Abstract
Biosynthesis of phosphatidylinositol (PI)-containing lipoarabinomannan (LAM) and lipomannan (LM) of Mycobacterium spp. follows a conserved pathway involving multiple membrane-associated, substrate-specific mannosyltransferases (ManTs) responsible for the sequential addition of α-mannopyranosyl (Manp) units donated by decaprenyl-P-Manp on the periplasmic side of the plasma membrane. Because of their receptor-binding and immunomodulatory properties, the α(1→2)-linked di- and tri-Manp motifs that functionalize the nonreducing arabinan termini of LAM (ManLAM) in Mycobacterium tuberculosis are of crucial importance. We now show that the M. tuberculosis ManT, Rv2181, is required for the addition of these α(1→2)-linked Manp residues but also at other locations of the LAM molecule. Structural analyses of the LM and LAM variants produced by a M. tuberculosis Rv2181 knockout mutant revealed the presence of but a single Manp residue on the nonreducing arabinan termini of LAM and also a complete absence of α(1→2)-linked Man branching on the mannan backbones of LM and LAM. A recombinant strain was constructed in ManLAM-deficient Mycobacterium smegmatis that coexpressed Rv2181 and Rv1635c—the ManT responsible for the addition of the first Manp capping residue of ManLAM. Analysis revealed LAM termini fully capped with di- and tri-Manp motifs in addition to α(1→2)Man branching on the mannan backbones of LM and LAM, confirming the involvement of the α(1→2)ManT Rv2181 in the dual role of Man capping and mannan-core branching, and in the process generated a rapidly growing, ManLAM-containing strain, a tool for the study of the role of ManLAM in the pathogenesis of tuberculosis.
Keywords: tuberculosis, glycosyltransferase
Mannose (Man) is a key component of several functionally important cell-wall and intracellular structures of mycobacteria, including the family of glycosylphosphoinositides consisting of phosphatidylinositol mannosides (PIMs), lipomannan (LM), and lipoarabinomannan (LAM), a cytoplasmic methylmannose polysaccharide (MMP), and a few O-mannosylated glycoproteins (1–3). The diversity of their structures is afforded by an unparalleled range of glycosyltransferases (GTs) using either GDP-Manp or decaprenyl (C50)-P-Manp donors. The most significant of these structures is LAM because it has been implicated in various aspects of the virulence and pathogenesis of M. tuberculosis (M. tb) such as induction of phagocytosis, phagosomal alteration and inhibition of fusion with lysosomes, and induction of innate, humoral, and acquired T cell-mediated immunity (4–6).
There are invariant aspects to the structure of all forms of LAM: a PIM base, an elongated α(1→6) linear α(1→2) branched mannan “core” (LM), and a highly branched D-arabinan attached to the terminus of the mannan core (LAM). Variability occurs in another aspect of the structure, in that the nonreducing termini of the arabinan of LAM may be “capped” to various degrees with short α(1→2) mannopyranosyl (Manp) chains consisting of 1–3 residues to yield the functionally important ManLAM [supporting information (SI) Fig. S1]. Mycobacterium smegmatis (M. smg) is devoid of Manp caps, partially replaced by inositol phosphate units (7, 8).
The early stages of PIM biosynthesis have been defined (9). The initial Manp residues are donated by GDP-Manp and involve mannosyltransferases (ManTs) of the GT-B superfamily (10–12). The mechanisms of membrane translocation of PIM2 or PIM3 are unknown, but subsequent steps require C50-P-Manp and GTs of the membranous GT-C superfamily; an example is the transmembrane protein (PimE) responsible for the synthesis of PIM5 (13). The linear α(1→6)-linked mannan backbone modulated by α(1→2) Manp side branches (Fig. S1) of LM and LAM is the product of C50-P-Manp and the GT-C ManTs, Rv2174 (14) and Rv2181 (15). EmbC of the Emb operon, combined with C50-P-arabinofuranose (Araf) as the Araf donor, is responsible for the synthesis of the arabinan of LAM (16). However, the genetics and the biosynthetic origins of the Manp caps that modulate LAM to a functional ligand responsible for such as the uptake of M. tb by macrophages and dendritic cells have not been defined.
We had shown that MT1671/Rv1635c from M. tb CDC1551 is responsible for the deposition of the first Manp residue on the nonreducing arabinan termini of LAM (17). However, distinct α(1→2) ManTs responsible for the formation of the immunomodulatory di- and tri-Manp motifs of ManLAM have not been identified. In light of the evidence that Rv2181 catalyzes the attachment of the α(1→2) Manp branches to the mannan backbone of LM/LAM (15) we postulated that this same enzyme may serve the additional role of completing the Man-capping events. Evidence is presented, based on phenotypic analysis of a knockout mutant of M. tb H37RvΔRv2181 and a recombinant strain of M. smg coexpressing Rv2181 and Rv1635c, that Rv2181 is required not only for Man capping but also for LM and LAM branching and perhaps other glycosylation events important for the pathogenesis of M. tb.
Results
Rationale for a Role of Rv2181 in Man-Capping.
Because Man-capping of LAM is thought to take place on the periplasmic side of the plasma membrane (18), members of the GT-C superfamily without an already assigned function represent prime candidates for this role. Based on this hypothesis and on the assumption that such enzymes should be missing from those Mycobacterium spp. devoid of LAM-based Man caps, e.g., M. smg, we previously identified MT1671 of M. tb CDC1551 (ortholog of Rv1635c of M. tb H37Rv) as responsible for the addition of the first Manp unit of the important Manp-containing capping motifs. The only other putative GT-C enzyme devoid of an ortholog in M. smg is Rv3779; however, it is present in the expected arabinogalactan biosynthetic gene cluster (18). An alternative hypothesis is that the Man caps could arise from the action of a promiscuous ManT, such as PimE (Rv1159) or Rv2181, responsible for the transfer of α-1,2-linked Manp units to PIM4 and the mannan backbone of LM and LAM, respectively (13, 15).
Construction of M. tb Rv2181 Knockout Mutant (H37RvΔRv2181) by Allelic Exchange and Growth Phenotype.
Attempts to generate a knockout mutant of Rv2181 in M. tb by homologous recombination using the thermosensitive vector pPR27 were successful. Analysis by Southern blot of candidate mutants confirmed gene replacement at the Rv2181 locus (data not shown). H37RvΔRv2181 grew normally in 7H9-OADC-Km broth and 7H11-OADC-Km plates at 37 °C indicating that Rv2181 is not required for optimal growth in vitro.
Structural Characterization of LM and ManLAM from M. tb with a Disrupted Copy of Rv2181.
Our earlier work demonstrated that the M. smg ΔMSMEG_4247 deficient in the expression of the ortholog of Rv2181 failed to synthesize LM as such and instead possessed an altered form of LAM different from that of the wild type (WT) strain (15). Thus we anticipated the absence of LM from H37RvΔRv2181. Surprisingly, SDS/PAGE demonstrated a product reduced in quantity and shorter in size (Fig. 1A). Also, the ManLAM from this mutant migrated faster than WT ManLAM, indicative of a smaller size. WT ManLAM and the product from the mutant both reacted to Con A (Fig. 1B) and CS-35 (Fig. 1C), suggesting that, other than the size, some aspects of the arabinan and mannan components of the structure are unaffected. Quantitative analysis of the alditol acetate derivatives of LM from WT M. tb demonstrated a Man:inositol ratio of 21:1 compared with 11:1 for LM from H37RvΔRv2181. Likewise, similar glycosyl compositional analysis of ManLAM from H37RvΔRv2181 showed Ara:Man:inositol in a ratio of 29:25:1, in comparison to 32:35:1 in the ManLAM from WT H37Rv. These results suggest that disruption of Rv2181 did not appreciably affect the assembly of arabinan in ManLAM but drastically reduced the Man content in both LM and ManLAM.
Fig. 1.
SDS/PAGE analysis of LM and ManLAM from M. tuberculosis H37Rv (WT) and H37RvΔRv2181 (Δ). Similar amounts of LM and ManLAM were extracted from cells of WT (lanes 1) and H37RvΔRv2181 (lanes 2), separated on a 10–20% Tricine gel, and visualized by periodic acid/Schiff reagent staining (A) and Western blot with lectin Con A (B) and monoclonal antibody CS-35 (C). Positions of molecular mass markers (kDa) are indicated by arrows.
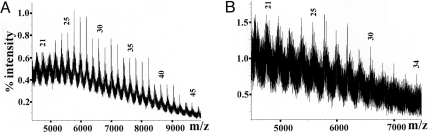
The MALDI-TOF/MS spectrum of LM from H37RvΔRv2181 in the positive mode was dominated by a set of peaks assigned to the mannan polymer as consisting of Manp21–34 units in length (Fig. 2B). For comparison, it was necessary to determine precisely the degree of mannosylation of the LM synthesized by WT M. tb, previously not accomplishable with precision (19). Permethylated WT LM, devoid of the heterogeneity associated with variable acyl attachments through deacylation, showed MALDI-TOF/MS spectra with a broad cluster of peaks centered on m/z 5985 (Fig. 2A). Within the overall cluster, individual peaks were each separated by ≈204 mass units, indicative of a mannan core varying in Manp residues from Man21–50. The most abundant species fell within m/z 5,781–6,393, corresponding to Man26–29. These results suggest that synthesis of the mannan segments up to the Man34 stage is independent of Rv2181. Only the steps beyond this stage, amounting to an additional Man12–15 residues, are affected by the mutation. Similar MALDI-TOF/MS analysis in the negative mode of ManLAM from WT M. tb showed a broad MW peak centered at ≈15.92 kDa, indicative of the heterogeneity of the molecule (Fig. S2A). ManLAM isolated from H37RvΔRv2181 also showed a broad peak at an average molecular mass of 12.47 kDa, again indicative of a smaller size (Fig. S2B). Apparently, the ManLAM from the mutant strain is a truncated form of WT ManLAM, incomplete in some respects within the mannan core and/or Manp capping motifs.
Fig. 2.
MALDI-TOF/MS analysis of purified LM from M. tuberculosis H37Rv and H37RvΔRv2181. Shown is positive mode MS analysis of permethylated WT LM (A) and mutant LM (B). The arabic numerals represent the number of Manp residues.
Per-O-methylation analysis of LM from the mutant as compared with WT demonstrated a preponderance of 6-linked Manp residues and the absence of 2,6-linked with few terminal t-Manp, suggestive of a α(1→6) Manp linear mannan polymer lacking α(1→2) Manp branching (Fig. 3 A and B). The glycosyl linkage pattern of ManLAM from the mutant showed t-Araf, 5-linked Araf, and 3,5-linked Araf similar to those of WT ManLAM, indicative of little or no alteration in the nature of the arabinan component (Fig. 3 C and D). There was an increase in the proportion of 6-linked Manp, no evident 2-linked and 2,6-linked Manp, and a decrease in t-Manp residues; t-Manp can be attributed to the Manp linked to the 2-position of inositol, to the t-Manp at the nonreducing end of the mannan core, and to Manp caps.
Fig. 3.
Glycosyl linkage analysis of per-O-methylated LM and ManLAM prepared from M. tuberculosis LM (A), H37RvΔRv2181 LM (B), M. tuberculosis ManLAM (C), and H37RvΔRv2181 ManLAM (D). Samples of purified LM and ManLAM were prepared as described in Materials and Methods, per-O-methylated, hydrolyzed, and reduced, and the per-O-acetylated glycosyl derivatives were analyzed by GC/MS.
Purified LM and ManLAM samples were also analyzed by 2-dimensional 1H-13C HSQC experiments, and resonances for anomeric regions were assigned by referring to a body of published NMR data on the structures of LM and LAM (8, 20, 21). The anomeric proton resonance region of LM from WT M. tb was dominated by signals assignable to 2,6-Manp, t-Manp, and 6-Manp, respectively (Fig. S3A). However, two of the signals attributed to t-Manp and 2,6-linked Manp of the LM are missing in the H37RvΔRv2181 LM (Fig. S3B). In the case of H37RvΔRv2181 ManLAM, 2D NMR analysis corroborated linkage analysis in that the overall arabinosylation pattern was retained; however, there is the virtual absence of signals attributed to 2,6-Manp + 2-Manp and t-Manp(core+cap) in the mutant ManLAM (Fig. S3C). Conversely, the volume of the cross-peaks for 6-Manp generated from the ManLAM of H37RvΔRv2181 increased 56-fold relative to those of WT ManLAM (Fig. S3 C and D). Apparently, disruption of Rv2181 results in the virtual absence of the branched Manp units normally attached to C-2 of the α(1→6) mannan backbone of LM and ManLAM and also to those normally attached to C2 of the first Manp unit of the Man caps.
ManLAM from the WT and mutant strains were also digested with endoarabinanase from Cellulomonas gelida and analyzed by MALDI-TOF/MS (Fig. 4 A and B). The MS profile of the digested WT ManLAM was dominated by di-Manp units as demonstrated by the prominent Man2Ara4 (peracetylated [M+Na]+ m/z = 1,565) and Man4Ara6 (peracetylated [M+Na]+ m/z = 2,573) peaks, a result consistent with previously published data for M. tb ManLAM (21, 22). However, the profile of the digested product from the mutant closely resembled that of a LAM in which the Ara4 and Ara6 motifs are substituted with a single Manp residue on each terminal β-Araf (peracetylated [M+Na]+ m/z values of 1,277 and 1,997, respectively) (Fig. 4B). Altogether, these results demonstrate that the α(1→2) ManT Rv2181 is a dual function ManT responsible for the deposition of the second α(1→2) Manp capping residues of ManLAM in addition to α(1→2) branches on the mannan cores of the LM and ManLAM.
Fig. 4.
MALDI-TOF/MS analysis of the endoarabinanase digestion products of ManLAM. Shown are ManLAM from M. tuberculosis H37Rv (A) and H37RvΔRv2181 (B). Samples were digested with endoarabinanase from C. gelida for 16 h at 37 °C, peracetylated, and analyzed by MALDI-TOF/MS to verify the identification of the peaks. The molecular ions were detected as [M + Na]+ and [M + K]+. All spectra contained a prominent Ara2 peak at the lower end of the spectrum; i.e., below 800 m/z (not shown). M, mannose; A, arabinose.
Construction and Analysis of three Recombinant Strains of M. smg ΔMSMEG_4247 Expressing Rv2181 and Rv1635c and Coexpressing Rv2181 and Rv1635c.
To further study the function of Rv2181, we sought to coexpress this gene in M. smg, which is normally devoid of Man-capped LAM, in conjunction with Rv1635c. The mutant M. smg ΔMSMEG_4247 was chosen as host strain because it is devoid of the Rv2181 ortholog (15). It was transformed with an empty expression plasmid, pVV16, or pVV-Rv2181, or pVV-Rv1635c and pVV-Rv2181-Rv1635c. The recombinant strain expressing both Rv2181 and Rv1635c demonstrated much smaller colonies and diminished growth compared with those expressing the individual genes; apparently coexpression of these two integral membrane proteins is partially toxic for the cells.
Analysis of LM and LAM from the 3 recombinant strains showed, as expected, the failure of ΔMSMEG_4247/pVV16 to synthesize LM, evident by lack of response to the PAS stain and Con A (Fig. S4 A and B). The strain complemented with Rv2181 restored the synthesis of LM. LAM of WT M. smg does not react with Con A because of the absence of Man caps. However, LAM from ΔMSMEG_4247 transformed with Rv1635c did react with Con A, indicating that the expression of this gene results in the addition of the first Manp of the capping unit. Examination of strain ΔMSMEG_4247/pVV-Rv2181-Rv1635c showed the characteristic M. tb H37Rv WT reactivity of both LM and LAM to Con A (Fig. S4B). Endoarabinanase digestion and MS analysis of the LAM populations produced by the different recombinant strains revealed that those from ΔMSMEG_4247/pVV16 (Fig. 5A) and ΔMSMEG_4247/pVV-Rv2181 (Fig. 5B) yielded 3 clusters of Aran signals centered at Ara2, Ara4, and Ara6. It is obvious from the signal intensity that Ara4 and Ara6 constitute the major digestion products in LAM from both strains. In contrast, the molecular ion signals afforded by LAM from ΔMSMEG_4247/pVV-Rv1635c (Fig. 5C) attributable to Man1Ara4 and Man2Ara6 are significantly higher in intensity in comparison with Ara4 and Ara6. Importantly, in LAM isolated from ΔMSMEG_4247/pVV-Rv2181-Rv1635c, the signal for Man2Ara6 decreased and new signals attributed to Man4Ara6, Man5Ara6, and Man6Ara6 appeared (Fig. 5D). It is apparent that the MS profile of the digestion products of this LAM is similar to those of WT M. tb demonstrating that the production of di-Manp caps depends on the presence of the α(1→2) ManT encoded by Rv2181.
Fig. 5.
MALDI-TOF/MS profile of the peracetyl derivatives of Cellulomonas arabinanase digested products of LAM variants. Shown are positive mode MS spectra of arabinanase digested and peracetylated products of LAM isolated from ΔMSMEG_4247/pVV16 (A), ΔMSMEG_4247/pVV-Rv2181 (B), ΔMSMEG_4247/pVV-Rv1635c (C), and ΔMSMEG_4247/pVV-Rv2181-Rv1635c (D).
These results were confirmed by methylation analysis of LM and LAM of the recombinant strains, demonstrating the presence of those 2,6-linked, 2-Manp (specific for M. tb ManLAM), and associated t-Manp. These findings are consistent with the body of evidence that the new product produced by ΔMSMEG_4247/pVV-Rv2181-Rv1635c has the same Manp capping motif and core branching as ManLAM from M. tb. These results clearly establish that Rv2181 catalyzes the addition of α(1→2) branching Manp residues to the mannan core of LM and LAM and also the second Manp capping residue of the nonreducing terminal Araf motif, resulting in the formation of a branched LM and LAM and di- and tri-Manp capped LAM in M. smg.
Discussion
Current knowledge on the structure of LAM has resulted primarily from detailed studies on a few selected laboratory strains of M. tb, Mycobacterium bovis bacillus Calmette–Guérin, and M. smg (4, 5). Considerable effort has been invested in correlating particular structural features with aspects of the immunopathogenesis of tuberculosis (23). An outcome of these efforts is the consensus that the mannose-containing caps of ManLAM constitute the single most important structural entity engaged in phagocytosis by macrophages and subsequent events such as inhibition of phagosome/lysosome fusion and immunomodulation. However, all of these studies were conducted with the purified molecule. Isogenic M. tb mutants devoid of Man-capped LAM are crucial to fully resolve the role of Man capping in disease pathogenesis. Indeed a promising start has been made in this direction but with M. bovis bacillus Calmette–Guérin mutants deficient in Man caps (24). This latter study revealed only marginal differences between WT and capless mutant strains in accord with the highly attenuated nature of M. bovis bacillus Calmette–Guérin.
Until recently, little was known of the enzymes involved in LM/LAM synthesis. Through genetic and biochemical studies, the membranous GT-C superfamily members relying on polyprenyl-linked sugar donors have emerged as the GTs responsible for the ultimate stages of their synthesis (14, 15, 17). The full genetics and enzymology of the Man capping events have remained undefined. Previously, availing of the fact that LAM from the saprophytic M. smg is devoid of Man caps we used a form of subtractive genomics to identify the GT-C enzyme MT1671/Rv1635c as responsible for the addition of the first Manp of the ManLAM caps. However, extension of this strategy failed to identify those enzymes responsible for further elongation, to the extent of di- and tri-saccharides. Of course, the α(1→2)-linkages of the di- and tri-Manp units on the nonreducing ends of ManLAM, namely the terminal mono- and di-Manp units of PIM5 and PIM6, the α(1→2) Manp branching residues of the mannan core of LM/LAM (Fig. S1), and the di- and tri-Manp units of the 45- to 47-kDa glycoprotein of Mycobacterium spp., are all identical. Moreover, the enzymes catalyzing the addition of these α(1→2)-linkages use lipid-linked sugar donors, belong to the GT-C superfamily, and catalyze the reactions on the extracytoplasmic side of the plasma membrane (13, 15, 25). Yet these ManTs have inherent specific features as evidenced by the fact that the branching of the mannan core of LM and LAM was unaffected by disruption of pimE in M. smg, and, conversely, PIM6 was synthesized at comparable levels in WT and mutant cells of M. smg MSMEG_4247 deficient in the Rv2181 equivalent (15). The structural basis for this specificity remains to be elucidated.
A prima facie case was made in the present study for the involvement of Rv2181 in α(1→2) Manp capping of ManLAM, and on this basis the mutant H37RvΔRv2181 was generated by allelic exchange. It accumulated two novel lipid-linked intermediates considerably shorter in size than the WT LM and ManLAM (Fig. 1). Linkage analysis and NMR, in particular, help demonstrate the complete loss of α(1→2) Manp branching residues from the mannan core of LM (Fig. 3 and Fig. S3). Moreover, there was the virtual absence of α(1→2) Manp branching of the mannan core and di- and tri-Manp caps of ManLAM, and the known arabinan motifs and the first Manp residue of the Man caps remained largely unaffected (Figs. 3D and 4B). From these results, it can be concluded that the primary lesion in M. tb ΔRv2181 resides in the arrest of branching of the mannan core and the Man-capping aspects of the LM/ManLAM biosynthetic pathway.
The exploitation of the “LM-less” phenotype of the mutant ΔMSMEG_4247, along with the use of a plasmid coexpressing Rv2181 and Rv1635c in this mutant, provided us with the opportunity to examine for the first time the formation of a complete “M. tb type ManLAM” in M. smg. It is intriguing that the expression of Rv1635c in M. smg (17) yielded a LAM population that had t-Araf residues capped only with a single Manp, despite M. smg expressing an ortholog of Rv2181 (MSMEG_4247), whereas both MSMEG_4247 and Rv2181 are still effective in mannan core branching (15). Yet only the expression of Rv2181 resulted in the addition of the second and perhaps third Manp capping residues. It is tempting to speculate that Rv2181 carries an additional recognition motif allowing the synthesis of Man capping of ManLAM. Clearly, further delineation of the structure–function aspects of Rv2181 is required to understand its role in the biosynthesis of LM and ManLAM.
In conclusion, the H37RvΔRv2181 mutant allowed the assignment of a new key function to Rv2181, which would have otherwise not been possible if similar studies had been conducted solely on M. smg. Altogether, these data indicate that M. tb Rv2181 endows dual α(1→2) ManT activities, adding α(1→2)-linked Manp residues to the mannan core of LM and LAM as well as addition of the second Manp cap to the Man capping motifs. It is also likely from the evidence that Rv2181 also mediates the addition of the third Manp in Man capping, which represents the final step of Man capping of ManLAM. Furthermore, the recombinant strains generated in the course of this study, whether H37RvΔRv2181 or the M. smg-synthesizing mono or di- and tri-Man-capped LAM, will be useful in studying the biological significance of varying degrees of Man capping in phagocytosis and other immunomodulatory events.
Materials and Methods
Culture Conditions.
M. smg mc2155 and M. tb H37Rv were grown in 7H9 medium as described (17). H37RvΔRv2181 was cultured in 7H9 with kanamycin (50 μg/mL), and M. smg mc2155 ΔMSMEG_4247/pVV16 (previously annotated as MSMEG4250) (15), ΔMSMEG_4247/pVV-Rv2181, ΔMSMEG_4247/pVV-Rv1635c, and ΔMSMEG_4247/pVV-Rv2181-Rv1635c were grown in the presence of kanamycin (Km) (50 μg/mL) and hygromycin (Hyg) (50 μg/mL). All bacterial strains were harvested at late log phase.
Construction of H37RvΔRv2181.
M. tb Rv2181 and its flanking regions were amplified by PCR with the forward 5′-TATAATCTAGAACAGCTTGATCCGCACC-3′ and reverse 5′-TATAATCTAGAGGTTCGAGGGGTTGGCC-3′ primers; XbaI sites are underlined. The 2.3-kb PCR fragment was subcloned into pUC19 (Invitrogen). pUC19-Rv2181 was digested with BspDI and NcoI resulting in the deletion of ≈429 bp of the Rv2181 coding sequence, blunt-ended with T4 DNA polymerase and ligated with a kanamycin resistance (Kmr) gene excised from pUC4K (Amersham Biosciences) by HincII digestion. A 3.1-kb fragment of Rv2181::Km was then excised from the resulting plasmid with XbaI and inserted into the XbaI site of the temperature-sensitive pPR27xylE (26), yielding pPR27xylERv2181::Km, the plasmid used for allelic replacement in M. tb H37Rv. Allelic replacement at the Rv2181 locus was confirmed by Southern hybridization. The blots were hybridized with a 2.3-kb 32P-labeled DNA probe generated by digestion of the PCR fragment subcloned in the pUC19 vector, as described above.
Coexpression of Rv2181 and Rv1635c in M. smg ΔMSMEG_4247.
To construct the expression plasmid pVV-Rv2181-Rv1635c coexpressing Rv2181 and Rv1635c, pVV-Rv2181 (15) was digested with SpeI, dephosphorylated with calf alkaline phosphatase, and ligated with the Rv1635c gene excised from pVV16-Rv1635c (17) by XbaI and NheI digestion. The resulting plasmid, pVV-Rv2181-Rv1635c, was transformed into the ΔMSMEG_4247 mutant strain, and transformants were selected on plates containing Km and Hyg at 37 °C.
Analysis of PIMs, LM, and LAM.
Cells were extracted with CHCl3/CH3OH (2:1) and CHCl3/CH3OH/H2O (10:10:3) (15). The residue was further extracted with equal volumes of water and PBS-saturated phenol at 80 °C for 2 h. The aqueous layer on cooling (containing ManLAM, LM, and PIMs) was dialyzed and analyzed by SDS/PAGE followed by periodic acid/Schiff (PAS) staining (27) on commercial 10–20% gradient Tricine SDS/polyacrylamide gels (Invitrogen). Blotting to nitrocellulose membranes was performed at 50 V for 1 h. Immunodetection was performed with monoclonal antibody CS-35, specific for the arabinan component of LAM (28) and Con A, which recognizes terminal Manp residues. The nitrocellulose membranes probed with Con A-peroxidase were subsequently developed with the 4-chloro-1-naphthol/3,3′-diaminobenzidine, tetrahydrochloride (CN/DAB) substrate kit (Pierce).
To obtain sufficient quantities of LM and ManLAM for structural analysis, the cell pellets from 5-L cultures of M. tb were delipidated by serial extractions of CHCl3/CH3OH (2:1) and CHCl3/CH3OH/H2O (10:10:3) followed by Triton X-114 extractions, ethanol precipitation, and pronase digestion, and LM and ManLAM were resolved on a sephacryl S-200 in tandem with S-100 column as described (22). Fractions containing the LM and LAM variants were monitored on Tricine gels by staining with the PAS reagent, pooled, and dialyzed.
Analytical Procedures.
To analyze monosaccharide composition, LM and LAM samples were hydrolyzed with 2 M trifluoroacetic acid, converted to alditol acetates, and analyzed by GC using scyllo-inositol as an internal standard. To determine linkage patterns, samples were per-O-methylated (29), and alditol acetates prepared as described (30), solubilized in CHCl3, and analyzed by GC/MS (Thermoquest GCQ Plus; Thermo Electron). For MALDI-TOF/MS, the matrix consisted of 2,5-dihydroxybenzoic acid at a concentration of 10 mg/mL in a mixture of water/acetonitrile (1:1, vol/vol) containing 0.1% TFA and 1.0 μg of sample mixed with 1.0 μl of the matrix solution. Analyses were performed on a Bruker Ultraflex MALDI-TOF/TOF instrument using reflector mode detection. Mass spectra were recorded in the negative mode for the underivatized material and positive mode for the permethylated and acetylated sample using a 30-ns time delay with a grid voltage of 94% and full accelerating voltage (25 kV). The mass spectra were mass assigned by using external calibration. NMR spectra were acquired after several lyophilizations in D2O of 1–4 mg/0.6 mL in 100% D2O. Two-dimensional 1H-13C-HSQC (heteronuclear single quantum correlation spectroscopy) spectra were acquired on a Varian Inova 500-MHz NMR spectrometer by using the supplied Varian pulse sequences.
Endoarabinanase Digestion of LAM Variants.
LAM (20 μg) was incubated for 16 h at 37 °C with an endoarabinanase isolated from C. gelida (4, 31). An aliquot of the digestion mixtures containing both the mannan core and the released oligosaccharides was analyzed by SDS/PAGE followed by PAS staining. The remaining mixtures were peracetylated and analyzed by MALDI-TOF/MS (17).
Supplementary Material
Acknowledgments.
We thank Christopher D. Rithner for the NMR, Jessica Prenni for MALDI-TOF/MS analysis, Anita Amin for endoarabinanase preparation, and Henrieta Skovierova for helpful discussions. This work was supported by National Institute of Allergy and Infectious Diseases/National Institutes of Health Grant AI-064798 and AI-018357.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807761105/DCSupplemental.
References
- 1.Brennan P, Ballou CE. Biosynthesis of mannophosphoinositides by Mycobacterium phlei. The family of dimannophosphoinositides. J Biol Chem. 1967;242:3046–3056. [PubMed] [Google Scholar]
- 2.Maitra SK, Ballou CE. Heterogeneity and refined structures of 3-O-methyl-D-mannose polysaccharides from Mycobacterium smegmatis. J Biol Chem. 1977;252:2459–2469. [PubMed] [Google Scholar]
- 3.Dobos KM, Khoo KH, Swiderek KM, Brennan PJ, Belisle JT. Definition of the full extent of glycosylation of the 45-kilodalton glycoprotein of Mycobacterium tuberculosis. J Bacteriol. 1996;178:2498–2506. doi: 10.1128/jb.178.9.2498-2506.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterjee D, Khoo KH. Mycobacterial lipoarabinomannan: an extraordinary lipoheteroglycan with profound physiological effects. Glycobiology. 1998;8:113–120. doi: 10.1093/glycob/8.2.113. [DOI] [PubMed] [Google Scholar]
- 5.Nigou J, Gilleron M, Puzo G. Lipoarabinomannans: From structure to biosynthesis. Biochimie. 2003;85:153–166. doi: 10.1016/s0300-9084(03)00048-8. [DOI] [PubMed] [Google Scholar]
- 6.Briken V, Porcelli SA, Besra GS, Kremer L. Mycobacterial lipoarabinomannan and related lipoglycans: From biogenesis to modulation of the immune response. Mol Microbiol. 2004;53:391–403. doi: 10.1111/j.1365-2958.2004.04183.x. [DOI] [PubMed] [Google Scholar]
- 7.Khoo KH, Dell A, Morris HR, Brennan PJ, Chatterjee D. Inositol phosphate capping of the nonreducing termini of lipoarabinomannan from rapidly growing strains of Mycobacterium. J Biol Chem. 1995;270:12380–12389. doi: 10.1074/jbc.270.21.12380. [DOI] [PubMed] [Google Scholar]
- 8.Gilleron M, et al. Mycobacterium smegmatis phosphoinositols-glyceroarabinomannans. Structure and localization of alkali-labile and alkali-stable phosphoinositides. J Biol Chem. 1997;272:117–124. doi: 10.1074/jbc.272.1.117. [DOI] [PubMed] [Google Scholar]
- 9.Berg S, et al. Roles of conserved proline and glycosyltransferase motifs of EmbC in biosynthesis of lipoarabinomannan. J Biol Chem. 2005;280:5651–5663. doi: 10.1074/jbc.M411418200. [DOI] [PubMed] [Google Scholar]
- 10.Kordulakova J, et al. Definition of the first mannosylation step in phosphatidylinositol mannoside synthesis. PimA is essential for growth of mycobacteria. J Biol Chem. 2002;277:31335–31344. doi: 10.1074/jbc.M204060200. [DOI] [PubMed] [Google Scholar]
- 11.Schaeffer ML, et al. The pimB gene of Mycobacterium tuberculosis encodes a mannosyltransferase involved in lipoarabinomannan biosynthesis. J Biol Chem. 1999;274:31625–31631. doi: 10.1074/jbc.274.44.31625. [DOI] [PubMed] [Google Scholar]
- 12.Kremer L, et al. Characterization of a putative alpha-mannosyltransferase involved in phosphatidylinositol trimannoside biosynthesis in Mycobacterium tuberculosis. Biochem J. 2002;363:437–447. doi: 10.1042/0264-6021:3630437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morita YS, et al. PimE is a polyprenol-phosphate-mannose-dependent mannosyltransferase that transfers the fifth mannose of phosphatidylinositol mannoside in mycobacteria. J Biol Chem. 2006;281:25143–25155. doi: 10.1074/jbc.M604214200. [DOI] [PubMed] [Google Scholar]
- 14.Kaur D, et al. New insights into the biosynthesis of mycobacterial lipomannan arising from deletion of a conserved gene. J Biol Chem. 2007;282:27133–27140. doi: 10.1074/jbc.M703389200. [DOI] [PubMed] [Google Scholar]
- 15.Kaur D, et al. Biosynthesis of mycobacterial lipoarabinomannan: Role of a branching mannosyltransferase. Proc Natl Acad Sci USA. 2006;103:13664–13669. doi: 10.1073/pnas.0603049103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang N, et al. The Emb proteins of mycobacteria direct arabinosylation of lipoarabinomannan and arabinogalactan via an N-terminal recognition region and a C-terminal synthetic region. Mol Microbiol. 2003;50:69–76. doi: 10.1046/j.1365-2958.2003.03681.x. [DOI] [PubMed] [Google Scholar]
- 17.Dinadayala P, et al. Genetic basis for the synthesis of the immunomodulatory mannose caps of lipoarabinomannan in Mycobacterium tuberculosis. J Biol Chem. 2006;281:20027–20035. doi: 10.1074/jbc.M603395200. [DOI] [PubMed] [Google Scholar]
- 18.Berg S, Kaur D, Jackson M, Brennan PJ. The glycosyltransferases of Mycobacterium tuberculosis—Roles in the synthesis of arabinogalactan, lipoarabinomannan, and other glycoconjugates. Glycobiology. 2007;17:35–56. doi: 10.1093/glycob/cwm010. [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee D, Hunter SW, McNeil M, Brennan PJ. Lipoarabinomannan. Multiglycosylated form of the mycobacterial mannosylphosphatidylinositols. J Biol Chem. 1992;267:6228–6233. [PubMed] [Google Scholar]
- 20.Nigou J, et al. The phosphatidyl-myo-inositol anchor of the lipoarabinomannans from Mycobacterium bovis bacillus Calmette Guerin. Heterogeneity, structure, and role in the regulation of cytokine secretion. J Biol Chem. 1997;272:23094–23103. doi: 10.1074/jbc.272.37.23094. [DOI] [PubMed] [Google Scholar]
- 21.Torrelles JB, et al. Truncated structural variants of lipoarabinomannan in Mycobacterium leprae and an ethambutol-resistant strain of Mycobacterium tuberculosis. J Biol Chem. 2004;279:41227–41239. doi: 10.1074/jbc.M405180200. [DOI] [PubMed] [Google Scholar]
- 22.Khoo KH, Tang JB, Chatterjee D. Variation in mannose-capped terminal arabinan motifs of lipoarabinomannans from clinical isolates of Mycobacterium tuberculosis and Mycobacterium avium complex. J Biol Chem. 2001;276:3863–3871. doi: 10.1074/jbc.M004010200. [DOI] [PubMed] [Google Scholar]
- 23.Nigou J, et al. Mycobacterial lipoarabinomannans: Modulators of dendritic cell function and the apoptotic response. Microbes Infect. 2002;4:945–953. doi: 10.1016/s1286-4579(02)01621-0. [DOI] [PubMed] [Google Scholar]
- 24.Appelmelk BJ, et al. The mannose cap of mycobacterial lipoarabinomannan does not dominate the Mycobacterium-host interaction. Cell Microbiol. 2008;10:930–944. doi: 10.1111/j.1462-5822.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- 25.VanderVen BC, Harder JD, Crick DC, Belisle JT. Export-mediated assembly of mycobacterial glycoproteins parallels eukaryotic pathways. Science. 2005;309:941–943. doi: 10.1126/science.1114347. [DOI] [PubMed] [Google Scholar]
- 26.Pelicic V, et al. Efficient allelic exchange and transposon mutagenesis in Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1997;94:10955–10960. doi: 10.1073/pnas.94.20.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prinzis S, Chatterjee D, Brennan PJ. Structure and antigenicity of lipoarabinomannan from Mycobacterium bovis BCG. J Gen Microbiol. 1993;139:2649–2658. doi: 10.1099/00221287-139-11-2649. [DOI] [PubMed] [Google Scholar]
- 28.Kaur D, Lowary TL, Vissa VD, Crick DC, Brennan PJ. Characterization of the epitope of anti-lipoarabinomannan antibodies as the terminal hexaarabinofuranosyl motif of mycobacterial arabinans. Microbiology. 2002;148:3049–3057. doi: 10.1099/00221287-148-10-3049. [DOI] [PubMed] [Google Scholar]
- 29.Dell A, et al. Mass spectrometry of carbohydrate-containing biopolymers. Methods Enzymol. 1994;230:108–132. doi: 10.1016/0076-6879(94)30010-0. [DOI] [PubMed] [Google Scholar]
- 30.McNeil M, Chatterjee D, Hunter SW, Brennan PJ. Mycobacterial glycolipids: Isolation, structures, antigenicity, and synthesis of neoantigens. Methods Enzymol. 1989;179:215–242. doi: 10.1016/0076-6879(89)79123-0. [DOI] [PubMed] [Google Scholar]
- 31.McNeil MR, Robuck KG, Harter M, Brennan PJ. Enzymatic evidence for the presence of a critical terminal hexa-arabinoside in the cell walls of Mycobacterium tuberculosis. Glycobiology. 1994;4:165–173. doi: 10.1093/glycob/4.2.165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.