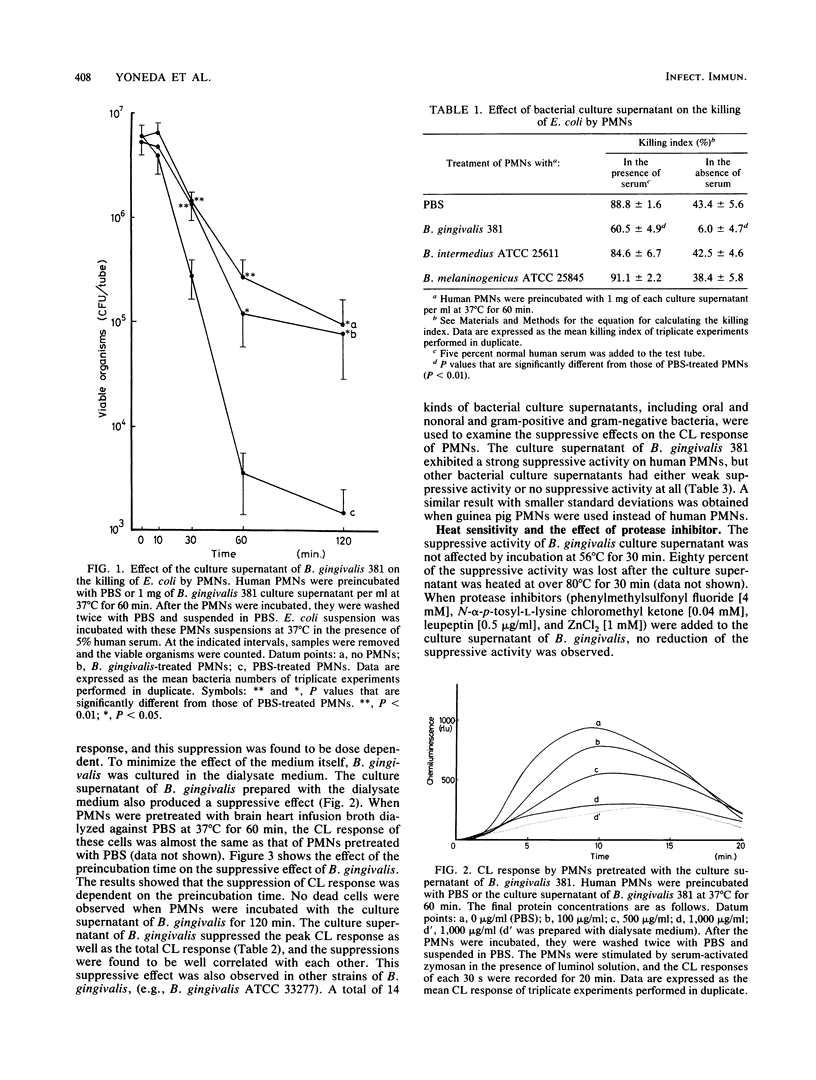
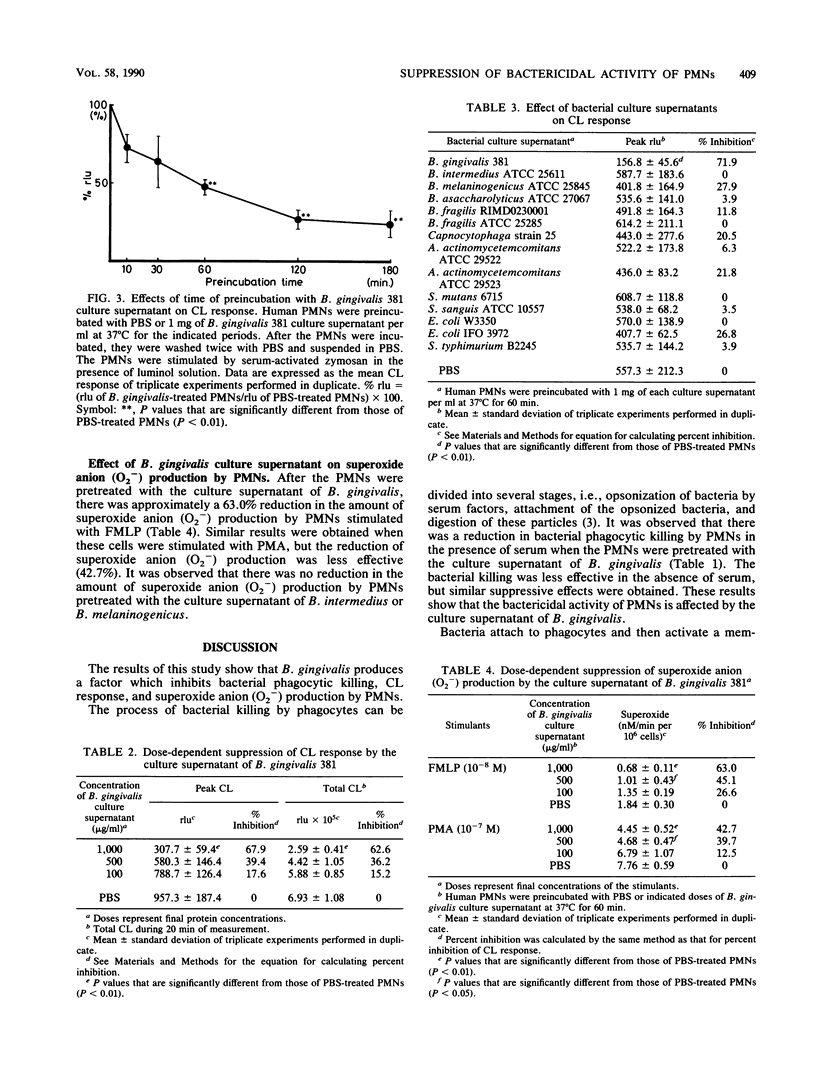
Abstract
The direct effects of the culture supernatant of oral microorganisms on the bactericidal activity of human polymorphonuclear leukocytes (PMNs) were investigated. The bactericidal activity of PMNs, which were preincubated with the supernatant of Bacteroides gingivalis, Bacteroides intermedius, Bacteroides melaninogenicus or phosphate-buffered saline, was examined by counting the surviving bacteria. B. gingivalis-treated PMNs were found to have a diminished ability for killing bacteria in the presence or absence of serum. The chemiluminescence response of PMNs, which were preincubated with the culture supernatant of B. gingivalis, was strikingly reduced compared with that of PMNs preincubated with phosphate-buffered saline or other bacterial culture supernatants. The production of superoxide anion (O2-) by PMNs stimulated with either formyl-methionyl-leucyl-phenylalanine or phorbol myristate acetate was reduced in both cases after the PMNs were preincubated with the culture supernatant of B. gingivalis. However, it was observed that there was more reduction in superoxide anion (O2-) production stimulated with formyl-methionyl-leucyl-phenylalanine compared with that stimulated with phorbol myristate acetate. These results suggest that B. gingivalis releases a factor which interferes with the bactericidal activity of PMNs by modulating the generation of reactive oxygen species. These suppressive effects on bactericidal activity may be important in the pathogenesis of this microorganism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Stjernholm R. L., Steele R. H. Evidence for the generation of an electronic excitation state(s) in human polymorphonuclear leukocytes and its participation in bactericidal activity. Biochem Biophys Res Commun. 1972 May 26;47(4):679–684. doi: 10.1016/0006-291x(72)90545-1. [DOI] [PubMed] [Google Scholar]
- Attström R., Tynelius-Bratthall G., Egelberg J. The effect of experimental leukopenia on chronic gingival inflammation in dogs. 2. Induction of leukopenia by heterologous anti-neutrophil serum. J Periodontal Res. 1971;6(3):200–210. doi: 10.1111/j.1600-0765.1971.tb00610.x. [DOI] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Cainciola L. J., Genco R. J., Patters M. R., McKenna J., van Oss C. J. Defective polymorphonuclear leukocyte function in a human periodontal disease. Nature. 1977 Feb 3;265(5593):445–447. doi: 10.1038/265445a0. [DOI] [PubMed] [Google Scholar]
- Degnan E. J., Perlov A. N. Infected oral lesions of cyclic neutropenia. J Oral Med. 1973 Jan-Mar;28(1):29–31. [PubMed] [Google Scholar]
- Di Murro C., Nisini R., Cattabriga M., Simonetti-D'Arca A., Le Moli S., Paolantonio M., Sebastiani L., D'Amelio R. Rapidly progressive periodontitis. Neutrophil chemotaxis inhibitory factors associated with the presence of Bacteroides gingivalis in crevicular fluid. J Periodontol. 1987 Dec;58(12):868–872. doi: 10.1902/jop.1987.58.12.868. [DOI] [PubMed] [Google Scholar]
- Dzink J. L., Socransky S. S., Haffajee A. D. The predominant cultivable microbiota of active and inactive lesions of destructive periodontal diseases. J Clin Periodontol. 1988 May;15(5):316–323. doi: 10.1111/j.1600-051x.1988.tb01590.x. [DOI] [PubMed] [Google Scholar]
- Fujita I., Irita K., Takeshige K., Minakami S. Diacylglycerol, 1-oleoyl-2-acetyl-glycerol, stimulates superoxide-generation from human neutrophils. Biochem Biophys Res Commun. 1984 Apr 30;120(2):318–324. doi: 10.1016/0006-291x(84)91256-7. [DOI] [PubMed] [Google Scholar]
- Genco R. J., Slots J. Host responses in periodontal diseases. J Dent Res. 1984 Mar;63(3):441–451. doi: 10.1177/00220345840630031601. [DOI] [PubMed] [Google Scholar]
- Grenier D., Chao G., McBride B. C. Characterization of sodium dodecyl sulfate-stable Bacteroides gingivalis proteases by polyacrylamide gel electrophoresis. Infect Immun. 1989 Jan;57(1):95–99. doi: 10.1128/iai.57.1.95-99.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Ebersole J., Felton J., Brunsvold M., Kornman K. S. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1988 Jan 1;239(4835):55–57. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- Ingham H. R., Sisson P. R., Tharagonnet D., Selkon J. B., Codd A. A. Inhibition of phagocytosis in vitro by obligate anaerobes. Lancet. 1977 Dec 17;2(8051):1252–1254. doi: 10.1016/s0140-6736(77)92662-9. [DOI] [PubMed] [Google Scholar]
- Iwahi T., Imada A. Interaction of Escherichia coli with polymorphonuclear leukocytes in pathogenesis of urinary tract infection in mice. Infect Immun. 1988 Apr;56(4):947–953. doi: 10.1128/iai.56.4.947-953.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lavine W. S., Maderazo E. G., Stolman J., Ward P. A., Cogen R. B., Greenblatt I., Robertson P. B. Impaired neutrophil chemotaxis in patients with juvenile and rapidly progressing periodontitis. J Periodontal Res. 1979 Jan;14(1):10–19. doi: 10.1111/j.1600-0765.1979.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Robinson J. P., Flynn M., Hudson J. L., Duque R. E. Reduced oxidative function in gingival crevicular neutrophils in periodontal disease. Infect Immun. 1988 Jan;56(1):156–160. doi: 10.1128/iai.56.1.156-160.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K., Hirofuji T., Chinju N., Tanigawa K., Iwamoto Y., Hatakeyama T., Aono M. The modulation of polymorphonuclear leukocyte function by Bacteroides gingivalis. Adv Dent Res. 1988 Nov;2(2):315–318. doi: 10.1177/08959374880020021901. [DOI] [PubMed] [Google Scholar]
- Mayrand D., Holt S. C. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988 Mar;52(1):134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Cato E. P., Smibert R. M., Burmeister J. A., Palcanis K. G., Ranney R. R. Comparative bacteriology of juvenile periodontitis. Infect Immun. 1985 May;48(2):507–519. doi: 10.1128/iai.48.2.507-519.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton C., Hammond P. G., Slots J., Genco R. J. Serum antibodies to oral Bacteroides asaccharolyticus (Bacteroides gingivalis): relationship to age and periondontal disease. Infect Immun. 1981 Jan;31(1):182–192. doi: 10.1128/iai.31.1.182-192.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y., Okuda K., Takazoe I. Detection of specific antibody in adult human periodontitis sera to surface antigens of Bacteroides gingivalis. Infect Immun. 1987 Mar;55(3):832–834. doi: 10.1128/iai.55.3.832-834.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namavar F., Verweij A. M., Bal M., van Steenbergen T. J., de Graaff J., MacLaren D. M. Effect of anaerobic bacteria on killing of Proteus mirabilis by human polymorphonuclear leukocytes. Infect Immun. 1983 Jun;40(3):930–935. doi: 10.1128/iai.40.3.930-935.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T., Carlsson J., Sundqvist G. Inactivation of key factors of the plasma proteinase cascade systems by Bacteroides gingivalis. Infect Immun. 1985 Nov;50(2):467–471. doi: 10.1128/iai.50.2.467-471.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omann G. M., Allen R. A., Bokoch G. M., Painter R. G., Traynor A. E., Sklar L. A. Signal transduction and cytoskeletal activation in the neutrophil. Physiol Rev. 1987 Jan;67(1):285–322. doi: 10.1152/physrev.1987.67.1.285. [DOI] [PubMed] [Google Scholar]
- Page R. C., Beatty P., Waldrop T. C. Molecular basis for the functional abnormality in neutrophils from patients with generalized prepubertal periodontitis. J Periodontal Res. 1987 May;22(3):182–183. doi: 10.1111/j.1600-0765.1987.tb01562.x. [DOI] [PubMed] [Google Scholar]
- Root R. K., Cohen M. S. The microbicidal mechanisms of human neutrophils and eosinophils. Rev Infect Dis. 1981 May-Jun;3(3):565–598. doi: 10.1093/clinids/3.3.565. [DOI] [PubMed] [Google Scholar]
- Rotstein O. D., Nasmith P. E., Grinstein S. The Bacteroides by-product succinic acid inhibits neutrophil respiratory burst by reducing intracellular pH. Infect Immun. 1987 Apr;55(4):864–870. doi: 10.1128/iai.55.4.864-870.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotstein O. D., Pruett T. L., Fiegel V. D., Nelson R. D., Simmons R. L. Succinic acid, a metabolic by-product of Bacteroides species, inhibits polymorphonuclear leukocyte function. Infect Immun. 1985 May;48(2):402–408. doi: 10.1128/iai.48.2.402-408.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K., Kato C., Teshigawara H. Saliva inhibits the chemiluminescence response, phagocytosis, and killing of Staphylococcus epidermidis by polymorphonuclear leukocytes. Infect Immun. 1988 Aug;56(8):2125–2132. doi: 10.1128/iai.56.8.2125-2132.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallay K., Listgarten M., Sanavi F., Ring I., Nowotny A. Bacterial invasion of oral tissues of immunosuppressed rats. Infect Immun. 1984 Mar;43(3):1091–1093. doi: 10.1128/iai.43.3.1091-1093.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandholm L. Proteases and their inhibitors in chronic inflammatory periodontal disease. J Clin Periodontol. 1986 Jan;13(1):19–26. doi: 10.1111/j.1600-051x.1986.tb01409.x. [DOI] [PubMed] [Google Scholar]
- Sato M., Otsuka M., Maehara R., Endo J., Nakamura R. Degradation of human secretory immunoglobulin A by protease isolated from the anaerobic periodontopathogenic bacterium, Bacteroides gingivalis. Arch Oral Biol. 1987;32(4):235–238. doi: 10.1016/0003-9969(87)90016-1. [DOI] [PubMed] [Google Scholar]
- Seim S. Production of reactive oxygen species and chemiluminescence by human monocytes during differentiation and lymphokine activation in vitro. Acta Pathol Microbiol Immunol Scand C. 1982 Jun;90(3):179–185. doi: 10.1111/j.1699-0463.1982.tb01436.x. [DOI] [PubMed] [Google Scholar]
- Sela M. N., Weinberg A., Borinsky R., Holt S. C., Dishon T. Inhibition of superoxide production in human polymorphonuclear leukocytes by oral treponemal factors. Infect Immun. 1988 Mar;56(3):589–594. doi: 10.1128/iai.56.3.589-594.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Genco R. J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984 Mar;63(3):412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- Socransky S. S., Haffajee A. D., Dzink J. L. Relationship of subgingival microbial complexes to clinical features at the sampled sites. J Clin Periodontol. 1988 Aug;15(7):440–444. doi: 10.1111/j.1600-051x.1988.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Tempel T. R., Kimball H. R., Kakenashi S., Amen C. R. Host factors in periodontal disease: periodontal manifestations of Chediak-Higashi Syndrome. J Periodontal Res. 1972;(10):26–27. [PubMed] [Google Scholar]
- Uchida T., Kanno T., Hosaka S. Direct measurement of phagosomal reactive oxygen by luminol-binding microspheres. J Immunol Methods. 1985 Feb 28;77(1):55–61. doi: 10.1016/0022-1759(85)90183-8. [DOI] [PubMed] [Google Scholar]
- Uitto V. J., Larjava H., Heino J., Sorsa T. A protease of Bacteroides gingivalis degrades cell surface and matrix glycoproteins of cultured gingival fibroblasts and induces secretion of collagenase and plasminogen activator. Infect Immun. 1989 Jan;57(1):213–218. doi: 10.1128/iai.57.1.213-218.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke T. E., Horoszewicz H. U., Cianciola L. J., Genco R. J. Neutrophil chemotaxis dysfunction in human periodontitis. Infect Immun. 1980 Jan;27(1):124–132. doi: 10.1128/iai.27.1.124-132.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke T. E. Neutrophil receptor modulation in the pathogenesis of periodontal diseases. J Dent Res. 1984 Mar;63(3):452–454. doi: 10.1177/00220345840630031701. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Nishikata M., Suzuki T., Hoover C. I., Newbrun E. Characterization of a trypsin-like protease from the bacterium Bacteroides gingivalis isolated from human dental plaque. Arch Oral Biol. 1984;29(7):559–564. doi: 10.1016/0003-9969(84)90078-5. [DOI] [PubMed] [Google Scholar]


