Abstract
Sodium is an essential nutrient whose deposition in rainfall decreases with distance inland. The herbivores and microbial decomposers that feed on sodium-poor vegetation should be particularly constrained along gradients of decreasing sodium. We studied the use of sucrose and NaCl baits in 17 New World ant communities located 4–2757 km inland. Sodium use was higher in genera and subfamilies characterized as omnivores/herbivores compared with those classified as carnivores and was lower in communities embedded in forest litter than in those embedded in abundant vegetation. Sodium use was increased in ant communities further inland, as was preference for the baits with the highest sodium concentration. Sucrose use, a measure of ant activity, peaked in communities 10–100 km inland. We suggest that the geography of ant activity is shaped by sodium toxicity near the shore and by sodium deficit farther inland. Given the importance of ants in terrestrial ecosystems, changing patterns of rainfall with global change may ramify through inland food webs.
Keywords: ants, biogeochemistry, geography, limitation, sodium
There are many reasons why the availability of sodium should impact population and ecosystem processes. Sodium is vital for maintaining osmotic balance, muscle activity, and nervous system function (1), and body sodium is tightly regulated (2). Terrestrial plants contain ca. 1.0 mg · kg−1 sodium (3), whereas the herbivores and decomposer microbes that eat them maintain sodium levels 100- to 1000-fold higher (2, 4). Because sodium (unlike, e.g., nitrogen) is readily lost through excretion, basal consumers often must expend considerable energy to find and harvest it (5–7).
Sodium supply varies geographically. It is carried inland by oceanic aerosols, and the sodium content in rainfall can drop up to 1000-fold with increasing distance from the ocean (8, 9). Furthermore, inland ecosystems with high rainfall may experience significant sodium loss through leaching (10). As a consequence, continental populations of moose (6), gorillas (11), parrots (12), butterflies (13), and bees (14) often search for “salt licks” (15) or resort to cannibalism (16) to maintain sodium balance. However, aside from the salient work of Blair-West et al. (17) on introduced rabbits in Australia, we know of no detailed studies of how attraction to sodium, and potentially sodium limitation, varies geographically.
Ant communities (Hymenoptera: Formicidae) are a model system for exploring the biogeography of salt limitation. Ants are common players in terrestrial ecosystems (18, 19). Moreover, ants are trophically diverse, with herbivores that feed on sugary exudates common in “green” food webs with abundant vegetation (e.g., forest canopies and grasslands) and predators that feed on the consumers of decomposer microbes, common in litter (or “brown”) food webs (20–23).
We quantified recruitment to NaCl and sucrose in 17 ant communities to test 2 hypotheses concerning the biogeography of salt limitation. The first hypothesis posits that decreased sodium inputs via rainfall should increase the sodium limitation of consumers. We predicted that the harvesting of NaCl solution by ants increases from the coastline to the interior of continents. The second hypothesis posits that herbivores are more sodium-limited than predators. We predicted that carnivorous taxa would use NaCl baits less frequently than herbivores and omnivores, and that communities embedded in green food webs would show more attraction to sodium than those from brown food webs.
Results
We sampled ant communities from a variety of ecosystems [supporting information (SI) Table S1], ranging from coastal forest and scrub 4 km inland to an Amazonian rain forest 2757 km inland. These communities yielded between 4 (Panama litter) and 18 genera (Peru rain forest litter; median, 8) and were sampled at temperatures from 26 °C (Peru rainforest) to 34 °C (Florida scrub; median, 28 °C).
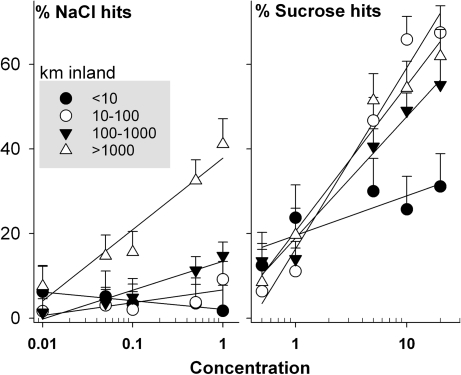
Ant recruitment to sucrose increased from concentrations of 0.5% to 20% (F1,66 = 83.14; P < 0.0001), with 20% sucrose (Fig. 1) uniformly trending the highest. Ant recruitment to sucrose also varied with distance inland (F3, 66 = 4.9; P < 0.004). A significant distance–concentration interaction (F3,66 = 6.8; P < 0.0005) indicated that the exponent of the recruitment curve varied with distance to the ocean. When communities in each distance bin were analyzed separately, the percentage of vial hits scaled as sucrose concentration0.15 at < 10 km inland (F1,8 =; P = 0.18; r2 = 0.21) up to 0.88 at 10–100 km inland (F1,17 = 5.7; P = 0.0001; r2 = 0.65), and down to 0.50 (F1,29 = 7.8; P = 0.0001; r2 = 0.68) and 0.48 (F1,12 = 9.71; P = 0.0001; r2 = 0.89), respectively, at 100–1000 and > 1000 km inland. The highest sucrose recruitment values (66% and 68%) were 10–100 km at concentrations of 10% and 20%, respectively. Further inland, these concentrations averaged 49% and 55%, and 54% and 61% hits (Fig. 1).
Fig. 1.
Community-wide use of NaCl and sucrose baits as a function of concentration and distance inland (least squares means plus 1 standard error).
Like sucrose, ant recruitment to NaCl baits showed significant interactions (F3,71 = 4.4; P = 0.007), along with the effects of concentration (F1,71 = 7.06; P = 0.0097) and distance inland (F3,71 = 10.2; P = 0.0001; Fig. 1). Within 10 km of the ocean, vial hits marginally decreased as NaCl concentration−0.27 (F1,8 = −2.02; P = 0.08; r2 = 0.34), failed to vary with concentration at 10–100 km (F1,18 = 1.4; P = 0.17; r2 = 0.10), increased with NaCl concentration0.33 at 100–1000 km (F1,33 = 3.7; P = 0.007; r2 = 0.30), and increased most strongly with NaCl concentration0.44 at > 1000 km inland (F1,12 = 3.3; P = 0.0024; r2 = 0.55). Unlike sucrose, the highest values for recruitment to NaCl were at the sites furthest inland.
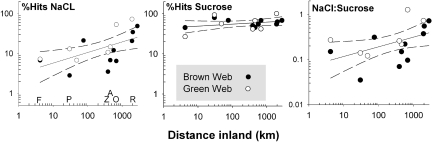
When distance inland was treated as a continuous variable, the maximum percentage of NaCl baits used by an ant community increased with distance inland (Fig. 2; F1,16 = 7.8; P = 0.013; y = 3.8x0.25; r2 = 0.35). The ratio of NaCl to sucrose use also increased with distance inland (F1,16 = 5.02; P = 0.041; y = 0.99x0.05; r2 = 0.26).
Fig. 2.
The maximum use of sucrose and NaCl baits by ant communities in 17 communities varies with distance inland and type of food web. Sites are classified as a brown food web if the majority of vegetation is in a closed canopy or as a green food web if vegetation is readily accessible from the litter. In six regions (F, Florida; P, Panama; Z, Arizona; A, Arkansas; O, Oklahoma; R, Peru; see Table 1), green and brown webs at the same distance inland were compared.
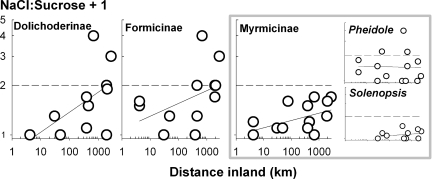
We decomposed the community-level pattern in Fig. 2 to that of its three dominant subfamilies and two most widespread genera (Fig. 3). Although the best-fit regression exponents varied two-fold, from 0.06 (Myrmicinae) to 0.17 (Dolichoderinae), the subfamilies consistently demonstrated increased sodium use relative to sucrose use with distance inland (F1,28 = 14.05, P = 0.0008; subfamily, F2,28 = 1.76, P = 0.19; interaction, F3,28 = 1.95, P = 0.16). Compared with whole communities, subfamilies yielded more triangular scatterplots, suggesting that inland locations were necessary, but not sufficient, to generate high recruitment to NaCl in subfamily-based ant communities. Proceeding further downward taxonomically, only 2 of 32 genera, both Myrmicines, met our inclusion criteria of 10 sites, with >5 hits per site. For Pheidole and Solenopsis, the distance effect disappeared (F1,21 = 0.06, P = 0.80; genus, F2,28 = 3.92, P = 0.06; interaction, F3,28 = 0.56, P = 0.46).
Fig. 3.
Taxonomic decomposition of salt use across 17 ant communities. The ratio of hits on NaCl baits and sucrose baits is compared among three common subfamilies and two common genera of the subfamily Myrmicinae. The dashed line represents equal use; the straight curve is the best fit by linear regression.
Finally, we tested the prediction that increasing a taxon's trophic level decreases salt recruitment. We did so in two ways. First, as predicted, six genera (from four subfamilies) classified as herbivore omnivores (24) had six-fold higher Na:CHO ratios than the 11 genera (from five subfamilies) classified as carnivores (8.7 vs. 1.5; Kruskal-Wallis χ211,6 = 8.0; P = 0.0024). When these genera were collapsed into subfamilies, the difference was nine-fold (6.9 vs. 0.75; Kruskal Wallis χ25,4 = 2.9; P = 0.0432). Second, across six paired communities, sodium use was consistently higher (average, 20%) in ant communities embedded in green food webs (signed-rank test, S = 10; P = 0.031; Fig. 1).
Discussion
Temperature and rainfall, two “master regulators” (25), vary geographically, with consequences for the structure and function of ecological communities (19, 26, 27). But despite the chemical diversity of living organisms (1) and spatial patterns of biogeochemistry (28), there have been few studies of the geography of nutrient limitation in consumers (17, 29). Here we show that ant communities increasingly recruit to sodium baits with increasing distance inland. That ants recruited to NaCl at all was somewhat of a surprise; in an Oklahoma grassland, the use of 1% NaCl baits exceeded the use of 10%–20% sugar solutions! This strongly suggests that sodium availability, via the deposition of NaCl in oceanic aerosols, can limit the activity and/or abundance of a dominant insect group.
Patterns of ant recruitment to our experimental gradients also suggest NaCl limitation further inland. The use of a commonly accepted carbohydrate—sucrose—roughly, and inversely, mirrors recruitment to NaCl. Between 10 and 100 km inland, ants appeared to be indifferent to the NaCl concentration gradient, suggesting the absence of NaCl limitation; in this same zone, the highest use of 10% and 20% sucrose baits was recorded. Further inland, ant communities increasingly preferred the highest concentrations of NaCl, while at the same time, maximal recruitment to sucrose dropped (Fig. 1). Intriguingly, <10 km inland, ants tended (P = 0.08) to use lower-concentration NaCl baits (i.e., 0.01%, 0.05%); in the same locales, the lowest community-wide recruitment to sucrose was recorded. This suggests possible sodium toxicity in ecosystems receiving regular exposure to saline oceanic aerosols (although these sites also were among the warmest during our bait trials; Table 1).
Table 1.
Seventeen ant communities sampled for sucrose and NaCl use
| Region | Name | Genera | Biome | Distance inland, km | Number | Latitude | Longitude | Food Web | Temperature, °C |
|---|---|---|---|---|---|---|---|---|---|
| Peru | Cicra | 18 | Rain forest | 2757 | 6 | 12.56 S | 70.10 W | Brown | 26.7 |
| Peru | Iquitos | 15 | Rain forest | 2028 | 5 | 3.95 S | 73.41 W | Brown | 25.5 |
| Peru1 | ACTS litter | 12 | Rain forest | 1920 | 5 | 3.25 S | 72.91 W | Brown | 26.7 |
| Peru1 | ACTS canopy | 4 | Rain forest | 1920 | – | 3.25 S | 72.91 W | Green | – |
| Oklahoma2 | Ranch | 8 | Grassland | 676 | 4 | 35.23 N | 97.19 W | Green | 25.6 |
| Oklahoma2 | Lake | 6 | Oak forest | 676 | 4 | 35.20 N | 97.13 W | Brown | 27.3 |
| Arkansas3 | Hayfield | 8 | Old field | 448 | 5 | 33.81 N | 94.20 W | Green | 30.3 |
| Arkansas3 | Cut | 8 | Mixed forest | 452 | 5 | 33.81 N | 91.28 W | Brown | 27.0 |
| Arkansas | Oak | 5 | Oak forest | 567 | 5 | 34.82 N | 92.49 W | Brown | 27.5 |
| Arizona4 | Stateline | 9 | Warm desert | 389 | 4 | 31.95 N | 109.16 W | Green | 28.5 |
| Arizona4 | SWRS | 6 | Pine forest | 389 | 4 | 31.88 N | 109.22 W | Brown | 29.2 |
| Costa Rica | La Selva | 9 | Rain forest | 76 | 5 | 10.43 N | 84.01 W | Brown | 27.5 |
| Florida | Everglades | 10 | Grassland | 48 | 5 | 25.38 N | 80.61 W | Green | 28.2 |
| Panama5 | BCI | 4 | Rain forest | 30 | 5 | 9.15 N | 79.86 W | Brown | 25.7 |
| Panama5 | BCI | 9 | Clearing | 30 | 1 | 9.15 N | 79.86 W | Green | 32.5 |
| Florida6 | Scrub | 11 | Pine scrub | 4 | 4 | 27.59 N | 80.37 W | Green | 34.1 |
| Florida6 | Hammock | 5 | Oak forest | 4 | 4 | 27.59 N | 80.37 W | Brown | 32 |
Number refers to the number of sampling transects run. Temperature refers to the mean temperature at start of trials. Sites sharing the same superscript are green and brown food webs paired by region. Temperature was not recorded in canopy studies, where 15 trees were sampled during mid-morning over 2 days.
Overall, these findings suggest that the availability of sodium (and/or other elements distributed through aerosols) may place metabolic limits on ant (and presumably, other consumer) activity. Ecosystems 10–100 km inland may occupy a sodium “sweet spot,” in which supplies are adequate to meet metabolic demand but not high enough to hinder ant activity (either through depression of plant productivity or by more direct effects). This working hypothesis makes clear predictions as to how experimental NaCl additions should affect the activity and standing crop of ants, in much the same way that “salt licks” increase the production of cattle in the continental interior (7) and irrigation with mined water depresses plant productivity (2, 3).
The simplest scenario underlying increasing NaCl use further inland is that all ant colonies have the same NaCl requirements and ability to harvest NaCl. If this were so, then we would expect to see self-similarity in patterns of ant recruitment to sodium as we decompose patterns for the family Formicidae into its constituent subfamilies, genera, and species. Within the limits of reasonable statistical power (10 sites with at least five hits per site), we show that three common subfamilies consistently increase NaCl use relative to sucrose further inland, but that two genera do not. Both genera, Pheidole and Solenopsis, may be buffered from NaCl deprivation by being carnivorous (16). Consistent with our second prediction, carnivorous ant genera and subfamilies are less likely to recruit to salt than those with lower delta 15N signatures (24). Sodium stress may also be tempered by reducing body sodium (17), increasing the efficiency of osmoregulation (30, 31), switching sodium with phosphate or potassium (2), or feeding at higher trophic levels (16). Ultimately, the increasingly triangular scatterplots at finer taxonomic resolution may represent individual taxa, populations, or individuals implementing a variety of tactics to ameliorate sodium stress (32, 33). The degree to which our community-wide pattern is shaped by taxonomic replacement by more or less sodium-hungry taxa, by local adaptation, and/or by phenotypic plasticity awaits further study.
Sodium availability also varies locally with soil type, topography, and irrigation by groundwater (34). Some variability in sodium and sucrose recruitment across sites is accounted for by the local changes in habitat; for omnivorous taxa, access to sodium (and various other micronutrients) is likely higher in brown food webs, where fungi and bacteria concentrate sodium from sodium-poor leaf litter (4, 35). Our results support the hypothesis that ants in habitats surrounded by green vegetation are more herbivorous and sodium-stressed; however, the use of sodium baits even in litter communities appears to increase rapidly beyond ca. 300 km inland (Fig. 2).
In conclusion, the availability of NaCl appears to generate a geographic signature on the activity of a dominant group of terrestrial consumers, the ants. Sodium has a clear importance as a limiting nutrient in animal husbandry (2, 7). It also is toxic to plants; one-third of irrigated land is less productive due to NaCl contamination (2). The biogeography of salt limitation also may play out at the ecosystem level (29); for example, if low sodium input has little effect on plants but hampers the activity of microbial decomposers (4), then ecosystems of the continental interior (e.g., much of the Amazon basin, the grasslands of North America) may store more plant production as undecomposed carbon compared with ecosystems closer (but not necessarily adjacent) to the ocean. Given the importance of oceanic aerosols in sodium deposition and the uncertainty in predicting rainfall in a changing climate (36), this may be another unexpected forcing factor on global carbon cycles.
Methods
We sampled the NaCl and sucrose use of 17 ant communities (Table S1). For each site, we measured the distance from the oceanic source of precipitation using Google Earth. Where this was ambiguous (i.e., the Everglades are in the center of the Florida peninsula), or where two oceans generated rainfall (i.e., the Chihuahua desert of Arizona), multiple measures were taken and averaged. In six localities (Arizona, Arkansas, Florida, Oklahoma, Panama, Peru; Table 1), we sampled pairs of communities at similar (± 10 km) distances inland. Each pair was characterized a priori as a brown food web (i.e., closed canopy forests with a thick litter layer and little vegetation in the understory) or a green food web (i.e., minimal canopy, if any, and abundant living vegetation accessible to the ground ant community). All brown communities were in forest litter. Green communities included grasslands, hayfields, lawns, and, in one case, the canopy of a tropical forest (see below).
Ant communities were sampled with transects of labeled Eppendorf vials. Each vial was half-stuffed with cotton saturated with sucrose or NaCl solution. Sucrose was used as a standard of overall ant activity, because sugars are widely used by ants and there was no a priori reason to expect geographic variation in sucrose use. Vials were baited with a series of four or five concentrations (sucrose: 0.1%, 0.5%, 1%, 5%, 10%, and 20% by mass; NaCl: 0.01%, 0.05%, 0.1%, 0.5%, and 1% by mass) plus a distilled water treatment. Fifteen vials of each concentration were snapped shut and thoroughly mixed in plastic bags. At each site, four or five linear transects were laid out (with one exception—“Panama clearing” had only one transect), and a vial was randomly selected, opened, and placed every 1 m. After 1 h, we collected the vials, snapping the cap shut and capturing the ants using the baits. These ants were identified to genus and counted in the laboratory.
In addition, at one site (ACTS in Peru), we used a canopy walkway to sample the ant communities of 15 tree crowns and the leaf litter communities beneath. Because vials could not be reliably placed and recovered from tree branches, we placed cotton balls soaked in sucrose, NaCl, and distilled water solutions in a row (with baits separated by 10 cm) on a large branch in the canopy or on the leaf litter. Solutions started at 0.1% NaCl and 1% sucrose and were observed for 15 min. Over successive 5-min intervals, concentrations were increased (NaCl: 0.05%, 0.1%, and 0.5%, sucrose: 5%, 10%, and 20%). We recorded the number of ants that drank from the solutions (assayed as 5 sec of continuous contact with the mandibles) over the period in which each concentration was presented. A total of 15 trees and 19 litter patches (10 m apart) were sampled in this way.
For each site analyzed with the vial method, sucrose and NaCl vials were scored, at each concentration, by the percentage (out of 15 vials) with ants minus the activity at distilled water. The cotton ball experiments were scored similarly as the percentage of trials (15 tree, 19 litter) in which sucrose and or NaCl was collected by ants. The ACTS litter sampled with the Eppendorf vials and cotton balls yielded similar values; only vial data are presented here.
To analyze patterns of recruitment with concentration and distance inland, we first calculated the average percentage of hits by ants for each concentration at a given site across each site's replicate transects. We used one-tailed tests to evaluate predictions of (1) higher sodium use further inland and (2) lower sodium use with increasing carnivory.
We tested prediction 1 in two ways. First, we used analysis of covariance to test the hypothesis that log10 (ant recruitment) increased with log10 (concentration for sucrose and NaCl), but that recruitment to NaCl, but not to sucrose, increased with distance inland. We did so by binning sites by distance inland (< 10 km [n = 2], 10–100 km [n = 4], 100–1000 [n = 7], and > 1000 km [n = 4]). We also evaluated maximum sucrose and NaCl recruitment across sites across the range of concentrations (typically at 1% NaCl and 10%–20% sucrose) with distance inland. Because sodium use may be confounded by overall activity (as gauged by the activity at sucrose baits), we also evaluated changes in the ratio of sodium use to sucrose use per site. We evaluated taxonomic self-similarity for NaCl and sucrose use for subfamilies and genera that met the minimum criteria of the presence at 10 or more sites and at least five bait hits per site. In each case, we used analysis of covariance, with taxonomic membership as the class variable and distance inland as the continuous variable.
We also tested prediction 2 (lower sodium use with increasing carnivory) in two ways. First, we characterized genera by the Na:CHO ratio based on the total number of hits on NaCl versus those on sucrose. (Two genera, Gigantiops and Pseudomyrmex were assigned arbitrary values of 2, given absence of any workers at sucrose baits.) We used Davidson's (22, 24) studies of Amazon ants to assign 17 of 32 genera as “herbivore-omnivore” (6 genera) or “carnivore” (11 genera), based on Davidson's threshold of 5.5% delta 15N. We compared Na:CHO ratios for these two trophic groups using a Kruskal-Wallis test. As before, we then took subfamily means and repeated the test at a coarser taxonomic resolution. Second, we used a signed-rank test to compare NaCl and sucrose use in ants of for six pairs of matched green food web and brown food web communities.
Supplementary Material
Acknowledgments.
We thank F. Azorsa, N. Clay, M. Koinig, S. Price, C. Schaller, and N. Zegarra for field and laboratory assistance and O. Acevedo, P. Bucur, P. Jensen, S. Madigosky, N. Pitman, and R. S. Sikes for logistical support. The Panamanian Autoridad Nacional del Ambiente (ANAM), the Costa Rican Ministerio del Ambiente y Energía (MINAE), and the Peruvian Instituto Nacional de Recursos Naturales (INRENA) provided permits. The Amazon Conservatory for Tropical Studies, Amazon Explorama Lodges, the Smithsonian Tropical Research Institute, and the Organization for Tropical Studies facilitated access to field sites. This research was supported in part by the National Geographic Society and the Amazon Conservation Association.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804528105/DCSupplemental.
References
- 1.Frausto da Silva JJR, Williams RJP. The Biological Chemistry of the Elements: The Inorganic Chemistry of Life. Oxford, UK: Oxford Univ Press; 2001. [Google Scholar]
- 2.National Research Council. Mineral tolerance of animals. Washington DC: National Academies Press; 2005. [Google Scholar]
- 3.Marschner H. Mineral Nutrition in Higher Plants. San Diego: Academic Press; 1995. [Google Scholar]
- 4.Cromack FJ, et al. In: The Role of Arthropods in Forest Ecosystems. Mattson WJ, editor. New York: Springer-Verlag; 1977. pp. 78–84. [Google Scholar]
- 5.Botkin DB, Jordan PA, Dominski AS, Lowendorf HS. Sodium dynamics in a northern ecosystem (moose, wolves, plants) Proc Natl Acad Sci U S A. 1973;70:2745–2748. doi: 10.1073/pnas.70.10.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belovsky GE, Jordan PA. Sodium dynamics and adaptations of a moose population. J Mammal. 1981;62:613–621. [Google Scholar]
- 7.National Research Council. Nutrient Requirements of Beef Cattle. 7th Rev Ed. Washington, DC: National Academies Press; 2000. [Google Scholar]
- 8.Stallard RF, Edmond JM. Geochemistry of the Amazon, 1: Precipitation chemistry and the marine contribution to the dissolved load at the time of peak discharge. J Geophys Res. 1981;86:9844–9858. [Google Scholar]
- 9.National Atmospheric Deposition Program. Sodium ion wet deposition. [Accessed August 5, 2008];2006 Available at: http://nadp.sws.uiuc.edu/isopleths/maps2000/nadep.pdf.
- 10.Vitousek PM, Sanford RL. Nutrient cycling in moist tropical forest. Ann Rev Ecol Sys. 1986;17:137–167. [Google Scholar]
- 11.Rothman JM, Van Soest PJ, Pell AN. Decaying wood is a sodium source for mountain gorillas. Biol Lett. 2006;2:321–324. doi: 10.1098/rsbl.2006.0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emmons LH, Stark NM. Elemental composition of a natural mineral lick in Amazonia. Biotropica. 1979;11:311–313. [Google Scholar]
- 13.Arms K, Feeny P, Lederhouse RC. Sodium: Stimulus for puddling behavior by tiger swallowtail butterflies, Papilio glaucus. Science. 1974;185:372–374. doi: 10.1126/science.185.4148.372. [DOI] [PubMed] [Google Scholar]
- 14.Barrows WM. Aggregation behavior and response to sodium chloride in females of a solitary bee, Augochlora pura (Hymenoptera: Halictidae) Florida Entom. 1974;57:189–193. [Google Scholar]
- 15.Tracy BF, McNaughton SJ. Elemental analysis of mineral lick soils from the Serengeti National Park, the Konza Prairie and Yellowstone National Park. Ecography. 1995;18:91–94. [Google Scholar]
- 16.Simpson SJ, Sword GA, Lorch PD, Couzin ID. Cannibal crickets on a forced march for protein and salt. Proc Natl Acad Sci U S A. 2006;103:4152–5156. doi: 10.1073/pnas.0508915103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blair-West JR, et al. Physiological, morphological, and behavioral adaptation to a sodium-deficient environment by wild native Australian and introduced species of animals. Nature. 1968;217:922–928. doi: 10.1038/217922a0. [DOI] [PubMed] [Google Scholar]
- 18.Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Belknap Press; 1990. [Google Scholar]
- 19.Kaspari M, Alonso L, O'Donnell S. Three energy variables predict ant abundance at a geographic scale. Proc R Soc Lond B. 2000;267:485–490. doi: 10.1098/rspb.2000.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yanoviak SP, Kaspari M. Community structure and the habitat templet: Ants in the tropical forest canopy and litter. Oikos. 2000;89:256–266. [Google Scholar]
- 21.Kaspari M. Using the Metabolic Theory of Ecology to predict global patterns of abundance. Ecology. 2004;85:1800–1802. [Google Scholar]
- 22.Davidson DW, Cook SC, Snelling RR, Chua TH. Explaining the abundance of ants in lowland tropical rainforest canopies. Science. 2003;300:969–972. doi: 10.1126/science.1082074. [DOI] [PubMed] [Google Scholar]
- 23.Tobin JE. In: Nourishment and Evolution in Insect Societies. Hunt JH, Napela C, editors. Boulder, CO: Westview Press; 1994. pp. 278–309. [Google Scholar]
- 24.Davidson DW. Ecological stoichiometry of ants in a New World rain forest. Oecologica. 2005;142:221–231. doi: 10.1007/s00442-004-1722-0. [DOI] [PubMed] [Google Scholar]
- 25.Krebs CJ. Ecology. 4th Ed. New York: Harper & Row; 1985. [Google Scholar]
- 26.Hawkins BA, et al. Energy, water, and broad-scale geographic patterns of species richness. Ecology. 2003;84:3105–3117. [Google Scholar]
- 27.Currie DJ, Paquin V. Large-scale biogeographical patterns of species richness of trees. Nature. 1987;329:326–327. [Google Scholar]
- 28.Chapin FS, Matson PA, Mooney HA. Principles of Ecosystem Ecology. New York: Springer-Verlag; 2002. [Google Scholar]
- 29.McNaughton SJ, Banyikwa FF, McNaughton MM. Promotion of the cycling of diet-enhancing nutrients by African grazers. Science. 1997;278:1798–1800. doi: 10.1126/science.278.5344.1798. [DOI] [PubMed] [Google Scholar]
- 30.Nakajima T, et al. Natural selection and population history in the human angiotensinogen gene (AGT): 736 complete AGT sequences in chromosomes from around the world. Am J Hum Genet. 2004;74:898–916. doi: 10.1086/420793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson EE, et al. CYP3A variation and the evolution of salt-sensitivity variants. Am J Hum Genet. 2004;75:1059–1069. doi: 10.1086/426406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lord J, Westoby M, Leishman MR. Seed size and phylogeny in six temperate floras: Constraints, niche conservatism, and adaptation. Am Naturalist. 1995;146:349–364. [Google Scholar]
- 33.Simpson SJ, Raubenheimer D. Feeding behaviour, sensory physiology and nutrient feedback: A unifying model. Entom Exp Appl. 1996;80:55–64. [Google Scholar]
- 34.Tan KH. Soil Sampling, Preparation, and Analysis. New York: CRC Press; 1995. [Google Scholar]
- 35.McBrayer JF, et al. Decomposer invertebrate populations in U.S. forest biomes. Pedobiology. 1977;17:89–96. [Google Scholar]
- 36.Wentz FJ, Ricciardulli L, Hilburn K, Mears C. How much more rain will global warming bring? Science. 2007;317:233–235. doi: 10.1126/science.1140746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.