Like EDTA, DHSA augmented the chemotactic response in plasma (rows 1 vs. 4). As in EDTA/plasma, oleic acid inhibited the augmentation (rows 2 vs 1). However, arachidonic acid, here in physiologic concentration, had no significant effect on the outsized chemotactic response induced by DHSA (row 3 vs. 1). Nor did it at 400 μM (not shown). Neither fatty acid affected the non-directed motile function of the PMN.
Hence, adding delipidated albumin to plasma had a similar effect to that of adding EDTA, augmentation of the chemotactic response.
Are Fatty Acid Effects on Chemotaxis Independent of EDTA in the Medium?
Because DHSA produces a similar increase in the dynamic range to that of EDTA, we could also use it to indicate whether effects seen with fatty acids are independent of the presence of EDTA. In Table 2, we confirmed the inhibitory effect of oleic acid on chemotaxis, employing delipidated albumin as the amplifier rather than EDTA. However, 50 μM arachidonic acid had no significant effect (Table 2); nor did it in excess, 400 μM (data not shown). Thus, the further amplification of chemotaxis by arachidonic acid in EDTA plasma is not a general effect.
Mechanism of EDTA-Dependent Augmentation of Chemotaxis in the Presence of Arachidonic Acid.
Given that human erythrocytes can convert 5-HPETE to LTA4 (via hemoglobin) and then to LTB4 (13), we wondered whether a donor cell with 5-lipoxygenase activity (e.g., leukocytes) could be generating 5-HPETE from the added arachidonic acid. The destroyed red cells might convert it to LTA4 and then to LTB4, thereby enriching the gradient with this powerful chemoattractant. EDTA would presumably keep 5-HPETE from being degraded in plasma.
We tested this hypothesis as follows. First, as seen in Table 3, the LTB4 receptor antagonist α-LTB4 prevented the augmentation of chemotaxis seen with arachidonic acid (row 3 vs. row 2, P = 0.009). Note that α-LTB4 did not reduce chemotaxis when no arachidonic acid (the presumed substrate for the augmented chemotactic response) had been added (row 4 vs. row 1). Thus, this was not a nonspecific effect, and it provides further confirmation that the increased chemotaxis seen when EDTA alone is added to plasma (row 1 vs. row 5) is not itself due to the generation of LTB4 (2).
Table 3.
Prevention of the augmentation of chemotaxis induced by arachidonic acid in EDTA/plasma, by an LTB4 receptor antagonist (a-LTB4)
| Mean diameter ± SEM, cm | n | P (vs. EDTA) | P (vs. EDTA + arach.) | ||
|---|---|---|---|---|---|
| 1. | EDTA control | 9.00 ± 0.29 | 4 | — | |
| 2. | EDTA + arachidonic acid | 11.63 ± 0.46 | 4 | 0.017 | — |
| 3. | EDTA + arach. + a-LTB4 | 8.25 ± 0.70 | 4 | 0.009 | |
| 4. | EDTA + a-LTB4 | 11.39 ± 1.14 | 4 | 0.102 | |
| 5. | No EDTA | 5.75 + 0.34 | 4 | 0.009 |
We thought that arachidonic acid might be acting as substrate for the generation of one of its known products, the powerful chemotactic agent leukotriene B4 (LTB4). In fact, an LTB4 receptor antagonist (a-LTB4) prevented the augmentation seen with arachidonic acid (row 3 vs. 2, P = 0.009). Hence, there appears to be a double gradient, of which the effect of 1 source, that of LTB4, can be blocked. Note that a-LTB4 does not reduce chemotaxis when no arachidonic acid (the presumed subsrate for the augmentation) has been added (row 4 vs. 1).
Second (Table 4), the addition of excess exogenous LTB4 prevented the further augmentation of the EDTA effect by arachidonic acid (row 4 vs. row 3; P = 0.02) without affecting the EDTA control (row 2 vs. row 1; P = 0.69). This result suggests distraction, by exogenous LTB4, of the contribution of endogenous LTB4 to the gradient.
Table 4.
Distraction of the chemotactic gradient by exogenous LTB4
| Mean diameter ± SEM, cm | n | P (vs. EDTA) | P (vs. Arach./EDTA) | ||
|---|---|---|---|---|---|
| 1 | EDTA control | 11.13 ± 1.22 | 5 | — | |
| 2 | + LTB4, 500 ng/ml | 10.40 ± 1.78 | 5 | 0.69 | 0.19 |
| 3 | + Arachidonic acid, 400 μM | 15.15 ± 2.17 | 5 | 0.11 | — |
| 4 | + Arach. + LTB4 | 8.05 ± 1.30 | 5 | 0.006 | 0.02 |
Exogenous LTB4 did not affect chemotaxis in EDTA/plasma when no arachidonic acid had been added (row 1 vs. 2). However, it did reverse the further augmentation of chemotaxis by arachidonic acid (row 3 vs. 4). This supports the presence of a double chemotactic gradient, in which only the one generated by arachidonic acid is distractable by LTB4. LTB4 did not affect the non-directed motile function of the PMN.
Third (Table 5), in plasma without EDTA, there was no effect on control chemotaxis of α-LTB4 (row 4 vs. row 1; P = 0.66), of excess arachidonic acid (row 2 vs. row 1; P = 0.85), or of both (row 3 vs. row 1; P = 0.61). This finding again suggests that EDTA is necessary in plasma for arachidonic acid to be converted to LTB4 in these circumstances.
Table 5.
In plasma without EDTA, a-LTB4 has no effect
| Mean diameter ± SEM, cm | n | P (vs. Control) | P (vs. Arach.) | ||
|---|---|---|---|---|---|
| 1 | Plasma Control (mean of 2) | 6.71 ± 0.73 | 6 | — | 0.85 |
| 2 | + Arachidonic, 400 μM | 6.96 ± 0.95 | 6 | 0.85 | — |
| 3 | + Arach. + a-LTB4 | 6.17 ± 0.75 | 6 | 0.61 | 0.26 |
| 4 | + a-LTB4, 50 μM | 6.96 ± 0.88 | 6 | 0.66 | 1.00 |
Without EDTA, arachidonic acid did not augment chemotaxis (row 2 vs. 1), nor did a-LTB4 diminish chemotaxis (rows 3 vs. 1 and 4 vs. 1). Neither reagent affected the non-directed motile function of the PMN. Thus, EDTA (Table 3) appears necessary for arachidonic acid to be converted to LTB4.
Oleic Acid Actions on Unamplified Chemotaxis.
As noted above, employing amplifiers of chemotaxis such as EDTA or excess albumin is convenient because, among plasmas that may contain quite different concentrations of the various endogenous fatty acids, amplifiers increase the scale in which inhibition can be observed, that is, the signal-to-noise ratio. However, they are not physiologic, and it is therefore important to confirm the effect of a putative inhibitor without them.
To determine whether we could show inhibition of chemotaxis by oleic acid in the absence of either amplifier, we performed a series of experiments in which duplicate assays of oleic acid using a physiologic concentration, 250 μM (normal plasma concentrations, 72.8–400.5 μM), and duplicate controls were used for each plasma/leukocyte sample (Table 6A). There was a highly significant inhibition of chemotaxis by oleic acid (n = 13, P = 0.001).
Table 6.
Inhibition of chemotaxis by oleic acid without amplifiers of the chemoactic response
| Mean diameter ± SEM, cm | n | P | ||
|---|---|---|---|---|
| A. | Controls, mean of 2 | 6.06 ± 0.23 | 13 | — |
| Oleic 250 μM, mean of 2 | 4.86 ± 0.22 | 13 | 0.001 | |
| B. | Control | 5.75 ± 0.33 | 8 | — |
| Oleic, 250 μM | 4.22 ± 0.31 | 8 | 0.0004 | |
| Palmitic, 200 μM | 5.44 ± 0.38 | 8 | 0.29 |
A. In 81% plasma without EDTA or delipidated albumin, oleic acid significantly inhibited the chemotactic response. B. In 16% plasma to minimize the effects of endogenous fatty acids, oleic acid again significantly inhibited the chemotactic response; palmitic acid did not. Neither fatty acid affected the non-directed motile function of the PMN.
To minimize endogenous fatty acid effects, we did an additional series of experiments in media containing only 16% plasma in NCTC, comparing oleic acid with palmitic acid, the next most abundant fatty acid in plasma (64.4–222.5 μM) (Table 6B). [As predicted from its lack of effect when delipidated albumin was used to augment chemotaxis (Table 2), arachidonic acid alone had no significant effect on chemotaxis (not shown)]. In 8 consecutive experiments, oleic acid again significantly decreased the chemotactic response (n = 8; P = 0.0004); palmitic acid did not (Table 6B).
Action of Oleic Acid in a Filter Assay with LTB4, fMLP, and IL-8.
This assay was used to confirm the videomicroscopic results in a standard system with defined chemoattractants. We began with LTB4. Results were negative for some time, until we realized that the tonic inhibitor must be by nature weak, so that significant chemotactic events in vivo are not impeded. Therefore, we (i) used the lowest concentration of LTB4 that reliably yielded a positive signal in triplicate (5 × 10−9 M), (ii) cut the time of incubation from 1.5–2.5 h to 20 min, when the negative controls (random locomotion) are just beginning to appear in the lower chamber, and (iii) used initially a high concentration of oleic acid (400 μM). In 5 consecutive experiments, oleic acid significantly inhibited chemotaxis toward LTB4: LTB4 alone, 15,630 PMNs/100 μL ± 3,651 (SEM); LTB4 + oleic acid, 1,800 ± 1,104; P = 0.02. In a second series of experiments, we used the middle physiologic concentration of oleic acid generally used in the videomicroscopic studies (250 μM); oleic acid continued to inhibit chemotaxis toward LTB4 (Table 7A). Similarly, we determined the lowest concentration of fMLP that reliably yielded a positive signal in duplicate (1 × 10−10 M). Oleic acid, 250 μM was again inhibitory; oleic acid 125 μM was not significantly so (for n = 5) (Table 7B). For IL-8, we reduced the duplicate incubation times to 15 min; the lowest reliably stimulatory concentration was 1.2 × 10−10 M. With a larger n than for LTB4, we show a dose–response inhibition of chemotaxis by oleic acid (250 μM, 125 μM, and 65 μM), and all of the differences are statistically significant (Table 7C).
Table 7.
Filter assay for chemotaxis
| Mean PMN/100 μl ± SEM | n | P | ||
|---|---|---|---|---|
| A. | LTB4, 5 × 10−9 M | 23,280 ± 2,713 | 5 | — |
| + Oleic, 250 μM | 14,260 ± 739 | 5 | 0.03 | |
| Negative control | 6,920 ± 1,231 | 5 | 0.005 | |
| B. | fMLP, 1 × 10−10 M | 27,000 ± 7,005 | 5 | — |
| + Oleic, 250 μM | 10,000 ± 3,275 | 5 | 0.01 | |
| + Oleic, 125 μM | 18,700 ± 8,394 | 5 | 0.25 | |
| Negative control | 7,700 ± 2,755 | 5 | 0.03 | |
| C. | IL-8, 1.2 × 10−10 M | 38,857 ± 7,347 | 7 | — |
| + Oleic, 250 μM | 9,714 ± 2,602 | 7 | 0.006 | |
| + Oleic, 125 μM | 20,214 ± 4,203 | 7 | 0.05 | |
| + Oleic, 65 μM | 27,286 ± 4,807 | 7 | 0.02 | |
| Negative control | 7,500 ± 2,449 | 7 | 0.004 |
Upper chambers in different experiments received 0.3–0.5 × 106 PMN/100 μl. A. LTB4, 5 × 10−9 M, 20 min. B. fMLP, 1 × 10−10 M, 20 min. C. IL-8, 1.2 × 10−10 M, 15 min.
Discussion
We appear to be dealing with a regulatory mechanism for chemotaxis present in human plasma—tonic inhibition by fatty acids— of which oleic acid is one example. The inhibitory action of oleic acid was observed when the dynamic range of the chemotactic response was increased through the use of EDTA (Figs. 2 and 3 and Table 1) or of delipidated albumin (Table 2), as well as when no such amplification was induced (Table 6). Oleic acid was identified as a potential neutrophil regulator through an unbiased lipidomics-based mass spectrometry approach, confirming earlier observations of its action on human neutrophil (10) and rat intestinal dendritic cell (11) responses in vitro.
Teleologically, this formulation makes a good deal of sense. Recruitment of PMNs cannot be allowed to continue once a bacterial invader, for example, has been disposed of; modulatory elements have to get the upper hand. It is likely that vascular minitraumas are frequent, activating cells to produce chemokines, and it is essential that the response to false alarms not get out of control. Reassertion of some baseline level of tonic inhibition would seem more efficient than simply waiting for such stimuli to dissipate. This formulation fits with the concept of inflammatory exudates, promulgated by one of us (C.N.S.) (7), that resolution of inflammation and maintenance of its absence are not passive events. A general view of this kind of restraint has been stressed by Nathan (14): “The non-inflammatory state does not arise passively from an absence of inflammatory stimuli; rather maintenance of health requires the positive actions of specific gene products to suppress reactions to potentially inflammatory stimuli that do not warrant a full response.”
The nature of the chemotactic gradient induced in irradiated erythrocytes and its amplification by EDTA is unknown. In previous studies, we were not able to suppress the response with antagonists of the promiscuous chemokine receptor CXCR2 (IL-8RB) and of receptors for LTB4 and platelet-activating factor; antibodies to the IL-8 receptors CXCR1 (IL-8RA), CXCR2, and the C5a receptor; or the serine protease inhibitors benzamidine and aminoethylbenzenesulfonyl fluoride (2). Whatever the chemoattractant, there is a long history of therapy employing lasers of appropriate wavelength to produce selective photothermolysis. In particular, these techniques have been widely applied to vascular lesions, targeting the erythrocytes that contain the chromophore molecule hemoglobin (15). Neutrophils are dominant in the early inflammatory response (16). Our in vitro system is simpler in that damaged adjacent tissue (endothelium, blood vessel wall) is not present, and ingress of neutrophils is attributable solely to chemoattraction from the lysed erythrocytes. This distinction in turn suggests that a portion of the chemoattraction emanating from in vivo lesions may be due to the lysed red cells.
The strength of this videomicroscopic system is that we are in total visual control. We pick the moment to create the chemotactic gradient and can keep track of every cell in the field, and the visual record is permanent. Thus we know, for example, that cell morphology and random locomotion are unaffected by oleic acid; only chemotaxis appears to be involved. Moreover, the sensitivity of the system allowed us to detect the weak modulatory effects that would have to obtain in vivo to allow responses to appropriate chemotactic stimuli to occur. In contrast, a standard filter assay for chemotaxis was unrevealing until we found a sufficiently brief time of measurement (20 min) and a sufficiently low concentration of chemoattractant (LTB4, 5 × 10−9 M) so that clear differences between oleic acid and controls became appreciable (Table 7). Similar results were seen when the chemoattractant was fMLP (1 × 10−10 M) or IL-8 (1.2 × 10−10 M).
The mechanism of action of the inhibitory effect of oleic acid on chemotaxis (Figs. 2 and 3, Table 1) is also unknown, but based on the findings described here, our hypothesis is that to operate most efficiently, the anionic form of the carboxylic acid is required to interact with divalent cations to bring about its interaction with specific protein moieties. Thus, by making divalent cations unavailable, EDTA would at least partially inhibit the inhibitor and thereby augment the chemotactic response. Augmentation of the chemotactic response by delipidated albumin, on the other hand (Table 2), would depend upon its binding to the fatty acid inhibitor(s), largely removing it from play. This interpretation is open to further study.
The initial observation of augmentation of chemotaxis by arachidonic acid in EDTA/plasma (Fig. 3 and Table 1) served as an excellent control for oleic acid and raised the prospect of an in situ countermodulatory effect to that of oleic acid. However, the effect of this minor component of plasma was not seen when DHSA was used as the amplifier of chemotaxis (Table 2) or when no amplifier was used (Table 6), and so it is not likely to be operant in normal circumstances.
Moreover, we have developed evidence, employing both an LTB4 receptor antagonist (α-LTB4) (Tables 3 and 5) and exogenous LTB4 (Table 4), that augmentation of chemotaxis by arachidonic acid in the presence of EDTA is attributable to enrichment of the chemotactic gradient with LTB4. This interpretation is largely in keeping with what we know. Leukocytes have the 5-lipoxygenase that can transform arachidonic acid into 5-HPETE, and red blood cells can transform 5-HPETE into LTA4 via hemoglobin, and LTA4 into LTB4 via a cytoplasmic epoxide hydrolase (13). The likely role of EDTA would be to support the presence of 5-HPETE in plasma, where lipid hydroperoxides are generally unstable. Thus, when arachidonic acid is added to EDTA/plasma there appears to be a doubly enriched gradient, the one amplified by EDTA, the other further amplified by arachidonic acid, of which both require EDTA but only the latter can be suppressed by an LTB4 receptor blocker (Tables 3 and 5) or distracted by exogenous LTB4 (Table 4).
The levels of total unesterified fatty acids have a wide range: They vary in human plasma from 280–890 μM, generally with oleic > palmitic > stearic > linoleic > arachidonic (12). Future work should extend to different doses and combinations of the fatty acids in plasma, in association with spectrometric determination of the actual concentrations of endogenous fatty acids or their derivatives in particular plasma samples.
In summary, we have uncovered a modulatory action in plasma that may return almost-spent chemotactic responses, for example, an infection when the bacteria have been removed, to baseline in an efficient manner, and prevent trivial (weak) stimuli (e.g., minor trauma) from getting going. Moreover, the level of tonic inhibition in individual plasmas might explain in part why some individuals respond more forcefully to chronic inflammatory stimuli (e.g., in acne, in autoimmune disease) than others. The manipulation of inhibitory elements in plasma would have potential therapeutic anti-inflammatory usefulness.
Materials and Methods
Reagents.
Reagents included EDTA (2%, Sigma), final concentration 10 mM, and DHSA (Sigma A3782–1G), final concentration 180–300 mg/ml. Sodium salts of fatty acids (Sigma), including oleic (O 7501), arachidonic (A 8798), and palmitic (P 9767), were dissolved first in ethanol 95% (Prolabo), then in autologous plasma at the final concentrations indicated (and ethanol ≤0.5%). PBS or, for the fatty acids, ethanol (≤0.5%), served as controls. LTB4 in EtOH (Sigma; 3 × 10−4 M), fMLP (Sigma; initially 1 × 10−2 M in DMSO, and IL-8 (Sigma; 1.2 × 10−5 M) were diluted to final concentrations in buffer (NCTC 135, Eurobio) ± autologous plasma. α-LTB4 (LTB4 receptor antagonist, Pfizer) was dissolved in DMSO at 50 mM, then in autologous plasma to 50 μM.
Leukocytes.
Three tubes of 7 ml each of blood from anonymous normal volunteers were taken in sodium heparin (10 μl/ml blood). After sedimentation on an incline of 60° at room temperature for 1.5–2 h, the buffy coat was recovered, concentrated in a microcentrifuge (Costar; Model #8455) for ≈5 s at 5,585 × g, and resuspended along with some red cells in autologous plasma containing platelets. In some preparations additional fatty acids were added with or without EDTA or after delipidated albumin had been added (15 min, 37 °C). The usual final concentration of plasma was ≈81%. In some preparations, plasma was diluted to 16% in NCTC 135.
Chemotaxis via Videomicroscopy.
A drop of this suspension sufficient only to wet an entire overlying 22 mm × 32 mm coverslip (≈4 μl) is deposited on a clean glass slide, and the preparation sealed with paraffin and removed to the warmed (33 °C) stage of a Zeiss Photomicroscope (objective, 25× phase contrast) linked to a video camera (C800, Desi-Novelec) and to a time-lapse video tape recorder (Panasonic). A field containing 3 contiguous red cells was chosen. A chemotactic gradient lasting several minutes was produced by destruction of the red cells by a ruby laser (6,943 Å) that emits 3 Joules in 500 μs and focuses (using the microscope's optics in reverse) to a diameter of 5 μm (17). Each experiment was recorded in time-lapse video with a speed of 16× normal and for a minimum duration of 24 min.
The maximum accumulation of PMNs around the destroyed target often occurs in the first 10–15 min. As noted above, in the presence of EDTA or delipidated albumin, PMNs keep coming and the maximum response is toward 30 min, sometimes longer. With the permanent video recordings, we fast-forward each sequence to the region of greatest density of arriving cells, select the frame of estimated maximum aggregation, and measure its mean diameter directly from the video monitor (magnification ≈ 1,250×). A set of video sequences of the experiment described in the legend to Fig. 3 (control, oleic acid, and arachidonic acid) is posted as supporting information (SI) Movies S1–S3.
Each of the 222 video sequences that generated the data for Tables 1–6 was examined by 2 observers. Measurements of mean diameter were made by 1 observer and, as a measure of intraobserver variability, were repeated many months later and blinded to the original data, for the experiments depicted in Table 6A. This series of experiments was chosen for validation because no amplifiers (EDTA or albumin) were used and the control and oleic acid values were therefore closest to each other. The 52 new measurements, 2 control and 2 experimental for each of 13 experiments, were analyzed with the following results: controls, 5.72 ± 0.24 cm; oleic acid, 4.43 ± 0.25 cm. Note (compare, Table 6) that the original and recalculated controls, and the original and recalculated oleic acid results, are each within 10% of each other. The P value was again 0.001.
Chemotaxis via Filter Assay.
We used 6.5-mm Transwell polycarbonate membranes, pore size 3.0 μm (Sigma–Aldrich). Media was 10% autologous plasma in NCTC 135 or in Hanks solution (Eurobio). Chambers (100 μl) and wells (600 μl) were preincubated in media for 1 h at 37 °C in a CO2 incubator. Neutrophils were purified by standard centrifugation through Lymphoprep (Nycomed) of buffy coats containing few erythrocytes, obtained as for the videomicroscopic studies. They were washed twice in buffer, counted, and suspended in media. The preincubated Transwell wells and chambers all received media, and the chambers received neutrophils, 0.3–0.5 × 106/100 μL. The wells received LTB4, fMLP, or IL-8, initially in multiple dilutions to determine the lowest reliably stimulatory concentrations of duplicate or triplicate samples incubated for various periods of time. With these dilutions determined, some samples received oleic acid, the potential inhibitor, above and below the filter.
Statistics.
Paired t tests were used (two-tailed). Each n is made up of results from different donors on different days.
Supplementary Material
Acknowledgments.
We thank Katherine Gotlinger for carrying out lipid extractions and analyses, and Pfizer for supplying the LTB4 receptor antagonist (CP105696). We are ever grateful to the late Marcel Bessis, who made this work possible. The work was supported in part by U.S. Public Health Service Grants AR10493, AI43558 (to S.E.M.), and GM38765 and P-50-DE016191 (to C.N.S.); the G. Harold & Leila Y. Mathers Charitable Foundation; and the ESHE Fund. This work began while S.E.M. was a fellow of the John Simon Guggenheim Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802572105/DCSupplemental.
References
- 1.Malawista SE, de Boisfleury Chevance A. Random locomotion and chemotaxis of human blood polymorphonuclear leukocytes (PMN) in the presence of EDTA: PMN in close quarters require neither leukocyte integrins nor external divalent cations. Proc Natl Acad Sci USA. 1997;94:11577–11582. doi: 10.1073/pnas.94.21.11577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malawista SE, Van Damme J, Smallwood JI, de Boisfleury Chevance A. Chemotactic activity of human blood leukocytes in plasma treated with EDTA: Chemoattraction of neutrophils about monocytes is mediated by the generation of NAP-2. J Leukoc Biol. 2002;72:175–182. [PubMed] [Google Scholar]
- 3.Malawista SE, de Boisfleury-Chevance A. Cryopreserved cytoplasts from human polymorphonuclear leukocytes (cytokineplasts) are chemotactic at speeds comparable to those of fresh intact cells. IJ Leuk Biol. 1991;50:313–315. doi: 10.1002/jlb.50.3.313. [DOI] [PubMed] [Google Scholar]
- 4.Malawista SE, de Boisfleury-Chevance A. Chemotaxis by human neutrophils and their cytokineplasts, treated with inhibitors of nitric oxide synthase: No suppression of orientation or trajectory. J Leuk Biol. 1997;61:58–62. doi: 10.1002/jlb.61.1.58. [DOI] [PubMed] [Google Scholar]
- 5.Van Damme J, et al. Separation and comparison of two monokines with lymphocyte-activating factor activity: IL-1 beta and hybridoma growth factor (HGF). Identification of leukocyte-derived HGF as IL-6. J Immunol. 1988;140:1534–1541. [PubMed] [Google Scholar]
- 6.Van Damme J, Van Beeumen J, Conings R, Decock B, Billiau A. Purification of granulocyte chemotactic peptide/interleukin-8 reveals N-terminal sequence heterogeneity similar to that of β-thromboglobulin. Eur J Biochem. 1989;181:337–344. doi: 10.1111/j.1432-1033.1989.tb14729.x. [DOI] [PubMed] [Google Scholar]
- 7.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: Signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 8.Serhan CN, et al. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 10.Higazi AR, Barghouti II. Regulation of neutrophil activation by oleic acid. Biochim Biophys Acta. 1994;1201:442–446. doi: 10.1016/0304-4165(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 11.Tsuzuki Y, et al. Differential modulation in the functions of intestinal dendritic cells by long- and medium-chain fatty acids. J Gastroenterol. 2006;41:209–216. doi: 10.1007/s00535-005-1747-0. [DOI] [PubMed] [Google Scholar]
- 12.Burtis CA, Ashwood ER. Tietz Textbook of Clinical Chemistry. Philadelphia: W. B. Saunders Co.; 1999. [Google Scholar]
- 13.Fitzpatrick F, et al. Metabolism of leukotriene A4 by human erythrocytes. A novel cellular source of leukotriene B4. J Biol Chem. 1984;259:11403–11407. [PubMed] [Google Scholar]
- 14.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 15.Anderson RR. Dermatologic history of the ruby laser: The long story of short pulses. Arch Dermatol. 2003;139:70–74. doi: 10.1001/archderm.139.1.70. [DOI] [PubMed] [Google Scholar]
- 16.Aghassi D, Anderson RR, Gonzalez S. Time-sequence histologic imaging of laser-treated cherry angiomas with in vivo confocal microscopy. J Am Acad Dermatol. 2000;43:37–41. doi: 10.1067/mjd.2000.105560. [DOI] [PubMed] [Google Scholar]
- 17.Malawista SE, Chevance de Boisfleury A. The cytokineplast: Purified, stable, and functional motile machinery from human blood polymorphonuclear leukocytes. J Cell Biol. 1982;95:960–973. doi: 10.1083/jcb.95.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.