Abstract
The heroin analogue 1-methyl-4-phenylpyridinium, MPP+, both in vitro and in vivo, produces death of dopaminergic substantia nigral cells by inhibiting the mitochondrial NADH dehydrogenase multienzyme complex, producing a syndrome indistinguishable from Parkinson's disease. Similarly, a fragment of amyloid protein, Aβ1–42, is lethal to hippocampal cells, producing recent memory deficits characteristic of Alzheimer's disease. Here we show that addition of 4 mM d-β-hydroxybutyrate protected cultured mesencephalic neurons from MPP+ toxicity and hippocampal neurons from Aβ1–42 toxicity. Our previous work in heart showed that ketone bodies, normal metabolites, can correct defects in mitochondrial energy generation. The ability of ketone bodies to protect neurons in culture suggests that defects in mitochondrial energy generation contribute to the pathophysiology of both brain diseases. These findings further suggest that ketone bodies may play a therapeutic role in these most common forms of human neurodegeneration.
Alzheimer's disease affects about 5 million people and Parkinson's disease about 500,000 people in the United States (1). The incidence of Alzheimer's is expected to increase as the population ages as its prevalence rises from 2.5% of those at 65 years of age to 47% of those over 85 years of age (2). Alzheimer's disease is multifactorial (3), characterized by loss of recent memory, decrease in brain acetyl choline (4), and death of hippocampal neurons. These changes result from the accumulation of a proteolytic (5) fragment of the β chain of amyloid precursor protein, Aβ1–42, (6) both intracellularly (7) and extracellularly in pathologically characteristic amyloid plaques. Recently, immunization against Aβ1–42 has been reported to prevent pathological change in transgenic mice overexpressing amyloid precursor protein (8). There is no general agreement on the pathophysiological mechanisms of amyloid toxicity. At present, approximately 20% of cases can be related to abnormalities of Aβ1–42 metabolism because of defects located on chromosome 1, 14, 19, or 21 (9), leaving approximately 80% of cases caused by other factors. Among those factors increasing amyloid accumulation are: brain trauma (10), ischemia (11), insulin resistance (12), or impairment of brain energy metabolism (13, 14).
Parkinson's disease clinically is characterized by muscle rigidity, tremor of the distal extremities, and bradykinesia and pathologically is characterized by eosinophilic Lewy-body inclusions comprised of the nucleoprotein α-synuclein and ubiquitin and by death of substantial nigral dopaminergic neurons (15). Parkinson's disease can be caused by genetic abnormalities, environmental toxins, or infections, and it can be treated, at least temporarily by l-dopa administration. Experimentally, a syndrome indistinguishable from Parkinsonism can be induced by administration of the heroin analogue 1-methyl-4-phenylpyridinium, MPP+ (15), which is taken up by the dopamine transporter of dopinergic neurons where it inhibits the activity of the mitochondrial NADH dehydrogenase multienzyme complex (EC 1.6.5.3) (16, 17).
Brain in normal adults entirely depends on the metabolism of glucose for its energy needs, being unable to use exogenous fatty or amino acids. The single exception is the ability of brain to derive a major portion of its energy needs from the metabolism of ketone bodies (18), referred to as ketones. In heart, we have shown that ketones decreased the need for glycolysis (19), bypassed the blockade of pyruvate dehydrogenase (PDH) multienzyme complex resulting from insulin deficiency, increased the concentration of metabolites of the first third of the tricarboxylic acid (TCA) cycle, reduced the mitochondrial [NAD+]/[NADH], oxidized mitochondrial coenzyme Q, thus increasing the Q/QH2 ratio, increased the ΔG of ATP hydrolysis, and increased metabolic efficiency (20, 21). Elevation of blood ketones, the brain's only alternative to glucose as an energy source (18), has been used for 50 years as a treatment for refractory epilepsy (22). In light of our findings on the effects of ketones in heart, we therefore asked whether they might be neuroprotective against MPP+ toxicity on cultured mesencephalic neurons and against Aβ1–42 toxicity on hippocampal neurons, both models for neurological disease associated with aging.
Materials and Methods
Mesencephalic Culture.
Primary serum-free culture of 14-day embryonic mesencephalic cells were prepared from the ventral, medial 1.0 mm3 volume block of tissue comprising the mesencephalic dopaminergic region as described (23). This dissection technique provides cell populations of >95% neurons including 20% dopaminergic neurons, tyrosine hydroxylase positive (TH+) cells, and <5% glial cells. Dissected tissue blocks were dispersed by pipetting in DMEM/F12 medium (Gibco) containing 10% FCS and 17.5 mM glucose to which was added 0.01% apo-transferrin, 5 μg/ml insulin, 30 nM l-thyroxin, 20 nM progesterone, 30 nM sodium selenite, 100 units/ml penicillin, and 100 mg/ml streptomycin. Twenty five microliters of the cell suspension containing 5 × 106 cells/ml was plated on 8-well chamber slides (LabTek, Nunc), coated with poly-d-lysine (Sigma). After 4 h incubation at 37°C, in 5% CO2 at 100% humidity, 375 μl of media was added. After 12 h incubation, the medium was aspirated and changed to serum-free medium, which substituted 0.01% BSA (Fraction V, Sigma) for the FCS. At the third day in culture, Na d-β-hydroxybutyrate (Sigma) was added to half the wells to make a final concentration of 4 mM. At the fifth day in culture, 0, 1.0, 5, or 10 μM MPP+ (Research Biochemicals-Sigma) was added. Survival of neurons was evaluated at the seventh day in culture by the double immunostaining of anti-TH (Boehringer) and anti-microtubular associated protein 2 (MAP2) (Boehringer) as described (24).
Hippocampal Cultures.
Hippocampal cells were dissected from 18-day embryonic rats for microisland cultures (23) and dispersed by gentle pipetting in neurobasal media (Life Technologies, Grand Island, NY) and centrifuged at 250 g for 10 min. Cells were suspended in neurobasal media containing 1:50 B27, 0.5 mM l-glutamine, 25 μM d,l-glutamate, 100 units/ml penicillin, and 100 mg/ml streptomycin at a cell density of 2 × 105 cells/ml. A 20-μl aliquot was placed in an eight-chamber LabTek (Nunc-Nalge) culture dish coated previously with poly-d-lysine and placed in an incubator for 4 h, after which 400 μl of media was added. On days 2 and 4, half the media was exchanged. On day 6, half the media was removed and mixed with 200 μl of DMEM/F12. Na d-β-hydroxybutyrate was added to the mixed media and 200 μl replaced in the well so as to create a concentration within the well of 4 mM. Twelve hours later, half of the media was replaced with DMEM/F12 with 100 μl of: media only, media containing ketones, media containing 15 μM fresh Aβ1–42 (Bachem), or a combination of the latter two. The final concentration of ketones in the media was 4 mM and of Aβ1–42 5 μM. The effect of diluting neurobasal media with DMEM/F12 was to raise the media Na+ concentration from 78.4 mM to 139.5 mM, within the physiologically normal range for extracellular fluid of 136 to 145 mM. At the same time, the insulin concentration present in neurobasal media was decreased to 1/3. These changes of inorganic ions toward more physiological levels in the media increased the rate of neuronal death. The cells were incubated from 1–36 h. The cells then were fixed with 4% paraformaldehyde in PBS for 10 min, permeabilized with 1% acetic acid in 95% ethanol at −4°C for 15 min, washed three times with Dulbecco's PBS, and blocked with BlockAce (Yukijirushi, Tokyo). Neurons were stained with anti-MAP2 for 60 min. Unbound antibody was removed by washing with PBS for 10 min twice. A total of 150 μl of 75× diluted Vector fluorescein anti-mouse IgG (Vector Laboratories) was added, and the wells were shaken in darkness for 1 h. The wells were washed twice with PBS. Ten minutes later the wells were mounted by using Vectashield mounting medium (Vector Laboratories). For staining of glia, antiglial fibrillary acidic protein (Boehringer) was used in a similar procedure.
Results
Effects of Ketone Bodies on MPP+ Toxicity in Mesencephalic Neuronal Cultures.
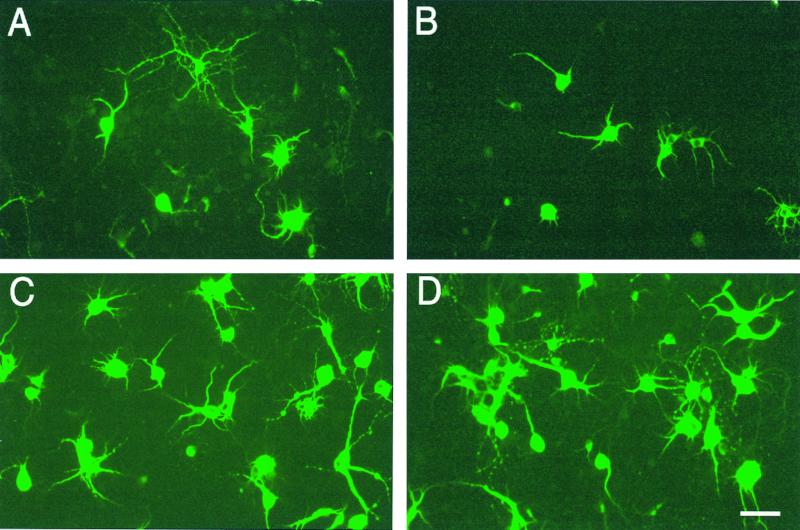
Addition of 1–10 μM MPP+ to cultured mesencephalic cells for 2 days decreased the mean cell count of TH+ cells at all concentrations tested (Table 1). Addition of 4 mM of Na d-β-hydroxybutyrate, the reduced form of the ketones, significantly increased the survival of TH+ neurons at all concentrations of MPP+ tested (Table 1). Because MPP+ only acts on neurons with a dopamine transporter, there was no effect of MPP+ or ketones on the number of MAP2-staining neurons in these mesencephalic cultures. In addition to decreasing the TH+ cell number, exposure to 5 μM MPP+ decreased the outgrowth of neurites, whereas ketones reversed this effect (Fig. 1).
Table 1.
The effects of MPP+ and ketone on cultured mesencephalic neuron count
| MPP+, μM | TH+ neurons, /mm2
|
MAP+ neurons, /mm2
|
||
|---|---|---|---|---|
| Control | Ketones | Control | Ketones | |
| 0 | 65 ± 6 | 64 ± 7 | 485 ± 32 | 476 ± 34 |
| 1 | 30 ± 3 | 46 ± 6* | 464 ± 23 | 466 ± 30 |
| 5 | 18 ± 3 | 48 ± 5* | 514 ± 25 | 549 ± 29 |
| 10 | 10 ± 3 | 30 ± 6* | 437 ± 29 | 540 ± 27 |
Values are mean cell counts/mm2 ± SEM (n = 20). ∗ indicates a significant difference from control at P < 0.05 as judged by Mann–Whitney U test.
Figure 1.
Anti-TH stain of day 7 of rat mesencephalic neuronal culture exposed to MPP+ and ketones for 2 days. (A) Control culture of anti-TH-stained mesencephalic neuronal cultures. (B) Cultures after addition of 5 μM MPP+, (C) after addition of MPP+ and 4 mM ketone bodies, and (D) after addition of 4 mM ketone bodies alone. Addition of 5 μM MPP+ to mesencephlic neuronal cultures resulted in a decrease in TH+ cells, a disappearance of neurites, and a shrinkage of cell body volume. Addition of 4 mM Na d-β-hydroxybutyrate to cultures containing 5 μM MPP+ reversed most of the effects of MPP+. The cell number and cell body volume did not differ significantly from control. (Scale bar = 20 μm.)
Table 1 shows that ketones act as neuroprotective agents against the toxicity of MPP+ on TH+ dopaminergic neurons in culture but have no effect on the more numerous MAP2+ neurons lacking the dopamine uptake system. Antiglial fibrillary acidic protein staining indicated that glial cells comprised fewer than 5% of the total cell number in both types of culture.
The Toxic Effects of Aβ1–42 on Hippocampal Neurons in Culture Were Reversed by d-β-Hydroxybutyrate.
In preliminary experiments, we tried doses of Aβ1–42 from 2.5, 5.0, 7.5, to 10 μM. A dose of 2.5 μM Aβ1–42 resulted in no difference in cell count from control after 8 h incubation; 5 μM Aβ1–42 decreased cell counts from 172 to 80 whereas control neurons without Aβ1–42 decreased from 170 to 110 over the same period. A dose of 10 μM Aβ1–42 decreased cell number from 170 to fewer than 10. Accordingly, we picked 5 μM Aβ1–42 to study the effects of ketone bodies because this dose of Aβ1–42 gave a 50% decrease in cell number in 8 h whereas control neurons decreased only 35%.
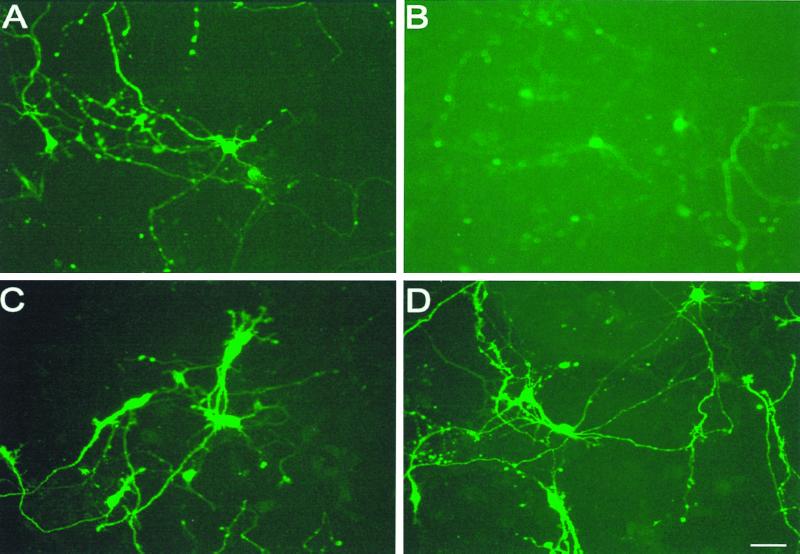
The exposure of 6-day cultured hippocampal neurons from 18-day-old embryos to 5 μM Aβ1–42 for 14 h decreased cell number (Fig. 2) and neurite number and length (Fig. 3B) in comparison to control (Fig. 3A). Addition of 4 mM d-β-hydroxybutyrate to cells exposed to Aβ1–42 doubled the surviving cell number (Fig. 2) and increased cell size and neurite outgrowth compared with cells exposed to Aβ1–42. This finding shows that ketones may act as neuroprotective agents against Aβ1–42 toxicity (Figs. 2 and 3 B versus C). In addition, exposure of cells to ketone bodies for 14 h increased both surviving cell number (Fig. 3) and neurite number (Fig. 3D) compared with control cells (Fig. 3A), suggesting that ketone bodies can act as growth factors to neurons in culture.
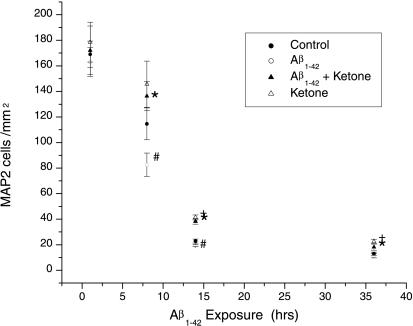
Figure 2.
Time course of the effects of 5 μM Aβ1–42, 4 mM ketones, or the combination on the survival of hippocampal neurons in culture. ●, The mean control cell number/mm2 with error bar indicating the SEM where n = 12. All statistical tests performed were Mann–Whitney U tests, and significance was taken to be P < 0.05. ○, The mean cell number after exposure to Aβ1–42; ▴ after exposure to 5 μM Aβ1–42 + 4 mM d-β-hydroxybutyrate and ▵ after exposure to 4 mM d-β-hydroxybutyrate alone. Exposure to 5 μM Aβ1–42 significantly decreased the cell number compared with controls at 8 and 14 h as indicated by #. Addition of 4 mM d-β-hydroxybutyrate to cells exposed to 5 μM Aβ1–42 increased the cell number compared with exposure of Aβ1–42 alone at 8, 14, and 36 h as indicated by *. Addition of ketone bodies alone increased the cell number compared with controls as indicated by +. Our study therefore confirms the previous reports of the toxicity of Aβ1–42 to cultured hippocampal neurons (28). In addition we show that ketones not only reverse the toxicity of Aβ1–42, but act as a growth factor for neurons in culture.
Figure 3.
The effects on cultured rat hippocampal cells of Aβ1–42, ketones, or the combination. (A) The 6-day control cultures of 18-day embryonic rat hippocampal tissue; (B) after 14 h exposure to 5 μM Aβ1–42, (C) after exposure to both Aβ1–42 and 4 mM d-β-hydroxybutyrate, and (D) the effects of ketone bodies alone. Addition of Aβ1–42 resulted in a decrease in neuronal number and number of neurites (B versus A). Addition of ketones to cells exposed to Aβ1–42 showed no decrease in neuron or neurite number, indicating that ketones act as neuroprotective agents against the toxicity of Aβ1–42 on cultured hippocampal neurons (C versus B).
Discussion
The protection by ketones of hippocampal neurons exposed to Aβ1–42 or of mesencephalic neurons exposed to MPP+ suggests that mitochondrial dysfunction plays a significant role in both of these common neurological diseases. MPP+ binds to one of two ubiquinone binding sites of the NADH multienzyme complex (17, 25), which is in the same domain as the rotenone and piericidin A sites. Inhibition of NADH dehydrogenase, in addition to decreasing cell respiration, decreases mitochondrial proton pumping (16) and increases free radical production, the latter effect being correlated with cell death (26). The major source of mitochondrial reactive oxygen species is the semiquinone form of reduced coenzyme Q (27). Ketone bodies not only reduce mitochondrial [NAD+]/[NADH] but increase mitochondrial [Q]/[QH2] (20, 21). The oxidation of the coenzyme Q couple should, by decreasing the semiquinone, decrease free radical production. In the perfused heart, addition of ketone bodies caused a reduction of the free cytosolic NADP couple (R.L.V., unpublished work), which controls the redox state of glutathione, the major detoxifying agent for H2O2. The oxidation of the coenzyme Q couple should decrease product inhibition of NADH dehydrogenase while decreasing free radical production, accounting for ketones' ability to decrease MPP+ toxicity.
How ketones overcome the toxicity of Aβ1–42 is not immediately obvious. There are reports however, suggesting that Aβ1–42 activates glycogen synthase 3β kinase (28, 29), which phosphorylates the E1α subunit of the pyruvate dehydrogenase (PDH) multienzyme complex. Our findings that ketones can ameliorate Aβ1–42 toxicity are compatible with the ability of ketones to bypass a block at mitochondrial PDH (Fig. 4). Ketones are the physiological means of overcoming PDH inhibition, resulting from a lack of insulin stimulation (20), and ensure the continuing function of the TCA cycle and hence the provision of NADH, the major substrate required for electron transport and ADP phosphorylation. Mitochondrial function has increasingly been recognized to play a central role in cell death. Opening of the mitochondrial voltage-dependent anion channel by the proapoptotic protein Bax is an early event in apoptosis (30). Mutations in mitochondrial DNA are known to result in a wide variety of rare neurological diseases with pleotropic manifestations (31). Abnormalities in mitochondrial proteins encoded by genomic DNA in Friedreich's ataxia lead to increased free radical formation and a deficiency of mitochondria ATP production (32). However, an increase in the efficiency of mitochondrial energy generation has not been thought to be important in the most common neurological diseases. Our observation that ketones offer neuroprotection in cell culture models of the two most common degenerative neurological diseases has several important implications.
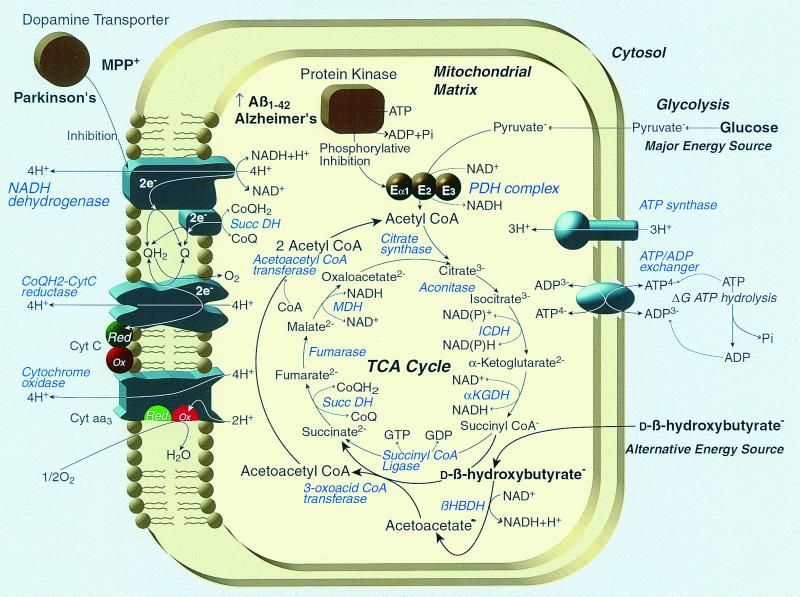
Figure 4.
The hypothesized effects of ketones on metabolic blocks induced by Aβ1–42 and MPP+. Usually brain entirely depends for energy on the mitochondrial metabolism of pyruvate produced from glucose by the glycolytic pathway. Aβ1–42 is reported to stimulate the phosphorylation of the E1a subunit of PDH by glycogen synthase kinase 3β (28). Phosphorylation of PDH blocks the conversion of pyruvate to acetyl CoA, which is required to fuel the TCA cycle, which provides mitochondrial NADH needed to power electron transport. Ketones provide the only alternative source of acetyl CoA for brain during inhibition of the PDH multienzyme complex. In so doing, ketone bodies not only increase mitochondrial acetyl CoA, citrate, and the first 1/3 of TCA cycle metabolites but also reduce the free mitochondrial NAD couple and oxidize the mitochondrial coenyzme Q couple, causing an increase in the ΔG of ATP hydrolysis (21). The oxidation of the coenzyme Q couple by ketones would tend to decrease the major source of mitochondrial reactive oxygen species, the semiquinone form of coenzyme Q (27), while at the same time relieving product inhibition of NADH dehydrogenase (EC 1.6.5.3), accounting for the ability of ketones to decrease MPP+ toxicity.
Despite the genetic and pathophysiological diversity in the etiology of Alzheimer's and Parkinson's diseases, our finding that ketones can protect neurons in culture models of these diseases is compatible with previous suggestions that the two conditions have common features (15). Clinically intermediate forms of dementia, specifically Lewy body dementia, share common features, and Parkinsonism is significantly associated with dementia and pathologically characterized by Lewy bodies in the substantia nigra. The Lewy body aggregates of α-synuclein and ubiquitin, while differing in protein composition from the β-amyloid plaque, suggest that both conditions share a common defect in protein degradative processing that may be related to defective mitochondrial energy generation. This inference is compatible with the earlier reports of increasing Aβ1–42 deposition associated with impairment of energy metabolism (13, 14), hypoperfusion (11), or trauma (10).
Another implication of our finding is that elevation of ketones may offer neuroprotection in the treatment or prevention of both Alzheimer's disease, where therapy is lacking, and Parkinson's disease, where therapy with l-dopa is time limited. The high-fat ketogenic diet used in childhood epilepsy may not be suitable for use in adults because of its atherogenic potential; however, alternative dietary sources of ketones produced biotechnologically (33) may overcome this difficulty and provide benefit without the undesirable side effects of current ketogenic diets.
Acknowledgments
This work was supported by grants from the Ministry of Education, Science and Culture of Japan to K.N. and from BTG PLC to K.C.
Abbreviations
- MPP+
1-methyl-4-phenylpyridinium
- TCA
tricarboxylic acid
- TH
tyrosine hydroxylase
- MAP2
microtubular associated protein 2
- PDH
pyruvate dehydrogenase
References
- 1.Price D L. Nature (London) 1999;399:A3–A5. doi: 10.1038/399a003. [DOI] [PubMed] [Google Scholar]
- 2.Evans D A, Funkenstein H H, Albert M S, Scherr P A, Cook N R, Chown M J, Hebert L E, Hennekens C H, Taylor J O. J Am Med Assoc. 1989;262:2551–2556. [PubMed] [Google Scholar]
- 3.Selkoe D J. Nature (London) 1999;399:A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- 4.Pope A, Hess H H, Lewin E. Trans Am Neurol Assoc. 1965;89:15–16. [PubMed] [Google Scholar]
- 5.Vassar R, Bennett B D, Babu-Khan S, Kahn S, Mendiaz E A, Denis P, Teplow D B, Ross S, Amarante P, Loeloff R, et al. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 6.Hardy J A, Higgins G A. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 7.Kuo Y M, Emmerling M R, Vigo-Pelfrey C, Kasunic T C, Kirkpatrick J B, Murdoch G H, Ball M J, Roher A E. J Biol Chem. 1996;271:4077–4081. doi: 10.1074/jbc.271.8.4077. [DOI] [PubMed] [Google Scholar]
- 8.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, et al. Nature (London) 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 9.Selkoe D J. Science. 1997;275:630–631. doi: 10.1126/science.275.5300.630. [DOI] [PubMed] [Google Scholar]
- 10.Graham D I, Gentleman S M, Nicoll J A, Royston M C, McKenzie J E, Roberts G W, Griffin W S. Acta Neurochir (Wien) Suppl. 1996;66:96–102. doi: 10.1007/978-3-7091-9465-2_17. [DOI] [PubMed] [Google Scholar]
- 11.Kalaria R N, Bhatti S U, Lust W D, Perry G. Ann NY Acad Sci. 1993;695:190–193. doi: 10.1111/j.1749-6632.1993.tb23050.x. [DOI] [PubMed] [Google Scholar]
- 12.Kuusisto J, Koivisto K, Mykkanen L, Helkala F L, Vanhunen M, Kervinen K, Kesaniemi Y A, Riekkinen P J, Laakso M. Br Med J. 1997;315:1045–1049. doi: 10.1136/bmj.315.7115.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabuzda D, Busciglio J, Chen L B, Matsudaira P, Yankner B A. J Biol Chem. 1994;269:13623–13628. [PubMed] [Google Scholar]
- 14.Lin L, Georgievska B, Mattsson A, Isacson O. Proc Natl Acad Sci USA. 1999;96:12108–12113. doi: 10.1073/pnas.96.21.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunnett S B, Bjorklund A. Nature (London) 1999;399:A32–A39. doi: 10.1038/399a032. [DOI] [PubMed] [Google Scholar]
- 16.Miyoshi H, Inoue M, Okamoto S, Ohshima M, Sakamoto K, Iwamura H. J Biol Chem. 1997;272:16176–16183. doi: 10.1074/jbc.272.26.16176. [DOI] [PubMed] [Google Scholar]
- 17.Sablin S O, Krueger M J, Yankovskaya V L, Tkachenko S E, Razdolsky A N, Bachurin S O, Ramsay R R, Singer T P. J Biochem Toxicol. 1996;11:33–43. doi: 10.1002/(SICI)1522-7146(1996)11:1<33::AID-JBT5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 18.Cahill G F, Jr, Aoki T T. In: Cerebral Metabolism and Neural Function. Passonneau J V, Hawkins R A, Lust W D, Welsh F A, editors. Baltimore: Williams & Wilkins; 1980. pp. 234–242. [Google Scholar]
- 19.Kashiwaya Y, Sato K, Tsuchiya N, Thomas S, Fell D A, Veech R L, Passonneau J V. J Biol Chem. 1994;269:25502–25514. [PubMed] [Google Scholar]
- 20.Kashiwaya Y, King M T, Veech R L. Am J Cardiol. 1997;80:50A–64A. doi: 10.1016/s0002-9149(97)00458-x. [DOI] [PubMed] [Google Scholar]
- 21.Sato K, Kashiwaya Y, Keon C A, Tsuchiya N, King M T, Radda G K, Chance B, Clarke K, Veech R L. FASEB J. 1995;9:651–658. doi: 10.1096/fasebj.9.8.7768357. [DOI] [PubMed] [Google Scholar]
- 22.Freeman J M, Vining E P G. Epilepsia. 1992;33:1132–1136. doi: 10.1111/j.1528-1157.1992.tb01770.x. [DOI] [PubMed] [Google Scholar]
- 23.Takeshima T, Shimoda K, Sauve Y, Commissiong J W. Neuroscience. 1994;60:809–823. doi: 10.1016/0306-4522(94)90506-1. [DOI] [PubMed] [Google Scholar]
- 24.Takeshima T, Johnston J M, Commissiong J W. J Neurosci. 1994;14:4769–4779. doi: 10.1523/JNEUROSCI.14-08-04769.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gluck M R, Krueger M J, Ramsay R R, Sablin S O, Singer T P, Nicklas W J. J Biol Chem. 1994;269:3167–3174. [PubMed] [Google Scholar]
- 26.Barrientos A, Moraes C T. J Biol Chem. 1999;274:16188–16197. doi: 10.1074/jbc.274.23.16188. [DOI] [PubMed] [Google Scholar]
- 27.Chance B, Sies H, Boveris A. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- 28.Hoshi M, Takashima A, Noguchi K, Murayama M, Sato M, Kondo S, Saitoh Y, Ishiguro K, Hoshino T, Imahori K. Proc Natl Acad Sci USA. 1996;93:2719–2723. doi: 10.1073/pnas.93.7.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoshi M, Takashima A, Murayama M, Yasutake K, Yoshida N, Ishiguro K, Hoshino T, Imahori K. J Biol Chem. 1997;272:2038–2041. doi: 10.1074/jbc.272.4.2038. [DOI] [PubMed] [Google Scholar]
- 30.Shimizu S, Narita M, Tsujimoto Y. Nature (London) 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 31.Wallace D C. Science. 1999;283:1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 32.Lodi R, Cooper J M, Bradley J L, Manners D, Styles P, Taylor D J, Schapira A H. Proc Natl Acad Sci USA. 1999;96:11492–11495. doi: 10.1073/pnas.96.20.11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerngross T U. Nat Biotechnol. 1999;17:541–542. doi: 10.1038/9843. [DOI] [PubMed] [Google Scholar]